Abstract
BACKGROUND
Although progenitor cells have been described in distinct anatomical regions of the lung, description of resident stem cells has remained elusive.
METHODS
Surgical lung-tissue specimens were studied in situ to identify and characterize human lung stem cells. We defined their phenotype and functional properties in vitro and in vivo.
RESULTS
Human lungs contain undifferentiated human lung stem cells nested in niches in the distal airways. These cells are self-renewing, clonogenic, and multipotent in vitro. After injection into damaged mouse lung in vivo, human lung stem cells form human bronchioles, alveoli, and pulmonary vessels integrated structurally and functionally with the damaged organ. The formation of a chimeric lung was confirmed by detection of human transcripts for epithelial and vascular genes. In addition, the self-renewal and long-term proliferation of human lung stem cells was shown in serial-transplantation assays.
CONCLUSIONS
Human lungs contain identifiable stem cells. In animal models, these cells participate in tissue homeostasis and regeneration. They have the undemonstrated potential to promote tissue restoration in patients with lung disease. (Funded by the National Institutes of Health.)
The fundamental properties of stem cells are self-renewal, clonogenicity, and multipotentiality. Although tissue-specific stem cells have been identified,1,2 they have not always been shown to exhibit stem-cell properties, particularly in the mammalian lung.3 Various cell populations with some of these features have been described in distinct anatomical regions of the lung,3–5 but the demonstration of a noncommitted cell without specialized functions remains elusive.
Basal epithelial cells, located in the trachea in mice and the distal airways in humans,5 express tumor protein 63 (p63) and cytokeratin 5 (CK5) and have been viewed as a class of lung stem cells. However, basal epithelial cells are not clonogenic, and their epithelial characteristics are consistent with their ability to differentiate only into tracheal or bronchiolar epithelial cells. Clara cells distributed in the tracheal epithelium and partly in the bronchioles in the mouse and in the small airways in humans can divide and contribute to the development and repair of proximal and more distal respiratory structures,6 but these properties are insufficient to classify them as stem cells. Clara cells secrete mucin, which is in opposition to the undifferentiated phenotype that defines stem cells. Similarly, bronchoalveolar stem cells generate small colonies in vitro and express molecular markers of Clara cells and epithelial cells.7,8 Type II alveolar epithelial cells divide and form type II and type I alveolar pneumocytes. On this basis, type II cells have been considered progenitors of the alveolar epithelium.9 Side population cells have been found, but their differentiation in vitro mimics that of mesenchymal stromal cells.10
To establish whether the human lung possesses a stem-cell pool, we used the stem-cell antigen c-kit11–13 as a marker of identification and characterization. Our criteria for human lung stem cells were self-renewal, clonogenicity, and multipotentiality in vitro and in vivo.
METHODS
A detailed description of the methods is provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.
HUMAN LUNG TISSUE
Samples of normal human lung tissue were obtained, by means of a protocol approved by the institutional review board of each institution, from 12 unused donor organs and with written informed consent for research use from each donor. Fetal lungs were obtained after nine cases of fetal death. Formalin-fixed, paraffin-embedded lung-tissue specimens were subjected to immunohistochemical analysis involving antibodies to identify putative stem cells (which are c-kit–positive), epithelial cells, cartilage, endothelial cells, fibroblasts, mast cells, and vascular and nonvascular smooth-muscle cells.13 Morphometric analysis was used to assess cellular features and to quantitate the number of stem cells per square millimeter and per unit mass.14
HUMAN LUNG STEM CELLS
Eight samples of normal lung tissue were obtained from the Brigham and Women’s Hospital Thoracic Surgery Tissue Bank by means of a protocol approved by the institutional review board. Single-cell suspensions were generated with the use of enzymatic digestion. Cells were expanded, sorted, immunophenotyped, and cultured in F-12 medium (GIBCO) plus 10% fetal-calf serum (GIBCO). Cells were analyzed by means of fluorescence-activated cell sorting, immunocytochemistry, Western blotting, and immunoprecipitation, and tissues were analyzed with the use of immunohistochemical analysis, in situ hybridization, quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR), spectral analysis, and two-photon microscopy.13 Human lung stem cells were labeled for in vivo tracking by means of infection with a lentivirus-carrying enhanced green fluorescent protein (EGFP).15 The mean (±SD) infection efficiency was 80±5% in seven separate experiments.
LUNG INJURY, REGENERATION, AND SERIAL TRANSPLANTATION
Anesthetized C57BL/6 female mice immunosuppressed with the use of cyclosporine underwent thoracotomy, and 2 to 3 mm2 of lung tissue was damaged with a steel probe that had been cooled in liquid nitrogen. The area adjacent to the damaged tissue then received six injections (with about 20,000 cells in each injection) of EGFP-expressing human lung stem cells mixed with 1% rhodamine-labeled polystyrene microspheres. The chests were then closed. Mice were killed at 12 hours, 2 days, or 10 to 14 days after surgery. In some mice, lung tissue that had regenerated was excised, and EGFP-expressing c-kit–positive human lung stem cells were reisolated by means of fluorescence-activated cell sorting and injected into another mouse with a cryodamaged lung.
RESULTS
IDENTIFICATION OF HUMAN LUNG STEM CELLS
Lung samples were digested and c-kit–positive cells were collected by means of immunosorting (Fig. 1 in the Supplementary Appendix). Human bone marrow cells exposed to collagenase and lung c-kit–negative cells were used as controls. CD34-positive human bone marrow cells treated with collagenase retained the surface antigen CD34 and expressed bone marrow lineage markers. Cells that were negative for c-kit failed to express the stem-cell antigen (Fig. 2A in the Supplementary Appendix).
