Abstract
The hormonal correlates of reproductive aging and the menopause transition reflect an initial loss of the follicle cohort, while a responsive ovary remains, and an eventual complete loss of follicle response, with persistent hypergonadotropic amenorrhea. The physiology of the process is described, along with key findings of relevant studies, with an emphasis on SWAN, the Study of Women’s Health Across the Nation. A clinical framework is provided to help clinicians forecast the major milestones of the menopausal transition and to predict potential symptoms or disease.
Keywords: Menopause, menopause transition, inhibin, AMH, LH, FSH, estrogen, progesterone
Introduction
Women undergo progressive follicle loss throughout life4. While the majority of follicles in an individual woman’s ovaries are lost in fetal life2, there is progressive, exponential loss of oocytes through a woman’s reproductive life span. Most mathematical models of oocyte loss indicate an exponential process, and an acceleration of follicle loss as women enter the menopausal transition11,33, indicating that a woman loses follicles at the fastest rates when she is the most reproductively aged. Moreover, there is evidence that the structural and functional quality of oocytes deteriorates with reproductive aging3.
In addition to changes in follicle numbers, which is the main driver of the menopausal transition, follicle dynamics also shift with the process of menopause16,37. The cardinal hormonal changes recognized to occur in concert with the menstrual cycle irregularity that herald entry into the menopausal transition also serve to accelerate the follicular growth process and may make it less effective. Finally, alterations in central neural processes that reliably produce an ovulatory LH surge in midreproductive life appear to become less reliable, and lead to further cycle dysfunction48.
This review will describe the hormonal changes that accompany the process of follicle loss and menopause. We shall draw primarily, when possible, from large-sample epidemiological studies, especially SWAN, the Study of Women’s Health Across the Nation42. SWAN is a multi-ethnic, longitudinal cohort study of 3300 US women from 7 national sites enrolled when they were between the ages of 40-52. Women were followed annually in SWAN, which is now in its 14th follow-up year. Thus, most of the SWAN participants have undergone menopause and their data constitutes the most representative community-based sample available to date. SWAN’s Daily Hormone Study will also be discussed, as it is the most comprehensive study of menstrual cycles in midlife US women that has ever been collected.
Staging the Menopausal Transition
The menopausal transition (MT) is preceded by approximately 35 years of regular, predictable menstrual cycles. During this time, women have a well-defined intermenstrual interval of 25-35 days. These cycles consist of a 14 day luteal phase, and a follicular phase that is at least 10-11 days in length. Prior to the appearance of a break in this characteristic menstrual rhythm, the oocyte supply has been dwindling, but has not yet reached a critical level. Nonetheless, although no signs are detectable to a woman, some subtle hormonal changes are happening in this midreproductive interval. Follicle-stimulating hormone, critical for the terminal stages of follicle growth, rises, albeit slowly, throughout the reproductive years1. In addition to this change, a woman’s follicle cohort size shrinks, even though she continues to reliably ovulate every month.
The MT has been broken down into two stages: early and late. The initiation of the transition is when a previously regularly cycling woman experiences a skipped menstrual cycle, or notes a variation of her intermenstrual interval >6 days15. She remains in the early transition until she experiences more than 60 days’ amenorrhea, at which time she transitions into the ‘late’ MT. The late MT, characterized by prolonged amenorrhea, is accompanied by less frequent cycling, and ends with the final menstrual period (FMP). The FMP is only defined once a year of amenorrhea has been experienced. Each of these menstrual cycle milestones is accompanied by hormonal alterations that help the clinician determine a woman’s progress through the MT.
Methods for Evaluating Reproductive Aging and Predicting Ovarian Function
Follicle Stimulating Hormone
A monotropic rise in FSH is considered the endocrinological hallmark of the MT. FSH rises appear to be intermittent as the follicle supply dwindles, but it is an accurate reflection of a woman’s ability to conceive. As such, it is more of an indicator of egg quantity than of egg quality in the early transition. FSH rises are more sustained over time in the late MT and the rise is most precipitous in the years bracketing the FMP32. Early follicular phase FSH, taken between cycle days 2-5, is the most sensitive and convenient time in the cycle to perform this measurement.
Estradiol
Along with the monotropic FSH rise characteristic of the MT, estradiol (E2) can also be elevated in the early follicular phase of the cycle, especially in the early MT34,35.This appears to be a reflection of the accelerated folliculogenesis and shortening of the follicular phase that occur in the early MT. Early follicular E2 levels are the last biomarker of the transition to irrevocably change, with a rapid decline beginning two years before the FMP and reaching stability two years afterwards12.
Inhibin B
The inhibins are peptides of the tgf-beta superfamily that are produced by the granulosa cells of ovarian follicles in their terminal stages of development. They consist of a common alpha subunit, with specificity conferred by the beta subunit. Inhibin B is produced by the granulosa cells of the growing follicle cohort and reflects both the health of the individual follicles and their overall numbers10. Inhibin A is a product of the dominant follicle and the corpus luteum. The shrinkage of the follicle cohort that accompanies aging is detectable by a reduction in inhibin B. Thus, early follicular phase inhibin B measurement has been proposed as way to measure ovarian reserve directly10,40. Inhibin B is the one of the earliest harbingers of the menopausal transition. Thus, loss of the follicle cohort appears to be the initiating step into the hormonal changes that ensue with the onset of the MT.
