Abstract
Bone morphogenetic protein-2 (BMP-2) increases oxidant stress and endoplasmic reticulum (ER) stress to stimulate differentiation of osteoblasts; however, the role of these signaling pathways in the transition of smooth muscle cells to a calcifying osteoblast-like phenotype remains incompletely characterized. We, therefore, treated human coronary artery smooth muscle cells (HCSMC) with BMP-2 (100 ng/ml) and found an increase in NADPH oxidase activity and oxidant stress that occurred via activation of the bone morphogenetic protein receptor 2 and Smad 1 signaling. BMP-2-mediated oxidant stress also increased endoplasmic reticulum (ER) stress demonstrated by increased expression of GRP78, phospho-IRE1α, and the transcription factor XBP1. Analysis of a 1 kb segment of the Runx2 promoter revealed an XBP1 binding site; electrophoretic mobility shift and chromatin immunoprecipitation assays demonstrated that XBP1 bound to the Runx2 promoter at this site in BMP-2-treated HCSMC. Inhibition of oxidant stress or ER stress decreased Runx2 expression, intracellular calcium deposition, and mineralization of BMP-2-treated HCSMC. Thus, in HCSMC, BMP-2 increases oxidant stress and ER stress to increase Runx2 expression and promote vascular smooth muscle cell calcification.
Keywords: bone morphogenetic protein-2, endoplasmic reticulum stress, oxidant stress, vascular calcification, vascular smooth muscle cells
1. Introduction
Vascular calcification increases arterial stiffness, pulse pressure, and is associated with increased adverse cardiovascular events [1,2]. Vascular calcification is a highly regulated process that recapitulates skeletal bone formation and involves dedifferentiation of vascular smooth muscle cells to assume an osteochondrogenic phenotype [3,4,5]. Runx2, a master transcription factor that is essential for osteoblast and chondrocyte differentiation, regulates the expression of bone-related proteins important for mineralization [6,7,8]. Runx2 is expressed by osteoblasts, and, once vascular smooth muscle cells express Runx2, they are considered to have acquired an osteochondrogenic phenotype [4,5,9].
Bone morphogenetic protein-2 (BMP-2), a member of the transforming growth factor-β superfamily, regulates osteoblast differentiation and bone formation [10,11,12]. BMP-2 has been identified in vivo in calcified vessels of diabetic LDLR−/− mice and has been shown to stimulate vascular calcification, in part, by regulating phosphate transport and increasing Runx2 mRNA levels in vascular smooth muscle cells in vitro [13,14].
Reactive oxygen species (ROS) and increased oxidant stress have also been implicated in the pathogenesis of vascular calcification [5,16,17,18,19]. Hydrogen peroxide was shown to increase Runx2 and alkaline phosphatase expression, calcium uptake, and vascular smooth muscle cell mineralization [15,16]. BMP2 may exert some of its effects on calcification by increasing oxidant stress; in murine 2T3 pre-osteoblast cells, BMP-2 increased oxidant stress to induce differentiation [20]. Endoplasmic reticulum (ER) stress, which may be activated as a consequence of increased oxidant stress or perturbed Ca2+ homeostasis, triggers the unfolded protein response (UPR) to limit cell damage. The UPR initiates distinct signaling pathways, including ER transmembrane inositol-requiring enzyme 1α (IRE1α) and the transcription factor XBP1, PKR-like ER kinase (PERK), and ATF6, to induce molecular chaperones and quality control protein expression. [21]. Recently, ER stress has been linked to murine osteoblast differentiation [22]; however, the relationship between BMP-2, oxidant stress, ER stress, and how these signals modulate Runx2 expression and vascular smooth muscle cell calcification remains incompletely characterized.
2. Materials and methods
2.1 Cell culture and siRNA transfection
Human coronary artery smooth muscle cells (HCSMC) (Lonza) were grown in Smooth Muscle Growth Medium-2 supplemented with SingleQuots® and experiments were conducted on cells from passages 3–5. Cells were treated with recombinant human BMP-2 (100 ng/ml) (R&D Systems) for up to 14 days. For calcium quantification studies or von Kossa staining, medium was supplemented with β-glycerophosphate (5 mmol/L). In select studies, cells were co-incubated with apocynin (3 × 10−5 mol/L). To decrease p22phox expression, HCSMC were transfected with Stealth Select RNAi™ (HSS141745) (Invitrogen) using Lipofectamine™ 2000 for 5 h in OptiMEM®I medium. Cells were then placed in full growth medium and experiments were performed after 48 h. Similar methodology was used to decrease the expression of bone morphogenetic protein receptor-2 (BMPR2) (Stealth RNAi™ HSS101067), Smad 1 (Stealth RNAi™ HSS106248), or XBP1 (Stealth RNAi™ HSS111391). (Invitrogen). Corresponding scrambled control siRNAs were selected based on the manufacturer's recommendation and transfections were carried out under similar conditions.
