Viral receptor identified
The transferrin receptor 1 (TfR1) has been identified as the cellular receptor for four New World arenaviruses — the Junin, Machupo, Guanarito and Sabia viruses. This class of arenaviruses is important because they cause fatal haemorrhagic fevers. Treating cultured cells with an antibody against TfR1 blocks viral entry and replication. Antibodies that limit arenavirus replication without interfering with host iron metabolism may be effective in controlling outbreaks of New World haemorrhagic fever.
Supplementary information
The online version of this article (doi:10.1038/nature05539) contains supplementary material, which is available to authorized users.
Abstract
At least five arenaviruses cause viral haemorrhagic fevers in humans. Lassa virus, an Old World arenavirus, uses the cellular receptor α-dystroglycan to infect cells1. Machupo, Guanarito, Junin and Sabia viruses are New World haemorrhagic fever viruses that do not use α-dystroglycan2. Here we show a specific, high-affinity association between transferrin receptor 1 (TfR1) and the entry glycoprotein (GP) of Machupo virus. Expression of human TfR1, but not human transferrin receptor 2, in hamster cell lines markedly enhanced the infection of viruses pseudotyped with the GP of Machupo, Guanarito and Junin viruses, but not with those of Lassa or lymphocytic choriomeningitis viruses. An anti-TfR1 antibody efficiently inhibited the replication of Machupo, Guanarito, Junin and Sabia viruses, but not that of Lassa virus. Iron depletion of culture medium enhanced, and iron supplementation decreased, the efficiency of infection by Junin and Machupo but not Lassa pseudoviruses. These data indicate that TfR1 is a cellular receptor for New World haemorrhagic fever arenaviruses.
Supplementary information
The online version of this article (doi:10.1038/nature05539) contains supplementary material, which is available to authorized users.
Main
Arenaviruses are enveloped, single-stranded, bisegmented RNA viruses3. The family Arenaviridae consists of a single genus (Arenavirus), which includes at least 23 recognized viruses4. Arenaviruses have been classified into two antigenically and geographically distinct groups, the Lassa–lymphocytic choriomeningitis serocomplex (‘Old World arenaviruses’) and the Tacaribe serocomplex (‘New World arenaviruses’). Five arenaviruses are known to cause acute viral haemorrhagic fever in humans, with case-fatality rates as high as 30%. Lassa virus (LASV) is an Old World arenavirus that causes Lassa fever. Machupo (MACV), Guanarito (GTOV), Junin (JUNV) and Sabia (SABV) viruses are New World arenaviruses that cause Bolivian, Venezuelan, Argentinian and Brazilian haemorrhagic fever, respectively. LASV, MACV, GTOV and JUNV have been classified as National Institute of Allergy and Infectious Diseases Category A Priority Pathogens, in part because of their lethality and significant potential for misuse4,5.
The arenaviral entry protein GP is processed into two subunits, GP1 and GP2 (ref. 6). Like other class 1 fusion proteins, GP1 is thought to mediate association with a cellular receptor7. After internalization of the virus to an acidified endosomal compartment, GP2 undergoes a conformational change that promotes fusion of the viral and cellular membranes7,8,9. We characterized a series of MACV GP1 truncation variants, fused at their carboxy termini to the Fc domain of human IgG1 (Fig. 1a). Only full-length MACV GP1 (residues 59–258) and a single truncation variant, GP1Δ (residues 79–258), efficiently bound MACV-permissive Vero African green monkey kidney cells, whereas GP1 variants expressing residues 102–258, 121–258, 59–170 or 59–205 did not (Supplementary Fig. 1a). GP1Δ bound Vero cells saturably and more efficiently than GP1 (Fig. 1b). GP1Δ, but not the receptor-binding domains of Lake Victoria marburgvirus or severe acute respiratory syndrome (SARS) coronavirus entry proteins, precipitated from Vero cells a protein of 95 kDa under reducing conditions, and approximately 200 kDa under non-reducing conditions (Fig. 1c). Mass-spectrometric analysis identified this protein as TfR1 (CD71). MACV GP1 efficiently bound a Chinese hamster ovary (CHO) cell line stably expressing human TfR1 (CHO-hTfR1)10 but did not bind parental CHO cells, confirming a high-affinity association between MACV GP1 and human TfR1 (Fig. 1d).
Figure 1. MACV GP1 and GP1Δ efficiently bind TfR1.
