Abstract
DNA from the am16 mutant of bacteriophage phi X174 may be replicated in vitro and expressed in vivo to give five classes of revertants. Each class may be specifically induced by the appropriate biasing of the concentrations of deoxynucleoside triphosphates in a predictable manner. The frequency of each reversion follows a kinetic rate equation relating it to the concentrations of the triphosphates involved in the substitution. The reversions corresponding to TAG leads to GAG, AAG, CAG, TGG, and TCG are calculated to occur with frequencies of 5 X 10(-7), 4 X 10(-7), 4 X 10(-7), approximately 2 X 10(-7), and approximately 5 X 10(-9), respectively, at the concentration of triphosphates found in vivo. The frequencies are in the range found for the reversion of the phage in vivo and so are consistent with errors in nucleotide selection by DNA polymerase (deoxynucleosidetriphosphate:DNA deoxynucleotidyltransferase, EC 2.7.7.7) III being largely responsible for the rate of spontaneous mutation in vivo. The relative frequency of mispairing leading to misincorporation is: purine.purine approximately purine.pyrimidine much greater than pyrimidine.pyrimidine, confirming predictions from model-building studies that transversions arise through purine.purine mismatches.
Full text
PDF

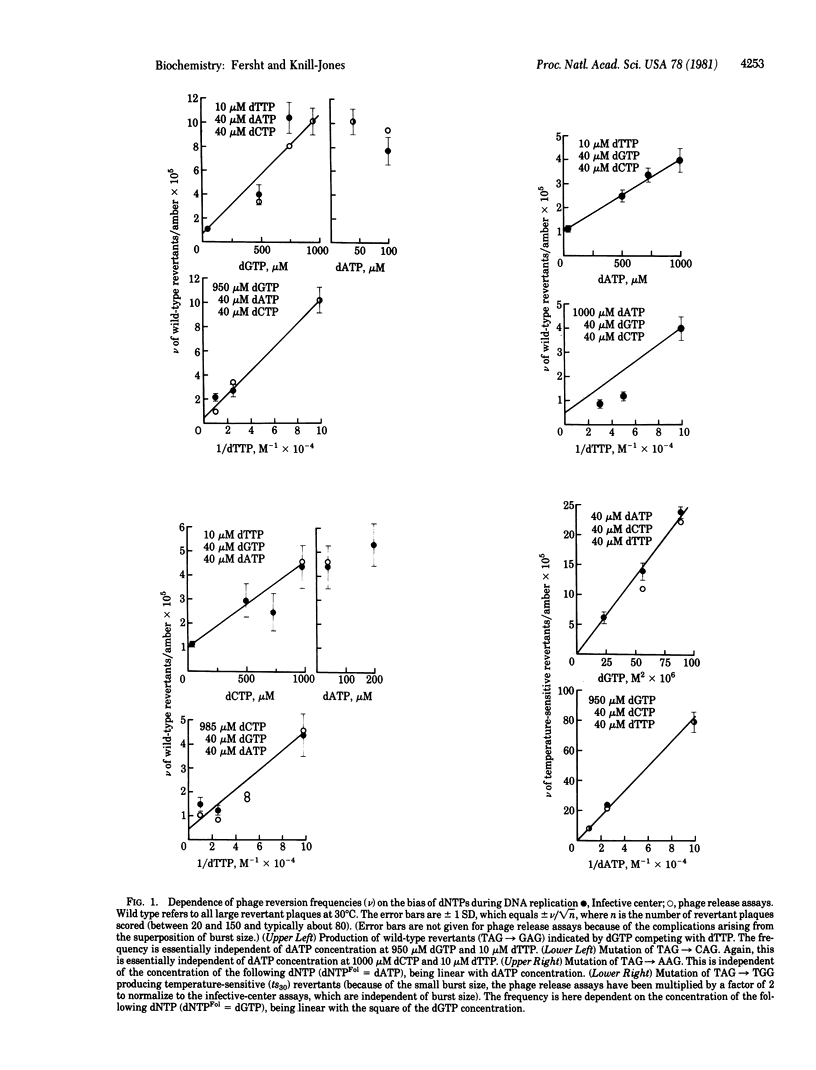
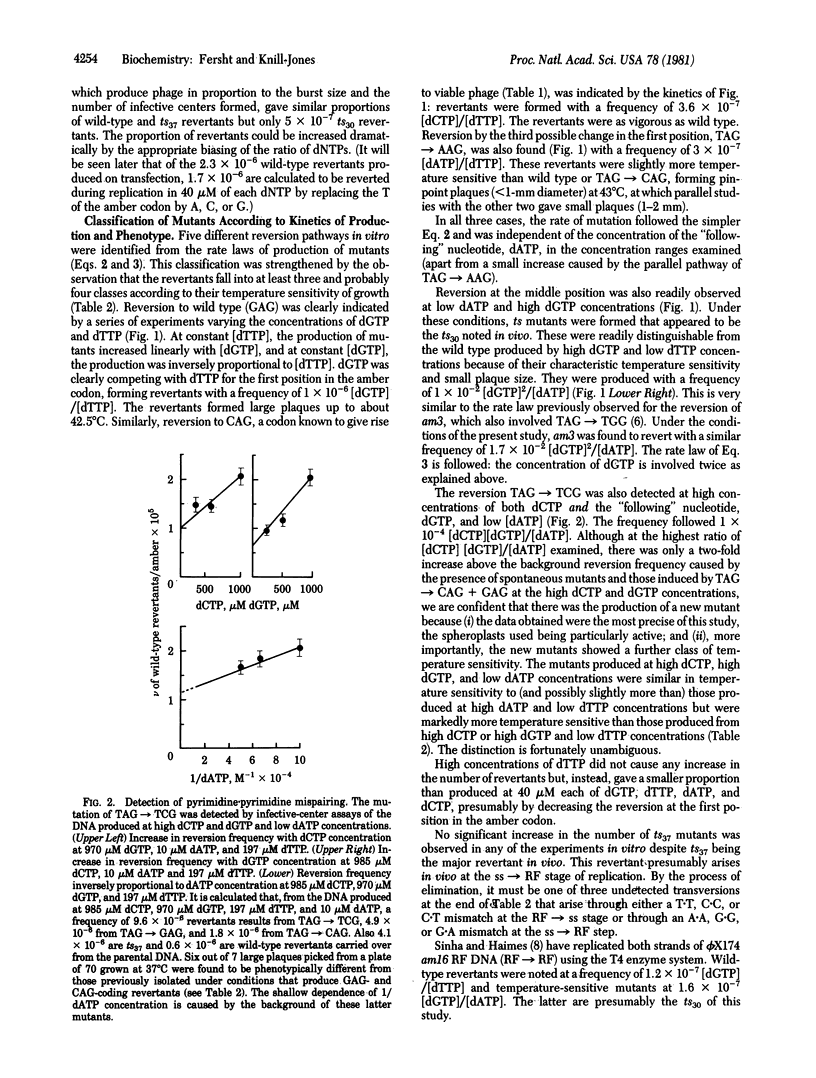

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benbow R. M., Hutchison C. A., Fabricant J. D., Sinsheimer R. L. Genetic Map of Bacteriophage phiX174. J Virol. 1971 May;7(5):549–558. doi: 10.1128/jvi.7.5.549-558.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. L., Smith M. The sequence of a region of bacteriophage phiX174 DNA coding for parts of genes A and B. J Mol Biol. 1977 Oct 15;116(1):1–28. doi: 10.1016/0022-2836(77)90115-2. [DOI] [PubMed] [Google Scholar]