2.2 Alkaline phosphatase activity
Alkaline phosphatase activity was determined using the QuantiChrom™ Alkaline Phosphatase Assay kit (BioAssay Systems) according to the manufacturer's instructions.
2.3 Intracellular calcium deposition
Cells were washed with PBS and decalcified with 0.6 mmol/L HCl at 4°C for 24 h. Calcium released from the cell cultures into the supernatant was determined colorimetrically by the ο-cresolphthalein method using the Calcium Colorimetric Assay (BioVision). Calcium content was normalized to total cell protein and expressed as μg/mg cell protein.
2.4 von Kossa staining
Cells were fixed with 10% formalin for 1 h at 25°C. The cells were treated with 5% silver nitrate (Sigma-Aldrich) and exposed to UV light for 60 min. The wells were then washed and incubated with sodium thiophosphate (5%) (Sigma-Aldrich) for 5 min. After this time, the cells were washed and calcium-phosphate deposits were observed as black stained areas. Densitometry was performed using a VersaDoc (BioRad) scanning system to quantitate density.
2.5 Dichlorodihyrdofluorescein fluorescence
Non-specific cellular ROS levels were determined as described previously using 20 μM 6-carboxy-2'-7'-dichlorodihydrofluorescein diacetate di(acetoxymethyl) ester (Molecular Probes)[23].
2.6 RNA isolation and quantitative real-time PCR
RNA isolation and quantitative real-time PCR were performed as described previously using TaqMan® Universal Master Mix, and 20× TaqMan® NOX1, NOX4, Runx2, or GAPDH Gene Expression Assays (Applied Biosystems) [23]. Samples were run on Applied Biosystems 7900 HT. Quantitation of data was performed using the ΔΔCT method using GAPDH gene expression as an endogenous reference.
2.7 NADPH oxidase activity
NADPH oxidase activity was measured in isolated membrane fractions using lucigenin (5 μmol/L) chemiluminescence. To perform the assay, membrane fractions were incubated in a 50 mmol/L phosphate buffer, pH 7.0, 1 mmol/L EGTA, 150 mmol/L sucrose, 5 μmol/L dark-adapted lucigenin, and 100 μmol/L NADPH. The reaction was initiated by the addition of the membrane fraction, and luminescence was measured using a ND-20/20 luminometer (Turner Biosystems) every 15 s for 5 min. Superoxide dismutase (SOD) (120 U/ml)-inhibitable superoxide production measured as luminescence was considered to be specific for NADPH oxidase activity. Results were standardized to protein content of the membrane fraction.
2.8 Immunoblotting and densitometry
Preparation of cell homogenates and SDS-polyacrylamide gel electrophoresis was performed as described previously [23]. The membranes were incubated with antibodies to Runx2 (R&D Systems), alkaline phosphatase, p22phox, bone morphogenetic protein receptor-2, XBP1, osteocalcin, osteopontin (Santa Cruz Biotechnology), Smad 1, BiP/GRP78, phospho-IRE1α, (Cell Signaling Technology). Blots were stripped and reprobed with an antibody to tubulin (Santa Cruz Biotechnology), which served as a loading control.
2.9 Cytosolic and ER Ca2+ Measurement
Cytosolic and ER Ca2+ measurements were made at 37°C with fura-2 AM (5 μmol/L)(Molecular Probes) by calculating the ratio at 340 nm/380 nm (340 nm/380 nm excitation; 510 emission) using a SpectraMax Gemini Spectrofluorometer (Molecular Devices) according to the method of Dickhout et al [24].
2.10 Electrophoretic mobility shift assay
Nuclear preparations were isolated from cells using the BDTM TransFactor Nuclear Extraction Kit (Clontech) according to manufacturer's instructions. The oligonucleotide probe was biotin-labeled with Biotin 3' End DNA Labeling kit (Pierce). The probe contained the XBP1 binding site (−596 - −591) from the Runx2 promoter and was included using the oligonucleotides: forward 5'-AAAAGCCATGACTCTATGAGTGTGTA-3'and reverse 5'-TACACACTCATAGAGTCATGGCTTTT-3'. Electrophoretic mobility shift assays were performed using the LightShift® Chemiluminescent EMSA kit (Pierce) according to manufacturer's instructions as described previously [23].