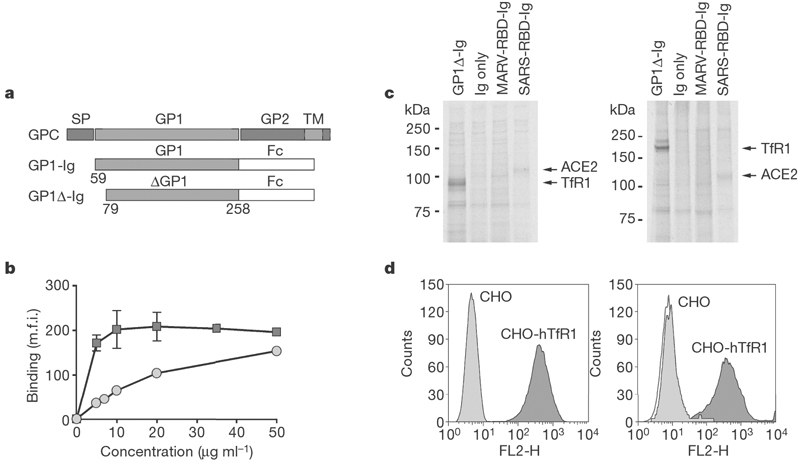
a, Schematic representation of MACV glycoprotein precursor (GPC), GP1-Ig and GP1Δ-Ig. Locations of the signal peptide (SP), the GP1 and GP2 domains and the transmembrane domain (TM) are indicated. b, Vero cells analysed by flow cytometry with the use of the indicated concentrations of purified GP1-Ig (circles) or GP1Δ-Ig (squares). m.f.i., mean fluorescence intensity. Error bars indicate standard deviation. c, Vero cells were metabolically labelled, incubated with GP1Δ-Ig or the indicated control Ig-fusion proteins, lysed, immunoprecipitated, and analysed by SDS–PAGE under reducing (left) or non-reducing (right) conditions. d, Binding of anti-human TfR1 antibody (left) and MACV GP1-Ig (right) to CHO (light grey) or CHO-hTfR1 (dark grey) cells analysed by flow cytometry. The unfilled curve in the right-hand panel is secondary antibody alone.
We then sought to identify cell lines that were non-permissive to infection by a murine retrovirus pseudotyped with MACV GP. Consistent with previous studies11,12 was our observation that murine NIH 3T3 cells and baby hamster kidney (BHK-S) and CHO cell lines were relatively non-permissive to MACV pseudovirus and did not bind MACV GP1. Vero, rhesus macaque LLC-MK2, and human HeLa and 293T cell lines efficiently bound GP1 and were permissive to MACV-GP-mediated infection (not shown). Consistent with the binding study in Fig. 1d was our observation that CHO-hTfR1 cells, but not parental CHO cells, were efficiently infected by MACV pseudoviruses, whereas LASV and lymphocytic choriomeningitis virus (LCMV) pseudoviruses infected CHO-hTfR1 and parental cells comparably (Fig. 2a). We investigated the ability of an anti-human TfR1 antibody and a control anti-HLA (anti-human leukocyte antigen) antibody to inhibit infection mediated by MACV and LASV GP proteins. Both antibodies efficiently bound HeLa and 293T cells (Fig. 2b, upper panels) but only the anti-human TfR1 antibody inhibited infection by MACV pseudovirus (Fig. 2b, lower panels). The anti-human TfR1 antibody had no effect on LASV pseudovirus infection. Together, these data indicate that TfR1 is a necessary receptor for MACV.
Figure 2. MACV pseudovirus entry depends on human TfR1.