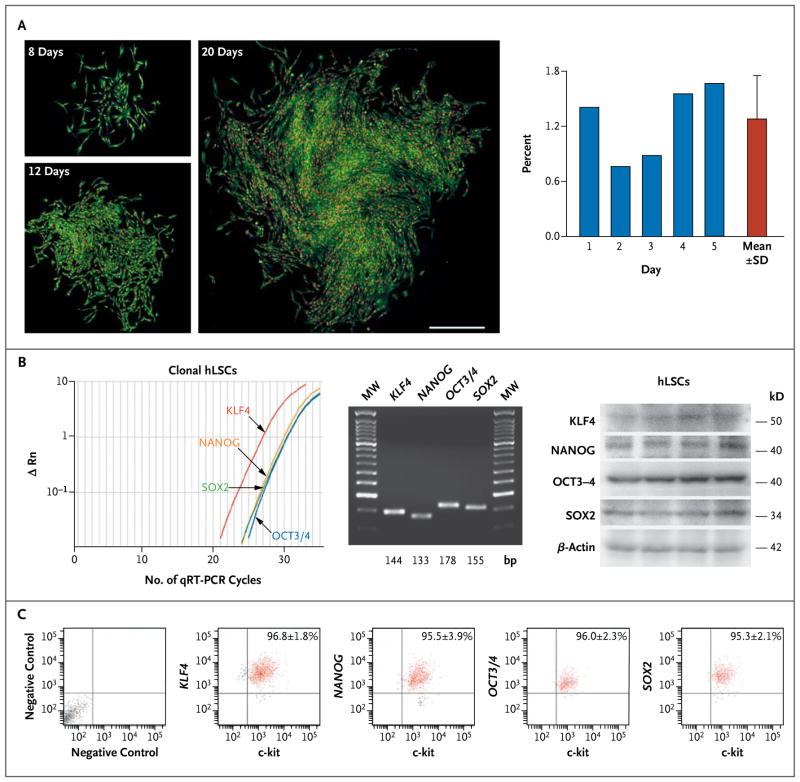
Cells that were positive for c-kit were negative for hematopoietic and mesenchymal markers, epitopes of mast cells (Fig. 2B in the Supplementary Appendix), and transcription factors and cytoplasmic proteins of epithelial cells, endothelial cells, and smooth-muscle cells (Fig. 2C in the Supplementary Appendix). For clonal analysis, c-kit–positive cells were subjected to fluorescence-activated cell sorting to place single cells in each well of Terasaki plates or seeded at a limiting dilution (1 cell per 20 mm2). After 3 or 4 weeks, multicellular clones were obtained; cells in the clones continued to express c-kit. Cloning efficiency (measured as the percentage of seeded cells) averaged 1% with both methods (Fig. 1A, and Fig. 2D in the Supplementary Appendix). Clonal human lung stem cells were essentially all positive for c-kit and negative for epithelial-cell, endothelial-cell, and smooth-muscle–cell markers (Fig. 2E in the Supplementary Appendix). The expression of c-kit was confirmed by means of qRT-PCR assay and Western blotting (Fig. 3A and 3B in the Supplementary Appendix). Messenger RNA (mRNA) transcripts for genes encoding thyroid transcription factor 1 (TTF1, p63, CK5, surfactant protein C [SP-C], Clara cell 10-kD secretory protein [CC10], and the epithelial-cell chloride channel CFTR [cystic fibrosis transmembrane conductance regulator]) were barely detectable, confirming the primitive state of the clonal human lung stem cells (Fig. 3C in the Supplementary Appendix).
Figure 1. Clonogenicity and Pluripotency of Human Lung Stem Cells (hLSCs).
Panel A shows hLSC clones obtained by limiting dilution. Individual c-kit–positive lineage-negative cells generated clones that increased in size with time. Cloning efficiency is shown in individual cases and as means ±SD. Panel B (left) shows representative results of quantitative reverse-transcriptase–polymerase-chain-reaction (qRT-PCR) assays (with ΔRn indicating the log10-transformed relative number of messenger RNA [mRNA] transcripts) of transcripts for Kruppel-like factor 4 gene (KLF4), the homeobox transcription factor Nanog gene (NANOG), the octamer-binding transcription factor 3–4 gene (OCT3/4), and the sex-determining-region Y–box 2 gene (SOX2) in undifferentiated clonal human lung stem cells. MW denotes molecular-weight DNA ladders. Sequences are listed in Figure 5B in the Supplementary Appendix. The expression of these four genes in undifferentiated clonal hLSCs is also shown (middle, with the transcript sizes shown along the bottom), and the expression of corresponding proteins is also shown (right, with protein sizes listed). β-Actin was used as an indicator of equal loading in all lanes. Panel C shows bivariate-distribution plots (with the x-axis units on a log10 scale and values within each quadrant indicating the mean [±SD] percentage of cells found in each quadrant) of c-kit KLF4, NANOG, OCT3/4, and SOX2 in undifferentiated clonal hLSCs.
Clonal human lung stem cells exposed to dexamethasone largely lost the c-kit epitope and expressed epithelial and vascular lineage proteins (Fig. 4A in the Supplementary Appendix). Progressive stages of commitment of epithelial and vascular cells were found, as indicated by morphologic characteristics (Fig. 4B and 4C in the Supplementary Appendix).