2.11 Chromatin immunoprecipitation assay
The chromatin immunoprecipitation assay was performed using the QuikChIP assay (Imgenex) according to manufacturer's instructions. Immunoprecipitation was performed with anti-XBP1 (Santa Cruz Biotechnology) or human IgG (Sigma) as a negative control. The DNA associated with the immunoprecipitate was utilized as a template for PCR to amplify the Runx2 promoter sequence containing the XBP1 binding site. The primers used were: forward 5'-CGAGCCAGGGATCTCTGTTA-3' and reverse 5'-CTGCATCCATTGCTGTCCTA-3'. As a specificity control, the tubulin promoter was similarly amplified using the primers: forward 5'-GGTCTGGACCAACAGGAAAA-3' and reverse 5'-CGAAGAGGAGAGGTTGTTGC-3'[23].
2.12 Statistical analysis
All experiments were performed a minimum of 3 times at least in duplicate. Continuous data were expressed as mean ± SEM. Comparison between groups was performed by Student's paired two-tailed t test. A one-way ANOVA was used to examine differences in response to treatments between groups, with post hoc analysis performed by the method of Student-Newman-Keuls using Origin 8.1 software (OriginLab, Northampton, MA). A p value of <0.05 was considered significant.
3. Results
3.1 BMP-2 promotes calcification of HCSMC
To examine the time course of Runx2 expression and calcification in BMP-2-stimulated HCSMC, we treated cells with BMP-2 (100 ng/ml) for up to 14 days. Runx2 protein expression was increased after 24 h and remained elevated for up to 14 days (Supplementary Fig. 1A). At this time-point, alkaline phosphatase protein expression was increased by 2.9-fold (p<0.01) and activity by 5.6-fold (p<0.01) (Supplementary Fig. 1B). This finding was associated with an increase in intracellular calcium deposition (Supplementary Fig. 1C) and calcification assessed by von Kossa staining (Supplementary Fig. 1D). Thus, in HCSMC, BMP-2 increases Runx2 expression early and calcification is evident after 14 days.
3.2 BMP-2 activates NADPH oxidase to increase oxidant stress
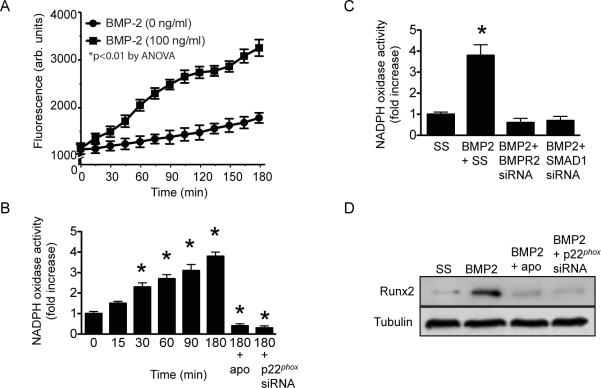
As increased oxidant stress has been shown to increase Runx2 expression, we next sought to determine if BMP-2 increased Runx2 expression in HCSMC by increasing ROS levels. We found that compared to untreated cells, BMP-2 increased ROS levels significantly after 30 min with a maximal effect observed by 180 min (1,623.8 ± 125.0 vs. 3,290.1 ± 207.2 arb. fluorescent units, p<0.01) (Fig. 1A). We next examined NADPH oxidase activity in the membrane fraction of BMP-2-treated HCSMC as NADPH oxidase has been implicated in vascular calcification [17]. Although we did not see an increase in NOX1 or NOX4 mRNA levels in BMP-2 treated HCSMC up to 24 h, BMP-2 increased NADPH oxidase activity by 1.5 ± 0.1-fold after 15 min and by 3.8 ± 0.2-fold (p<0.01) after 180 min. Co-incubation of the cells with apocynin or transfection with an siRNA to the p22phox subunit of NADPH oxidase, to decrease protein expression by 74%, resulted in diminished BMP-2-stimulated NADPH oxidase activity (Fig. 1B).
Figure 1.