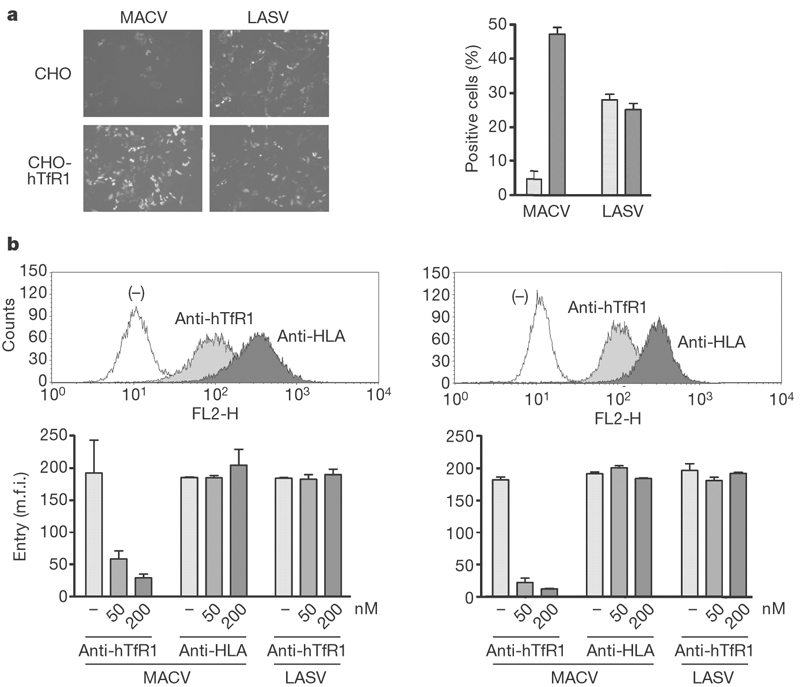
a, Parental CHO or CHO-hTfR1 cells were incubated with MACV or LASV pseudoviruses expressing GFP. Infection level was assessed two days later by flow cytometry. Left: fluorescent micrographs of infected cells. Right: results of flow cytometric analysis of the same cells; light grey bars, CHO; dark grey bars, CHO-hTfR1. b, Top: analysis of HeLa cells (left) and 293T cells (right) with anti-human TfR1 and anti-HLA-A,B,C antibodies. Bottom: infection of these cells incubated with the MACV or LASV pseudoviruses in the presence of the indicated concentrations of antibodies, measured as GFP fluorescence. m.f.i., mean fluorescence intensity. Error bars indicate standard deviation.
To determine whether TfR1 contributed to infection by other New World arenaviruses, we investigated infection by JUNV pseudovirus, as well as replication of infectious arenaviruses. Infection of CHO-hTfR1 cells by JUNV, but not LASV, pseudovirus was substantially more efficient than infection of parental CHO cells (Fig. 3a). Like MACV pseudovirus, JUNV pseudovirus infection of 293T cells was inhibited by anti-human TfR1 antibody (Fig. 3b). Replication of infectious MACV, GTOV, JUNV and SABV in 293T cells was markedly inhibited by anti-human TfR1 antibody, but not by anti-HLA antibody (Fig. 3c). Nearly identical results were obtained with HeLa cells (not shown). These data indicate that TfR1 is an obligate receptor for each of these New World haemorrhagic fever arenaviruses, despite the diversity of their GP1 proteins, but are consistent with previous studies indicating that these viruses use a common cellular receptor11,12.
Figure 3. JUNV, GTOV and SABV use human TfR1.
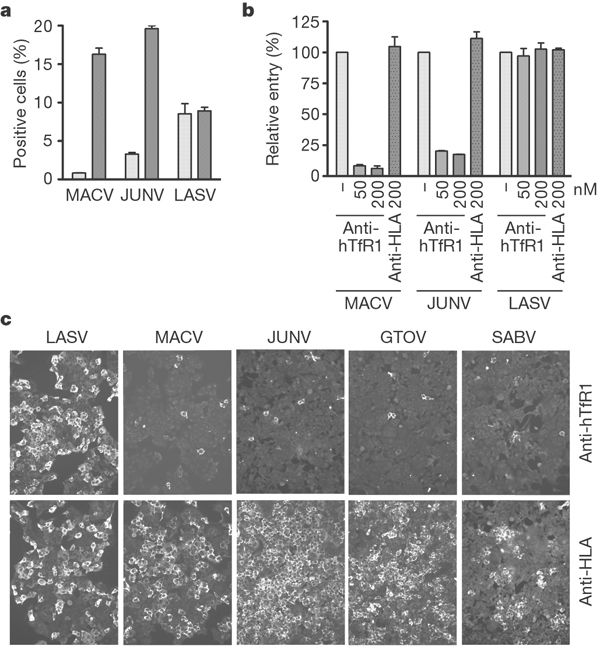
a, CHO cells (light grey bars) and CHO-hTfR1 cells (dark grey bars) were incubated with indicated pseudoviruses, and infection level was assessed two days later by flow cytometry. b, 293T cells were incubated with the indicated concentrations of anti-human TfR1 or control antibody, pseudoviruses were added, and infection was measured as described for Fig. 2b. c, 293T cells were incubated in the presence of the indicated antibody at 200 nM, infectious viruses were added, and incubation was continued for 24 h. Cells were washed, fixed and stained as described in Methods. Error bars indicate standard deviation.