The presence of c-kit has been used to identify a pool of self-renewing, clonogenic, and multi-potent human cardiac stem cells13 (Fig. 3A in the Supplementary Appendix). However, human lung stem cells and human cardiac stem cells acquired only cell phenotypes of the organ of origin. Under identical differentiating conditions, human lung stem cells did not generate cardiomyocytes and human cardiac stem cells failed to form lung epithelial cells (Fig. 4D in the Supplementary Appendix). Collectively, these data indicate that human lung stem cells are distinct from human cardiac stem cells and differentiate into structures of endodermal origin (epithelial cells) and mesodermal origin (vessels). Clonal human lung stem cells express the homeobox transcription factor Nanog (NANOG), octamer-binding transcription factor 3–4 (OCT3/4), sex-determining-region Y–box 2 (SOX2), and Kruppel-like factor 4 (KLF4) (Fig. 1B and 1C, and Fig. 5 in the Supplementary Appendix). The genes encoding these four proteins promote reprogramming of fibroblasts into pluripotent stem cells,16 suggesting that adult human lung stem cells are multi-potent cells with a high degree of plasticity.
DIVISION OF HUMAN LUNG STEM CELLS
Stem cells divide symmetrically and asymmetrically. In the case of symmetric division, the uniform localization of the numb homologue NUMB and α-adaptin at the two poles of a dividing stem cell leads to the generation of two identical daughter cells. Conversely, with asymmetric division, the localization of these proteins at one pole results in two cells with differing fates.13
To define the growth behavior of human lung stem cells, the partitioning of α-adaptin was monitored, and TTF1, GATA binding protein 6 (GATA6), and v-ets erythroblastosis virus E26 oncogene homologue 1 (ETS1) were used as markers of cell commitment. Human lung stem cells were found to divide symmetrically and asymmetrically, although symmetric division predominated. Asymmetric division of human lung stem cells gave rise to one daughter cell that expressed TTF1, GATA6, or ETS1 and one daughter cell that retained the stem-cell phenotype. However, symmetric division generated two lineage-negative or two lineage-positive daughter cells (Fig. 6 in the Supplementary Appendix). These in vitro results suggest that the human lung harbors stem cells that are self-renewing, clonogenic, and multi-potent.
BEHAVIOR OF CLONAL AND NONCLONAL HUMAN LUNG STEM CELLS IN VIVO
The confirmation of human lung stem cells requires serial transplantation in vivo to establish whether these cells create functionally integrated structures in the relevant tissue microenvironment. To test this possibility, cryoinjury was induced in the left lung of immunosuppressed mice (Fig. 7 in the Supplementary Appendix). Shortly after lung injury, clonal or nonclonal human lung stem cells were administered in the region adjacent to the area of damage.
These in vivo studies had three objectives. The first was to show that single cell–derived clonal human lung stem cells generate a multicomponent, structurally organized tissue in the recipient mouse lung. This aimed to fulfill the criteria required to prove the function of stem cells in vivo. The second objective was to show that clonal human lung stem cells self-renew in the damaged lung and can be harvested and reimplanted in another set of animals with lung injury, promoting lung repair. This aimed to mimic the serial transplantation assay commonly used for the analysis of the self-renewal property of hematopoietic stem cells. The third objective was to show that nonclonal (non–single-cell-derived), lineage-negative human lung stem cells engraft and form human lung parenchyma in the recipient mouse organ. This aimed to understand the clinical importance of sorted lineage-negative human lung stem cells; it would be unrealistic to use clonal human lung stem cells in patients in view of the low efficiency of clonal formation and the cost of this cell-culture approach.
Symmetric and asymmetric division of clonal and nonclonal human lung stem cells in vivo was identified 12 to 48 hours after lung injury and cell delivery. The bipolar and unipolar localization of α-adaptin, together with the concurrent expression of TTF1, GATA6, or ETS1, confirmed the ability of human lung stem cells to form new stem cells and cells destined to acquire specialized functions. After 2 days, approximately 30% of the delivered cells were present within the damaged tissue and the bordering region, and approximately 25% were cycling (Fig. 8 in the Supplementary Appendix). After 10 to 14 days, clonal human lung stem cells had formed human bronchioles, alveoli, and pulmonary vessels, partly restoring the structural integrity of the recipient parenchyma (Fig. 2A). Thus, the clonal human lung stem cells showed self-renewal and multipotentiality in vivo. Sorted nonclonal human lung stem cells performed a similar regeneration of the various components of the distal airways and their vasculature (Fig. 9 in the Supplementary Appendix). The restored EGFP-positive structures were not restricted to the injured portion of the lung.
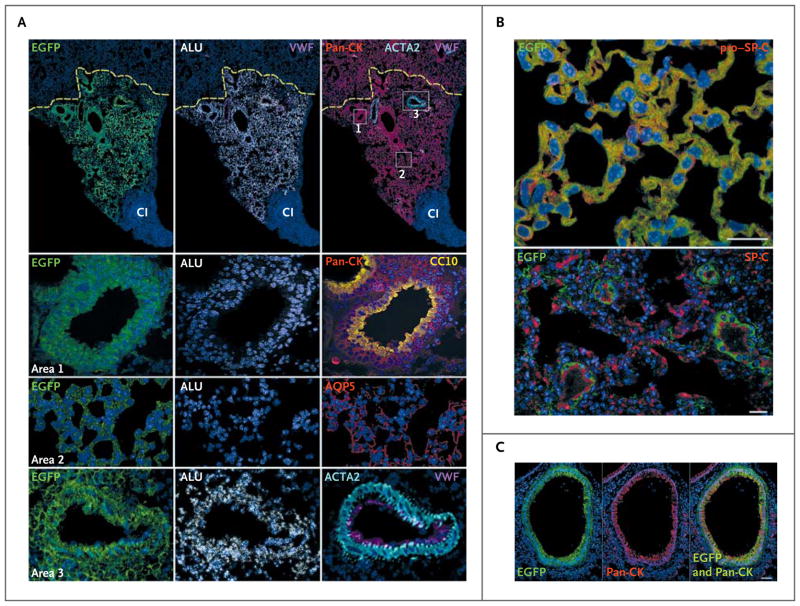
Figure 2. Lung Regeneration by Clonal and Nonclonal Human Lung Stem Cells.