NADPH oxidase is activated by BMP-2. HCSMC were treated with BMP-2 (0 or 100 ng/ml) and (A) ROS levels were measured by 2'7'-dichlorodihydrofluorescein fluorescence for up to 180 min (n=6). (B) NADPH oxidase activity was measured in the membrane fraction of BMP-2-treated cells. In select studies, HCSMC were co-incubated with apocynin (3 × 10−5 mol/L) or transfected with an siRNA to p22phox (n=3). (C) NADPH oxidase activity was also determined in HCSMC transfected with siRNA to decrease bone morphogenetic protein receptor 2 (BMPR2) and Smad 1 (n=3). (D) Runx2 expression was examined in BMP-2-treated HCSMC in the presence or absence of co-incubation with apocynin (3 × 10−5 mol/L) or transfection with an siRNA to p22phox (n=3). *p<0.01 vs. 0, SS. Representative blots are shown. apo, apocynin.
Next, to determine if BMPR2 and Smad 1 signaling were involved in BMP-2 activated NADPH oxidase activity, we transfected HCSMC with BMPR2 siRNA, to decrease protein levels by 72%, or with Smad 1 siRNA, to decrease protein levels by 83%. Here, NADPH oxidase activity was abrogated in HCSMC with decreased expression of BMPR2 (0.7 ± 0.2-fold, p<0.05) or Smad 1 (0.8 ± 0.2-fold, p<0.05) compared to scrambled control-transfected cells (3.9 ± 0.4-fold, p<0.01) (Fig. 1C). To demonstrate that NADPH oxidase activity was involved in the observed increase in Runx2 expression, Runx2 protein levels were examined in HCSMC treated with BMP-2 for 24 h that had been co-incubated with apocynin or transfected with p22phox siRNA. Here, decreased NADPH oxidase activity resulted in diminished expression of Runx2 (Fig. 1D) indicating that BMP-2 activates NADPH oxidase via BMPR2 and Smad 1 to increase Runx2 expression in HCSMC.
3.3 BMP-2 activates endoplasmic reticulum stress to increase Runx2 expression
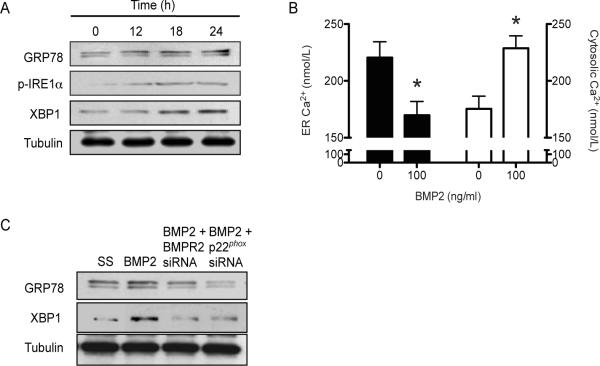
We then examined the effect of BMP-2 on endoplasmic reticulum (ER) stress as ER stress is activated by ROS and has been observed in calcified vessels [25]. After 18 h of BMP-2 treatment, HCSMC demonstrated increased ER stress as evidenced by increased expression of the ER chaperone, GRP78/BiP, and the ER stress markers, phospho-IRE1α and the transcription factor XBP1 that was sustained at 24 h (Fig. 2A). In addition, BMP-2-treated HCSMC demonstrated evidence of Ca2+ perturbation as levels of ER Ca2+ were significantly lower than that observed in untreated cells (217.32 ± 7.82 vs. 151.66 ± 5.45 nmol/L, p<0.01) and cytosolic Ca2+ levels (165.68 ± 6.94 vs. 223.33 ± 5.18 nmol/L, p<0.01) were increased consistent with ER stress (Fig. 2B).
Figure 2.
BMP-2 increases ER stress. ER stress was examined in BMP-2 (0 or 100 ng/ml)-treated HCSMC by (A) examining the expression of the ER chaperone GRP78, the phosphorylation state of IRE1α (p-IRE1α), and XBP1, a transcription factor that is activated by p-IRE1α (n=3). (B) ER and cytosolic calcium were measured using fura-2 by calculating the ratio of values obtained at 340 nm/380 nm (n=6). (C) ER stress markers were examined in HCSMC transfected with an siRNA to BMPR2, p22phox, or a scrambled sequence (SS) control to examine the contribution of BMPR2 or NADPH oxidase activation, respectively to ER stress (n=3). *p<0.001 vs. 0. Representative blots are shown.
To demonstrate that BMP-2 activated ER stress by signaling through the BMPR2 receptor and NADPH oxidase, we examined GRP78 and XBP1 expression in HCSMC transfected with BMPR2 siRNA or p22phox siRNA. Here, we found that decreased BMPR2 expression or NADPH oxidase activity reduced ER stress as shown by diminished expression of GRP78 and XBP1 in BMP-2-treated HCSMC (Fig. 2C).
