Abstract
Use of drugs of abuse in combination is common among recreational users and addicts. The combination of a psychomotor stimulant with an opiate, known as a ‘speedball’, reportedly produces greater effects than either drug alone and has been responsible for numerous deaths. Historically, the most popular speedball combination is that of cocaine and heroin. However, with the growing popularity of methamphetamine in recent years, there has been increased use of this drug in combination with other drugs of abuse, including opiates. Despite this, relatively little research has examined interactions between methamphetamine and opiates. In the current research, behavioral interactions between methamphetamine and the prototypical opiate, morphine, were examined across a variety of dose combinations in Sprague-Dawley rats. The combination of methamphetamine and morphine produced stimulation of behavior that was dramatically higher than either drug alone; however, the magnitude of the interaction was dependent on the dose of the drugs and the specific behaviors examined. The results demonstrate complex behavioral interactions between these drugs, but are consistent with the idea that this combination is used because it produces a greater effect than either drug alone.
Keywords: Speedball, Methamphetamine, Morphine, Locomotor behavior, Polydrug abuse, Synergism
Introduction
Polydrug use, in which individuals administer combinations of different drugs, is common among drug abusers. One of the most popular combinations for injection drug users is that of a psychomotor stimulant with an opiate, often referred to as a “speedball” or “bombita” (Ellinwood et al., 1976; Leri et al., 2003a). The extent of speedball use is high in injection drug users, with up to 92% of heroin addicts reporting concomitant use of cocaine (for review see Leri et al., 2003a). Speedball use and its dangers have been brought to the attention of the general public through the high profile deaths of celebrities from such drug combinations, including John Belushi in 1982, River Phoenix in 1993 and Chris Farley in 1997 (Burnett et al., 2010).
Opiates and psychomotor stimulants produce their neural and behavioral effects through distinct actions on the brain. The behavioral and subjective effects of opiates are produced principally via actions at mu opioid receptors, while those of psychomotor stimulants are due to enhancement of synaptic levels of dopamine (Leri et al., 2003a; Trujillo et al., 1993; Watson et al., 1989). However, there is significant overlap between mu opioid receptors and dopamine, especially in the nucleus accumbens, a brain region that is critically involved in both the stimulant effects and the rewarding effects of opiates and psychomotor stimulants (Trujillo et al., 1993; Watson et al., 1989).
There are a variety of reasons individuals self-administer opiate/stimulant combinations. Three of the more likely are: 1) administration of the combination produces effects greater than either drug alone (such as a greater high or greater rush); 2) administration of one decreases the side-effects of another (such as the opiate decreasing cocaine-induced agitation or anxiety, or conversely, cocaine tempering opiate-induced sedation); or 3) the combination produces unique subjective effects desired by the user (Ellinwood et al., 1976; Leri et al., 2003a). Although a number of studies have examined these possibilities, there is still an incomplete understanding of the phenomenon, and of the behavioral consequences of coadministration of opiates and psychomotor stimulants.
The most commonly abused and most widely researched speedball combination is that of heroin and cocaine (Cornish et al., 2005; David et al., 2001; Duvauchelle et al., 1998; Guzman and Ettenberg, 2004; Lamas et al., 1998; Leri et al., 2003b; Leri and Stewart, 2001; Malow et al., 1992; Martin et al., 2006; Mattox et al., 1997; Mello and Negus, 1998; Mello et al., 1995; Negus, 2005; Ranaldi and Munn, 1998; Roberts et al., 1997; Rowlett et al., 2007; Rowlett et al., 2005; Rowlett and Woolverton, 1995, 1997; Smith et al., 2006; Torrens et al., 1991). Considerably less is known about coadministration of other stimulants and other opiates. However, due to the current popularity and availability of stimulants other than cocaine (especially methamphetamine), it is likely that drug combinations using other stimulants will increase in popularity. For example, the U.S. Drug Abuse Warning Network indicates that in 2007, 5% of drug-related emergency room visits were due to use of methamphetamine (Substance Abuse and Mental Health Services Administration, 2010). Moreover, over 40% of injection methamphetamine users have reported an opiate as their second drug of choice upon admission to treatment (Substance Abuse and Mental Health Services Administration, 2003).
To better understand concomitant use of methamphetamine and an opiate, the current study investigated the behavioral effects of morphine/methamphetamine combinations on behavioral activity in rats. Six dose combinations were examined in laboratory rats, including three morphine doses (1.0, 5.0 and 10.0 mg/kg) and two methamphetamine doses (0.3 and 1.0 mg/kg). Previous research on cocaine/heroin combinations demonstrates that such drug combinations produce greater stimulant effects than either drug alone (Masukawa et al., 1993). Although the specific neurochemical actions of cocaine and methamphetamine have been found to differ (Izawa et al., 2006; Shimada et al., 1996; Zhang et al., 2001), the stimulant effect of each is thought to be mediated by increased synaptic levels of dopamine in the nucleus accumbens (Gold et al., 1989; Mori et al., 2004; Swerdlow et al., 1986). Due to the similarities between cocaine and methamphetamine, we hypothesized that animals receiving the “speedball” combination of methamphetamine and morphine would exhibit a much more potent behavioral response than animals receiving either drug alone.