Regions of cryoinjury (CI) in mouse lung were injected with human lung stem cells, and newly formed human pulmonary structures are visible because the cells express various proteins in various colors (with the protein indicated as the color used in its label in the figure): enhanced green fluorescent protein (EGFP); Alu repeat sequences (ALU); pan-cytokeratin (pan-CK); von Willebrand factor (VWF); Clara cell 10-kD secretory protein (CC10); aquaporin 5 (AQP5); actin, alpha 2, smooth muscle, aorta (ACTA2); and pro–surfactant protein C (pro–SP-C) and SP-C. Lung regeneration was discernible 14 days after delivery of clonal human lung stem cells (Panel A), with the human cells having largely replaced the CI region, adjacent to an area of residual injury. Recipient mouse epithelial cells, positive for pan-CK but negative for EGFP, are present at the periphery of the regenerated tissue; the yellow dashed lines define this boundary (Panel A). Areas within the rectangles are shown at a higher magnification in the lower panels: area 1 corresponds to a human bronchiole composed of epithelial cells positive for EGFP, ALU, and pan-CK and CC10; area 2, human alveoli positive for EGFP, ALU, and AQP5; area 3, a human pulmonary arteriole positive for EGFP, ALU, and ACTA2 and von Willebrand factor (VWF) (Panel A). Panel B shows alveoli, formed by clonal human lung stem cells, that express EGFP and pro–SP-C or EGFP and SP-C (scale bar, 20 μm). Panel C shows newly formed human bronchioles with epithelial cells positive for EGFP and pan-CK (with the merged image shown at the right; scale bar, 50 μm).
With both clonal and nonclonal human lung stem cells, epithelial cells organized in well-defined alveolar structures were present throughout the affected lobe (Fig. 2B, and Fig. 10 in the Supplementary Appendix). Human lung stem cells generated bronchioles, approximately 30 to 250 μm in diameter, as well as small and intermediate-sized pulmonary arterioles approximately 20 to 70 μm in diameter (Fig. 2C, and Fig. 11 and 12 in the Supplementary Appendix). Similar degrees of lung repair were found with clonal and nonclonal human lung stem cells, suggesting that these cells had roughly equivalent efficacy. Among both clonal and nonclonal cells, the fraction of epithelial cells labeled by bromodeoxyuridine averaged 90%. The newly formed human lung parenchyma replaced more than 30% of the original damaged tissue (Fig. 13 in the Supplementary Appendix). The specificity of the signal for native and labeled EGFP; pan-cytokeratin (pan-CK); pro–SP-C; SP-C; CC10; aquaporin 5 (AQP5); Alu repeat sequences (Alu); actin, alpha 2, smooth muscle, aorta (ACTA2); and von Willebrand factor (VWF) was validated by means of spectral analysis (Fig. 14 in the Supplementary Appendix).
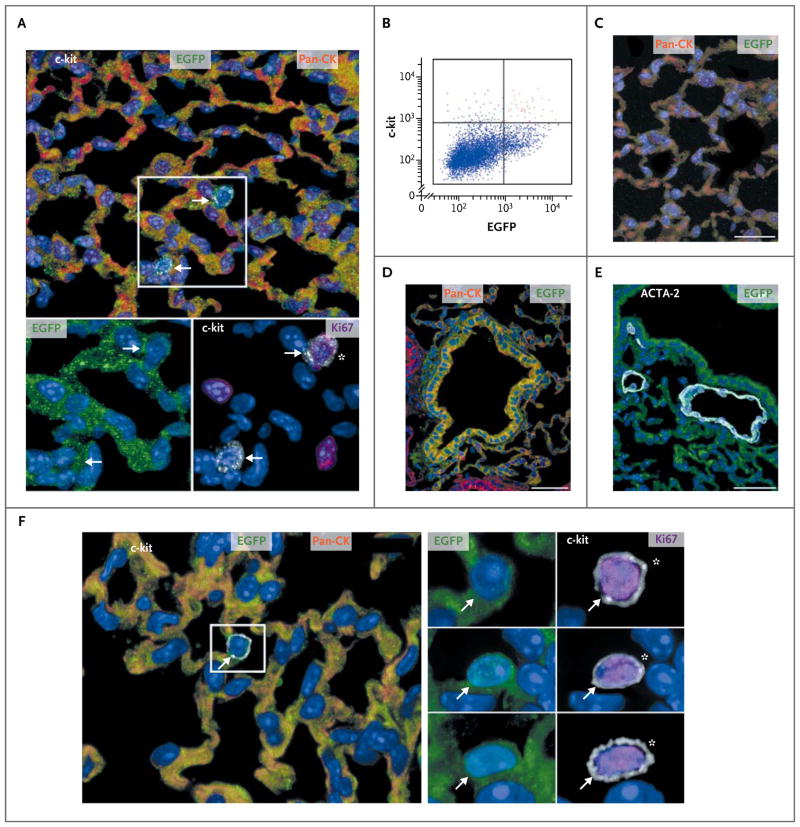
Ten to 14 days after cryoinjury and cell implantation, undifferentiated, cycling c-kit–positive human lung stem cells were identified within the regenerated human lung parenchyma and in the adjacent, intact recipient mouse lung (Fig. 3A). Approximately 20,000 human lung stem cells were present in each treated mouse. After enzymatic digestion of the damaged lung, cells were sorted for detection of c-kit and EGFP (Fig. 3B) and delivered immediately to the cryoinjured portion of the lung of another recipient mouse. Ten days after cell treatment, all eight treated mice were killed, and human bronchi, alveoli, and vessels were identified, documenting that the newly formed human lung structures derived from the serially transplanted human lung stem cells (Fig. 3C, 3D, and 3E). Undifferentiated, cycling, c-kit–positive human lung stem cells were detected in these mice (Fig. 3F), providing further evidence in support of the self-renewal and long-term proliferation of human lung stem cells in vivo.