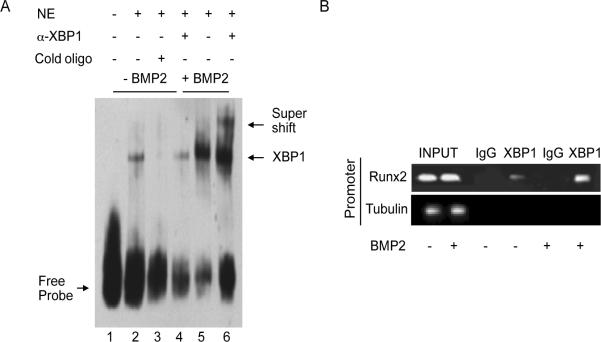
Next, to determine if ER stress was sufficient to increase Runx2 expression, we treated HCSMC with the ER stress inducer tunicamycin (0.5 μg/ml) for 24 h and examined Runx2 mRNA levels. Compared to vehicle-treated cells, tunicamycin increased Runx2 mRNA levels significantly (1.1 ± 0.1 vs. 3.8 ± 0.3-fold, p<0.01). Analysis of a 1 kb segment of the Runx2 promoter upstream of the transcription start site revealed a single XBP1 binding site at (−596 - −591). We performed a mobility shift assay to determine if XBP1 protein-DNA binding occurred in BMP-2-treated cells. In untreated cells, there was a specific protein-DNA complex (lane 2) that was abolished by the addition of excess cold oligonucleotide (lane 3). In BMP-2-treated cells, there was an increase in the protein-DNA complex (lane 5), and the addition of an XBP1 antibody supershifted this complex (lane 6) (Fig. 3A). We confirmed these findings by performing a chromatin immunoprecipitation assay to demonstrate the association of XBP1 to the Runx2 promoter. Compared to untreated cells, there was an increase in XBP1 association with the Runx2 promoter in BMP-2-treated cells (Fig. 3B). Taken together, these findings demonstrate that BMP-2 increases ER stress, which, in turn, leads to increased expression of Runx2.
Figure 3.
XBP1 binds to the Runx2 promoter. (A) Electrophoretic mobility shift assays (n = 4) were performed using an oligonucleotide containing the XBP1 binding site sequence from the Runx2 promoter (5'-AAAAGCCATGACTCTATGAGTGTGTA-3') and nuclear extract from BMP-2 (0 ng/ml)- (lane 2) or BMP-2 (100 ng/ml)-treated cells (lane 6). Supershift assays were carried out with an antibody to XBP1 (lanes 4 and 6). A competition assay (lane 3) was performed with 100-fold excess of cold oligonucleotide. (B) Chromatin immunoprecipitation (n = 4) of lysates using an antibody to XBP1 was followed by PCR amplification of the XBP1 binding site in the Runx2 promoter; immunoglobulin G (IgG) and amplification of the tubulin promoter by PCR served as controls.
3.4 Inhibition of the ER stress limits BMP-2-mediated mineralization of HCSMC
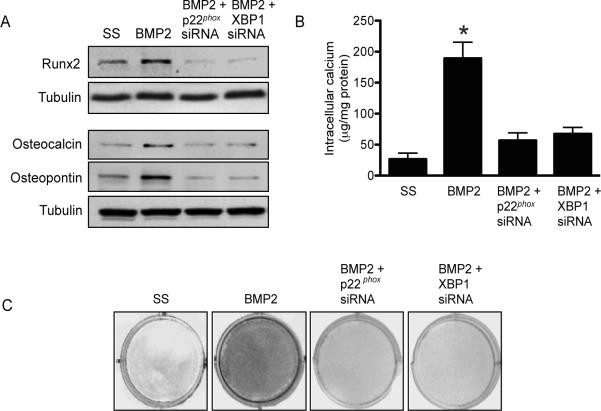
We next sought to determine if inhibiting oxidant stress and/or ER stress would decrease Runx2 protein expression, and, thereby, limit HCSMC mineralization. Runx2 protein expression was abrogated in BMP-2-stimulated HCSMC transfected with p22phox siRNA to limit both oxidant stress and ER stress, or XBP-1 siRNA (Fig.4A). We also examined the effect of decreased oxidant stress or ER stress on osteocalcin and osteopontin expression, which are Runx2-dependent target proteins involved in calcification. After 14 days, osteocalcin and osteopontin expression were decreased in HCSMC with reduced oxidant stress and ER stress (Fig. 4A). These findings were associated with decreased intracellular calcium deposition compared to BMP-2 stimulated HCSMC (189.6 ± 32.1 vs. 62.4 ± 11.4 vs. 71.5 ± 9.6 μg/mg protein, p<0.01) (Fig. 4B) as well as decreased calcification assessed by von Kossa staining (Fig. 4C).