Methods
Animals
Seventy-two adult male Sprague-Dawley rats weighing 200–225 g at time of purchase (Harlan) were used in these studies. Animals were housed three per cage, in standard plastic rat cages, with no restrictions on food and water. A 12- hour light/dark cycle was maintained and animals were allowed to acclimate in the vivarium for at least one week before experimentation. Experimental protocols were approved by the California State University San Marcos Institutional Animal Care and Use Committee and are in compliance with the National Institutes of Health Guide for Care and Use of Laboratory Animals. This work is in compliance with the Uniform Requirements for manuscripts submitted to Biomedical journals.
Instruments
A Kinder Scientific Open Field Motor Monitor System was used to assess locomotor activity. This system consists of eight Plexiglas enclosures (16″ × 16″ × 15″) fitted with two arrays of photocells: the first, 5 cm above the floor measures horizontal activity; the second 12.5cm above the floor measures vertical (rearing) activity. Photocell arrays are interfaced with a personal computer for the collection of data, and the following measurements can be obtained: total photocell beam breaks, distance traveled, time at rest, ambulations (interruptions of a successive photocell beams, characteristic of forward locomotion), fine movements (interruptions of a single photocell beam, characteristic of stereotypic behavior) and rearing. In the experimental room in which the enclosures reside continuous sound was provided by a white noise generator (Lafayette Instruments, Lafayette, IN), and a ceiling incandescent light was set at a low level.
Drugs
Methamphetamine HCl and morphine sulfate were generous gifts from the National Institute on Drug Abuse Drug Supply Program. Drugs were dissolved in 0.9% saline and administered together subcutaneously in a ‘cocktail’ in a volume of 1.0 ml/kg at the respective dose combinations.
Procedure
Three experiments were performed covering the six dose combinations, each with 24 animals. In each experiment, two doses of methamphetamine were tested (0.3 and 1.0 mg/kg), together with a single dose of morphine (1.0, 5.0 or 10.0 mg/kg). Each test consisted of four groups at a single dose combination: 1) saline (control), 2) methamphetamine alone, 3) morphine alone, and 4) speedball. During the first week of each experiment, the lower dose of methamphetamine was tested with the selected dose of morphine; one week later, the higher dose of methamphetamine was tested with the same dose of morphine. This design (using the same group of animals to test two dose combinations) allowed for reduction in the number of animals. However, to minimize the impact of repeated injections and the potential for drug/environment conditioning, each animal was tested only twice. Additionally, three approaches were used to minimize the opportunity for crossover effects from one dose to the next: 1) at least one week separated injections, 2) testing of the lower dose of methamphetamine (0.3 mg/kg) preceded testing of the higher dose (1.0 mg/kg), and 3) animals were reassigned to groups across weeks (animals from each initial treatment group were redistributed across the four groups for the second test).
For each test, animals were placed individually into an enclosure and allowed to habituate to the apparatus for 30 minutes prior to injection. The behavioral response to the injection was followed over the next 210 minutes.
Data Analysis
Activity data was assessed at 10-minute intervals for a total of 210 minutes following injection. Three measures of activity were used to determine the response to the drug combinations: ambulations, rears and fine-movements, since these provided contrasting and enlightening information about the response to the drugs. Each measure was analyzed using a two-way repeated measures ANOVA (treatment group × time) to determine the overall effect of the drug combinations. Two distinct phases of activity were observed in the experiments: an early phase characterized by very high levels of activity during the first 90 minutes in the speedball groups, and a late phase characterized by a delayed increase in activity, peaking at lower levels, during the second 90 minutes. Secondary analyses of the total activity during the first 90 minute interval and the second 90 minute interval were performed by one-way factorial ANOVA followed by Tukey’s posthoc test to assess differences among groups.
Results
Methamphetamine 0.3 mg/kg and Morphine
Methamphetamine (0.3 mg/kg) produced a modest increase in activity, relative to the saline control group, that peaked at approximately 20–30 minutes post-injection. This was evident for both ambulations and fine movements, although relatively greater for the latter (Figures 1, 2, 3 and 4). Morphine produced a more complex dose-dependent effect on activity: at 1.0 mg/kg and 5.0 mg/kg, a mild stimulant effect was seen during the first 90 minutes post-injection; at 10 mg/kg, no change or a mild depressant effect was seen during this phase. At 5.0 and 10.0 mg/kg, a second slowly-developing stimulant phase was observed late in the session, peaking at approximately 120–180 minutes post-injection (Figures 1, 2, 3 and 4).
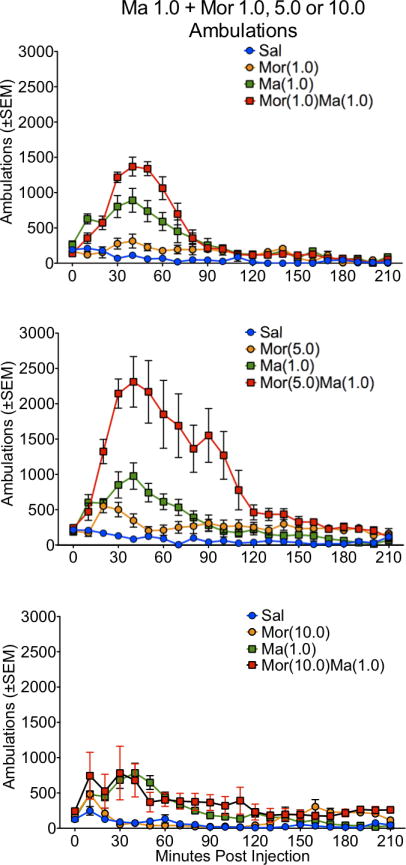
Figure 1.