Transferrin receptor 2 (TfR2) is similar (54% identity) to TfR1 and binds transferrin. We examined its role in MACV, GTOV or JUNV GP-mediated entry. Infection by MACV, JUNV, LASV, GTOV or LCMV pseudoviruses was not enhanced by the introduction of human TfR2 in CHO or BHK cells (Fig. 4a) despite its efficient expression (Supplementary Fig. 1b). LCMV has been proposed to use a receptor in addition to α-dystroglycan11, but no enhancement of LCMV pseudovirus entry was observed in the presence of human TfR1, indicating that this alternative receptor is not human TfR1. Transferrin is present at micromolar concentrations in human plasma13. We investigated whether iron-bound (holo) transferrin, which binds TfR1 with high affinity, or apo-transferrin, which does not, could modulate the infection of MACV pseudoviruses. Holo-transferrin did not interfere with the binding of MACV-GP1 or LASV-GP1 to 293T cells (not shown). Neither holo-transferrin nor apo-transferrin interfered with or enhanced infection with MACV pseudoviruses (Fig. 4b). Soluble TfR1 is also found in plasma at concentrations of about 20 nM, and up to 150 nM in anaemic individuals14. Soluble TfR1 inhibited the infection of 293T cells by JUNV and, to a smaller extent, MACV pseudoviruses (Fig. 4c). No inhibition of LASV pseudovirus infection was observed.
Figure 4. Soluble human TfR1, but not transferrin or human TfR2, modulates New World arenavirus infection.
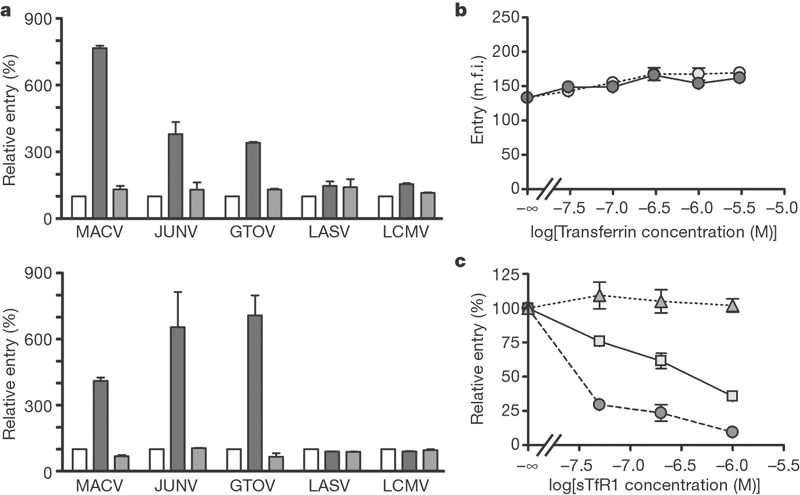
a, CHO cells (top) and BHK cells (bottom) were transfected with plasmids expressing human TfR1 (dark grey bars) or TfR2 (light grey bars) or with vector alone (white bars), and incubated with the indicated pseudoviruses. Entry was measured as in Fig. 2a. b, 293T cells were incubated for 1 h with MACV pseudovirus produced in serum-free medium and with the indicated amounts of human apo-transferrin (white circles) or holo-transferrin (grey circles) and then washed; the infection level was assessed two days later. c, 293T cells were infected for 1 h with LASV (triangles), MACV (squares) or JUNV (circles) preincubated with increasing concentrations of soluble human TfR1 for 30 min. Cells were washed, and GFP fluorescence was measured two days later. Error bars indicate standard deviation.
Depletion of iron in culture medium or in vivo has been shown to upregulate the cell-surface or tissue expression of TfR1 (refs 15–17). We investigated the effect of iron depletion on MACV, JUNV and LASV GP-mediated infection. JUNV pseudovirus more efficiently infected 293T cells and SLK human endothelial cells preincubated with the iron chelator deferoxamine (Fig. 5a), used clinically to treat iron toxicity18. However, treatment with deferoxamine consistently had a more modest effect on MACV pseudovirus infection. The basis of the lower sensitivity of MACV pseudovirus infection to deferoxamine is not clear, but it may indicate a role for an additional factor, limiting in these assays, in the entry of MACV. LASV infection was unaffected by deferoxamine at all concentrations. We also investigated the ability of excess iron to modulate GP-mediated infection. Ferric ammonium citrate, which downregulates TfR1 (ref. 19), efficiently inhibited MACV and JUNV pseudovirus infection but not that of LASV or LCMV. These data show that iron concentrations can modulate the efficiencies of MACV and JUNV infection.
Figure 5. Iron concentration modulates MACV and JUNV pseudovirus entry.
