Abstract
We and others previously demonstrated that Protein Kinase D1 (PKD1) is down regulated in several cancers including prostate, interacts with E-cadherin, a major cell adhesion epithelial protein and causes increased cell aggregation and decreased motility of prostate cancer cells. In this study, we demonstrate that PKD1 complexes with β3-integrin resulting in activation of Mek-Erk pathway, which causes increased production of MMP-2 and -9, that is associated with shedding of soluble 80 kDa E-cadherin extracellular domain. Interestingly, decreased cell proliferation following PKD1 transfection was rescued by MMP-2 and MMP-9 inhibitors and augmented by recombinant MMP-2 and MMP-9 proteins, suggesting an anti-proliferative role for MMPs in prostate cancer. Translational studies by in silico analysis of publicly available DNA microarray data sets demonstrate a significant direct correlation between PKD1 and MMP-2 expression in human prostate tissues. The study demonstrates a novel mechanism for anti-proliferative effects of PKD1, a protein of emerging translational interest in several human cancers, through increased production of MMP-2 and -9 in cancer cells.
Keywords: PKD1, E-cadherin shedding, MMP-2, MMP-9, Prostate Cancer, Protein Kinase
Introduction
The basement membrane and transmembrane proteins contain signals for cell survival and growth, but loss of these signal results in cell death or suppression of proliferation in both normal and cancerous cells (1, 2). Matrix Metalloproteinases (MMPs) belong to a family of proteinases that play a role in degradation of basement membrane or transmembrane proteins that can lead to loss of cell survival and growth signals (3). MMPs are neutral proteinases secreted from various cells as inactive zymogens and activated by Zn or Ca ion-dependent proteolytic cleavage that catalyze the destruction of extracellular matrix (ECM) and transmembrane proteins (4, 5). There are 24 known isoforms of MMPs to date (6) and most have been shown to contribute to cancer progression and metastasis (7). However, MMPs might also negatively regulate cancer-cell survival; MMP-3 and MMP-7 have been shown to have pro-apoptotic function in epithelial cells (8–10). Other MMPs, such as MMP-9 can suppress the proliferation of T lymphocytes through disruption of IL-2Rα signaling (11), MMP-8 deficient mice exhibit an increased tumor susceptibility compared to wild-type mice (12) and active MMP-2 promotes apoptosis of hepatic stellate cells by cleavage of N-cadherin (13). Moreover, mice deficient in MMP-2, MMP-3 or MMP-9 have lower level of apoptosis induced by TNF-α (14).
Several studies have shown an association between increased MMPs production and malignant progression of prostate cancer, with increased MMP-2 and MMP-9 associated with higher grade prostate cancer (15). However, whether MMP-2 and MMP-9 play a pro-apoptotic or pro-survival function in prostate cancer cells is unclear. In addition, there is paucity of data with regard to regulators of MMP secretion and activity in prostate cancer. In this study, we describe Protein Kinase D1 (PKD1) as a novel regulator of increased secretion of MMP-2 and -9 in prostate cancer cells, which causes suppression of prostate cancer cell proliferation and colony formation suggesting a tumor suppressive role for MMP-2 and -9 in prostate cancer.
PKD1 was originally cloned and termed PKCmu and latter reclassified as charter member of new proteins family called PKD, which comprises of a family of two other structurally closely related isoforms PKD2 and PKD3. PKD1 is associated with plethora of cellular functions including cell growth, cell survival, invadopodia formation, Golgi organization and trafficking (16). Additionally, PKD1 interacts with integrin, a heterodimeric transmembrane receptors that mediates interactions between cells and extracellular membrane (ECM) and transmit signals across the plasma membrane that influence a range of biological processes, including cell proliferation, migration and apoptosis (17) by modulating signaling pathways (18–20). Previously, we have reported that Protein Kinase D1 (PKD1) is downregulated in advanced prostate cancer (21) and interacts with E-cadherin, a major transmembrane cell-cell adhesion protein and a classic marker of epithelial cells (22). Our laboratory has also shown that PKD1 inhibits cell proliferation, motility and invasion in prostate cancer cells and functions epistatically with E-cadherin, which is mediated by β-catenin, a major binding partner in cadherin-catenin complex of proteins (23).
E-cadherin, a member of classical cadherins, plays an important role in tissue morphogenesis, wound healing and maintenance of tissue integrity (24, 25). The extracellular domain of E-cadherin interacts homotypically with cadherins on the surface of neighboring cells to form calcium-dependent adherence junctions. Cadherin-mediated adhesion must be dynamic to accommodate epithelial growth and remodeling during development and to facilitate wound healing and in turnover of epithelia in mature tissues, which can be accomplished by E-cadherin shedding (25). The shedding occurs following cleavage of extracellular domain of E-cadherin by proteases including ADAM, γ-secretase and MMPs (26–28). However, the exact isoforms of MMPs involved in E-cadherin shedding is yet to be defined. Proteolytic cleavage of E-cadherin has been suggested a cause of rapid changes in cell adhesion, signaling, anoikis and apoptosis (26–28).
In this study, we demonstrate that PKD1 is a novel regulator in MMP-2 and -9 production as well as shedding of E-cadherin in prostate cancer cells. PKD1 interacts with β3-integrin to regulate Erk phosphorylation resulting in increased secretion of MMP-2 and -9, suppresses prostate cancer cell proliferation as well as colony formation and increases E-cadherin shedding. In silico analysis of publicly available human tissue gene expression datasets confirmed a significant direct correlation between PKD1 and MMP-2 expression.
Materials and Methods
Cell culture, plasmid preparation and cell transfection
Human prostate cancer cell lines PC3 and DU145 (ATCC) were grown in RPMI 1640 media with 10% FBS. pEGFP and pEGFP-PKD1 plasmids were prepared as previously described (29). PC3 and DU145 cells were transfected with pEGFP and pEGFP-PKD1 plasmids by Lipofectamine-2000 (Invitrogen) and clones were selected by Geneticin (Invitrogen). The knockdown of PKD1 by shRNA has been described previously (29, 30). Erk was knocked down by transfection of SignalSilence p44/42 MAPK siRNA (cell Signaling) and SignalSilence Control siRNA (Cell Signaling) was used as control siRNA.
Reagents and chemicals
Erk, p-Erk(Thr202/Tyr204), p-Mek1/2 (ser217/221) and β-integrin antibodies were purchased from Cell Signaling; GFP, PKD1(clone C-20), IgG and E-cadherin (H-108) antibodies from Santa Cruz Biotechnologies and Type-A Gelatin from Sigma-Aldrich Inc. HRP and fluorescence conjugated anti-IgGs were purchased from Jackson ImmunoResearch Laboratories, Anti-MMP-2 and anti-MMP-9 from Chemicon International, recombinant proteins of MMP-2 and MMP-9 as well as inhibitor of MMP-2 and MMP-9 were from Calbiochem and MTS reagents from Promega.
Assay for MMP-2 and MMP-9 activity by zymography
The activities of MMP-2 and -9 in the conditioned media were assayed by zymography as described previously (31). Briefly, conditioned media were subjected to gel electrophoresis containing 0.3% gelatin. Then gels were washed, incubated for 18 h at 37°C in the reaction buffer (50 mM Tris-HCl, pH 7.4, 10 mM CaCl2) followed by staining and destaining.