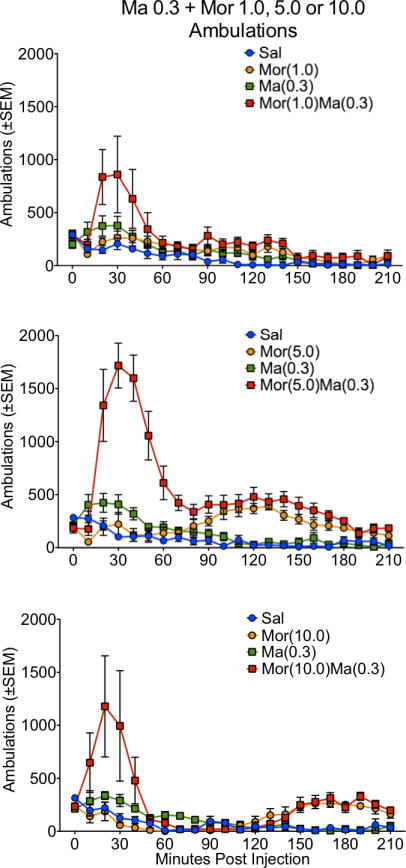
Locomotor activity, as expressed in ambulations, for the 210 minute time course in rats treated with saline (Sal), methamphetamine (Ma; 0.3 mg/kg), morphine (Mor; 1.0, 5.0 or 10 mg/kg s.c.) or the combination of Ma and Mor. Two-way repeated measures ANOVA for the time course showed a significant effect of treatment, time and interaction at all three dose combinations (p≤0.01).
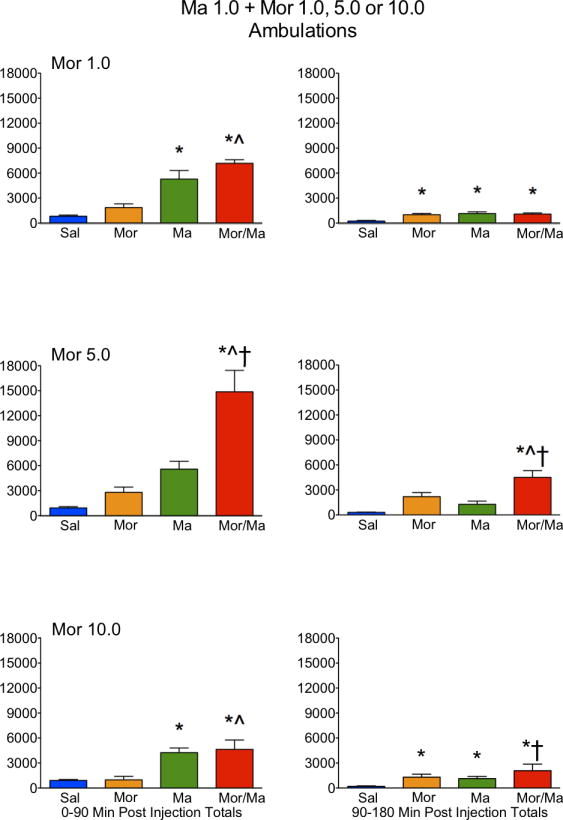
Figure 2.
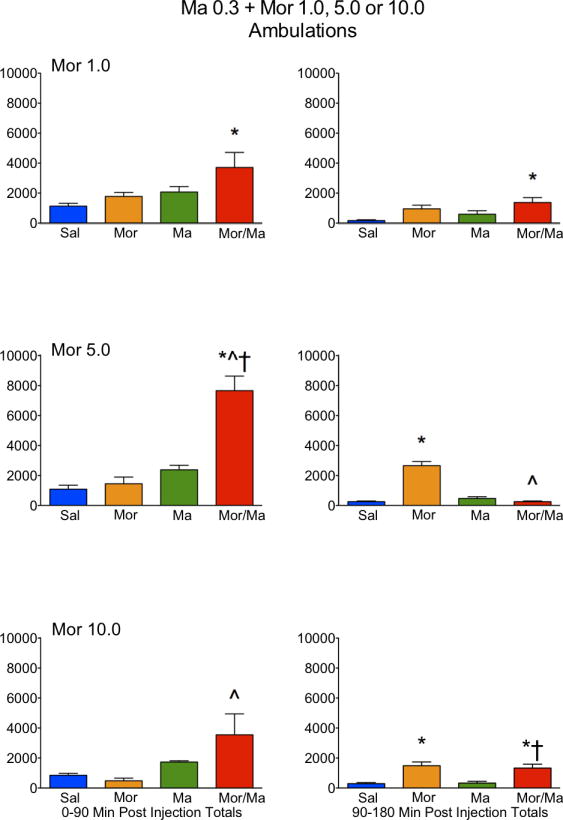
Locomotor activity, as expressed in ambulations, in rats treated with saline (Sal), methamphetamine (Ma; 0.3 mg/kg), morphine (Mor; 1.0, 5.0 or 10 mg/kg s.c.) or the combination of Ma and Mor. Left panels = total ambulations during the first 90 minutes post-injection. Right panels = total ambulations during the second 90 minutes post-injection. *=significantly different from Sal; ^=Mor/Ma significantly different from Mor alone; †=Mor/Ma significantly different from Ma alone.