Antimullerian Hormone or Mullerian Inhibiting Substance (AMH; MIS)
AMH/MIS are also tgf-beta superfamily peptides, and are produced by granulosa cells. However, they are not just produced by follicles in their terminal stages of growth but also by primary, secondary and early antral follicles and therefore reflect more completely the follicle cohort. Circulating concentrations of AMH/MIS decline throughout reproductive life and theoretically would constitute the earliest and most effective way to measure a woman’s progress towards menopause. While some studies indicate that AMH/MIS holds promise as a predictor of time to menopause, the current sensitivity of the measurement method is such that estimates of time to the FMP cannot be accomplished within a time frame less than 4 years43. It is hoped that eventual development of a sufficiently sensitive assay for AMH/MIS will become a ‘menopause test’.
Evaluations of Annual Serum Hormones Across the Transition
A primary directive of the RFA that led to the SWAN study was to ‘characterize the endocrinology/physiology of the perimenopause’, including the assessment and discovery of biomarkers of ovarian aging. Prior longitudinal studies by McKinlay21 and Burger5 had begun to describe the patterns of change in serum FSH and E2 across the MT, establishing them as candidate markers of ovarian function over time that tracked both ovarian secretion (E2) and central regulation of that secretion (FSH). Both were included as annual measures in the longitudinal design of SWAN, and mandated a standardized collection protocol anchored to a reproducible timeframe in the menstrual cycle due to significant variation in levels across the cycle. The early follicular phase was deemed the most reproducible and biologically comparable as women lost menstrual cyclicity through the MT, recognizing that changes in other menstrual cycle phases would not be evaluable with this sampling scheme. Longitudinal analyses of the accrued annual serum hormone levels included variables for day of cycle and loss of cyclicity to control for cycle variability.
An initial longitudinal analysis of FSH and E2 through five years of follow-up29 noted that serum E2 concentrations decreased and FSH concentrations increased significantly with age, with more rapid change at higher ages. As the Study matured, and more women had observed natural FMPs, it became possible to analyze these data anchored to the most widely utilized biomarker of ovarian aging, the FMP31,44. Utilizing analyses that describe change over time, previously able to decompose the MT into ‘epochs’ of FSH and E2 change around the FMP in a parallel study of somewhat younger women44, the FSH pattern across the menopause transition began with an increase about 6 years prior to the FMP, accelerated 2 years before the FMP, decelerated beginning 0.20 years before the FMP and attained stable levels 2 years after the FMP, independent of age at the FMP, race/ethnicity or smoking status. The mean E2 concentration did not change until 2 years prior to the FMP when it began decreasing, achieving maximal rate of change at the FMP, and then decelerating to achieve stability 2 years after the FMP. The timespans and overall patterns of change in serum FSH and E2 across the menopausal transition were not related to age at FMP.
In contrast to the maintenance of E2 levels until two years prior to the FMP, inhibin B and AMH concentrations fall to become undetectable by available assays 4-5 years before cessation of bleeding43, consistent with a decrease in both the size of the primordial follicle pool and the cohort of follicles recruited from that pool for each menstrual cycle. The fall in inhibin B mirrors the rise in FSH, supporting the primary role of the inhibins in the negative feedback regulation of FSH secretion.
Taken together, the dynamics of the early menopausal transition appear to be based upon follicle attrition and a loss of the follicle cohort, rather than complete follicle failure. The reduction in follicle cohort size appears to be the earliest event in the transition, as evidenced by a detectable drop in inhibin B6. Thus, while there are still responsive follicles available, inhibin restraint of FSH secretion is lost. The resulting FSH rise leads to faster and possibly dysregulated folliculogenesis, and the menstrual cycle may grow shorter. Occasionally, the cohort size is insufficient to develop an ovulatory follicle, and the cycle is skipped, but there is no period of amenorrhea longer than 60 days (i.e., two skipped cycles). By the late menopausal transition, follicle numbers become critically low and follicle competency (either due to abnormal regulation or to anatomical or functional inferiority of follicles that grow later in reproductive life) is reduced such that anovulatory cycles become more common and cycles in which folliculogenesis cannot even be initiated become more common. Women then undergo a period of time of ‘prolonged amenorrhea’ of 2-11 months’ duration. Eventually, at a follicle complement of approximately 1,00033, the FMP is attained and hypergonadotropic amenorrhea becomes permanent. Although the definition of the FMP as fully defined after 12 months’ amenorrhea and the process is widely believed to be irreversible, it is age related and not an absolute boundary. Women over aged 45 who experience 12 months’ amenorrhea have a 10% probability of having a subsequent menstrual episode47.
Menstrual Cycle Hormone Changes Across the MT
While numerous worldwide studies have reported findings on annual or semi-annual changes in serum hormones across the MT8,13,32, which are usually measured in the early follicular phase of the cycle, day to day hormone levels across a menstrual cycle have been less well characterized.