Electrophoresis, immunoblotting and immunoprecipitation
Electrophoresis, immunoblotting and immunoprecipitation were performed as described elsewhere (29, 32). Briefly, proteins were subjected to SDS-PAGE and transferred to polyvinylidene difluoride membrane. The membrane was blocked and incubated with respective antibody followed by incubation with secondary antibody. Proteins were visualized by enhanced chemiluminescence (Amersham). For immunprecipitation of specific proteins, cells lysates were incubated with respective antibody followed by incubation with protein-A sepharose beads (Thermo scientific). Beads were subsequently washed and extracted the protein with 2× sample buffer.
Cell proliferation assay (MTS)
Cells were seeded at a density of 1,000 cells per well in 96-well plates for 24 hrs and MTS assay was performed according to Manufacturers’ recommendation (Promega).
[3H]-Thymidine incorporation assay
GFP or PKD1 stably expressed DU145 cells were plated (5×103 cells per well) in 24-well plates with complete medium, serum starved overnight, treated with DMSO, 5µM MMP-2 inhibitor, 200nM MMP-9 inhibitor or recombinant MMP-2 and -9 (300ng/ml) proteins, labeled with [3H]-thymidine (0.25 µci/well), incubated for 48 hrs, washed sequentially with ice-cold phosphate-buffered saline (PBS), 5% ice-cold trichloroacetic acid (TCA) with 5 minutes incubation on ice, ice-cold PBS, solubilized in a solution of 200 µl 0.5% SDS/0.5N NaOH. Then 150 µl of cell solution was mixed with 3 ml scintillation fluid and counted by liquid scintillation counter.
RNA isolation and semi-quantitative RT-PCR
Total RNA was isolated from respective cells using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. RNA (1 µg) from each sample was reverse-transcribed using Superscript II (Invitrogen). Relative expression level of proliferating cell nuclear antigen (PCNA) was determined by semi-quantitative PCR. The PCNA primers were 5'-GGCGTGAACCTCACCAGTAT-3' (forward) 5'-TGTCCCATATCCGCAATTTT-3' (reverse). Specific primers for GAPDH were 5'-TCACCATCTTCCAGGAGC-3' (forward) and 5'-GGATGATGTTCTGGAGAGCC-3' (reverse). Platinum-Taq DNA polymerase (Invitrogen) was used in all amplification reactions to minimize nonspecific product amplification. The number of amplification cycles was 24 for PCNA and GAPDH.
Condensation of conditioned media
Condensation of conditioned media was performed as described previously (33). Briefly, cells were seeded on 6 cm dish at a concentration of 1×106 cells per dish. After 24 hrs incubation, medium was changed to serum free RPMI and incubated for additional 24 hrs. Then the conditioned media were collected and secreted proteins were ethanol precipitated, dissolved in 2× sample buffers for western blotting.
Immunoflurosence
Cells were cultured on cover slips until subconfluence and processed for immunofluorescence study. The coverslips were incubated with primary antibodies in 10% FBS in PBS. After washing with PBS, coverslips were incubated with fluorescein isothiocynate, conjugated secondary antibodies (Jackson ImmunoResearch Laboratories) for 1 h. The cells were washed in PBS and mounted on slides with Slow Fade Antifade reagent (with DAPI). Photographs were taken at 400× magnification using Olympus fluorescent microscope followed by analysis with imaging software (Photoshop).
Soft agar colony assay
Cells (5×104) were mixed with 0.36% agar in RMPI supplemented with 10% FBS and overlaid onto a 0.72% agar layer in 6-well plates. After 3 weeks of incubation, colonies in randomly selected fields (400× magnification) were counted.
Data mining and bioinformatics analysis
Three data sets (GSE3325, GSE6919 and GSE6099) were downloaded from http://www.ncbi.nlm.nih.gov/geo/ (last accessed on November 11, 2009: 6 PM) and analyzed using the statistical language R (34). All data were log transformed using RMA (Robust Multichip Average) algorithm. Normalized expression values were used to plot the data. Scatter plot were over laid on linear regression lines.
Results
PKD1 increases production of MMP-2 and -9 in DU145 and PC3 cells
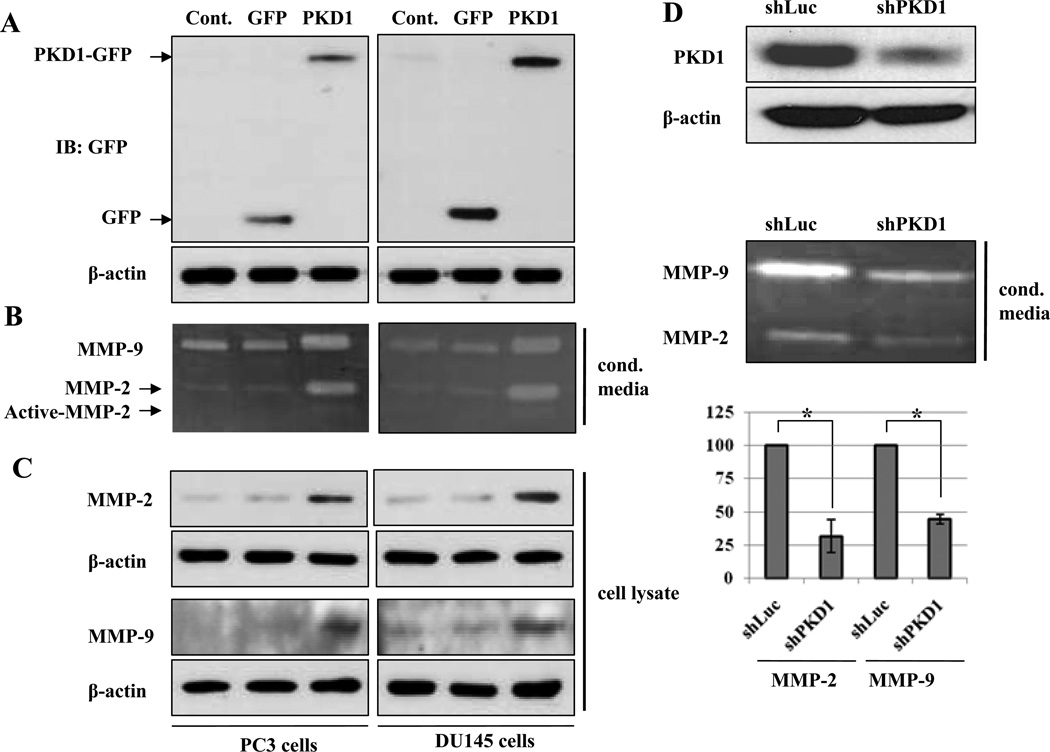
To explore the role of PKD1 in production of MMP-2 and -9, we first examined the condition media from DU145 and PC3 cells that were stably expressed GFP or PKD1-GFP (Fig. 1A) for MMP-2 and -9 secretion by zymography. As shown in Fig.1B, PKD1-GFP transfected cells demonstrate increased secretion and activation of MMP-2 or -9, compared to GFP-transfected and parental cells. These results strongly suggest that PKD1 is a positive modulator for secretion and activation of MMP-2 or -9 in DU145 and PC3 prostate cancer cells. To clarify whether the PKD1-induced secretion of MMP-2 and -9 in prostate cancer cells is associated with intracellular expression of MMP-2 and -9, we immunoblotted cell lysates with MMP-2 and -9 antibodies. As shown in Fig.1C, PKD1 increased expression of MMP-2 and -9 in DU145 and PC3 cells compared to GFP-transfected and parent cells. Our results suggest that PKD1 up regulated the expression and secretion of MMP-2 and -9. As a corollary, DU145 cells were transfected with shLuc as control or shPKD1 to knockdown expression of PKD1 demonstrated that secretion of MMP-2 and -9 were suppressed in the PKD1 knockdown DU145 cells by 68% and 55%, respectively, compared to shLuc transfected control cells (Fig. 1D).
