Abstract
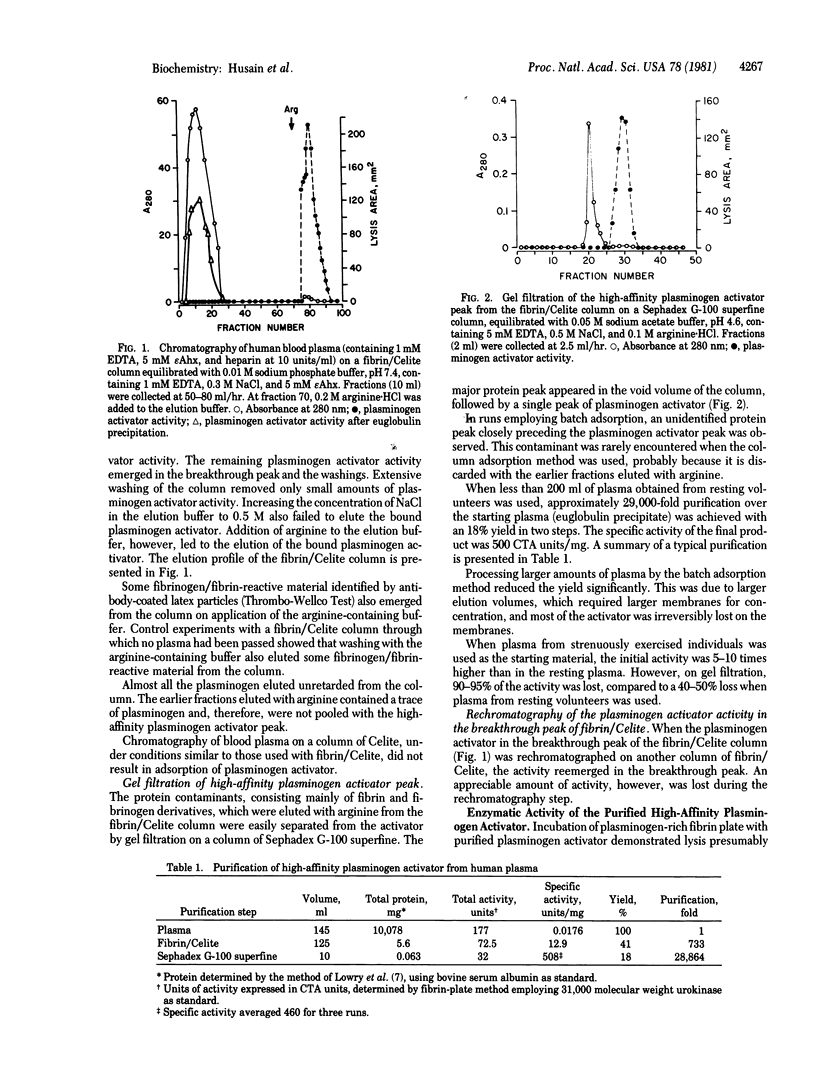
A preparation of fibrin precipitated over a solid Celite (diatomaceous earth) matrix that selectively binds 50-70% of the plasminogen activator present in human blood plasma is described. Affinity chromatography of plasma on fibrin/Celite followed by gel filtration led to a 29,000-fold purification of the plasminogen activator. The activator, referred to as the high-affinity plasminogen activator, is characterized by its ability to be strongly adsorbed by fibrin. Smaller amounts of other plasminogen activators and essentially all plasminogen were not bound to fibrin. The high-affinity plasminogen activator is a single-chain unstable protease with a molecular weight of 65,000-70,000. The high-affinity plasminogen activator has a low specific activity (500 CTA units/mg) compared to tissue or urine plasminogen activators (100,000-200,000 CTA units/mg) (CTA, Committee on Thrombolytic Agents).