The first studies of menstrual cycle dynamics across the menopausal transition were performed by Metcalf, from New Zealand. To characterize hormone patterns over time, these investigators developed methods suitable for the collection and analysis of daily, urinary samples for gonadotropins and sex steroids. In a series of small sample size, in both cross sectional and longitudinal studies22-26, the Metcalf group provided the framework that has informed much current research. By examining cycles longitudinally, they observed that the proportion of ovulatory cycles decreased as women approached the FMP. Once the FMP was attained, variable estrogen excretion was observed for the first year afterwards, but no further progesterone excretion was measurable. These observations have been confirmed by subsequent investigators.
Others have since added pertinent findings by examining larger sample sizes and uncovering novel relationships. In the SWAN Daily Hormone Study, 990 women were invited to complete a daily urine collection for an entire menstrual cycle or up to 50 days if menses did not occur, and to repeat the collection annually until the FMP. Of the original 990, 848 women (86%) had interpretable data available for analysis in the baseline collection38. Over a three-year follow-up period, several findings became evident from this large sample. First, a previously-unappreciated relationship between hormones and body mass index (BMI) became evident in this large sample size. With increasing BMI, LH, FSH, estrogen and progesterone metabolites all were observed to decrease significantly. Over time, progesterone metabolite excretion was reduced, both by age and by year on study. Moreover, Chinese and Japanese-American women demonstrated lower estrogen metabolite excretion compared to the other ethnic groups in SWAN.
In the BIMORA (Biodemographic Models of Reproductive Aging) project, samples of 17-134 women per reproductive stage group were assessed with 6-month, daily collections of urine27. These data confirmed prior, smaller studies35 indicating an increase in estrogen exposure and increased potential for unopposed estrogen exposure as women traverse the menopause. Others have examined the hormone patterns during a menstrual cycle be performing tri-weekly serum sampling14. To explain some of the erratic estrogen levels observed in perimenopausal women, these investigators coined the phrase ‘luteal out-of-phase’ cycles, or LOOP to describe a specific pattern of irregular cycling. In these cycles, the secondary, luteal rise of estrogen is maintained and even exaggerated, and leads to a subsequent cycle in which progesterone production is deficient. The investigators attributed this pattern to sustained FSH elevation, which ‘drove’ estrogen production in the subsequent cycle and likely caused folliculogenesis at an inappropriately early time (i.e., the preceding luteal phase).
Androgens and the Transition
The ovary, the periphery (particularly adipose tissue) and the adrenal gland are the sources of androgen production in women. A detailed picture of the patterns of androgen secretion and their correlates will be covered in another section and thus will not be developed in detail in this review.
Briefly, the contributions of the ovary, periphery and adrenal gland change over the course of the menopausal transition. As the ovarian contribution of testosterone declines with menopause, concurrent decreases in sex hormone binding globulin (SHBG), the principal, high-affinity serum carrier protein for both testosterone and estradiol, offsets the drop in testosterone, resulting in an overall increase in free androgen index (FAI, a measure of androgens that takes into account SHBG) in many women7. The decrease in SHBG that accompanies the menopausal transition is due to the dual influence of declining estradiol levels, a function of progress to menopause, and increased age-related insulin resistance. Adiposity also appears to contribute, via increased peripheral conversion of androstenedione via enhanced 17 beta hydroxysteroid dehydrogenase Type V28. Thus, women who acquire obesity in midlife or who experience a substantial gain in weight are likely to experience relative hyperandrogenemia. The relative androgen excess, defined as the molar ration of testosterone to estradiol, was observed to increase by 10.1% per year in women in the SWAN Study, and its increase correlated with the development of the metabolic syndrome over time45.
Adrenal androgens follow a different time course from ovarian androgens, with a general decline beginning in young adulthood and continuing through the menopausal transition. DHEAS is the most commonly measured adrenal androgen in most studies of the menopausal transition. The decline in DHEAS was found to be reversed in most women during the late menopausal transition18 , with 85% demonstrating an increase in the late transition9. Circulating DHEAS varies with ethnicity as Chinese women have the highest levels, and is significantly related to BMI. Women with higher DHEAS reported greater overall quality of life, physical functioning and fewer depressive symptoms39.
Relationships Between Cycle Characteristics and Patterns to Bleeding, Sleep, Symptoms, and Other Correlates of Health in Midlife Women
Menstrual cycle hormone patterns have been related to several other symptoms, signs and risk factors in a series of studies performed in SWAN. The patterns of menstrual bleeding have been related to urinary hormone excretion patterns46. For the most part, changes in cycle timing (unusually long or short cycles) were associated with a failure of ovulation. Short and long duration of menstrual bleeding were also associated with anovulation. However, the relative self-reported heaviness of menstrual bleeding was more likely to be associated with leiomyomata and obesity. These findings suggest that unusually heavy menstrual bleeding typically does not have a hormonal basis, and such patterns, especially when they are persistent, should be investigated for an underlying anatomical, gynecologic cause.
Women who collected daily urine samples in the SWAN Study also completed a daily diary in which they reported mood, sexual interest, and sleep quality. Women recorded whether or not they had had trouble sleeping during the previous night. Overall, a 29% increase in the odds of reporting trouble sleeping was observed as women progressed from regular cycling into the early transition17. Sleep quality was worst at the beginning and at the end of the menstrual cycle. The occurrence of vasomotor symptoms was also observed across the transition within a menstrual cycle.