Figure 1.
PKD1 increases production of MMP-2 and MMP-9 in prostate cancer cells. (A) Non-transfected (Cont.), GFP transfected (GFP) and PKD1-GFP transfected (PKD1) PC3 and DU145 cells were lysed and immunoblotted with GFP and β-actin antibodies, respectively. (B) Conditioned media were collected from same cells and subjected to gelatin zymography. (C) Same cell lysates as in (A) were immunoblotted with MMP-2, MMP-9 and β-actin, antibodies. (D) DU145 cells were transfected with shLuc and shPKD1 constructs. After 24h, cells were lysed and immunoblotted with PKD1 and β-actin antibodies, respectively (top). Conditioned media were collected from the same cell types and subjected to gelatin zymography (middle). Mean value of densitometric analysis of three independent experiments was shown in chart (bottom). Bar; standard deviation (SD) of mean, * P<0.05.
Phosphorylation of Mek and Erk is required for PKD1-induced secretion of MMP-2 and -9
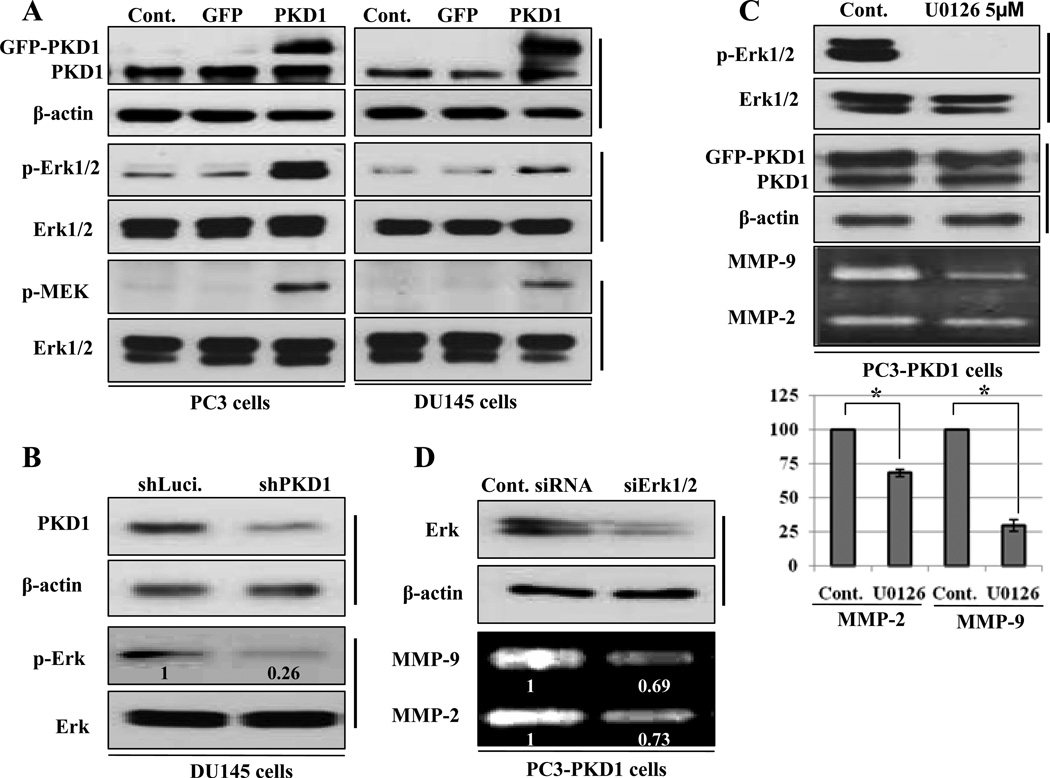
Previous reports suggest that MMP-2 or MMP-9 secretion is regulated by Ras-dependent Erk pathway (31, 35). To find out the downstream effectors of PKD1 signaling on secretion of MMP-2 and -9, we studied the activation of Mek and Erk in PKD1-GFP, GFP-expressing and parent PC3 and DU145 cells by immunoblotting with phospho specific antibodies for Mek and Erk proteins. The activated form of Mek and Erk proteins was increased in PKD1-GFP expressing cells compared to parent and GFP-expressing PC3 and DU145 cells (Fig. 2A). Alternatively, knock down of PKD1 by shRNA in DU145 cells decreased the phosphorylation of Erk (Fig. 2B), suggesting that PKD1 increases activation of Erk and Mek proteins.
Figure 2.
Mek-Erk phosphorylation is necessary for PKD1-induced MMP-2 and MMP-9 secretion in PC cells (A) Non-transfected (Cont.), GFP and PKD1-GFP transfected PC3 and DU145 cells lysates were immunoblotted with indicated antibodies. (B) DU145 cells were transfected with shLuc and shPKD1 constructs. After 24h, the cells were lysed and immunoblotted with PKD1, β-actin, p-Erk or Erk antibodies, respectively. The relative densitometric data of band intensity for p-Erk is shown in bottom of each panel. (C) PKD1 transfected stable PC3 cells were incubated with DMSO (Cont.) or U0126 (5µM) for 1h and cell lysates were immunoblotted with p-Erk, Erk, PKD1 or β-actin antibodies. Conditioned media were collected from each condition and subjected to gelatin zymography. Mean value of densitometric analysis of three independent experiments was shown in chart (bottom). Bar; standard deviation (SD) of mean, * P<0.05 (D) PC3-PKD1 cells were transfected with control siRNA or siErk. After 24 hours of transfection, cells were lysed and immunoblotted with Erk and β-actin antibodies. Conditioned media were collected from each condition and subjected to gelatin zymography (bottom of each panel). Relative densitometric data of band intensity were shown in bottom of each panel.
To confirm the role of Erk in PKD1-induced secretion of MMP-2 and -9, we used specific MEK inhibitor as well as siErk to examine the effect on MMP-2 and -9 secretion in PC3-PKD1 cells. Incubation of PC3-PKD1 cells with U0126, a specific inhibitor of MEK completely blocked the PKD1-induced activation of Erk without altering PKD1 expression (Fig. 2C). The secretion of MMP-2 and -9 was also suppressed by 31% and 70%, respectively with UO126 respectively compared to control cells (Fig. 2C). Similarly, knockdown of Erk suppressed MMP-2 and -9 secretion remarkably (Fig. 2D). These biochemical and genomic experimental data clearly demonstrate that PKD1-induced Erk activity influences MMP-2 and -9 secretion.