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of molecular size and molecular weights of biological compounds by gel filtration. Methods Biochem Anal. 1970;18:1–53. [PubMed] [Google Scholar]
- Aoki N., Sakata Y., Matsuda M., Tateno K. Fibrinolytic states in a patient with congenital deficiency of alpha 2-plasmin inhibitor. Blood. 1980 Mar;55(3):483–488. [PubMed] [Google Scholar]
- Binder B. R., Spragg J., Austen K. F. Purification and characterization of human vascular plasminogen activator derived from blood vessel perfusates. J Biol Chem. 1979 Mar 25;254(6):1998–2003. [PubMed] [Google Scholar]
- Bouma B. N., Miles L. A., Beretta G., Griffin J. H. Human plasma prekallikrein. Studies of its activation by activated factor XII and of its inactivation by diisopropyl phosphofluoridate. Biochemistry. 1980 Mar 18;19(6):1151–1160. doi: 10.1021/bi00547a018. [DOI] [PubMed] [Google Scholar]
- Broze G. J., Jr, Majerus P. W. Purification and properties of human coagulation factor VII. J Biol Chem. 1980 Feb 25;255(4):1242–1247. [PubMed] [Google Scholar]
- Cole E. R., Bachmann F. W. Purification and properties of a plasminogen activator from pig heart. J Biol Chem. 1977 Jun 10;252(11):3729–3737. [PubMed] [Google Scholar]
- Davie E. W., Fujikawa K., Kurachi K., Kisiel W. The role of serine proteases in the blood coagulation cascade. Adv Enzymol Relat Areas Mol Biol. 1979;48:277–318. doi: 10.1002/9780470122938.ch6. [DOI] [PubMed] [Google Scholar]
- FEARNLEY G. R. Fibrinolysis by adsorption. Nature. 1953 Sep 19;172(4377):544–545. doi: 10.1038/172544b0. [DOI] [PubMed] [Google Scholar]
- Ferguson E. W., Barr C. F., Bernier L. L. Fibrinogenolysis and fibrinolysis with strenuous exercise. J Appl Physiol Respir Environ Exerc Physiol. 1979 Dec;47(6):1157–1161. doi: 10.1152/jappl.1979.47.6.1157. [DOI] [PubMed] [Google Scholar]
- Gurewich V., Hyde E., Lipinski B. The resistance of fibrinogen and soluble fibrin monomer in blood to degradation by a potent plasminogen activator derived from cadaver limbs. Blood. 1975 Oct;46(4):555–565. [PubMed] [Google Scholar]
- KOWALSKI E., KOPEC M., NIEWIAROWSKI An evaluation of the euglobulin method for the determination of fibrinolysis. J Clin Pathol. 1959 May;12(3):215–218. doi: 10.1136/jcp.12.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liu C. Y., Nossel H. L., Kaplan K. L. The binding of thrombin by fibrin. J Biol Chem. 1979 Oct 25;254(20):10421–10425. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Radcliffe R., Heinze T. Isolation of plasminogen activator from human plasma by chromatography on lysine-sepharose. Arch Biochem Biophys. 1978 Jul;189(1):185–194. doi: 10.1016/0003-9861(78)90131-5. [DOI] [PubMed] [Google Scholar]
- Rákóczi I., Wiman B., Collen D. On the biological significance of the specific interaction between fibrin, plasminogen and antiplasmin. Biochim Biophys Acta. 1978 May 3;540(2):295–300. doi: 10.1016/0304-4165(78)90142-3. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Bissell E. R., Mitchell A. R., Pearson K. W. Direct photometric or fluorometric assay of proteinases using substrates containing 7-amino-4-trifluoromethylcoumarin. Thromb Res. 1980 Feb 1;17(3-4):393–402. doi: 10.1016/0049-3848(80)90074-2. [DOI] [PubMed] [Google Scholar]
- Thorsen S., Glas-Greenwalt P., Astrup T. Differences in the binding to fibrin of urokinase and tissue plasminogen activator. Thromb Diath Haemorrh. 1972 Aug 31;28(1):65–74. [PubMed] [Google Scholar]
- White W. F., Barlow G. H., Mozen M. M. The isolation and characterization of plasminogen activators (urokinase) from human urine. Biochemistry. 1966 Jul;5(7):2160–2169. doi: 10.1021/bi00871a003. [DOI] [PubMed] [Google Scholar]