Lipid profiles and inflammatory markers in women with varying cycle lengths in SWAN, when controlled for body size, showed no difference except for triglycerides which increased with increasing cycle length20. Lower mean cycle estrone conjugates and pregnanediol glucuronide were associated with higher triglycerides, insulin and inflammatory markers. In longitudinal SWAN studies, total perimenopause/early postmenopause and with falling E2 and rising FSH independently of age, while HDL peaked in the late perimenopause19. However, when referenced to an observed FMP, only total cholesterol, LDL c, and apolipoprotein B increased with menopause while all other lipid, inflammatory and glycemic markers changed with chronologic age19.
Factors, including BMI and race/ethnicity that influence hormones and patterns of hormones
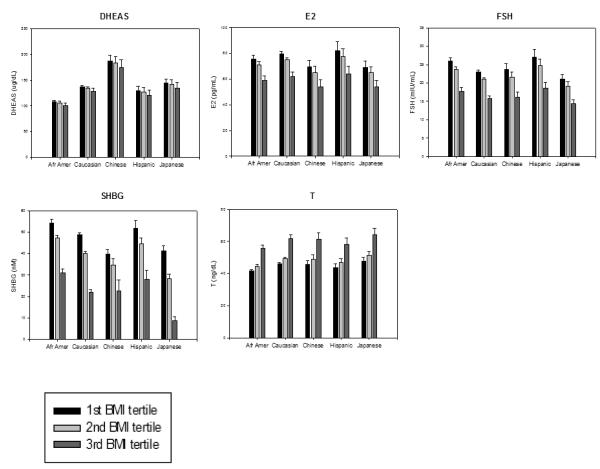
Initial cross-sectional analyses of baseline annual serum hormone levels demonstrated variation in all hormones by body size, positively for T and negatively for all others30. Ethnic variation in hormones was noted but was highly confounded by body size, and only FSH correlated with menopausal stage.
The longitudinal analyses in SWAN of FSH and E2 noted similar patterns in the decline of E2 and the increase in FSH with age across ethnic groups, but the levels of these hormones differed by race/ethnicity29. The ethnic differences in E2 and FSH were independent of menopausal status while the effect of BMI on serum E2 and FSH levels varied by menopausal status. Obesity markedly attenuated the FSH rise and delayed the initial increase29, while obesity, smoking behavior, and being Chinese or Japanese were associated with some variation in E2 levels, but not the pattern of E2 change. Thus, timespans and overall patterns of change in serum FSH and E2 across the menopausal transition were not related to age at FMP or smoking, while timespans but not overall patterns were related to obesity and race/ethnicity.
Clinical implications
With the maturation of SWAN and the transition to postmenopause by the vast majority of participants, both observed and obscured by hormone use or surgery, it has become possible to disentangle many of the coincident effects of both ovarian and chronologic aging on the health of women at midlife. Moreover, the complex patterns of change throughout the MT in both the cyclic and longitudinal hormone profiles are now in sharper focus and will help direct clinical care as well as guide future research. It is important to note that they describe a variable pattern of change from regular, predictable menses to the absence of cyclicity with the end of bleeding.
The management of perimenopausal abnormal uterine bleeding is informed by the evidence that variation in reproductive hormones and progressively more frequent anovulatory cycles contribute primarily to cycle length changes but not hypermenorrhea. Heavy bleeding in non-obese women requires anatomic evaluation while ‘normal’ but irregular bleeding can be managed hormonally once endometrial pathology has been ruled out. Heavier but normally cyclic bleeding in the early MT is usually transient and may not require any intervention unless sustained.
Not surprisingly, frequently reported symptoms such as VMS, sleep complaints and mood changes are experienced more perimenstrually, in the early and late phases of the cycle, and commonly occur well before the increasing variability in cycle length of the late MT. This confirms the practice of cyclic therapy for specific symptoms in the later luteal phase into the onset of menses when women prefer intermittent rather than continuous treatment.
There appears to be a clear menopausal effect on cardiovascular risk factors, most prominently on total cholesterol, LDL cholesterol and apolipoprotein B, but most such factors are more related to chronologic aging. Together with the menopausal effect on body composition41, this suggests that strategies to limit such an increase in cholesterol and fat mass, either by lifestyle modification or medications, would be most effective if initiated during the early MT before they had taken place rather than waiting for a confirmed FMP. Whether such interventions can ultimately affect the subsequent onset and development of cardiovascular disease remains to be demonstrated by prospective trials.
Perhaps most surprisingly, SWAN longitudinal hormone studies describe a remarkably consistent pattern of change around the FMP for women who were in their mid-to-late forties and cycling regularly at the onset of observation. While absolute hormone levels vary particularly by body size but also by ethnicity, and symptoms vary dramatically, the timespan of the most active hormone changes is about four years centered on the FMP. This time corresponds to the most probable time of reporting symptoms, and coincides with the onset of measurable changes in bone12 and body composition. It suggests that the late MT may be much more predictable than previously believed, and that interventions to optimize health in midlife and into old age could be reliably initiated well before the observed cessation of bleeding.