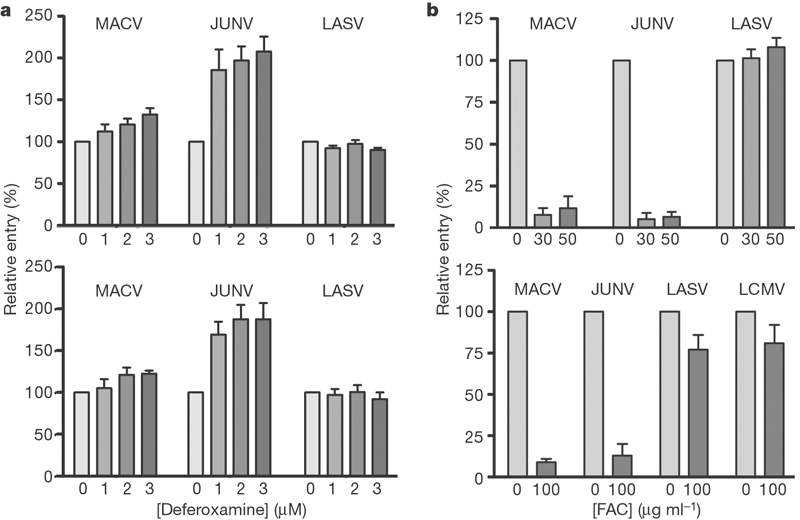
a, Human fibroblast 293T cells (top) or endothelial SLK cells (bottom) were incubated in cell culture medium containing 10% fetal bovine serum and the indicated amounts of deferoxamine. After 24 h, cells were infected with the indicated pseudoviruses at 4 °C. Cells were washed, complete medium was added, and infection level was assessed by GFP fluorescence. Entry is normalized to that of VSV pseudovirus. Results are shown as means ± s.d. for five experiments for both cell lines. b, Human fibroblast 293T cells (top) or epithelial HeLa cells (bottom) were incubated for 1 h with the indicated concentrations of ferric ammonium citrate (FAC) at 37 °C, infected with the indicated pseudovirus containing FAC. Cells were washed 1 h later and infection level was assessed by GFP fluorescence two days later. Error bars indicate standard deviation.
Collectively our data indicate that TfR1 is a necessary cellular receptor for the four New World arenaviruses that cause haemorrhagic fevers in humans. Several properties of TfR1 indicate its possible role in arenaviral replication and disease. It is rapidly and constitutively endocytosed to an acidic compartment, which is consistent with the pH dependence of arenavirus entry8. It is expressed ubiquitously, and at high levels on activated or rapidly dividing cells, including macrophages and activated lymphocytes, major targets of arenaviral infection20,21. TfR1 is also highly expressed on endothelial cells22,23, which are thought to be central to the pathogenesis of haemorrhagic fever24. TfR1 upregulation on immune cells activated in response to infection may accelerate viral replication in these cells and may in part explain the higher lethality of New World haemorrhagic fevers compared with Lassa fever. Some of these properties may also be useful to canine and feline parvoviruses and to mouse mammary tumour virus, animal viruses that also use TfR1 (refs 25, 26).
It is well established that cytosolic iron regulates TfR1 expression16. We show here that infection mediated by the JUNV GP was increased by depleting the culture medium of iron. These data raise the possibility that iron deficiency might therefore enhance susceptibility to or the severity of Argentine haemorrhagic fever in particular. However, soluble TfR1, which we have shown to inhibit MACV and JUNV GP-mediated infection, is also elevated in iron-deficient animals and can be found in anaemic individuals at concentrations that may inhibit JUNV replication14. Further studies will be necessary to clarify the role of iron deficiency as a risk factor in haemorrhagic fevers caused by New World arenaviruses. Our data also raise the possibility that iron supplementation may be useful in moderating these haemorrhagic fevers, because ferric ammonium citrate, which is used to treat anaemia in humans, markedly inhibited infection mediated by MACV and JUNV GP molecules.
Our studies suggest another approach to the treatment of, or prophylaxis for, New World haemorrhagic fevers, namely with a humanized anti-human TfR1 antibody. Several anti-human TfR1 antibodies have been developed and are currently under investigation as anti-tumour therapeutics21. Some of these antibodies, like the antibody used in this study, do not compete with transferrin, indicating that an anti-TfR1 antibody can limit arenaviral replication in an infected individual without interfering with iron metabolism. If so, such antibodies may be employed to limit a natural or intentional outbreak of New World haemorrhagic fever.