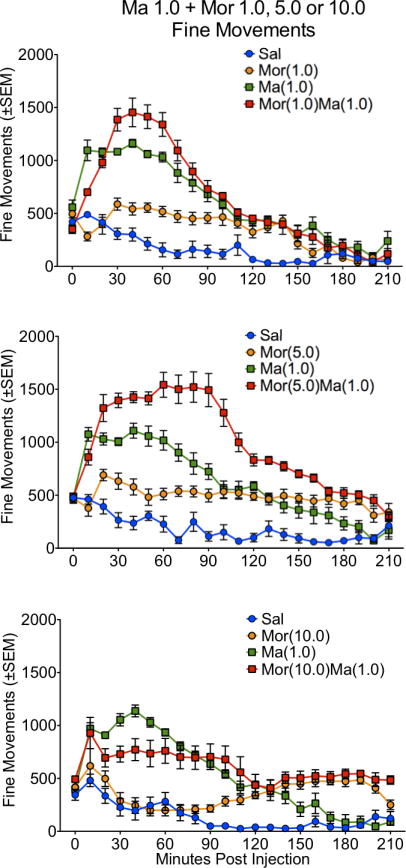
Figure 3.
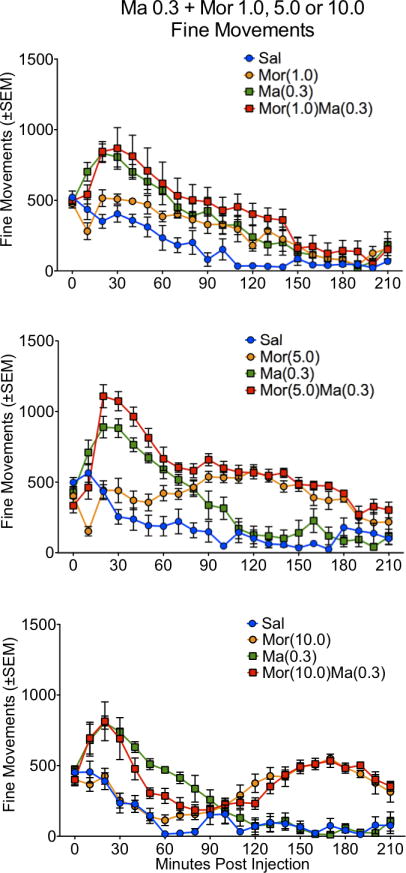
Locomotor activity, as expressed in fine movements, for the 210 minute time course in rats treated with saline (Sal), methamphetamine (Ma; 0.3 mg/kg), morphine (Mor; 1.0, 5.0 or 10 mg/kg s.c.) or the combination of Ma and Mor. Two-way repeated measures ANOVA for the time course showed a significant effect of treatment, time and interaction at all three dose combinations (p≤0.01).
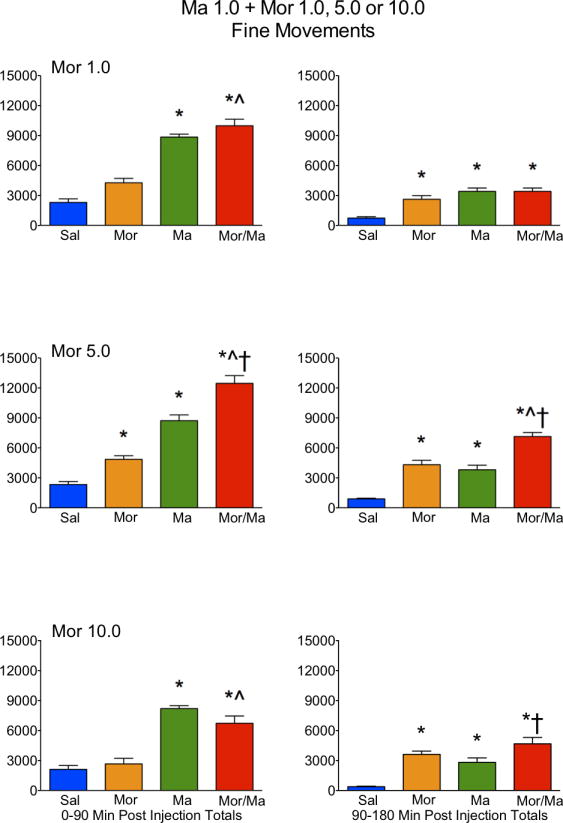
Figure 4.
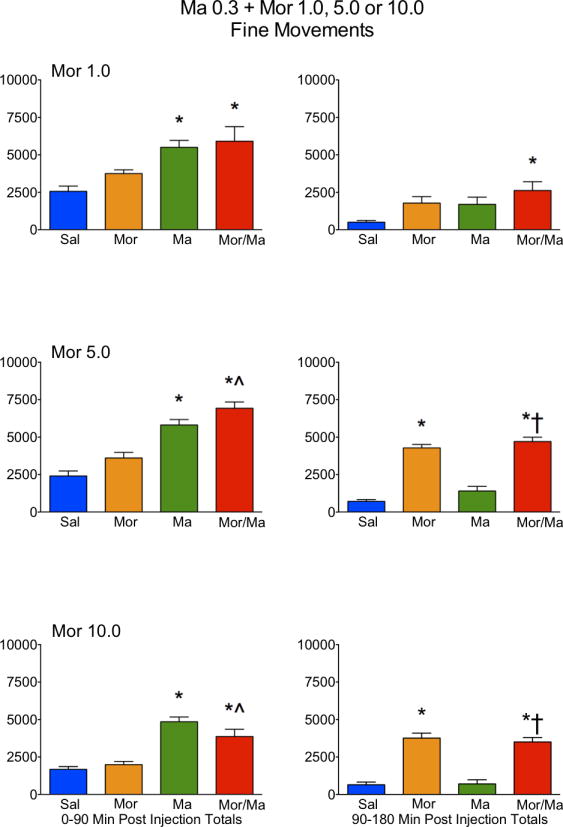
Locomotor activity, as expressed in fine movements, in rats treated with saline (Sal), methamphetamine (Ma; 0.3 mg/kg), morphine (Mor; 1.0, 5.0 or 10 mg/kg s.c.) or the combination of Ma and Mor. Left panels = total fine movements during the first 90 minutes post-injection. Right panels = total fine movements during the second 90 minutes post-injection. *=significantly different from Sal; ^=Mor/Ma significantly different from Mor alone; †=Mor/Ma significantly different from Ma alone.