For the clinician, the current convention is that the MT has begun when a previously regularly cycling woman, regardless of age, develops increasing cycle variability such that her longest and shortest cycles differ by a week. Hormone changes are gradual and superimposed on progressively increasing cycle variability, and they typically last for several years. The late MT is entered when a women has an episode without bleeding for at least 60 days, indicating the progressive dysregulation of individual follicle development and frequently near the time when hormone changes accelerate about two years prior to the FMP. Bleeding ceases when follicles can no longer produce sufficient E2 to stimulate endometrial proliferation, and hormone levels stabilize again about two years after the FMP.
Figure 1.
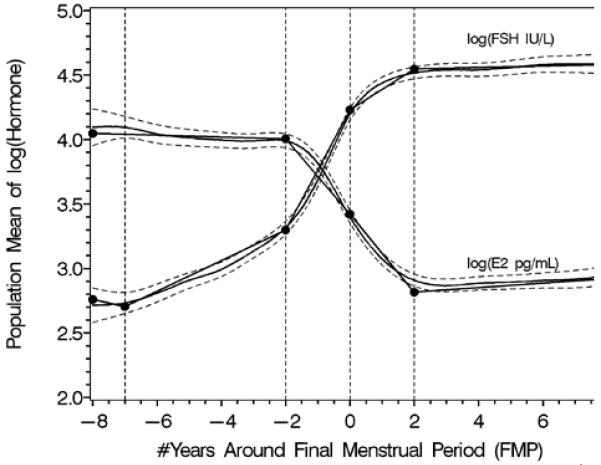
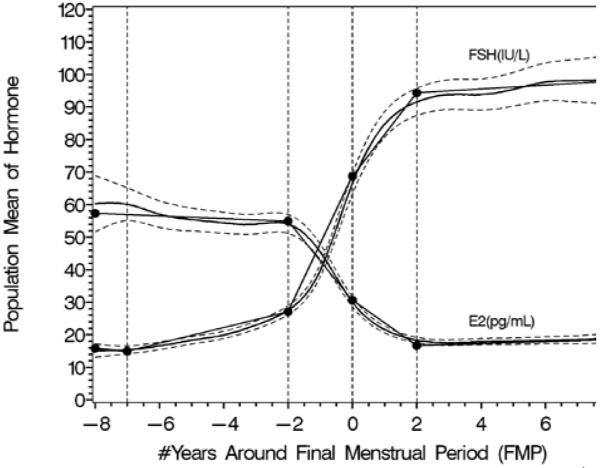
Rates of change in FSH and estradiol shown anchored to the final menstrual period. Panel A shows the log transformed values, panel B shows back-transformed values. Reprinted with permission31.
A. Segmented mean profiles for natural logarithm-transformed FSH and E2.
B. Segmented mean profiles for FSH and E2.
Figure 2.
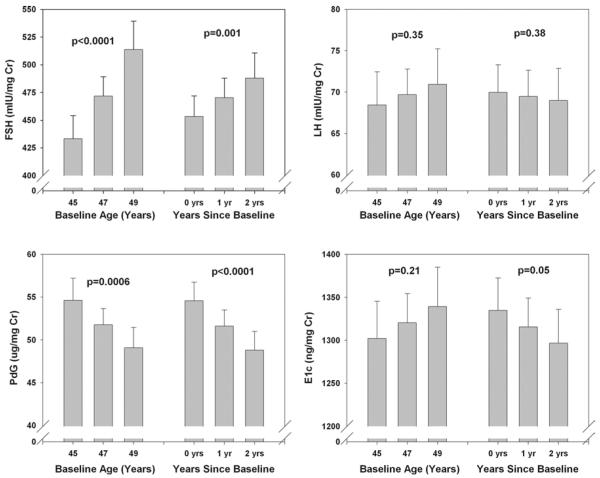
Adjusted mean whole-cycle urinary reproductive hormones (with CI) by age and elapsed years since baseline (ovulatory cycles only). Note the prominent rise in FSH and decrease in Pdg with both age and years on study. A more modest rise in LH is observed, and E1c does not decline with age. Reprinted with permission.36
Figure 3.
Baseline serum hormones in the SWAN Study sample. Each of the ethnic groups in SWAN are shown as clusters of bars; within each cluster, individual bars show BMI tertiles. Reprinted with permission30.
Figure 4.