- Cox E. C. Bacterial mutator genes and the control of spontaneous mutation. Annu Rev Genet. 1976;10:135–156. doi: 10.1146/annurev.ge.10.120176.001031. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T., Silver R. B. An analysis of the clone size distribution of phi-X-174 mutants and recombinants. Virology. 1966 Sep;30(1):10–19. doi: 10.1016/s0042-6822(66)81004-8. [DOI] [PubMed] [Google Scholar]
- Eisenberg S., Harbers B., Hours C., Denhardt D. T. The mechanism of replication of phiX174 DNA. XII. Non-random location of gaps in nascent phiX174 RF II DNA. J Mol Biol. 1975 Nov 25;99(1):107–123. doi: 10.1016/s0022-2836(75)80162-8. [DOI] [PubMed] [Google Scholar]
- Fersht A. R. Fidelity of replication of phage phi X174 DNA by DNA polymerase III holoenzyme: spontaneous mutation by misincorporation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4946–4950. doi: 10.1073/pnas.76.10.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler R. G., Degnen G. E., Cox E. C. Mutational specificity of a conditional Escherichia coli mutator, mutD5. Mol Gen Genet. 1974;133(3):179–191. doi: 10.1007/BF00267667. [DOI] [PubMed] [Google Scholar]
- Gillam S., Smith M. Site-specific mutagenesis using synthetic oligodeoxyribonucleotide primers: I. Optimum conditions and minimum ologodeoxyribonucleotide length. Gene. 1979 Dec;8(1):81–97. doi: 10.1016/0378-1119(79)90009-x. [DOI] [PubMed] [Google Scholar]
- Glickman B. W., Radman M. Escherichia coli mutator mutants deficient in methylation-instructed DNA mismatch correction. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1063–1067. doi: 10.1073/pnas.77.2.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulian M., Kornberg A., Sinsheimer R. L. Enzymatic synthesis of DNA, XXIV. Synthesis of infectious phage phi-X174 DNA. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2321–2328. doi: 10.1073/pnas.58.6.