Methods
Cells and plasmids
Vero cells (African green monkey kidney epithelial; CCL-81; ATCC), 293T cells (human kidney epithelial; CRL-11268; ATCC), HeLa 229 cells (human cervical epithelial; CCL-2.1; ATCC) and 3T3-Swiss cells (mouse embryo fibroblast; CCL-92; ATCC) were grown in DMEM medium; LLC-MK2 cells (rhesus monkey kidney epithelial; CCL-7; ATCC) in medium 199; BHK-21 cells (Chinese hamster kidney fibroblast; CCL-10; ATCC) and EJG cells (bovine capillary endothelial; CRL-8659; ATCC) in minimal essential medium (Eagle); SLK cells (human endothelial; NIH AIDS Research & Reference Regent Program) in RPMI medium; CHO cells (Chinese hamster ovary epithelial) in F12 medium; CHO-hTfR1 cells (a gift from T. McGraw) in F12 medium containing 400 μg ml-1 G418. All cell lines were grown in 10% fetal bovine serum.
MACV GPC coding sequence (residues 1–496 of Carvallo strain) was built from overlapping oligonucleotides by PCR and cloned into a pcDNA3.1 expression plasmid. MACV GP1-Ig was generated by PCR amplification of GP1 codons 59–258 and cloning into a previously described pcDM8-based plasmid expressing the CD5 signal sequence and the Fc region of human IgG1 (ref. 27). Truncation variants of GP1-Ig were generated by a modified QuickChange method (Stratagene). JUNV (MC2), GTOV (VINH-9551) and LASV (Josiah) GPC have been described previously28,29, as have SARS-RBD-Ig and MARV-RBD-Ig (ref. 30). LCMV (Armstrong) GPC-expressing plasmid was provided by J. de la Torre. Coding regions of human TfR1 and TfR2 were cloned into pCAGGS expression plasmid. Human TfR2 gene was provided by M. Wessling-Resnick. Soluble human TfR1 (residues 121–760) with an amino-terminal Myc tag was cloned into the modified pcDM8 plasmid described above.
Immunoprecipitation and flow cytometry
Ig-fusion proteins used for immunoprecipitation and flow cytometry were produced in SFM II medium (Invitrogen) from 293T cells transfected with the appropriate plasmids. Ig-fusion proteins, bound to Protein A–Sepharose, were eluted in 3 M MgCl2 and dialysed against PBS. Anti-human TfR1 (clone M-A712) and anti-HLA-A,B,C (clone G46-2.6) were purchased from BD Biosciences, and anti-human TfR2 (9F8-1C11) was purchased from Cell Sciences. Goat anti-human and anti-mouse secondary antibodies, conjugated with phycoerythrin, were purchased from Jackson Immunological Laboratories. Flow cytometry was performed in PBS containing 2% goat serum, except in experiments with apo-transferrin and holo-transferrin (Sigma), which were performed in 1% BSA.
To identify GP1Δ-Ig-binding proteins, Vero cells were labelled with [35S]-Express (New England Nuclear) for one day, scraped in the presence of a protease inhibitor cocktail (Sigma), incubated on ice with GP1Δ-Ig for 1 h, lysed in 1% decyl maltopyranoside (Anatrace), precipitated with Protein–A Sepharose (Pfizer-Pharmacia) and analysed by SDS–polyacrylamide-gel electrophoresis (SDS–PAGE). For mass-spectrometric analysis, immunoprecipitates were prepared in a similar manner from unlabelled cells. Coomassie-stained SDS–PAGE bands were excised and then digested in trypsin; tryptic fragments were identified by tandem mass spectrometry (LTQ-FT; ThermoElectron) and the SEQUEST algorithm.
Pseudovirus infection
Pseudoviruses were produced from 293T cells by transfecting at 1:1:1 ratio of plasmids expressing murine leukaemia virus gag/pol, arenaviral GP and pQCXIX transduction vector (BD Biosciences) expressing enhanced green fluorescent protein (EGFP), as described previously30. Virus-containing culture supernatant was harvested two days later, and filtered through 0.45-μm filter disks. Anti-human TfR1 and anti-HLA-A,B,C antibodies were dialysed against PBS. Cells were incubated with each of these antibodies at the indicated concentrations for 30 min at 37 °C. Pseudoviruses were added, cells were washed 16 h after infection, and entry level was measured by flow cytometry. To study the effect of tranferrin on viral entry, pseudoviruses were produced in serum-free medium (FreeStyle; Invitrogen). The role of human TfR2 was assessed in BHK cells transfected with pCAGGS-human TfR2 plasmid complexed with Lipofectamine 2000 (Invitrogen). Transfected cells were infected the next day, and the infection level was assessed two days later.