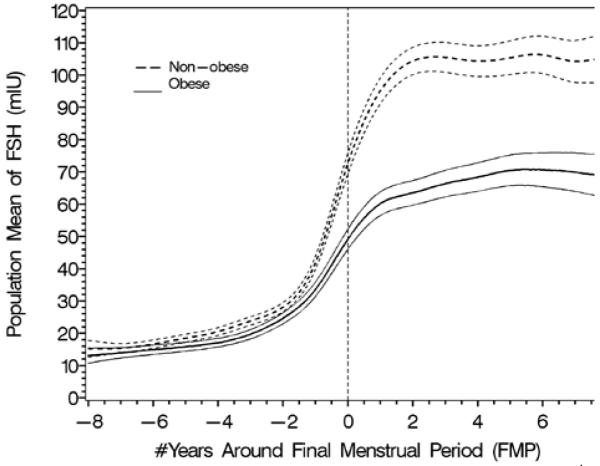
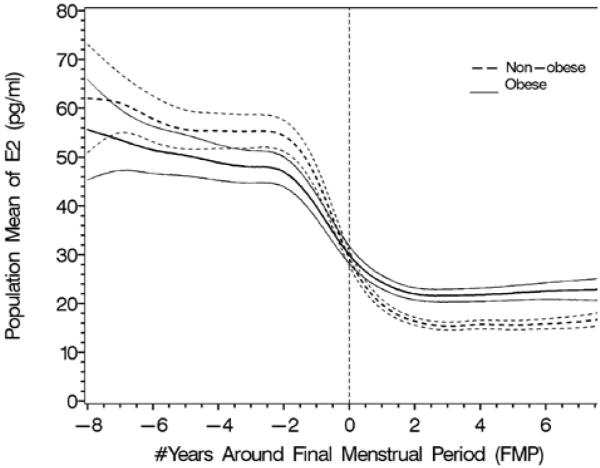
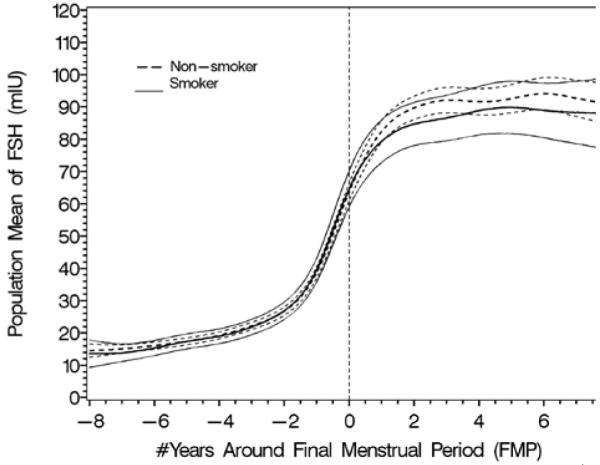
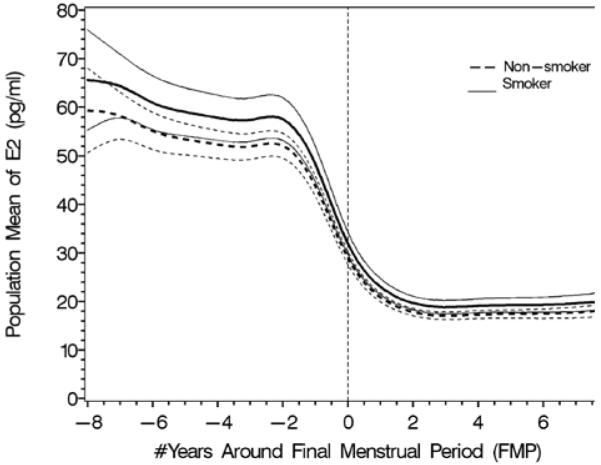
The effect of obesity (4A and B) and smoking (4C and D) on FSH and estradiol. Data are shown anchored to the FMP. Reprinted with permission31.
A. Population mean and 95% CI for mean FSH: obese vs. non-obese.
B. Population mean and 95% CI for mean E2: obese vs. non-obese.
C. Population mean and 95% CI for mean FSH: smoker vs. non-smoker.
D. Population mean and 95% CI for mean E2: smoker vs. non-smoker.
Acknowledgments
This work was funded by AG-12535 and NR-04061
Footnotes
Disclosures: Dr. Nanette Santoro is a consultant to Menogenix
Contributor Information
Nanette Santoro, University of Colorado School of Medicine, 12631 E 17th Avenue, Mail Stop B-198, AO1-Room 4010, Aurora, Colorado 80045 303-724-2041 303-724-2061 FAX Nanette.Santoro@ucdenver.edu.
John F Randolph, Jr., Division of Reproductive Endocrinology, University of Michigan, L4100 Women’s Medical Hospital, 1500 East Medical Center Drive, Ann Arbor, MI 48109-0276 734-763-4323 FAX 734-647-9727 JFRandol@umich.edu.
