Abstract
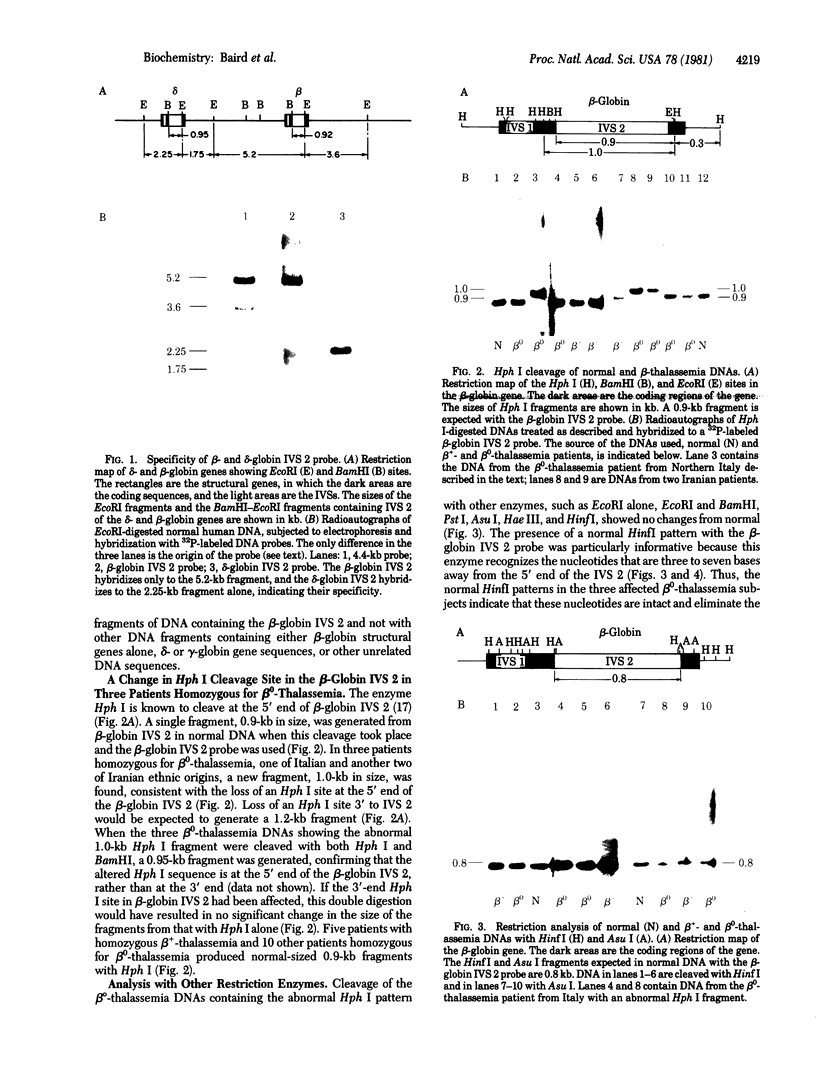
beta 0-Thalassemia is a heterogeneous group of disorders associated with absence of beta-globin. In a survey of DNAs from patients with beta 0-thalassemia of diverse ethnic origins, a change at the splice junction at the 5' end of the large intervening sequence (IVS 2) of the human beta-globin gene has been found in one patient of Italian and another two of Iranian ethnic origins. The enzyme Hph I recognizes a change at this site and generates a large-than-normal fragment of DNA, which hybridizes specifically to a beta-globin IVS 2 probe. No other changes in beta-globin gene DNA structure or organization are detectable by extensive restriction endonuclease analysis. The enzyme HinfI which recognizes a sequence beginning three nucleotides from the 5' end of the IVS 2 splice junction, produces normal fragments and localizes the defect to a G-G-T sequence at the 5'-end IVS 2 splice junction. This sequence is known to be remarkably conserved in all globin genes from many species and in most other genes examined to date. Thus, in at least some beta 0-thalassemia patients, the beta 0-thalassemia defect is associated with a nucleotide change at a splice junction. These patients provide unique examples of naturally occurring defects in splice junctions of eukaryotic genes associated with absence of specific gene function.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bank A., Mears J. G., Ramirez F. Disorders of human hemoglobin. Science. 1980 Feb 1;207(4430):486–493. doi: 10.1126/science.7352255. [DOI] [PubMed] [Google Scholar]
- Bank A. The thalassemia syndromes. Blood. 1978 Mar;51(3):369–384. [PubMed] [Google Scholar]
- Benz E. J., Forget B. G., Hillman D. G., Cohen-Solal M., Pritchard J., Cavallesco C., Prensky W., Housman D. Variability in the amount of beta-globin mRNA in beta0 thalassemia. Cell. 1978 Jun;14(2):299–312. doi: 10.1016/0092-8674(78)90116-2. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns A. L., Spence S., Kosche K., Ramirez F., Mears J. G., Schreiner H., Miller C., Baird M., Leibowitz D., Giardina P. Isolation and characterization of cloned DNA: the delta and beta globin genes in homozygous beta + thalassemia. Blood. 1981 Jan;57(1):140–146. [PubMed] [Google Scholar]
- Chang J. C., Kan Y. W. beta 0 thalassemia, a nonsense mutation in man. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2886–2889. doi: 10.1073/pnas.76.6.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Flavell R. A., Bernards R., Kooter J. M., de Boer E., Little P. F., Annison G., Williamson R. The structure of the human beta-globin gene in beta-thalassaemia. Nucleic Acids Res. 1979 Jun 25;6(8):2749–2760. doi: 10.1093/nar/6.8.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget B. G., Benz E. J., Jr, Skoultchi A., Baglioni C., Housman D. Absence of messenger RNA for beta globin chain in beta(0) thalassaemia. Nature. 1974 Feb 8;247(5440):379–381. doi: 10.1038/247379a0. [DOI] [PubMed] [Google Scholar]
- Gannon F., O'Hare K., Perrin F., LePennec J. P., Benoist C., Cochet M., Breathnach R., Royal A., Garapin A., Cami B. Organisation and sequences at the 5' end of a cloned complete ovalbumin gene. Nature. 1979 Mar 29;278(5703):428–434. doi: 10.1038/278428a0. [DOI] [PubMed] [Google Scholar]
- Hamer D. H., Leder P. Expression of the chromosomal mouse Beta maj-globin gene cloned in SV40. Nature. 1979 Sep 6;281(5726):35–40. doi: 10.1038/281035a0. [DOI] [PubMed] [Google Scholar]
- Hamer D. H., Leder P. Splicing and the formation of stable RNA. Cell. 1979 Dec;18(4):1299–1302. doi: 10.1016/0092-8674(79)90240-x. [DOI] [PubMed] [Google Scholar]