Figure 4.
Inhibition of ER stress limits HCSMC calcification. HCSMC were transfected with an siRNA to p22phox, XBP-1, or a scrambled sequence control (SS) and (A) expression of Runx2 expression and the Runx2-dependent calcification proteins was examined after 24 h (n=3); (B) Intracellular calcium deposition was determined after 14 days (n=6); and, (C) mineralization was assessed by von Kossa staining (n=3). Representative images and blots are shown. *p<0.01 vs. SS.
4. Discussion
In the present study, we identified one mechanism that links BMP-2, oxidant stress, and ER stress to increased Runx2 expression and vascular smooth muscle cell calcification. The role of BMP-2 in vascular calcification has been established [14,26]. Expression of BMPR2 has been shown in human vascular smooth muscle cells [4], and we now provide evidence that this receptor is involved in BMP-2-mediated calcification of HCSMC. Smad signaling has also been implicated in BMP-2 induced osteoblast differentiation and, in C2C12 myoblast cells, a constitutively active form of Smad 1, together with Smad 4 or Runx2, was shown to induce dedifferentiation of the myoblasts to acquire an osteoblastic phenotype [27,28]. Here, we demonstrate that Smad 1 is involved in the calcification process upstream of NADPH oxidase activation in BMP-2-treated HCSMC.
Increased oxidant stress has been implicated previously in the pathogenesis of vascular calcification [5,16]. Calcifying vascular cells exposed to hydrogen peroxide or xanthine/xanthine oxidase demonstrated increased oxidant stress, alkaline phosphatase activity, and [45Ca] incorporation [16]. Exogenous hydrogen peroxide (0.1 — 0.4 mmol/L) has been shown to increase murine aortic vascular smooth muscle cell calcification through a mechanism involving Akt signaling and Runx2 expression [15]. Although these investigators demonstrated an increase in phospho-Akt up to 60 min after exposure to hydrogen peroxide (0.4 mmol/L), and the presence of phospho-Akt in calcified smooth muscle cells, the intermediate events between Akt activation and Runx2 expression were not explored.
Our finding that NADPH oxidase is activated to increase ROS is consistent with prior studies: investigators have shown that uremic serum and β-glycerophosphate increased levels of the p22phox subunit of NADPH oxidase in A7r5 cells after 24 h and this finding was associated with an increase in Runx2 expression [17]. Similarly, NADPH oxidase activity was also shown to facilitate BMP-2-induced osteoblastic differentiation of murine 2T3 pre-osteoblasts. Here, the Nox4 isoform of NADPH oxidase was responsible for NADPH oxidase-mediated osteoblastic differentiation [20]. In our study, we implicate directly NADPH oxidase in the observed increase in Runx2 expression by using siRNA to decrease p22phox to limit NADPH oxidase activity. We did not, however, identify a select NADPH oxidase isoform as the principal source of ROS generation as both NOX1 and NOX4 mRNA were expressed in HCSMC, and we did not see a change in expression levels after BMP-2 stimulation for up to 24 h. Nonetheless, suppression of p22phox expression, which would inhibit both NADPH oxidase isoforms, resulted in a decrease in BMP-2-stimulated NADPH oxidase activity and Runx2 expression.
We also found that BMP-2-mediated NADPH oxidase activation increased ER stress leading to an increase in Runx2 expression. This finding is in line with studies in osteoblasts that demonstrated that ER stress induced by thapsigargin and tunicamycin increased Runx2 levels [29]. Similarly, ER stress was shown to modulate the effects of BMP2 by activating the PERK-eIF2α-ATF4 pathway to increase osteoblast differentiation [22]. In this study, the investigators focused on osteocalcin and bone sialoprotein, which are targets of ATF4 and essential for osteogenesis, but did not examine the effect of ER stress on Runx2 protein expression. Interestingly, these investigators also observed an increase in spliced XBP1 expression; however, the role of XBP1 on Runx2 expression was not examined [22]. We now provide evidence to demonstrate that XBP1 signaling increases Runx2 expression to modulate HCSMC calcification. In vivo, ER stress has been associated with vascular calcification. In a rat vitamin D3 plus nicotine model of vascular calcification, investigators found a 43.9% increase in GRP78 expression in calcified aortas [25]. Importantly, in our study, we examined ER stress in HCSMC at a time point prior to the development of calcification allowing us to conclude that ER stress contributes to, and is not merely a consequence of, vascular calcification.