For ambulations, the speedball combination produced the greatest activity, which was dependent on the dose of morphine, with the greatest stimulation at 5.0 mg/kg (Figures 1 and 2). This was particularly evident in the examination of the first 90 minutes of the session, where the drug combination produced a nearly 4-fold increase in activity over either drug alone (Figure 2). For fine movements, the combination produced effects that were not significantly different from methamphetamine alone (Figure 3 and 4). Repeated measures ANOVAs of the time course data confirmed significant effects for treatment, time, and an interaction for ambulations and fine movements at all doses (p≤0.01).
Methamphetamine 1.0 mg/kg and Morphine
The effects of the higher dose of methamphetamine (1.0 mg/kg) roughly paralleled those of the lower dose. Methamphetamine alone produced a more potent stimulant effect than the lower dose, which peaked at approximately 40 minutes post-injection. As above, the stimulant effect of methamphetamine was greater for fine movements, when compared to ambulations (Figures 5, 6, 7 and 8). The dose-dependent effects of morphine alone paralleled those described above.
Figure 5.
Locomotor activity, as expressed in ambulations, for the 210 minute time course in rats treated with saline (Sal), methamphetamine (Ma; 1.0 mg/kg), morphine (Mor; 1.0, 5.0 or 10 mg/kg s.c.) or the combination of Ma and Mor. Two-way repeated measures ANOVA for the time course showed a significant effect of treatment, time and interaction at all three dose combinations (p≤0.01).
Figure 6.
Locomotor activity, as expressed in ambulations, in rats treated with saline (Sal), methamphetamine (Ma; 1.0 mg/kg), morphine (Mor; 1.0, 5.0 or 10 mg/kg s.c.) or the combination of Ma and Mor. Left panels = total ambulations during the first 90 minutes post-injection. Right panels = total ambulations during the second 90 minutes post-injection. *=significantly different from Sal; ^=Mor/Ma significantly different from Mor alone; †=Mor/Ma significantly different from Ma alone.
Figure 7.
Locomotor activity, as expressed in fine movements, for the 210 minute time course in rats treated with saline (Sal), methamphetamine (Ma; 1.0 mg/kg), morphine (Mor; 1.0, 5.0 or 10 mg/kg s.c.) or the combination of Ma and Mor. Two-way repeated measures ANOVA for the time course showed a significant effect of treatment, time and interaction at all three dose combinations (p≤0.01).
Figure 8.
Locomotor activity, as expressed in fine movements, in rats treated with saline (Sal), methamphetamine (Ma; 1.0 mg/kg), morphine (Mor; 1.0, 5.0 or 10 mg/kg s.c.) or the combination of Ma and Mor. Left panels = total fine movements during the first 90 minutes post-injection. Right panels = total fine movements during the second 90 minutes post-injection. *=significantly different from Sal; ^=Mor/Ma significantly different from Mor alone; †=Mor/Ma significantly different from Ma alone.
Similar to the lower dose of methamphetamine, a potent interaction was observed in ambulations for the drug combination, which was dependent on the dose of morphine, with the greatest stimulation at 5.0 mg/kg (Figures 5 and 6). This was particularly evident in the examination of the first 90 minutes of the session, where the drug combination produced a nearly 3-fold increase in activity over either drug alone (Figure 6). For fine movements, the combination produced effects that were not significantly different from methamphetamine alone (Figures 7 and 8). Interestingly, at the highest dose of morphine, the combination resulted in effects slightly lower than methamphetamine alone (Figure 8). Repeated measures ANOVAs of the time course data confirmed significant effects for treatment, time, and an interaction for ambulations and fine movements at all doses (p<0.01).
Dose Response Summary
To further explore the magnitude of the speedball response, the first 90 minutes of activity were compared across doses for ambulations, fine movements and rears. In addition, a theoretical ‘additive’ response was determined by calculating a simple sum of the response to methamphetamine alone and morphine alone. Morphine by itself produced a modest inverted U-shaped dose-response, with the highest level of activity at 1.0 or 5.0 mg/kg (depending on the behavioral measure) and lower levels of activity at the 10.0 mg/kg dose. This inverted U-shaped dose-response was exaggerated in the speedball groups, with the 5.0 mg/kg morphine showing more a more potent increase relative to both the low and high dose of morphine in combination with methamphetamine (Figure 9).
Figure 9.