Figure 3. Human Lung Stem Cells in the Serial Transplantation Assay.
Cells expressing various proteins in various colors (with the protein indicated as the color used in its label in the figure) are shown, as are scatter plots of cells after flow analysis. Panel A shows newly formed human alveoli positive for enhanced green fluorescent protein (EGFP) and pan-cytokeratin (pan-CK) (top). Two c-kit–positive cells are visible (arrows within the rectangle). The c-kit–positive cells are EGFP-positive (bottom left, arrows) and one is cycling, as indicated by its Ki67-positive status (bottom right, asterisk). Panel B shows a scatter plot indicating c-kit–positive, EGFP-positive human lung stem cells (red dots); these were isolated after lung regeneration. After serial transplantation, regenerated alveoli (Panel C; scale bar, 20 μm) and a bronchiole (Panel D; scale bar, 50 μm) are positive for EGFP and pan-CK, whereas a newly formed arteriole (Panel E; scale bar, 50 μm) is positive for EGFP and actin, alpha 2, smooth muscle, aorta (ACTA2). In a lung in which serial transplantation was performed, EGFP-positive, pan-CK–positive human alveoli (Panel F) contain one c-kit–positive cell (arrow within the rectangle). This c-kit–positive cell (and two others, shown individually below the first in the panel on the right) is EGFP-positive (left, arrow) and is cycling (right, asterisk).
The c-kit epitope has been used to identify human cardiac stem cells and hematopoietic stem cells. However, the in vitro progeny of human lung stem cells and human cardiac stem cells are dramatically different, in spite of having the same differentiating conditions.13 In addition, hematopoietic stem cells generate blood cells almost exclusively. To test whether the microenvironment of the mouse lung dictated specific cell phenotypes in vivo, human c-kit–positive hematopoietic stem cells and human cardiac stem cells were delivered to the cryoinjured region of the lung; c-kit–negative human lung cells were used as the control. Ten days after cell administration, a small number of human, undifferentiated hematopoietic stem cells and human cardiac stem cells were found in the damaged area or its proximity (Fig. 15 in the Supplementary Appendix). Neither type of cells formed human lung structures. No cells were identified after injection of c-kit–negative human lung cells.
INTEGRATION OF HUMAN PULMONARY STRUCTURES
Seven to 15 days after cryoinjury and delivery of clonal human lung stem cells, qRT-PCR was used to detect mRNA transcripts for several human epithelial-cell genes (TTF1, TP63 [encoding p63], CFTR, KRT18 [encoding cytokeratin 18], CC10, SFTPC [encoding surfactant protein C], AQP5, and T1A [encoding podoplanin]), endothelial-cell genes (ETS1 and PECAM1 [encoding platelet–endothelial cell adhesion molecule]), and a gene encoding smooth-muscle–cell transforming growth factor β receptor 1 (TGFBR1) (Fig. 16 in the Supplementary Appendix). In addition, fusion events between human lung stem cells and mouse epithelial cells, smooth-muscle cells, and endothelial cells were ruled out through the identification of human and mouse X chromosomes in EGFP-positive bronchioles, alveoli, and pulmonary vessels (Fig. 17, 18, and 19 in the Supplementary Appendix).
To document the integration of human structures with the recipient mouse lung, an ex vivo preparation was used. The mouse lung was examined 10 to 14 days after cryoinjury and the injection of EGFP-positive human lung stem cells. The trachea, bronchi, bronchioles, and alveoli were perfused with rhodamine-labeled dextran to visualize the airways by means of two-photon microscopy17 (Fig. 20 in the Supplementary Appendix). Resident and regenerated alveoli were distinguished by the absence and presence of EGFP labeling, respectively. EGFP-positive alveoli were found, supporting their origin from the injected human lung stem cells (Fig. 21A and 21B in the Supplementary Appendix). Newly formed alveoli and vessels (EGFP-positive but rhodamine-negative) were in close proximity to each other, a finding that was consistent with the presence of integrated human respiratory domains in the mouse lung.
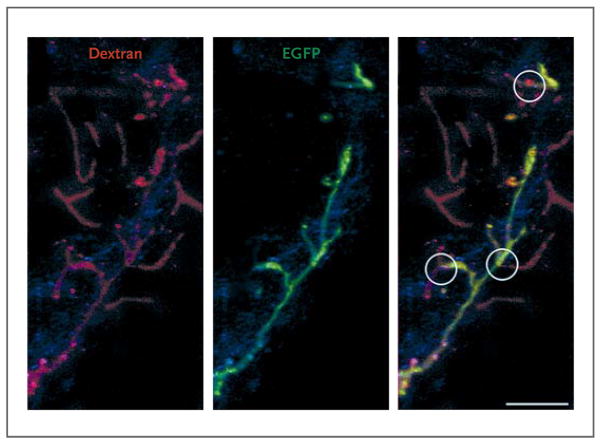
Perfusion of the pulmonary artery with dextran revealed a relevant number of vascular profiles with EGFP-positive walls, indicating their origin from the delivered human lung stem cells (Fig. 21C and 21D in the Supplementary Appendix). Human vessels and human alveoli (EGFP-positive but rhodamine-negative) were in close proximity with one another, reflecting integrated respiratory units involved in gas exchange. Direct connections were found between preexisting pulmonary vessels (EGFP-negative–walled) and regenerated pulmonary vessels (EGFP-positive–walled) (Fig. 4), documenting the integration of temporally distinct preexisting (mouse) and new (human) segments of the pulmonary vasculature.