PKD1-β3-integrin complex regulates the secretion of MMP-2 and -9
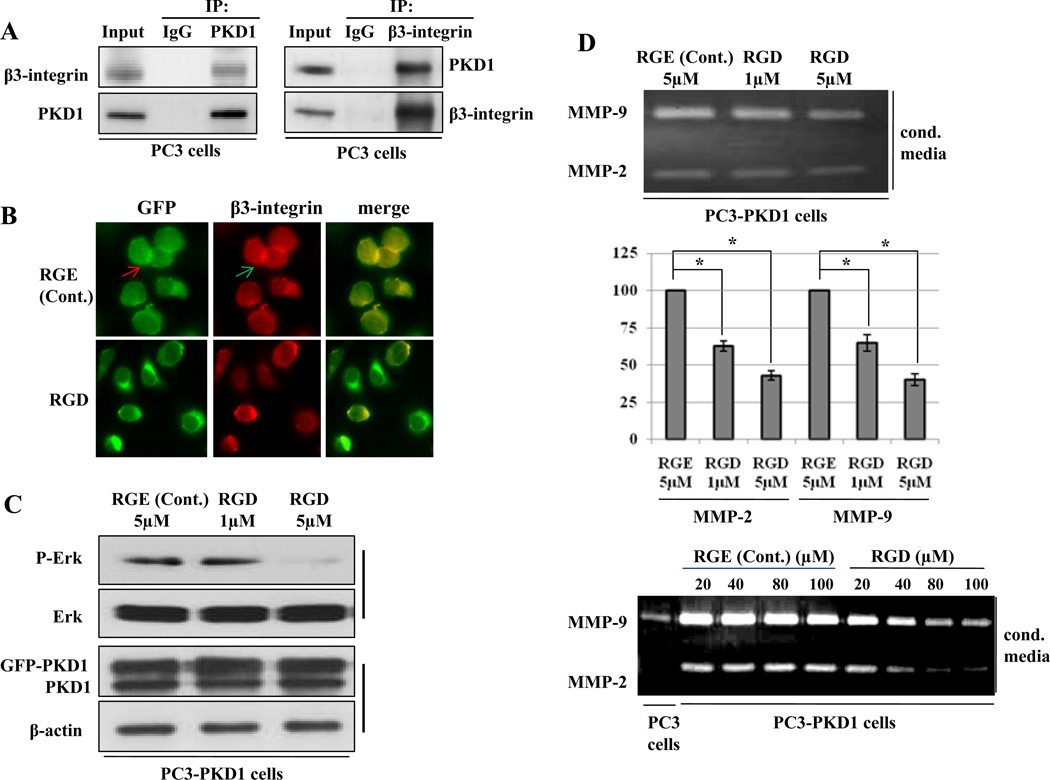
Several groups have reported that PKD1 complexes with β3-integrin and regulates various signaling pathways (19, 20), including the Erk and Akt activation (36, 37). We explored the presence of PKD1-β3-integrin protein complex in PC3 cells by immunoprecipitation and found that PKD1 and β3-integrin antibodies could immunoprecipitate β3-integrin and PKD1, respectively (Fig. 3A) in prostate cancer cells. Moreover, PKD1 and β3-integrin colocalized in the presence of β3-integrin mimetic RGE-peptide, which was abolished following treatment with RGD-peptide, a highly specific β3-integrin inhibitor (Fig. 3B), suggesting that PKD1 and β3-integrin exists in the same protein complex in vivo.
Figure 3.
PKD1 and β3-integrin form a protein complex and regulate the secretion of MMP-2 and -9 in PC3 cells. (A) Equal amount of proteins were immunoprecipitated with anti-PKD1 or anti-β3-integrin and immunoblotted with β3-integrin and PKD1 antibodies. (B) PKD1-GFP transfected PC3 cells were incubated with RGE-peptide, a control peptide, and RGD-peptide, a specific inhibitor of β3-integrin, immunostained with β3-integrin (red), PKD1-GFP (green) and merged (Yellow). (C) RGE-peptide or RGD-peptide incubated PKD1 overexpressed PC3 cells were lysed and immunoblotted with p-Erk, Erk, PKD1 and β-actin antibodies. (D) Conditioned media were collected and subjected to gelatin zymography (top and bottom). Mean value of densitometric analysis of three independent experiments for top-figure was shown in chart (middle). Bar; standard deviation (SD) of mean, * P<0.05.
We also observed that the inhibitory RGD-peptide suppressed the phosphorylation of Erk in PC3-PKD1 cells in a dose-dependent manner without altering PKD1 expression (Fig. 3C), whereas treatment with control RGE-peptide did not demonstrate any such effect. The secretion of MMP-2 and -9 was also suppressed by the RGD-peptide, but not by RGE-peptide (Fig. 4D). Quantitative analysis showed that 1µM and 5µM RGD-peptide suppressed MMP-2 and -9 by 37% and 35%; 57% and 59%, respectively compared to control RGE-peptide, suggesting a dose dependent response. Collectively, our results suggest that PKD1-β3-integrin complex is a signaling molecular complex which induces phosphorylation of Erk resulting in increased the secretion of MMP-2 and -9 in prostate cancer cells.
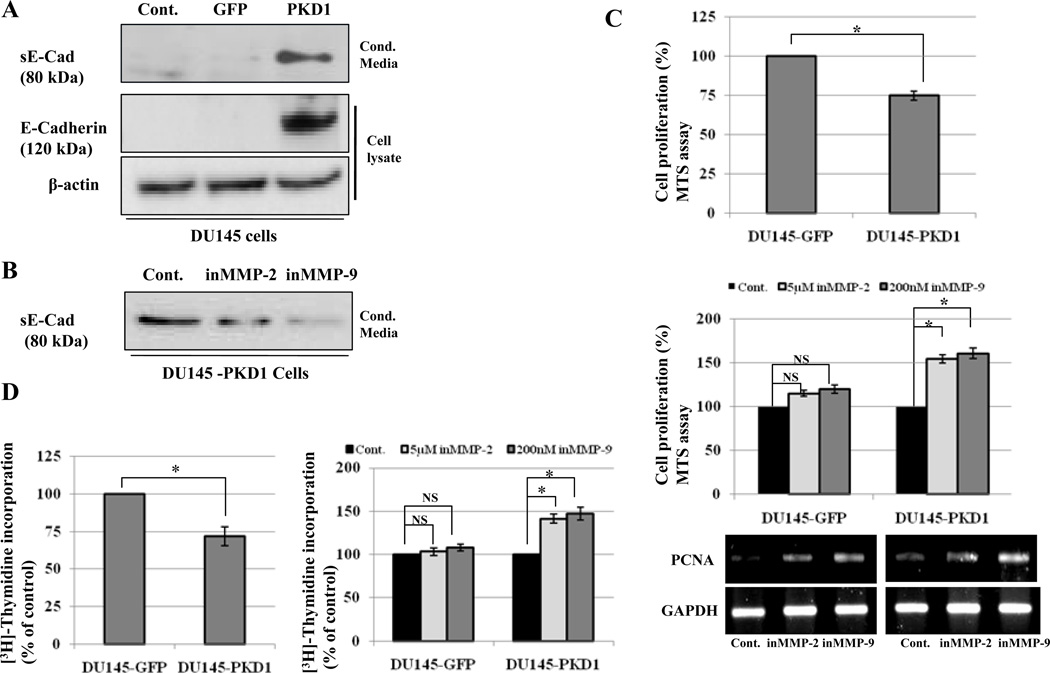
Figure 4.
PKD1 enhances E-cadherin shedding through MMP-2 and MMP-9 and suppresses cell proliferation. (A) Conditioned media were collected from Control, GFP and PKD1-GFP over expressing DU145 cells and immunoblotted with E-cadherin antibody (first panel). Lysates from same cells were immunoblotted with E-cadherin and β-actin antibodies, respectively. (B) PKD1 over expressing DU145 cells were incubated with DMSO (Cont.), 5µM MMP-2 inhibitor or 200nM MMP-9 inhibitor for 24 hrs and conditioned media were immunoblotted with E-cadherin antibody. (C and D) MTS and [3H]-thymidine incorporation assay was performed with GFP and PKD1-GFP over expressing DU145 cells. The cells were incubated with DMSO (Cont.), inhibitor of MMP-2 or -9 for 48 h. Cell proliferation was measured by MTS and [3H]-thymidine incorporation assay and expressed as a percentage of control. Data are the means of three independent experiments with triplicate samples. Bar; standard deviation (SD) of mean, * P<0.05. Results from RT-PCR of PCNA and GAPDH from the cells treated with MMP-2 and -9 inhibitors are also shown (Fig.4C bottom).