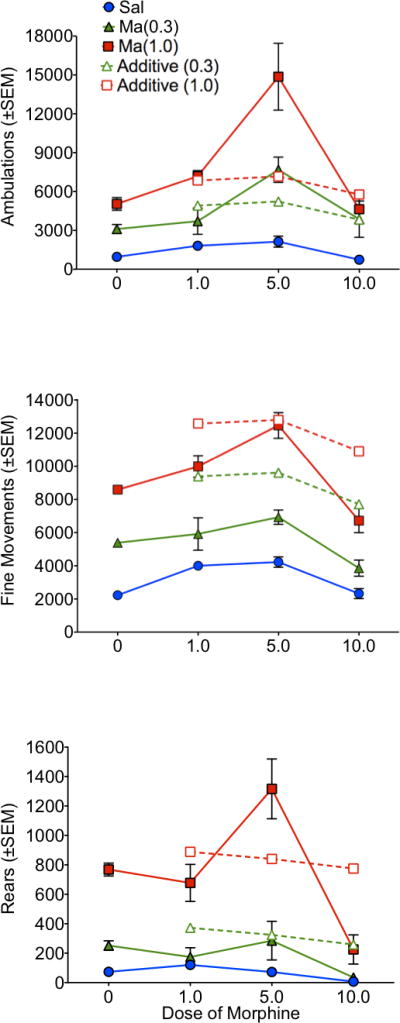
Dose-response summary. Total ambulations (top panel), fine-movements (middle panel) and rears (bottom panel) for the first 90 minutes post-injection at each dose combination. Solid lines show the dose-response for morphine when combined with saline or methamphetamine (0.3 or 1.0 mg/kg). Dashed lines show theoretical additive response for each dose of methamphetamine in combination with each dose of morphine. The theoretical additive lines were calculated by adding the activity counts from methamphetamine (Ma) alone and morphine (Mor) alone at the respective doses.
At the lowest dose of morphine (1.0 mg/kg) and at the highest dose of morphine (10.0 mg/kg), the magnitude of the response to the speedball approximated an additive effect for ambulations. The response for rears and fine-movements at these doses of morphine was less-than additive – activity in the speedball group did not exceed the sum of morphine alone and methamphetamine alone (Figure 9). At the middle dose of morphine, the speedball response depended on the dose of methamphetamine and the specific behavioral measure: for ambulations, the response was much greater than additive – approximately 50% higher at 0.3 mg/kg of methamphetamine and more than twice as high at 1.0 mg/kg of methamphetamine; for rears the response was additive at 0.3 mg/kg and approximately 50% greater than additive at 1.0 mg/kg; and for fine movements the response was less-than additive at 0.3 mg/kg of methamphetamine and approximated an additive response 1.0 mg/kg. Thus, for ambulations and rears the speedball combination produced effects greater than additive at 1–2 dose combinations, while fine movements did not.
Discussion
The key finding from this work is that methamphetamine and morphine, when administered together, produce a potent interaction in behavior. The interaction is dependent on the doses of the drugs used and the specific behavior examined, with ambulations (horizontal activity) and rearing (vertical activity) showing dramatic interactions, and fine movements (stereotypy) showing less evidence of interaction. Of particular note, the combination of 5.0 mg/kg of morphine with 1.0 mg/kg of methamphetamine produced an effect that was more than double for ambulations and more than 50% higher for rearing than predicted by summing the effects of each drug alone. This finding indicates that the interaction between methamphetamine and morphine is synergistic – that is, the effect of the combination is significantly greater than the sum of each. Although isobolographic analysis would help strengthen the argument that a synergistic interaction occurred, the shallow, inverted U-shaped dose response for morphine prevented this approach. The differing results for ambulations and rears versus fine movements are of interest, suggesting that the speedball effect represents a complicated behavioral interaction -- rather than an increase in all behaviors equally, forward locomotion and vertical activity are dramatically enhanced, while stereotypy is not.
The effects of the combination were clearly time-dependent, with the most potent interaction occurring during the first 90 minutes post-injection and less evidence of interaction in the later portion of the session. It is likely that a different time course for interactive effects would have been observed if the drugs had been administered sequentially with an interval between injections. However, the current studies were designed to model the street use of opioid/stimulant combinations where they are often injected in a single syringe (Leri et al., 2003a; Leri et al., 2004). The results demonstrate, that when administered together, there is a rapid onset for the interaction of morphine and methamphetamine, with the peak effects occurring by 30–60 minutes post-injection.
The current results complement and extend previous work by others, which examined behavioral interactions between methamphetamine and morphine in different strains of mice: ddY (Mori et al., 2004) and BALB/c (Ito et al., 2007). In each of these studies synergistic locomotor interactions between morphine and methamphetamine were seen at lower dose combinations (Ito et al., 2007; Mori et al., 2004). It is important to note that it’s difficult to directly compare these studies with the present findings, since these strains of mice respond very differently to morphine and methamphetamine than do the Sprague-Dawley rats used in the current study. For example, Sprague-Dawley rats show a complex morphine dose-response, with modest locomotor stimulation at doses up to 5.0 mg/kg and locomotor depression, followed by stimulation, at higher doses (Babbini and Davis, 1972; Brady and Holtzman, 1981, present data). Moreover, doses above 20 mg/kg are generally lethal to Sprague-Dawley rats (Trujillo and Akil, 1991). In contrast, ddY mice show robust dose-dependent locomotor stimulation, without depression, up to 200 mg/kg of morphine (Mori et al., 2004). Similarly, Sprague-Dawley rats show strong dose-dependent methamphetamine-induced locomotor stimulation, however BALB/c mice are resistant to this effect (Ito et al., 2007). Nevertheless, despite differences in response to individual drugs, studies in all three strains observed synergistic interactions between morphine and methamphetamine. The results illustrate that the interaction between methamphetamine and morphine is a robust phenomenon.