Figure 4. Integration of Human Pulmonary Structures.
Shown is pulmonary vasculature perfused with rhodamine-labeled dextran. Pulmonary vessels (left, shown in red) that are preexisting have enhanced green fluorescent protein (EGFP)–negative walls (middle), whereas those that are regenerated have EGFP-positive walls (middle). Sites of anastomosis between the preexisting and regenerated pulmonary vessels are shown (circles, right image).
HUMAN LUNG STEM CELL NICHES
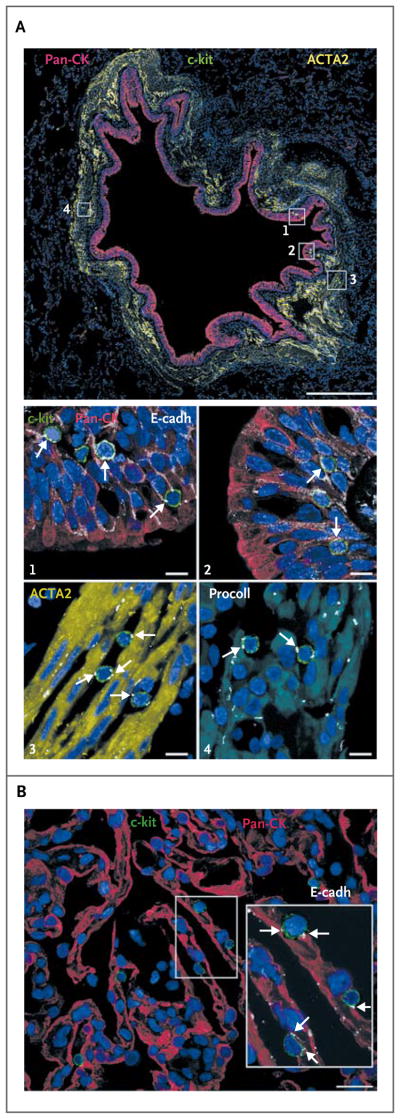
Typically, stem cells are located in particular anatomical niches where they are connected to the supporting cells by means of gap junctions and adherens junctions consisting of connexins and cadherins, respectively.18 In 12 adult and 9 fetal human lung-tissue specimens, we found structures with the characteristics of stem-cell niches in bronchioles from 25 to 1200 μm in diameter, as well as within the alveolar wall (Fig. 5A and 5B, and Fig. 22 and 23 in the Supplementary Appendix). E-cadherin was detected at the interface between human lung stem cells and epithelial cells, smooth-muscle cells, and fibroblasts, suggesting that the latter three cell types may function as supporting cells in the lung stem-cell niches. The anatomical proximity and structural connections between human lung stem cells and epithelial cells, smooth-muscle cells, and fibroblasts is consistent with the architectural organization of a niche, although the function of these potential supporting cells in the fate of human lung stem cells remains to be defined.
Figure 5. Localization of Human Lung Stem Cells.
Cells expressing various proteins in various colors (with the protein indicated as the color used in its label in the figure) are shown. Panel A shows a bronchiole, approximately 900 μm in diameter (scale bar, 0.5 mm in length), with epithelial cells positive for pan-cytokeratin (pan-CK) and smooth-muscle cells expressing actin, alpha 2, smooth muscle, aorta (ACTA2). Several c-kit–positive cells are present within the bronchiolar wall (within the four rectangles); in the images labeled with numerals, these rectangles are shown at higher magnification (scale bar, 10 μm). These show that c-kit–positive cells are connected by E-cadherin (E-cadh, arrows) to bronchiolar epithelial cells (images 1 and 2), smooth-muscle cells (as indicated by ACTA2; image 3, arrows), and fibroblasts (as indicated by procollagen [procoll]; arrows, image 4). The c-kit–positive cells were negative for the markers of mast cells CD45 and tryptase (not shown). Panel B (scale bar, 20 μm) shows that alveoli positive for pan-CK contain c-kit–positive cells in the cell walls. The area within the rectangle is shown at higher magnification in the inset. C-kit–positive cells are connected by E-cadherin (arrows) to epithelial cells.
In human bronchioles approximately 1.2 mm in diameter, basal epithelial cells expressed p63 in their nuclei and CK5 in their cytoplasm; and c-kit–positive cells within the basal-cell layer expressed p63 and CK5, suggesting a lineage relationship between human lung stem cells and basal epithelial cells. The basal epithelium also contained c-kit–positive cells negative for p63 and CK5, reflecting uncommitted cells (Fig. 22A in the Supplementary Appendix); p63-positive and CK5-positive epithelial cells made up 14±4% of bronchiolar epithelial cells.
Progenitor epithelial cells retained the c-kit epitope and expressed TTF119 in the absence of specialized cytoplasmic proteins. Precursor epithelial cells were positive for c-kit, TTF1, and pro–SP-C or cytokeratin. Similarly, progenitor endothelial cells and smooth-muscle cells expressing ETS1 and GATA6, respectively, were identified (Fig. 24 in the Supplementary Appendix).