Supplementary Material
Highlights.
Highlights for Liberman et al. Bone Morphogenetic Protein-2 Activates NADPH Oxidase to Increase Endoplasmic Reticulum Stress and Human Coronary Artery Smooth Muscle Cell Calcification
-
1
BMP-2 increases NADPH oxidase activity in human coronary smooth muscle cells
-
2
NADPH oxidase activity is increased via BMPR2 and Smad 1
-
3
Increased NADPH oxidase activity upregulates Runx2
-
4
BMP-2 stimulates ER stress and XBP1 binding to the Runx2 promoter
-
5
Inhibition of oxidant or ER stress abrogates smooth muscle cell calcification
Acknowledgements
This work was supported by funds from Conselho Nacional de Pesquisa e Tecnologia and Fundacao de Amparo a Pesquisa do Estado de Sao Paulo (ML), a grant from the Stanley J. Sarnoff Foundation (RCJ), and NIH grants HL061795, HL048743, HL107192, and HL108630 (J.L.), and NIH grants HL070819 and HL105301 (J.A.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dao HH, Essalihi R, Bouvet C, Moreau P. Evolution and modulation of age-related medial elastocalcinosis: impact on large artery stiffness and isolated systolic hypertension. Cardiovasc. Res. 2005;66:307–317. doi: 10.1016/j.cardiores.2005.01.012. [DOI] [PubMed] [Google Scholar]
- [2].Rennenberg RJ, Kessels AG, Schurgers LJ, van Engelshoven JM, de Leeuw PW, Kroon AA. Vascular calcifications as a marker of increased cardiovascular risk: a meta-analysis. Vasc. Health and Risk Management. 2009;5:185–197. doi: 10.2147/vhrm.s4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ. Res. 2006;99:1044–1059. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- [4].Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, Frutkin A, Dichek D, Giachelli CM. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ. Res. 2009;104:733–741. doi: 10.1161/CIRCRESAHA.108.183053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ. Res. 2001;89:1147–1154. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- [6].Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- [7].Inada M, Yasui T, Nomura S, Miyake S, Deguchi K, Himeno M, Sato M, Yamagiwa H, Kimura T, Yasui N, Ochi T, Endo N, Kitamura Y, Kishimoto T, Komori T. Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev. Dyn.: an official publication of the American Association of Anatomists. 1999;214:279–290. doi: 10.1002/(SICI)1097-0177(199904)214:4<279::AID-AJA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- [8].Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- [9].Speer MY, Li X, Hiremath PG, Giachelli CM. Runx2/Cbfa1, but not loss of myocardin, is required for smooth muscle cell lineage reprogramming toward osteochondrogenesis. J. Cell. Biochem. 2010;110:935–947. doi: 10.1002/jcb.22607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- [11].Abe E, Yamamoto M, Taguchi Y, Lecka-Czernik B, O'Brien CA, Economides AN, Stahl N, Jilka RL, Manolagas SC. Essential requirement of BMPs-2/4 for both osteoblast and osteoclast formation in murine bone marrow cultures from adult mice: antagonism by noggin. J. Bone Min. Res. 2000;15:663–673. doi: 10.1359/jbmr.2000.15.4.663. [DOI] [PubMed] [Google Scholar]
- [12].Mishina Y, Starbuck MW, Gentile MA, Fukuda T, Kasparcova V, Seedor JG, Hanks MC, Amling M, Pinero GJ, Harada S, Behringer RR. Bone morphogenetic protein type IA receptor signaling regulates postnatal osteoblast function and bone remodeling. J. Biol. Chem. 2004;279:27560–27566. doi: 10.1074/jbc.M404222200. [DOI] [PubMed] [Google Scholar]
- [13].Al-Aly Z, Shao JS, Lai CF, Huang E, Cai J, Behrmann A, Cheng SL, Towler DA. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr-/- mice. Arterioscler. Thromb. and Vasc. Biol. 2007;27:2589–2596. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- [14].Li X, Yang HY, Giachelli CM. BMP-2 promotes phosphate uptake, phenotypic modulation, and calcification of human vascular smooth muscle cells. Atherosclerosis. 2008;199:271–277. doi: 10.1016/j.atherosclerosis.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J. Biol. Chem. 2008;283:15319–15327. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Rad. Biol. & Med. 2001;31:509–519. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- [17].Sutra T, Morena M, Bargnoux A, Caporiccio B, Canaud B, Cristol J. Superoxide production: a procalcifying cell signalling event in osteoblastic differentiation of vascular smooth muscle cells exposed to calcification media. Free Rad. Res. 2009;42:789–797. doi: 10.1080/10715760802400766. [DOI] [PubMed] [Google Scholar]