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibner U., Alberts B. M. Fidelity of DNA replication catalysed in vitro on a natural DNA template by the T4 bacteriophage multi-enzyme complex. Nature. 1980 May 29;285(5763):300–305. doi: 10.1038/285300a0. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Loeb L. A. On the fidelity of DNA replication. The accuracy of Escherichia coli DNA polymerase I in copying natural DNA in vitro. J Biol Chem. 1980 Oct 25;255(20):9961–9966. [PubMed] [Google Scholar]
- Liu C. C., Burke R. L., Hibner U., Barry J., Alberts B. Probing DNA replication mechanisms with the T4 bacteriophage in vitro system. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):469–487. doi: 10.1101/sqb.1979.043.01.053. [DOI] [PubMed] [Google Scholar]
- Mathews C. K. Biochemistry of deoxyribonucleic acid-defective amber mutants of bacteriophage T4. 3. Nucleotide pools. J Biol Chem. 1972 Nov 25;247(22):7430–7438. [PubMed] [Google Scholar]
- Munch-Petersen A. Deoxyribonucleoside catabolism and thymine incorporation in mutants of Escherichia coli lacking deoxyriboaldolase. Eur J Biochem. 1970 Jul;15(1):191–202. doi: 10.1111/j.1432-1033.1970.tb00994.x. [DOI] [PubMed] [Google Scholar]
- Neuhard J., Thomassen E. Altered deoxyribonucleotide pools in P2 eductants of Escherichia coli K-12 due to deletion of the dcd gene. J Bacteriol. 1976 May;126(2):999–1001. doi: 10.1128/jb.126.2.999-1001.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topal M. D., Fresco J. R. Complementary base pairing and the origin of substitution mutations. Nature. 1976 Sep 23;263(5575):285–289. doi: 10.1038/263285a0. [DOI] [PubMed] [Google Scholar]
- Weymouth L. A., Loeb L. A. Mutagenesis during in vitro DNA synthesis. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1924–1928. doi: 10.1073/pnas.75.4.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]