NUMBERS OF HUMAN LUNG STEM CELLS
Stem cells are relatively uncommon in solid and nonsolid organs. In humans, there is 1 stem cell per approximately 10,000 to 20,000 cells in the bone marrow and per approximately 30,000 cells in the heart.13,20 Undifferentiated human lung stem cells negative for nuclear and cytoplasmic proteins of lung cells were found at a frequency of 1 per 24,000 cells; counts in the bronchioles and alveoli were 1 per 6000 cells and 1 per 30,000 cells, respectively. We estimate that 79% of human lung stem cells were nested in bronchioles and 21% in alveoli (Fig. 25A in the Supplementary Appendix). Human lung stem cells showed a preferential localization in small bronchioles lacking cartilage and alveoli, decreasing progressively in number in the larger airways (Fig. 25B in the Supplementary Appendix). Collectively, there were 7700 human lung stem cells per 10 cm3 of collapsed tissue volume.
The availability of nine samples of fetal lung tissue from 12 to 36 weeks of gestation allowed us to count the number of human lung stem cells during this phase of rapid prenatal growth. The four pluripotency genes NANOG, OCT3/4, SOX2, and KLF4 were also detected in c-kit–positive cells of the fetal human lung (Fig. 26A in the Supplementary Appendix). Uncommitted human lung stem cells were found in combination with epithelial, endothelial, and smooth-muscle cell progenitors (Fig. 23 and 26B in the Supplementary Appendix), strongly suggesting that fetal human lung stem cells acquired the cell phenotypes needed for the generation of functioning lung tissue. The frequency of human lung stem cells varied from 1 per 11,000 cells to 1 per 600 cells in the fetal lung, resulting in an average count of 1 per 4100 cells (Fig. 26C in the Supplementary Appendix).
DISCUSSION
Our results suggest that the human lung possesses a pool of c-kit–positive cells that have the fundamental properties of stem cells: they are self-renewing, clonogenic, and multipotent in vitro and in vivo. The ability of human lung stem cells to create human bronchioles, alveoli, and pulmonary vessels in the mouse provides evidence in favor of the crucial role that human lung stem cells may have in lung homeostasis and tissue regeneration after injury. These observations challenge the suggestion that the lung is an organ lacking a hierarchical cellular organization21 regulated by a compartment of resident stem cells.13,22–26
Our findings do not rule out or challenge the notion that basal epithelial cells, bronchoalveolar stem cells, Clara cells, side population cells, and type II alveolar epithelial cells are involved in the epithelial-cell response to inflammation or injury caused by aromatic hydrocarbons.6,10,21,27 Similarly, the presence of a human lung stem cell does not rule out the possibility that mature cells within the adult lung dedifferentiate or reprogram themselves to form a committed progeny, a phenomenon shown to be operative in other organs.28–30
For restoration of damaged tissue to occur, cells must be capable of the coordinated formation of both distal airways and distal pulmonary vasculature. Unipotent progenitors with distinct differentiation potential would have to be simultaneously activated to form functionally competent gas-exchange units. This limitation is shared by bronchoalveolar stem cells, Clara cells, side population cells, and type II alveolar epithelial cells, since they generate only type I and type II pneumocytes3,4,6–9,27 or stromal cells.10 Similarly, bone marrow cells expressing Clara-cell secretory protein transdifferentiate and acquire the phenotype of epithelial-cell lineages31 but lack the ability to replace and integrate the other components of the gas-exchange unit.
Our results indicate that human lung stem cells give rise to different populations of epithelial cells of endodermal origin and to pulmonary vessels derived from the mesoderm.32 However, human lung stem cells express four genes — NANOG, OCT3/4, SOX2, and KLF4 — that constitute the network of transcription factors that governs the pluripotency of human embryonic stem cells.33 The gene products have been found in c-kit–positive cells of the fetal human lung, strengthening the notion that adult human lung stem cells retain characteristics typical of stem cells residing in the developing organ. These transcription factors also promote reprogramming of adult somatic cells into pluripotent stem cells.16
Our study was not designed to improve the respiratory function of animals with pulmonary failure by means of cell implantation. The objective was to identify a class of human lung stem cells that had the ability to form distal airways and vessels in a mouse model of lung injury and to document that the newly formed structures of human origin replaced, in part, the damaged parenchyma and were connected with the vasculature and respiratory system of the recipient mouse organ. These findings, together with the results of our in vitro studies, provide evidence of a resident multipotent stem cell in the human lung. In addition, the in vivo demonstration that resident human lung stem cells divide both symmetrically and asymmetrically, do not express any markers typical of hematopoietic cells, and reconstitute lung tissue where hematopoietic stem cells fail to do so argues against the bone marrow origin of this stem-cell class.
In conclusion, we provide several lines of evidence suggesting the existence of human lung stem cells. Clonal human lung stem cells divided asymmetrically and generated bronchioles, alveoli, and pulmonary vessels of various dimensions, including capillaries, in vivo in a mouse model. Furthermore, human lung stem cells obtained from regenerated lung tissue were able to self-renew and create lung parenchyma in vivo in another mouse with lung damage. The immuno-histochemical identification of newly regenerated pulmonary structures is strengthened by the recognition of human sex chromosomes and human transcripts of epithelial and vascular genes within the regenerated mouse lung.13,25,34 The human X chromosome was detected in regenerated type II alveolar epithelial cells, suggesting the capacity of the human lung stem cells to terminally differentiate in vivo into functional, highly specialized cells that produce and secrete SP-C, a critical determinant of alveolar function and lung performance. Thus, c-kit–positive human lung stem cells show self-renewal, clonogenicity, and multipotentiality.