To study the role of iron in arenaviral infection, cells were incubated in complete medium containing indicated concentrations (1–3 μM) of the iron chelator deferoxamine (Sigma) for 24 h, or ferric ammonium citrate (30–100 μg ml-1) for 1 h. Cells were cooled on ice and infected with pseudoviruses by centrifugation (2,000g) at 4 °C for 30 min. Cells were washed, and GFP expression level was assessed 24 h (293T and HeLa cells) or 48 h (SLK cells) after infection.
Antibody inhibition of infectious arenaviruses
All infections of 293T and HeLa cells with LASV (Josiah), MACV (Carvallo), JUNV (XJ-13) GTOV (VINH-9551) or SABV were performed in the BSL-4 laboratory at the Special Pathogens Branch, Centers for Disease Control and Prevention, Atlanta. Infections and immunofluorescence staining were performed as described previously12. HeLa and 293T cells were grown on gelatin-treated glass coverslips, preincubated for 30 min with the indicated antibody at 200 nM, and then infected with virus at a multiplicity of infection of 0.1–1.0. At 24 h after infection, cells were washed, fixed with 3% formaldehyde, permeabilized with 0.1% Triton X-100 and incubated with mouse hyperimmune sera specific for New World or Old World arenaviruses. Cells were then washed and incubated with fluorescein isothiocyanate-conjugated secondary antibody.
Supplementary information
This file contains Supplementary Figure S1 with legend. (PDF 88 kb)
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Footnotes
Sheli R. Radoshitzky and Jonathan Abraham: These authors contributed equally to this work.
References
- 1.Cao W, et al. Identification of α-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science. 1998;282:2079–2081. doi: 10.1126/science.282.5396.2079. [DOI] [PubMed] [Google Scholar]
- 2.Spiropoulou CF, Kunz S, Rollin PE, Campbell KP, Oldstone MB. New World arenavirus clade C, but not clade A and B viruses, utilizes α-dystroglycan as its major receptor. J. Virol. 2002;76:5140–5146. doi: 10.1128/JVI.76.10.5140-5146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oldstone MB. Arenaviruses. I. The epidemiology molecular and cell biology of arenaviruses. Introduction. Curr. Top. Microbiol. Immunol. 2002;262:v–xii. [PubMed] [Google Scholar]
- 4.Charrel RN, de Lamballerie X. Arenaviruses other than Lassa virus. Antiviral Res. 2003;57:89–100. doi: 10.1016/S0166-3542(02)00202-4. [DOI] [PubMed] [Google Scholar]
- 5.Borio L, et al. Hemorrhagic fever viruses as biological weapons: medical and public health management. J. Am. Med. Assoc. 2002;287:2391–2405. doi: 10.1001/jama.287.18.2391. [DOI] [PubMed] [Google Scholar]
- 6.Buchmeier MJ, Southern PJ, Parekh BS, Wooddell MK, Oldstone MB. Site-specific antibodies define a cleavage site conserved among arenavirus GP-C glycoproteins. J. Virol. 1987;61:982–985. doi: 10.1128/jvi.61.4.982-985.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kunz S, Borrow P, Oldstone MB. Receptor structure, binding, and cell entry of arenaviruses. Curr. Top. Microbiol. Immunol. 2002;262:111–137. doi: 10.1007/978-3-642-56029-3_5. [DOI] [PubMed] [Google Scholar]
- 8.Castilla V, Mersich SE. Low-pH-induced fusion of Vero cells infected with Junin virus. Arch. Virol. 1996;141:1307–1317. doi: 10.1007/BF01718832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burns JW, Buchmeier MJ. Protein–protein interactions in lymphocytic choriomeningitis virus. Virology. 1991;183:620–629. doi: 10.1016/0042-6822(91)90991-J. [DOI] [PubMed] [Google Scholar]
- 10.McGraw TE, Greenfield L, Maxfield FR. Functional expression of the human transferrin receptor cDNA in Chinese hamster ovary cells deficient in endogenous transferrin receptor. J. Cell Biol. 1987;105:207–214. doi: 10.1083/jcb.105.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reignier T, et al. Receptor use by pathogenic arenaviruses. Virology. 2006;353:111–120. doi: 10.1016/j.virol.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 12.Rojek JM, Spiropoulou CF, Kunz S. Characterization of the cellular receptors for the South American hemorrhagic fever viruses Junin, Guanarito, and Machupo. Virology. 2006;349:476–491. doi: 10.1016/j.virol.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 13.Kasvosve I, Delanghe J. Total iron binding capacity and transferrin concentration in the assessment of iron status. Clin. Chem. Lab. Med. 2002;40:1014–1018. doi: 10.1515/CCLM.2002.176. [DOI] [PubMed] [Google Scholar]