PKD1-induced secretion of MMP-2 and -9 increases E-cadherin shedding and suppresses cell proliferation
MMPs are known to cause E-cadherin shedding by proteolysis of extracellular domain (38). We explored whether increased secretion of MMP-2 and -9 by PKD1 resulted in shedding of soluble E-cadherin (sE-cad) in conditioned media, which can be used as a read out of MMP activity. Overexpression of PKD1 enhanced production of sE-cad in conditioned media of DU145 cells compared to GFP-transfected and parental cells (Fig. 4A). Incubation of PKD1-DU145 cells with DMSO, MMP-2 or MMP-9 inhibitors suppressed sE-cad in conditioned media (Fig. 4B). PKD1 suppressed proliferation of DU145 cells, which was interestingly rescued by treatment with MMP-2 and MMP-9 inhibitors (Fig. 4C and D). In contrast, MMPs inhibitor has no effect in GFP-DU145 cells (Fig. 4C and D). These results were corroborated by RT-PCR of PCNA, a cell proliferation marker under same conditions (Fig. 4C, lower panel)). The results suggest that MMP-2 and -9 could mediate PKD1-induced suppression of cell proliferation in DU145 cells.
Recombinant MMP-2 and MMP-9 proteins suppress anchorage-independent and dependent growth of PKD1-overexpressing prostate cancer cells
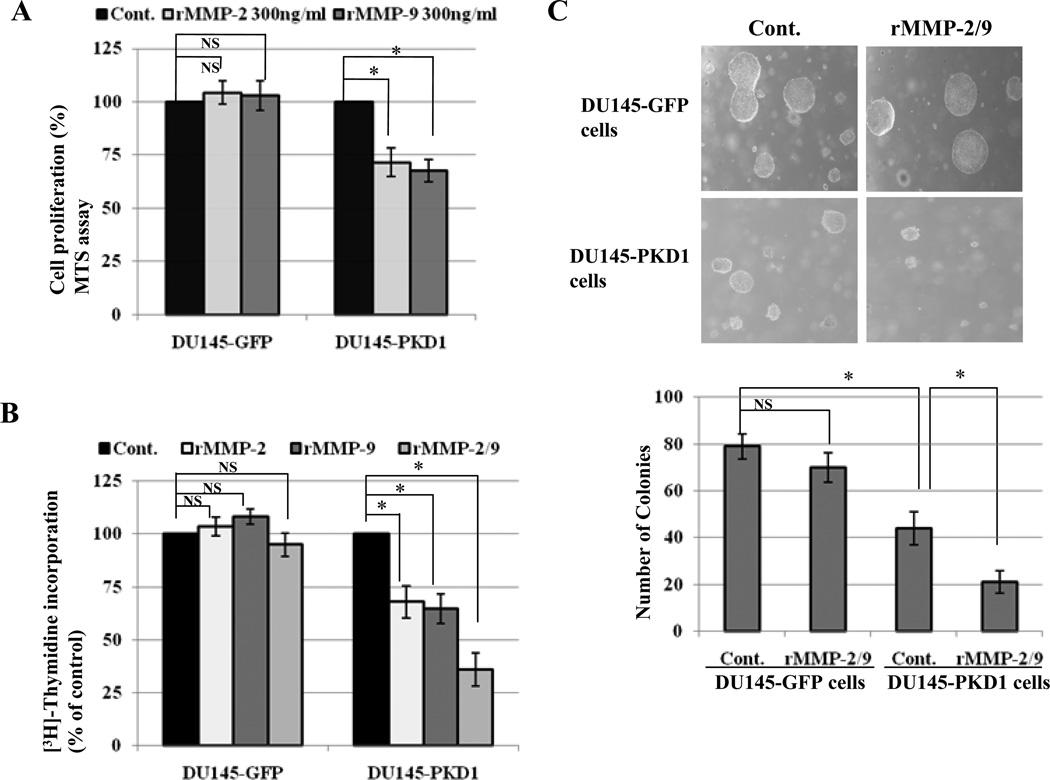
To further confirm the inhibitory role of MMP-2 and MMP-9 on cell proliferation, GFP-overexpressed and PKD1-overexpressed DU145 cells were incubated with recombinant MMP-2 and MMP-9 (rMMP-2, rMMP-9). The results showed that rMMP-2 and rMMP-9 suppressed the proliferation of PKD1 overexpressing cells only (Fig. 5A and B) and also inhibited anchorage-independent growth on soft agar by significant reduction in number of colonies in PKD1-DU145 cells compared to controls (Fig. 5C).
Figure 5.
Recombinant MMP-2 and MMP-9 suppressed proliferation and soft agar colony formation of PKD1-overexpressed PC cell. (A and B) GFP and PKD1-GFP over expressing DU145 cells were incubated with DMSO (Cont.), 300ng/ml rMMP-2, 300ng/ml rMMP-9 or both (rMMP-2/9) for 48 hrs. Cell proliferation was measured by MTS and [3H]-thymidine incorporation assay and percentage of cell proliferation was calculated compared to control. Bars; standard deviation (SD) of mean, * P<0.05. (B) Soft agar colony formation assay of same cells with control or rMMP-2 and rMMP-9 (rMMP-2/9) treatment. Picture was taken at 400X magnification. The numbers of colonies were counted and mean values of three independent experiments are shown. Bar; standard deviation (SD) of mean, * P<0.05.