References
- 1.Ebbiary NA Ahmed, Lenton EA, Cooke ID. Hypothalamic-pituitary ageing: progressive increase in FSH and LH concentrations throughout the reproductive life in regularly menstruating women. Clin Endocrinol (Oxf) 1994;41:199. doi: 10.1111/j.1365-2265.1994.tb02530.x. [DOI] [PubMed] [Google Scholar]
- 2.Baker TG. A Quantitative and Cytological Study of Germ Cells in Human Ovaries. Proc R Soc Lond B Biol Sci. 1963;158:417. doi: 10.1098/rspb.1963.0055. [DOI] [PubMed] [Google Scholar]
- 3.Battaglia DE, Goodwin P, Klein NA, et al. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum Reprod. 1996;11:2217. doi: 10.1093/oxfordjournals.humrep.a019080. [DOI] [PubMed] [Google Scholar]
- 4.Block E. Quantitative morphological investigations of the follicular system in women; variations at different ages. Acta Anat (Basel) 1952;14:108. doi: 10.1159/000140595. [DOI] [PubMed] [Google Scholar]
- 5.Burger HG. The endocrinology of the menopause. J Steroid Biochem Mol Biol. 1999;69:31. doi: 10.1016/s0960-0760(98)00145-9. [DOI] [PubMed] [Google Scholar]
- 6.Burger HG, Cahir N, Robertson DM, et al. Serum inhibins A and B fall differentially as FSH rises in perimenopausal women. Clin Endocrinol (Oxf) 1998;48:809. doi: 10.1046/j.1365-2265.1998.00482.x. [DOI] [PubMed] [Google Scholar]
- 7.Burger HG, Dudley EC, Cui J, et al. A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone-binding globulin levels through the menopause transition. J Clin Endocrinol Metab. 2000;85:2832. doi: 10.1210/jcem.85.8.6740. [DOI] [PubMed] [Google Scholar]
- 8.Burger HG, Hale GE, Robertson DM, et al. A review of hormonal changes during the menopausal transition: focus on findings from the Melbourne Women’s Midlife Health Project. Hum Reprod Update. 2007;13:559. doi: 10.1093/humupd/dmm020. [DOI] [PubMed] [Google Scholar]
- 9.Crawford S, Santoro N, Laughlin GA, et al. Circulating dehydroepiandrosterone sulfate concentrations during the menopausal transition. J Clin Endocrinol Metab. 2009;94:2945. doi: 10.1210/jc.2009-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danforth DR, Arbogast LK, Mroueh J, et al. Dimeric inhibin: a direct marker of ovarian aging. Fertil Steril. 1998;70:119. doi: 10.1016/s0015-0282(98)00127-7. [DOI] [PubMed] [Google Scholar]
- 11.Faddy MJ, Gosden RG, Gougeon A, et al. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod. 1992;7:1342. doi: 10.1093/oxfordjournals.humrep.a137570. [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab. 2008;93:861. doi: 10.1210/jc.2007-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freeman EW, Sammel MD, Gracia CR, et al. Follicular phase hormone levels and menstrual bleeding status in the approach to menopause. Fertil Steril. 2005;83:383. doi: 10.1016/j.fertnstert.2004.06.066. [DOI] [PubMed] [Google Scholar]
- 14.Hale GE, Hughes CL, Burger HG, et al. Atypical estradiol secretion and ovulation patterns caused by luteal out-of-phase (LOOP) events underlying irregular ovulatory menstrual cycles in the menopausal transition. Menopause. 2009;16:50. doi: 10.1097/GME.0b013e31817ee0c2. [DOI] [PubMed] [Google Scholar]
- 15.Harlow SD, Mitchell ES, Crawford S, et al. The ReSTAGE Collaboration: defining optimal bleeding criteria for onset of early menopausal transition. Fertil Steril. 2008;89:129. doi: 10.1016/j.fertnstert.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein NA, Battaglia DE, Miller PB, et al. Ovarian follicular development and the follicular fluid hormones and growth factors in normal women of advanced reproductive age. J Clin Endocrinol Metab. 1996;81:1946. doi: 10.1210/jcem.81.5.8626862. [DOI] [PubMed] [Google Scholar]
- 17.Kravitz HM, Janssen I, Santoro N, et al. Relationship of day-to-day reproductive hormone levels to sleep in midlife women. Arch Intern Med. 2005;165:2370. doi: 10.1001/archinte.165.20.2370. [DOI] [PubMed] [Google Scholar]
- 18.Lasley BL, Santoro N, Randolf JF, et al. The relationship of circulating dehydroepiandrosterone, testosterone, and estradiol to stages of the menopausal transition and ethnicity. J Clin Endocrinol Metab. 2002;87:3760. doi: 10.1210/jcem.87.8.8741. [DOI] [PubMed] [Google Scholar]
- 19.Matthews KA, Crawford SL, Chae CU, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54:2366. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matthews KA, Santoro N, Lasley B, et al. Relation of cardiovascular risk factors in women approaching menopause to menstrual cycle characteristics and reproductive hormones in the follicular and luteal phases. J Clin Endocrinol Metab. 2006;91:1789. doi: 10.1210/jc.2005-1057. [DOI] [PubMed] [Google Scholar]
- 21.McKinlay SM. The normal menopause transition: an overview. Maturitas. 1996;23:137. doi: 10.1016/0378-5122(95)00985-x. [DOI] [PubMed] [Google Scholar]
- 22.Metcalf MG. The approach of menopause: a New Zealand study. N Z Med J. 1988;101:103. [PubMed] [Google Scholar]
- 23.Metcalf MG, Donald RA, Livesey JH. Classification of menstrual cycles in preand perimenopausal women. J Endocrinol. 1981;91:1. doi: 10.1677/joe.0.0910001. [DOI] [PubMed] [Google Scholar]
- 24.Metcalf MG, Donald RA, Livesey JH. Pituitary-ovarian function before, during and after the menopause: a longitudinal study. Clin Endocrinol (Oxf) 1982;17:489. doi: 10.1111/j.1365-2265.1982.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 25.Metcalf MG, Donald RA, Livesey JH. Pituitary-ovarian function in normal women during the menopausal transition. Clin Endocrinol (Oxf) 1981;14:245. doi: 10.1111/j.1365-2265.1981.tb00193.x. [DOI] [PubMed] [Google Scholar]