- 14.Raya G, Henny J, Steinmetz J, Herbeth B, Siest G. Soluble transferrin receptor (sTfR): biological variations and reference limits. Clin. Chem. Lab. Med. 2001;39:1162–1168. doi: 10.1515/CCLM.2001.183. [DOI] [PubMed] [Google Scholar]
- 15.Anderson GJ, Powell LW, Halliday JW. Transferrin receptor distribution and regulation in the rat small intestine. Effect of iron stores and erythropoiesis. Gastroenterology. 1990;98:576–585. doi: 10.1016/0016-5085(90)90276-7. [DOI] [PubMed] [Google Scholar]
- 16.Andrews NC, Fleming MD, Levy JE. Molecular insights into mechanisms of iron transport. Curr. Opin. Hematol. 1999;6:61–64. doi: 10.1097/00062752-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Templeton DM, Liu Y. Genetic regulation of cell function in response to iron overload or chelation. Biochim. Biophys. Acta. 2003;1619:113–124. doi: 10.1016/S0304-4165(02)00497-X. [DOI] [PubMed] [Google Scholar]
- 18.Mattia E, Rao K, Shapiro DS, Sussman HH, Klausner RD. Biosynthetic regulation of the human transferrin receptor by desferrioxamine in K562 cells. J. Biol. Chem. 1984;259:2689–2692. [PubMed] [Google Scholar]
- 19.Ward JH, Kushner JP, Kaplan J. Regulation of HeLa cell transferrin receptors. J. Biol. Chem. 1982;257:10317–10323. [PubMed] [Google Scholar]
- 20.Oldstone MB. Arenaviruses. II. The molecular pathogenesis of arenavirus infections. Introduction. Curr. Top. Microbiol. Immunol. 2002;263:V–XII. [PubMed] [Google Scholar]
- 21.Daniels TR, Delgado T, Rodriguez JA, Helguera G, Penichet ML. The transferrin receptor, part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin. Immunol. 2006;121:144–158. doi: 10.1016/j.clim.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Soda R, Tavassoli M. Liver endothelium and not hepatocytes or Kupffer cells have transferrin receptors. Blood. 1984;63:270–276. [PubMed] [Google Scholar]
- 23.Jefferies WA, et al. Transferrin receptor on endothelium of brain capillaries. Nature. 1984;312:162–163. doi: 10.1038/312162a0. [DOI] [PubMed] [Google Scholar]
- 24.Peters CJ, Zaki SR. Role of the endothelium in viral hemorrhagic fevers. Crit. Care Med. 2002;30:S268–S273. doi: 10.1097/00003246-200205001-00016. [DOI] [PubMed] [Google Scholar]
- 25.Parker JS, Murphy WJ, Wang D, O’Brien SJ, Parrish CR. Canine and feline parvoviruses can use human or feline transferrin receptors to bind, enter, and infect cells. J. Virol. 2001;75:3896–3902. doi: 10.1128/JVI.75.8.3896-3902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross SR, Schofield JJ, Farr CJ, Bucan M. Mouse transferrin receptor 1 is the cell entry receptor for mouse mammary tumor virus. Proc. Natl Acad. Sci. USA. 2002;99:12386–12390. doi: 10.1073/pnas.192360099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.York J, Nunberg JH. Role of the stable signal peptide of Junin arenavirus envelope glycoprotein in pH-dependent membrane fusion. J. Virol. 2006;80:7775–7780. doi: 10.1128/JVI.00642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunz S, Rojek JM, Perez M, Spiropoulou CF, Oldstone MB. Characterization of the interaction of lassa fever virus with its cellular receptor α-dystroglycan. J. Virol. 2005;79:5979–5987. doi: 10.1128/JVI.79.10.5979-5987.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong SK, Li W, Moore MJ, Choe H, Farzan M. A 193-amino acid fragment of the SARS coronavirus S protein efficiently binds angiotensin-converting enzyme 2. J. Biol. Chem. 2004;279:3197–3201. doi: 10.1074/jbc.C300520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains Supplementary Figure S1 with legend. (PDF 88 kb)