- Kacian D. L., Gambino R., Dow L. W., Grossbard E., Natta C., Ramirez F., Spiegelman S., Marks P. A., Bank A. Decreased globin messenger RNA in thalassemia detected by molecular hybridization. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1886–1890. doi: 10.1073/pnas.70.6.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan Y. W., Holland J. P., Dozy A. M., Varmus H. E. Demonstration of non-functional beta-globin mRNA in homozygous beta (0) thalassemia. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5140–5144. doi: 10.1073/pnas.72.12.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantor J. A., Turner P. H., Nienhuis A. W. Beta Thalassemia: mutations which affect processing of the beta-Globin mRNA precursor. Cell. 1980 Aug;21(1):149–157. doi: 10.1016/0092-8674(80)90122-1. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Efstratiadis A., O'Connell C., Maniatis T. The nucleotide sequence of the human beta-globin gene. Cell. 1980 Oct;21(3):647–651. doi: 10.1016/0092-8674(80)90428-6. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Leder P., Hansen J. N., Konkel D., Leder A., Nishioka Y., Talkington C. Mouse globin system: a functional and evolutionary analysis. Science. 1980 Sep 19;209(4463):1336–1342. doi: 10.1126/science.7414319. [DOI] [PubMed] [Google Scholar]
- Maquat L. E., Kinniburgh A. J., Beach L. R., Honig G. R., Lazerson J., Ershler W. B., Ross J. Processing of human beta-globin mRNA precursor to mRNA is defective in three patients with beta+-thalassemia. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4287–4291. doi: 10.1073/pnas.77.7.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears J. G., Ramirez F., Leibowitz D., Bank A. Organization of human delta--and beta-globin genes in cellular DNA and the presence of intragenic inserts. Cell. 1978 Sep;15(1):15–23. doi: 10.1016/0092-8674(78)90079-x. [DOI] [PubMed] [Google Scholar]
- Mears J. G., Ramirez F., Leibowitz D., Nakamura F., Bloom A., Konotey-Ahulu F., Bank A. Changes in restricted human cellular DNA fragments containing globin gene sequences in thalassemias and related disorders. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1222–1226. doi: 10.1073/pnas.75.3.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Howard B. H., Berg P. Synthesis of rabbit beta-globin in cultured monkey kidney cells following infection with a SV40 beta-globin recombinant genome. Nature. 1979 Jan 11;277(5692):108–114. doi: 10.1038/277108a0. [DOI] [PubMed] [Google Scholar]
- Nienhuis A. W., Turner P., Benz E. J., Jr Relative stability of alpha- and beta-globin messenger RNAs in homozygous beta+ thalassemia. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3960–3964. doi: 10.1073/pnas.74.9.3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old J. M., Proudfoot N. J., Wood W. G., Longley J. I., Clegg J. B., Weatherall D. J. Characterization of beta-globin mRNA in the beta0 thalassemias. Cell. 1978 Jun;14(2):289–298. doi: 10.1016/0092-8674(78)90115-0. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Old J. M., Weatherall D. J., Nathan D. G. Partial deletion of beta-globin gene DNA in certain patients with beta 0-thalassemia. Proc Natl Acad Sci U S A. 1979 May;76(5):2400–2404. doi: 10.1073/pnas.76.5.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolenghi S., Comi P., Giglioni B., Williamson R., Vullo G., Conconi F. Direct demonstration of beta-globin mRNA in homozygous Ferrara betaO-thalassaemia patients. Nature. 1977 Mar 17;266(5599):231–234. doi: 10.1038/266231a0. [DOI] [PubMed] [Google Scholar]
- Ramirez F., O'Donnell J. V., Marks P. A., Bank A., Musumeci S., Schilirò G., Pizzarelli G., Russo G., Luppis B., Gambino R. Abnormal or absent beta mRNA in betao Ferrara and gene deletion in delta beta thalassaemia. Nature. 1976 Oct 7;263(5577):471–475. doi: 10.1038/263471a0. [DOI] [PubMed] [Google Scholar]
- Spritz R. A., Jagadeeswaran P., Choudary P. V., Biro P. A., Elder J. T., deRiel J. K., Manley J. L., Gefter M. L., Forget B. G., Weissman S. M. Base substitution in an intervening sequence of a beta+-thalassemic human globin gene. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2455–2459. doi: 10.1073/pnas.78.4.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson T., Chua C. G., Carrell R. W., Robin H., Exner T., Lee K. M., Kronenberg H. Haemoglobin Camperdown beta104(G6) arginine leads to serine. Biochim Biophys Acta. 1975 May 30;393(1):195–200. doi: 10.1016/0005-2795(75)90231-7. [DOI] [PubMed] [Google Scholar]