Expression of PKD1 directly correlates with MMP-2 expression in human prostate tissue
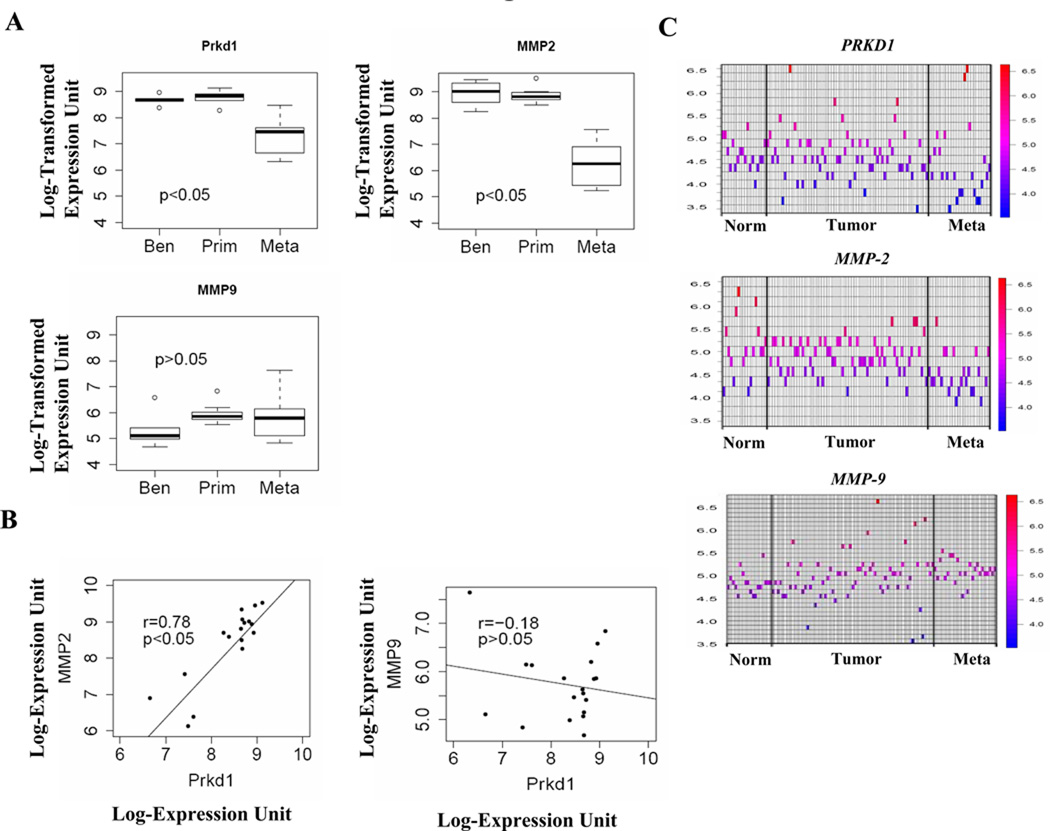
We interrogated publicly available DNA microarray expression data sets derived from human prostate benign and cancerous tissues using search terms PRKD1 (PKD1), MMP-2 and MMP-9 in NCBI’s Gene Expression Omnibus (GEO). We focused initially on data sets studying benign, primary and metastatic prostate cancer. Three data sets (GSE3325, GSE6919 and GSE6099) were identified and the gene expression data compared. As we previously reported (21), expression of PRKD1 was reduced in metastatic samples in all data sets analysis, but not in benign and primary tumor samples (Fig 6; data from 2 of 3 data sets shown). Similarly, MMP-2 gene expression was also down regulated in prostate metastatic samples only. As shown in Fig. 6B, we observed a significant positive correlation between PRKD1 and MMP-2 across all samples with r = 0.78 and p<0.05. Surprisingly, the expression of MMP-9 did not significantly correlate with PKD1 gene expression (r = −0.18, p>0.05).
Figure 6.
The expression of MMP-2 positively correlates with PKD1 (PRKD1) in human prostate cancer tissue. (A) Box plots of PRKD1, MMP-2 and MMP-9 expression levels in Benign (Ben), Primary (Prim) and Metastatic (Meta) human prostate cancer tissue microarray data set (GSE3325). The box represents the interquartile range of data with various samples and line through that box represents the median of the distribution. The range is indicated by the whiskers on the plot. Benign tumor, n = 6; Primary tumor, n = 7; and Metastatic tumor samples, n = 6. (B) Scattered plots of PRKD1 versus MMP-2 and MMP-9 of all samples displayed in (A). (C) The heat map presentation of PRKD1, MMP-2 and MMP-9 expression in Normal (Norm), Primary tumor and Metastatic (Meta) prostate tissue from microarray data set GSE6919.
The heat map plots the expression values (GSE6919) for the genes under consideration in each of the microarray chips used in this study. It confirms the behavior of PRDK1 stated earlier, in that the individual heat spots (expression values) tend towards the blue color (lower values) when expressed in normal, tumor or metastatic prostate tissues. Similarly, the expression signals for MMP-2 tend to be become smaller as the prostate tissue transforms to the metastatic state. In the case of MMP-9, only a weak trend in the opposite direction can be observed (Fig. 6C). There are more red spots as the tissue moves to the metastatic state.
Discussion
The association between MMPs and cancer can be traced back to 1970s and in fact, several MMP inhibitors have been evaluated clinically with very limited success (39). Arguably, several reasons are cited for failure of MMP inhibitors in clinical setting including incomplete understanding of various MMP isoforms and their functions. While initially MMPs were thought to play a major role in cancer progression, there is emerging data that several isoforms of MMPs also demonstrate anti-tumor properties (9, 10). MMPs can perform anti-tumor functions by stimulating apoptosis, regulating inflammation, angiogenesis inhibition, metastasis suppression, and altered hormonal signaling (40). In this study we demonstrate that MMP-2 and -9 inhibit proliferation of prostate cancer cells. MMP-9 along with MMP-3, -11 and -19 are examples of dual proteases that show pro- and anti-tumor roles, which are context dependent (39). We have identified MMP-2 as another MMP that shows anti-tumor properties in prostate cancer.
MMPs are well known to cleave cell surface proteins including adhesion molecules, apoptotic protein, cell surface receptors, cytokines, growth factors, proteases, intercellular junctional and structural proteins with important consequences on cellular phenotypic behavior (41). Of particular interest in cancer is the major cell adhesion protein, E-cadherin, which can be shed following cleavage of extracellular domain by MMPs, although the exact isoforms involved are unknown. Current study suggests that MMP-2 and -9 may be involved in the process. In fact, a 80 kDa shedding E-cadherin fragment is detectable in urine and serum of patients with variety of cancers and serum levels have been shown to correlate positively with metastatic prostate cancer and disease recurrence (42, 43). The consequence of sE-cadherin shedding can be manifold. For one, E-cadherin shedding causes loss of cell to cell adhesion and lead to epithelial mesenchymal transition (44). In addition, β-catenin bound to cytoplasmic domain of E-cadherin is disengaged and can translocate to nucleus with resultant effects on β-catenin dependent transcription and cell proliferation (45).
On the other hand, the direct role of shed sE-cadherin fragment on cells is unclear. Using immunoprecipitation technique to remove sE-cadherin from media, we were unable to demonstrate difference in growth of DU145 cells either in presence or absence of sE-cadherin (data not shown). While other studies have demonstrated that sE-cadherin shedding through induction of Kallikrein 6 in skin cancer cells or treatment with hepatocyte growth factor in stomach cancer cells is associated with increased cell proliferation and motility, whether the effects are directly due to of shedding of sE-cadherin fragment is unclear (46, 47). The data in the literature suggests sE-cadherin shedding is an association rather than causal in cellular phenotypic effects observed and that other mechanisms may be involved. However, other cleavage substrates of MMPs have been shown to affect cellular phenotype. MMPs might negatively regulate cancer-cell growth by regulating pro-apoptotic molecules like Fas ligand (FasL) and TNFα, which have a tumor suppressor effect in early phase of oncogenesis (7). The Fas ligand/Fas receptor (FasL/Fas) is an important mediator for apoptosis and has also been shown to be involved in apoptosis of prostate epithelial tissues. MMP-7 and MMP-3 can shed soluble FasL (sFasL) that induces the apoptosis in epithelial cells through FasL/Fas pathway (10, 41).
It is also interesting in this study that MMP-2 and -9 demonstrate antiproliferative effect in PKD1 transfected cells but not in controls, suggesting over expression of PKD1 is necessary for anti-proliferative effects of MMP-2 and -9. We have previously demonstrated that PKD1 and E-cadherin epistatically influence cell proliferation and motility (23) and PKD1 increases E-cadherin expression (Fig. 4A). It is possible that increased E-cadherin expression provides necessary substrate for MMP-2 and -9 dependent sE-cadherin shedding. We also demonstrated that other proteins such as β-catenin and Heat Shock Protein 27 (Hsp27) are mediators of PKD1 effect on cell proliferation (23, 30). PKD1 is also known to interact with several other proteins, of which, integrins are a family of heterodimer proteins that are also known to influence MMP secretion in cells (48).