- 26.Metcalf MG, Livesey JH. Gonadotrophin excretion in fertile women: effect of age and the onset of the menopausal transition. J Endocrinol. 1985;105:357. doi: 10.1677/joe.0.1050357. [DOI] [PubMed] [Google Scholar]
- 27.O’Connor KA, Ferrell RJ, Brindle E, et al. Total and unopposed estrogen exposure across stages of the transition to menopause. Cancer Epidemiol Biomarkers Prev. 2009;18:828. doi: 10.1158/1055-9965.EPI-08-0996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quinkler M, Sinha B, Tomlinson JW, et al. Androgen generation in adipose tissue in women with simple obesity--a site-specific role for 17betahydroxysteroid dehydrogenase type 5. J Endocrinol. 2004;183:331. doi: 10.1677/joe.1.05762. [DOI] [PubMed] [Google Scholar]
- 29.Randolph JF, Jr., Sowers M, Bondarenko IV, et al. Change in estradiol and follicle-stimulating hormone across the early menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab. 2004;89:1555. doi: 10.1210/jc.2003-031183. [DOI] [PubMed] [Google Scholar]
- 30.Randolph JF, Jr., Sowers M, Gold EB, et al. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. J Clin Endocrinol Metab. 2003;88:1516. doi: 10.1210/jc.2002-020777. [DOI] [PubMed] [Google Scholar]
- 31.Randolph JF, Jr., Zheng H, Sowers MR, et al. Change in follicle-stimulating hormone and estradiol across the menopausal transition: effect of age at the final menstrual period. J Clin Endocrinol Metab. 96:746. doi: 10.1210/jc.2010-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Randolph JF, Jr., Zheng H, Sowers MR, et al. Change in Follicle-Stimulating Hormone and Estradiol Across the Menopausal Transition: Effect of Age at the Final Menstrual Period. J Clin Endocrinol Metab. doi: 10.1210/jc.2010-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab. 1987;65:1231. doi: 10.1210/jcem-65-6-1231. [DOI] [PubMed] [Google Scholar]
- 34.Santoro N, Brockwell S, Johnston J, et al. Helping midlife women predict the onset of the final menses: SWAN, the Study of Women’s Health Across the Nation. Menopause. 2007;14:415. doi: 10.1097/gme.0b013e31802cc289. [DOI] [PubMed] [Google Scholar]
- 35.Santoro N, Brown JR, Adel T, et al. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81:1495. doi: 10.1210/jcem.81.4.8636357. [DOI] [PubMed] [Google Scholar]
- 36.Santoro N, Crawford SL, Lasley WL, et al. Factors related to declining luteal function in women during the menopausal transition. J Clin Endocrinol Metab. 2008;93:1711. doi: 10.1210/jc.2007-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santoro N, Isaac B, Neal-Perry G, et al. Impaired folliculogenesis and ovulation in older reproductive aged women. J Clin Endocrinol Metab. 2003;88:5502. doi: 10.1210/jc.2002-021839. [DOI] [PubMed] [Google Scholar]
- 38.Santoro N, Lasley B, McConnell D, et al. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The Study of Women’s Health across the Nation (SWAN) Daily Hormone Study. J Clin Endocrinol Metab. 2004;89:2622. doi: 10.1210/jc.2003-031578. [DOI] [PubMed] [Google Scholar]
- 39.Santoro N, Torrens J, Crawford S, et al. Correlates of circulating androgens in mid-life women: the study of women’s health across the nation. J Clin Endocrinol Metab. 2005;90:4836. doi: 10.1210/jc.2004-2063. [DOI] [PubMed] [Google Scholar]
- 40.Seifer DB, Lambert-Messerlian G, Hogan JW, et al. Day 3 serum inhibin-B is predictive of assisted reproductive technologies outcome. Fertil Steril. 1997;67:110. doi: 10.1016/s0015-0282(97)81865-1. [DOI] [PubMed] [Google Scholar]
- 41.Sowers M, Zheng H, Tomey K, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab. 2007;92:895. doi: 10.1210/jc.2006-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sowers MF, Crawford SL, Sternfeld B, et al. SWAN: a multi-center, mulitiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. Academic Press; San Diego: 2000. p. 175. [Google Scholar]
- 43.Sowers MR, Eyvazzadeh AD, McConnell D, et al. Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008;93:3478. doi: 10.1210/jc.2008-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sowers MR, Zheng H, McConnell D, et al. Follicle stimulating hormone and its rate of change in defining menopause transition stages. J Clin Endocrinol Metab. 2008;93:3958. doi: 10.1210/jc.2008-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torrens JI, Sutton-Tyrrell K, Zhao X, et al. Relative androgen excess during the menopausal transition predicts incident metabolic syndrome in midlife women: study of Women’s Health Across the Nation. Menopause. 2009;16:257. doi: 10.1097/gme.0b013e318185e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Voorhis BJ, Santoro N, Harlow S, et al. The relationship of bleeding patterns to daily reproductive hormones in women approaching menopause. Obstet Gynecol. 2008;112:101. doi: 10.1097/AOG.0b013e31817d452b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallace RB, Sherman BM, Bean JA, et al. Probability of menopause with increasing duration of amenorrhea in middle-aged women. Am J Obstet Gynecol. 1979;135:1021. doi: 10.1016/0002-9378(79)90729-4. [DOI] [PubMed] [Google Scholar]
- 48.Weiss G, Skurnick JH, Goldsmith LT, et al. Menopause and hypothalamicpituitary sensitivity to estrogen. JAMA. 2004;292:2991. doi: 10.1001/jama.292.24.2991. [DOI] [PubMed] [Google Scholar]