Supplementary Material
Acknowledgments
Supported by grants from the National Institutes of Health and Cardiocentro Ticino (to Dr. Bardelli).
Dr. Anversa reports being a board member of Autologous and that a patent application has been filed by Partners HealthCare (which includes Brigham and Women’s Hospital) for this class of human lung stem cells.
We thank Drs. Ana Castano and Claudia Fiorini.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Laugwitz KL, Moretti A, Lam J, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–53. doi: 10.1038/nature03215. [Erratum, Nature 2007;446:934.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Domian IJ, Chiravuri M, van der Meer P, et al. Generation of functional ventricular heart muscle from mouse ventricular progenitor cells. Science. 2009;326:426–9. doi: 10.1126/science.1177350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kotton DN, Fine A. Lung stem cells. Cell Tissue Res. 2008;331:145–56. doi: 10.1007/s00441-007-0479-2. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Matsumoto K, Stripp BR. Bronchiolar progenitor cells. Proc Am Thorac Soc. 2009;6:602–6. doi: 10.1513/pats.200907-078RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rock JR, Onaitis MW, Rawlins EL, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A. 2009;106:12771–5. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stripp BR, Reynolds SD. Maintenance and repair of the bronchiolar epithelium. Proc Am Thorac Soc. 2008;5:328–33. doi: 10.1513/pats.200711-167DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim CF, Jackson EL, Woolfenden AE, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–35. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 8.Rawlins EL, Okubo T, Xue Y, et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4:525–34. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brody JS, Williams MC. Pulmonary alveolar epithelial cell differentiation. Annu Rev Physiol. 1992;54:351–71. doi: 10.1146/annurev.ph.54.030192.002031. [DOI] [PubMed] [Google Scholar]
- 10.Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol. 2002;161:173–82. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orlic D, Fischer R, Nishikawa S, Nienhuis AW, Bodine DM. Purification and characterization of heterogeneous pluripotent hematopoietic stem cell populations expressing high levels of c-kit receptor. Blood. 1993;82:762–70. [PubMed] [Google Scholar]
- 12.Beltrami AP, Barlucchi L, Torella D, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–76. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 13.Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–73. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loud AV, Anversa P. Morphometric analysis of biologic processes. Lab Invest. 1984;50:250–61. [PubMed] [Google Scholar]
- 15.Hosoda T, D’Amario D, Cabral-DaSilva MC, et al. Clonality of mouse and human cardiomyogenesis in vivo. Proc Natl Acad Sci U S A. 2009;106:17169–74. doi: 10.1073/pnas.0903089106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamanaka S, Blau HM. Nuclear reprogramming to a pluripotent state by three approaches. Nature. 2010;465:704–12. doi: 10.1038/nature09229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tillmanns J, Rota M, Hosoda T, et al. Formation of large coronary arteries by cardiac progenitor cells. Proc Natl Acad Sci U S A. 2008;105:1668–73. doi: 10.1073/pnas.0706315105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie T, Li L. Stem cells and their niche: an inseparable relationship. Development. 2007;134:2001–6. doi: 10.1242/dev.002022. [DOI] [PubMed] [Google Scholar]
- 19.Kimura S, Hara Y, Pineau T, et al. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996;10:60–9. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- 20.Craig W, Kay R, Cutler RL, Lansdorp PM. Expression of Thy-1 on human hematopoietic progenitor cells. J Exp Med. 1993;177:1331–42. doi: 10.1084/jem.177.5.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snyder JC, Teisanu RM, Stripp BR. Endogenous lung stem cells and contribution to disease. J Pathol. 2009;217:254–64. doi: 10.1002/path.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fuchs E, Horsley V. More than one way to skin…. Genes Dev. 2008;22:976–85. doi: 10.1101/gad.1645908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eriksson PS, Perfilieva E, Björk-Eriksson T, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 24.Orford KW, Scadden DT. Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9:115–28. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- 25.Bearzi C, Leri A, Lo Monaco F, et al. Identification of a coronary vascular progenitor cell in the human heart. Proc Natl Acad Sci U S A. 2009;106:15885–90. doi: 10.1073/pnas.0907622106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Giangreco A, Arwert EN, Rosewell IR, Snyder J, Watt FM, Stripp BR. Stem cells are dispensable for lung homeostasis but restore airways after injury. Proc Natl Acad Sci U S A. 2009;106:9286–91. doi: 10.1073/pnas.0900668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol. 2004;164:577–88. doi: 10.1016/S0002-9440(10)63147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X, Mao Z, Liu S, et al. Dedifferentiation of adult human myoblasts induced by ciliary neurotrophic factor in vitro. Mol Biol Cell. 2005;16:3140–51. doi: 10.1091/mbc.E05-03-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonner-Weir S, Weir GC. New sources of pancreatic beta-cells. Nat Biotechnol. 2005;23:857–61. doi: 10.1038/nbt1115. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–32. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong AP, Keating A, Lu WY, et al. Identification of a bone marrow-derived epithelial-like population capable of repopulating injured mouse airway epithelium. J Clin Invest. 2009;119:336–48. doi: 10.1172/JCI36882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chambers I, Tomlinson SR. The transcriptional foundation of pluripotency. Development. 2009;136:2311–22. doi: 10.1242/dev.024398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leri A. Human cardiac stem cells: the heart of a truth. Circulation. 2009;120:2515–8. doi: 10.1161/CIRCULATIONAHA.109.911107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.