Interestingly, the interaction between methamphetamine and morphine followed an inverted U-shaped dose-response, with the greatest effect at 5.0 mg/kg of morphine in combination with methamphetamine, and lesser effects at 1.0 or 10.0 mg/kg of morphine. This dose-response curve paralleled the dose-response for morphine alone. The inverted U-shaped dose-response was somewhat surprising, especially within the selected dose-range. It is unclear why the stimulant effects of the combination diminished at 10 mg/kg of morphine, rather than increasing beyond those seen at 5.0 mg/kg. One possibility is that the depressant effects of morphine in this dose range overwhelm the stimulant effects of methamphetamine. Alternatively, this high dose combination may produce ‘behavioral toxicity’, interfering with the ability of the animal to move effectively. Yet another possibility is that the high dose combination caused the animals to shift from more ambulatory behavior to greater stereotypy. Indeed, such a shift has been reported for psychomotor stimulants at high doses -- as stereotypy increases, horizontal locomotion decreases due to behavioral competition (Joyce and Iversen, 1984; Lyon and Robbins, 1975; Sharp et al., 1987). However, the latter alternative is contradicted by the decrease in fine movements seen at the high dose combination. If the higher dose combination caused the animals to shift from ambulations to stereotypy, then this should have been reflected in an increase in fine movements. Instead, both ambulations and fine movements decreased in a parallel manner. Behavioral toxicity is also an unlikely explanation, since the response to the high dose combination was greater than in the saline control group – animals were moving around the apparatus, albeit less than at the 5 mg/kg dose of morphine – and there was no visible evidence of toxicity in the animals. Together, these findings lead to the conclusion that at the higher dose of morphine the depressant effects of this drug overwhelm the stimulant effects of methamphetamine.
Although we do not have evidence of toxicity in the current studies, it is important to comment on potentially toxic interactions between morphine and methamphetamine. Increased lethality from methamphetamine/morphine combinations has been observed in laboratory animals (Funahashi et al., 1988; Ginawi et al., 1997; Namiki et al., 2005), and there has also been at least one report indicating synergistic lethal effects of methamphetamine/morphine combinations in humans (Uemura et al., 2003). Thus, at dose combinations higher than those used in the present studies, enhanced lethality would likely occur.
The very dramatic increase in horizontal locomotion and rearing produced by the methamphetamine/morphine combination has implications for combined use of these drugs. This observation is consistent with the idea that individuals use such combinations to achieve enhanced effects from the drugs. In particular, the brain substrates of horizontal locomotion are closely related to those of drug reward (Robinson and Berridge, 2001; Trujillo et al., 1993; Wise and Bozarth, 1987). Thus, the increase in locomotor behavior produced by the combination may be a reflection (although indirect and nonspecific) of increased reward. Indeed, enhanced rewarding effects have been reported from combinations of methamphetamine and heroin (Ranaldi and Wise, 2000). Of course this doesn’t rule out other possibilities – in addition to an increased effects of the combination, methamphetamine and morphine may be used together because they produce unique subjective effects and/or the effects of one may diminish unwanted side-effects of the other. Polydrug abuse is a complex issue, likely involving several potential interactions of drug combinations. However, the current results demonstrate that combinations of morphine and methamphetamine produce synergistic effects that likely contribute to the use and abuse of such combinations.
Acknowledgments
This research was supported by the National Institute on Drug Abuse (DA 19859) and the National Institute of General Medical Sciences (GM 81069). Melissa Guaderrama was supported by the Research Initiative for Scientific Enhancement from NIGMS (GM 64783). These institutes had no involvement in study design; in the collection, analysis or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babbini M, Davis WM. Time-dose relationships for locomotor activity effects of morphine after acute or repeated treatment. Br J Pharmacol. 1972;46:213–224. doi: 10.1111/j.1476-5381.1972.tb06866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady LS, Holtzman SG. Locomotor activity in morphine-dependent and post-dependent rats. Pharmacol Biochem Behav. 1981;14:361–370. doi: 10.1016/0091-3057(81)90403-2. [DOI] [PubMed] [Google Scholar]
- Burnett LB, Roldan CJ, Adler J. Toxicity, Cocaine emedicine. 2010 Available online: http://emedicine.medscape.com/article/813959-overview.