PKD1 complexes with β3-integrin protein that is important for activation of Erk kinase and consequently increased secretion of MMP-2 and -9 in PC cells. Notably, PKD1 influences cell migration by promoting the recycling of αvβ3-integrin to form a polarized distribution of focal adhesions at the leading edge of migrating cells (19) and also promotes β1-integrin activation in T cells by regulating Rap1 activation (20). The data from this study provides in vivo evidence of active PKD1-integrin-Erk pathway in prostate cancer that accounts for increased MMP-2 and -9 secretion. The mechanistic understanding of signaling pathway in MMP-2 and -9 secretion may be important in designing therapeutic strategies and biomarker evaluation in prostate cancer. In order to successfully transfer the knowledge gained from this study to patient care, validation in human tissue samples is critical.
In silico analysis demonstrates a significant direct correlation between PKD1 and MMP-2 expression but not with MMP-9. While the inconsistency in the data with regard to MMP-9 expression may highlight the potential limitations of cell line studies, the expression of MMP-9 in study tissue is about half that of MMP-2, which may have resulted in false negative results. Moreover, MMP-9 expression is associated with favorable prognosis in node negative breast cancer and inversely with liver metastasis in colorectal cancer, thereby validation the protective role of this enzyme in other cancers (49, 50). However, relationship between PKD1 and MMP-2 expression is validated in human tissues and the tumor suppressive effects of MMP-2 in prostate cancer are clearly novel. Further mechanistic and validation studies with large cohort are necessary to assess the clinical utility of PKD1-MMP-2 and -9 expression in prostate cancer.
In summary, our study demonstrates that PKD1 increases MMP-2 and -9 secretion resulting in decreased prostate cancer cell proliferation and increased sE-cadherin shedding. The β3 integrin-ERK pathway is involved in PKD1 mediated increased MMP-2 and -9 secretion and expression; PKD1 correlates directly with MMP-2 secretion in human tissues, which may be potential protein targets in human prostate cancer for either biomarker development or therapeutics. The tumor suppressive effect of MMP-2 and -9 in prostate cancer is novel and provides impetus for further mechanistic studies.
Acknowledgment
We thank Dr. F.J. Johannes, Fraunhofer Institute for Interfacial Engineering, Stuttgart, Germany for providing the PKD1 expression vector and Dr. Gary Stein’s Lab, University of Massachusetts Medical School, USA for radioisotope facility. This work was supported by Institutional Grant from Department of Surgery, University of Massachusetts Medical School (KCB, HB, CD and CZ); NIH grants R01CA109874 (LRL) and DK032520 (JS).
References
- 1.Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- 2.Kantak SS, Kramer RH. E-cadherin regulates anchorage-independent growth and survival in oral squamous cell carcinoma cells. J Biol Chem. 1998;273:16953–16961. doi: 10.1074/jbc.273.27.16953. [DOI] [PubMed] [Google Scholar]
- 3.Alexander CM, Howard EW, Bissell MJ, Werb Z. Rescue of mammary epithelial cell apoptosis and entactin degradation by a tissue inhibitor of metalloproteinases-1 transgene. J Cell Biol. 1996;135:1669–1677. doi: 10.1083/jcb.135.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stetler-Stevenson WG, Krutzsch HC, Wacher MP, Margulies IM, Liotta LA. The activation of human type IV collagenase proenzyme. Sequence identification of the major conversion product following organomercurial activation. J Biol Chem. 1989;264:1353–1356. [PubMed] [Google Scholar]
- 5.McCawley LJ, Matrisian LM. Matrix metalloproteinases: they're not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 6.Puente XS, Sanchez LM, Overall CM, Lopez-Otin C. Human and mouse proteases: a comparative genomic approach. Nat Rev Genet. 2003;4:544–558. doi: 10.1038/nrg1111. [DOI] [PubMed] [Google Scholar]
- 7.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 8.Kayagaki N, Kawasaki A, Ebata T, et al. Metalloproteinase-mediated release of human Fas ligand. J Exp Med. 1995;182:1777–1783. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witty JP, Lempka T, Coffey RJ, Jr, Matrisian LM. Decreased tumor formation in 7,12-dimethylbenzanthracene-treated stromelysin-1 transgenic mice is associated with alterations in mammary epithelial cell apoptosis. Cancer Res. 1995;55:1401–1406. [PubMed] [Google Scholar]
- 10.Powell WC, Fingleton B, Wilson CL, Boothby M, Matrisian LM. The metalloproteinase matrilysin proteolytically generates active soluble Fas ligand and potentiates epithelial cell apoptosis. Curr Biol. 1999;9:1441–1447. doi: 10.1016/s0960-9822(00)80113-x. [DOI] [PubMed] [Google Scholar]
- 11.Sheu BC, Hsu SM, Ho HN, Lien HC, Huang SC, Lin RH. A novel role of metalloproteinase in cancer-mediated immunosuppression. Cancer Res. 2001;61:237–242. [PubMed] [Google Scholar]
- 12.Balbin M, Fueyo A, Tester AM, et al. Loss of collagenase-2 confers increased skin tumor susceptibility to male mice. Nat Genet. 2003;35:252–257. doi: 10.1038/ng1249. [DOI] [PubMed] [Google Scholar]
- 13.Hartland SN, Murphy F, Aucott RL, et al. Active matrix metalloproteinase-2 promotes apoptosis of hepatic stellate cells via the cleavage of cellular N-cadherin. Liver Int. 2009;29:966–978. doi: 10.1111/j.1478-3231.2009.02070.x. [DOI] [PubMed] [Google Scholar]
- 14.Wielockx B, Lannoy K, Shapiro SD, et al. Inhibition of matrix metalloproteinases blocks lethal hepatitis and apoptosis induced by tumor necrosis factor and allows safe antitumor therapy. Nat Med. 2001;7:1202–1208. doi: 10.1038/nm1101-1202. [DOI] [PubMed] [Google Scholar]
- 15.Lokeshwar BL. MMP inhibition in prostate cancer. Ann N Y Acad Sci. 1999;878:271–289. doi: 10.1111/j.1749-6632.1999.tb07690.x. [DOI] [PubMed] [Google Scholar]
- 16.Jaggi M, Du C, Zhang W, Balaji KC. Protein kinase D1: a protein of emerging translational interest. Front Biosci. 2007;12:3757–3767. doi: 10.2741/2349. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz MA, Baron V. Interactions between mitogenic stimuli, or, a thousand and one connections. Curr Opin Cell Biol. 1999;11:197–202. doi: 10.1016/s0955-0674(99)80026-x. [DOI] [PubMed] [Google Scholar]
- 18.Shattil SJ. Signaling through platelet integrin alpha IIb beta 3: inside-out, outside-in, and sideways. Thromb Haemost. 1999;82:318–325. [PubMed] [Google Scholar]
- 19.Woods AJ, White DP, Caswell PT, Norman JC. PKD1/PKCmu promotes alphavbeta3 integrin recycling and delivery to nascent focal adhesions. EMBO J. 2004;23:2531–2543. doi: 10.1038/sj.emboj.7600267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medeiros RB, Dickey DM, Chung H, et al. Protein kinase D1 and the beta 1 integrin cytoplasmic domain control beta 1 integrin function via regulation of Rap1 activation. Immunity. 2005;23:213–226. doi: 10.1016/j.immuni.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Jaggi M, Rao PS, Smith DJ, Hemstreet GP, Balaji KC. Protein kinase C mu is down-regulated in androgen-independent prostate cancer. Biochem Biophys Res Commun. 2003;307:254–260. doi: 10.1016/s0006-291x(03)01161-6. [DOI] [PubMed] [Google Scholar]