- [18].Muteliefu G, Enomoto A, Jiang P, Takahashi M, Niwa T. Indoxyl sulphate induces oxidative stress and the expression of osteoblast-specific proteins in vascular smooth muscle cells. Neph., Dial., Transpl. 2009;24:2051–2058. doi: 10.1093/ndt/gfn757. [DOI] [PubMed] [Google Scholar]
- [19].Hofmann Bowman MA, Gawdzik J, Bukhari U, Husain AN, Toth PT, Kim G, Earley J, McNally EM. S100A12 in vascular smooth muscle accelerates vascular calcification in apolipoprotein E-null mice by activating an osteogenic gene regulatory program. Arterioscler., Thromb., and Vasc. Biol. 2011;31:337–344. doi: 10.1161/ATVBAHA.110.217745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Mandal CC, Ganapathy S, Gorin Y, Mahadev K, Block K, Abboud HE, Harris SE, Ghosh-Choudhury G, Ghosh-Choudhury N. Reactive oxygen species derived from Nox4 mediate BMP2 gene transcription and osteoblast differentiation. Biochem. J. 2010;433:393–402. doi: 10.1042/BJ20100357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response,Nature reviews. Mol. Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- [22].Saito A, Ochiai K, Kondo S, Tsumagari K, Murakami T, Cavener DR, Imaizumi K. Endoplasmic reticulum stress response mediated by the PERK-eIF2(alpha)-ATF4 pathway is involved in osteoblast differentiation induced by BMP2. J. Biol. Chem. 2011;286:4809–4818. doi: 10.1074/jbc.M110.152900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Leopold JA, Dam A, Maron BA, Scribner AW, Liao R, Handy DE, Stanton RC, Pitt B, Loscalzo J. Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nature Med. 2007;13:189–197. doi: 10.1038/nm1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dickhout JG, Hossain GS, Pozza LM, Zhou J, Lhotak S, Austin RC. Peroxynitrite causes endoplasmic reticulum stress and apoptosis in human vascular endothelium: implications in atherogenesis. Arterioscler., Thromb., and Vasc. Biol. 2005;25:2623–2629. doi: 10.1161/01.ATV.0000189159.96900.d9. [DOI] [PubMed] [Google Scholar]
- [25].Duan X, Zhou Y, Teng X, Tang C, Qi Y. Endoplasmic reticulum stress-mediated apoptosis is activated in vascular calcification. Biochem. Biophys. Res. Comm. 2009;387:694–699. doi: 10.1016/j.bbrc.2009.07.085. [DOI] [PubMed] [Google Scholar]
- [26].Chen NX, Duan D, O'Neill KD, Wolisi GO, Koczman JJ, Laclair R, Moe SM. The mechanisms of uremic serum-induced expression of bone matrix proteins in bovine vascular smooth muscle cells. Kid. Int. 2006;70:1046–1053. doi: 10.1038/sj.ki.5001663. [DOI] [PubMed] [Google Scholar]
- [27].Javed A, Afzal F, Bae JS, Gutierrez S, Zaidi K, Pratap J, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Specific residues of RUNX2 are obligatory for formation of BMP2-induced RUNX2-SMAD complex to promote osteoblast differentiation. Cells, Tissues, Organs. 2009;189:133–137. doi: 10.1159/000151719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nojima J, Kanomata K, Takada Y, Fukuda T, Kokabu S, Ohte S, Takada T, Tsukui T, Yamamoto TS, Sasanuma H, Yoneyama K, Ueno N, Okazaki Y, Kamijo R, Yoda T, Katagiri T. Dual roles of smad proteins in the conversion from myoblasts to osteoblastic cells by bone morphogenetic proteins. J. Biol. Chem. 2010;285:15577–15586. doi: 10.1074/jbc.M109.028019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hamamura K, Yokota H. Stress to endoplasmic reticulum of mouse osteoblasts induces apoptosis and transcriptional activation for bone remodeling. FEBS letters. 2007;581:1769–1774. doi: 10.1016/j.febslet.2007.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.