- 22.Jaggi M, Rao PS, Smith DJ, et al. E-cadherin phosphorylation by protein kinase D1/protein kinase C{mu} is associated with altered cellular aggregation and motility in prostate cancer. Cancer Res. 2005;65:483–492. [PubMed] [Google Scholar]
- 23.Syed V, Mak P, Du C, Balaji KC. Beta-catenin mediates alteration in cell proliferation, motility and invasion of prostate cancer cells by differential expression of E-cadherin and protein kinase D1. J Cell Biochem. 2008;104:82–95. doi: 10.1002/jcb.21603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 25.Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- 26.Steinhusen U, Weiske J, Badock V, Tauber R, Bommert K, Huber O. Cleavage and shedding of E-cadherin after induction of apoptosis. J Biol Chem. 2001;276:4972–4980. doi: 10.1074/jbc.M006102200. [DOI] [PubMed] [Google Scholar]
- 27.Fouquet S, Lugo-Martinez VH, Faussat AM, et al. Early loss of E-cadherin from cell-cell contacts is involved in the onset of Anoikis in enterocytes. J Biol Chem. 2004;279:43061–43069. doi: 10.1074/jbc.M405095200. [DOI] [PubMed] [Google Scholar]
- 28.Noe V, Fingleton B, Jacobs K, et al. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci. 2001;114:111–118. doi: 10.1242/jcs.114.1.111. [DOI] [PubMed] [Google Scholar]
- 29.Du C, Jaggi M, Zhang C, Balaji KC. Protein kinase D1-mediated phosphorylation and subcellular localization of beta-catenin. Cancer Res. 2009;69:1117–1124. doi: 10.1158/0008-5472.CAN-07-6270. [DOI] [PubMed] [Google Scholar]
- 30.Hassan S, Biswas MH, Zhang C, Du C, Balaji KC. Heat shock protein 27 mediates repression of androgen receptor function by protein kinase D1 in prostate cancer cells. Oncogene. 2009 doi: 10.1038/onc.2009.291. [DOI] [PubMed] [Google Scholar]
- 31.Ruhul Amin AR, Uddin Biswas MH, Senga T, et al. A role for SHPS-1/SIRPalpha in Concanavalin A-dependent production of MMP-9. Genes Cells. 2007;12:1023–1033. doi: 10.1111/j.1365-2443.2007.01115.x. [DOI] [PubMed] [Google Scholar]
- 32.Mak P, Jaggi M, Syed V, et al. Protein kinase D1 (PKD1) influences androgen receptor (AR) function in prostate cancer cells. Biochem Biophys Res Commun. 2008;373:618–623. doi: 10.1016/j.bbrc.2008.06.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biswas MH, Hasegawa HH, Rahman MA, et al. SHP-2-Erk signaling regulates concanavalin A-dependent production of TIMP-2. Biochem Biophys Res Commun. 2006;348:1145–1149. doi: 10.1016/j.bbrc.2006.07.173. [DOI] [PubMed] [Google Scholar]
- 34.Gentleman RC, Carey VJ, Bates DM, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu E, Thant AA, Kikkawa F, et al. The Ras-mitogen-activated protein kinase pathway is critical for the activation of matrix metalloproteinase secretion and the invasiveness in v-crk-transformed 3Y1. Cancer Res. 2000;60:2361–2364. [PubMed] [Google Scholar]
- 36.Zheng DQ, Woodard AS, Tallini G, Languino LR. Substrate specificity of alpha(v)beta(3) integrin-mediated cell migration and phosphatidylinositol 3-kinase/AKT pathway activation. J Biol Chem. 2000;275:24565–24574. doi: 10.1074/jbc.M002646200. [DOI] [PubMed] [Google Scholar]
- 37.Eliceiri BP, Klemke R, Stromblad S, Cheresh DA. Integrin alphavbeta3 requirement for sustained mitogen-activated protein kinase activity during angiogenesis. J Cell Biol. 1998;140:1255–1263. doi: 10.1083/jcb.140.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ito K, Okamoto I, Araki N, et al. Calcium influx triggers the sequential proteolysis of extracellular and cytoplasmic domains of E-cadherin, leading to loss of beta-catenin from cell-cell contacts. Oncogene. 1999;18:7080–7090. doi: 10.1038/sj.onc.1203191. [DOI] [PubMed] [Google Scholar]
- 39.Lopez-Otin C, Palavalli LH, Samuels Y. Protective roles of matrix metalloproteinases: From mouse models to human cancer. Cell Cycle. 2009;8 doi: 10.4161/cc.8.22.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez-Otin C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800–808. doi: 10.1038/nrc2228. [DOI] [PubMed] [Google Scholar]
- 41.Cauwe B, Van den Steen PE, Opdenakker G. The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit Rev Biochem Mol Biol. 2007;42:113–185. doi: 10.1080/10409230701340019. [DOI] [PubMed] [Google Scholar]
- 42.Protheroe AS, Banks RE, Mzimba M, et al. Urinary concentrations of the soluble adhesion molecule E-cadherin and total protein in patients with bladder cancer. Br J Cancer. 1999;80:273–278. doi: 10.1038/sj.bjc.6690351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuefer R, Hofer MD, Gschwend JE, et al. The role of an 80 kDa fragment of E-cadherin in the metastatic progression of prostate cancer. Clin Cancer Res. 2003;9:6447–6452. [PubMed] [Google Scholar]
- 44.Zheng G, Lyons JG, Tan TK, et al. Disruption of E-cadherin by matrix metalloproteinase directly mediates epithelial-mesenchymal transition downstream of transforming growth factor-beta1 in renal tubular epithelial cells. Am J Pathol. 2009;175:580–591. doi: 10.2353/ajpath.2009.080983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marambaud P, Shioi J, Serban G, et al. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J. 2002;21:1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klucky B, Mueller R, Vogt I, et al. Kallikrein 6 induces E-cadherin shedding and promotes cell proliferation, migration, and invasion. Cancer Res. 2007;67:8198–8206. doi: 10.1158/0008-5472.CAN-07-0607. [DOI] [PubMed] [Google Scholar]
- 47.Lee KH, Choi EY, Hyun MS, et al. Association of extracellular cleavage of E-cadherin mediated by MMP-7 with HGF-induced in vitro invasion in human stomach cancer cells. Eur Surg Res. 2007;39:208–215. doi: 10.1159/000101452. [DOI] [PubMed] [Google Scholar]
- 48.Munshi HG, Stack MS. Reciprocal interactions between adhesion receptor signaling and MMP regulation. Cancer Metastasis Rev. 2006;25:45–56. doi: 10.1007/s10555-006-7888-7. [DOI] [PubMed] [Google Scholar]
- 49.Scorilas A, Karameris A, Arnogiannaki N, et al. Overexpression of matrix-metalloproteinase-9 in human breast cancer: a potential favourable indicator in node-negative patients. Br J Cancer. 2001;84:1488–1496. doi: 10.1054/bjoc.2001.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takeha S, Fujiyama Y, Bamba T, Sorsa T, Nagura H, Ohtani H. Stromal expression of MMP-9 and urokinase receptor is inversely associated with liver metastasis and with infiltrating growth in human colorectal cancer: a novel approach from immune/inflammatory aspect. Jpn J Cancer Res. 1997;88:72–81. doi: 10.1111/j.1349-7006.1997.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]