- Cornish JL, Lontos JM, Clemens KJ, McGregor IS. Cocaine and heroin (‘speedball’) self-administration: the involvement of nucleus accumbens dopamine and mu-opiate, but not delta-opiate receptors. Psychopharmacology (Berl) 2005;180:21–32. doi: 10.1007/s00213-004-2135-9. [DOI] [PubMed] [Google Scholar]
- David V, Polis I, McDonald J, Gold LH. Intravenous self-administration of heroin/cocaine combinations (speedball) using nose-poke or lever-press operant responding in mice. Behav Pharmacol. 2001;12:25–34. doi: 10.1097/00008877-200102000-00003. [DOI] [PubMed] [Google Scholar]
- Duvauchelle CL, Sapoznik T, Kornetsky C. The synergistic effects of combining cocaine and heroin (“speedball”) using a progressive-ratio schedule of drug reinforcement. Pharmacol Biochem Behav. 1998;61:297–302. doi: 10.1016/s0091-3057(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Ellinwood EH, Jr, Eibergen RD, Kilbey MM. Stimulants: interaction with clinically relevant drugs. Ann N Y Acad Sci. 1976;281:393–408. doi: 10.1111/j.1749-6632.1976.tb27948.x. [DOI] [PubMed] [Google Scholar]
- Funahashi M, Kohda H, Shikata I, Kimura H. Potentiation of lethality and increase in body temperature by combined use of d-methamphetamine and morphine in mice. Forensic Sci Int. 1988;37:19–26. doi: 10.1016/0379-0738(88)90103-x. [DOI] [PubMed] [Google Scholar]
- Ginawi OT, al-Shabanah OA, Bakheet SA. Increased toxicity of methamphetamine in morphine-dependent mice. Gen Pharmacol. 1997;28:727–731. doi: 10.1016/s0306-3623(96)00308-4. [DOI] [PubMed] [Google Scholar]
- Gold LH, Geyer MA, Koob GF. Neurochemical mechanisms involved in behavioral effects of amphetamines and related designer drugs. NIDA Res Monogr. 1989;94:101–126. [PubMed] [Google Scholar]
- Guzman D, Ettenberg A. Heroin attenuates the negative consequences of cocaine in a runway model of self-administration. Pharmacol Biochem Behav. 2004;79:317–324. doi: 10.1016/j.pbb.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Ito S, Mori T, Namiki M, Suzuki T, Sawaguchi T. Complicated interaction between psychostimulants and morphine in expression of phenotype of behavior in the dopaminergic system of BALB/c mice. J Pharmacol Sci. 2007;105:326–333. doi: 10.1254/jphs.fp0070653. [DOI] [PubMed] [Google Scholar]
- Izawa J, Yamanashi K, Asakura T, Misu Y, Goshima Y. Differential effects of methamphetamine and cocaine on behavior and extracellular levels of dopamine and 3,4-dihydroxyphenylalanine in the nucleus accumbens of conscious rats. Eur J Pharmacol. 2006;549:84–90. doi: 10.1016/j.ejphar.2006.08.031. [DOI] [PubMed] [Google Scholar]
- Joyce EM, Iversen SD. Dissociable effects of 6-OHDA-induced lesions of neostriatum on anorexia, locomotor activity and stereotypy: the role of behavioural competition. Psychopharmacology (Berl) 1984;83:363–366. doi: 10.1007/BF00428546. [DOI] [PubMed] [Google Scholar]
- Lamas X, Negus SS, Gatch MB, Mello NK. Effects of heroin/cocaine combinations in rats trained to discriminate heroin or cocaine from saline. Pharmacol Biochem Behav. 1998;60:357–364. doi: 10.1016/s0091-3057(98)00021-5. [DOI] [PubMed] [Google Scholar]
- Leri F, Bruneau J, Stewart J. Understanding polydrug use: review of heroin and cocaine co-use. Addiction. 2003a;98:7–22. doi: 10.1046/j.1360-0443.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rajabi H, Stewart J. Effects of cocaine in rats exposed to heroin. Neuropsychopharmacology. 2003b;28:2102–2116. doi: 10.1038/sj.npp.1300284. [DOI] [PubMed] [Google Scholar]
- Leri F, Stewart J. Drug-induced reinstatement to heroin and cocaine seeking: a rodent model of relapse in polydrug use. Exp Clin Psychopharmacol. 2001;9:297–306. doi: 10.1037//1064-1297.9.3.297. [DOI] [PubMed] [Google Scholar]
- Leri F, Stewart J, Tremblay A, Bruneau J. Heroin and cocaine co-use in a group of injection drug users in Montreal. J Psychiatry Neurosci. 2004;29:40–47. [PMC free article] [PubMed] [Google Scholar]
- Lyon M, Robbins TW. The action of central nervous system stimulant drugs: A general theory concerning amphetamine effects. In: Essman WB, Valzelli L, editors. Current Developments in Psychopharmacology. Vol. 2. New York: Spectrum Publications; 1975. [Google Scholar]
- Malow RM, West JA, Corrigan SA, Pena JM, Lott WC. Cocaine and speedball users: differences in psychopathology. J Subst Abuse Treat. 1992;9:287–291. doi: 10.1016/0740-5472(92)90021-f. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Kahn W, Cannon DG, Smith JE. Self-administration of heroin, cocaine and their combination under a discrete trial schedule of reinforcement in rats. Drug Alcohol Depend. 2006;82:282–286. doi: 10.1016/j.drugalcdep.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Masukawa Y, Suzuki T, Misawa M. Differential modification of the rewarding effects of methamphetamine and cocaine by opioids and antihistamines. Psychopharmacology (Berl) 1993;111:139–143. doi: 10.1007/BF02245515. [DOI] [PubMed] [Google Scholar]
- Mattox AJ, Thompson SS, Carroll ME. Smoked heroin and cocaine base (speedball) combinations in rhesus monkeys. Exp Clin Psychopharmacol. 1997;5:113–118. doi: 10.1037//1064-1297.5.2.113. [DOI] [PubMed] [Google Scholar]
- Mello NK, Negus SS. The effects of buprenorphine on self-administration of cocaine and heroin “speedball” combinations and heroin alone by rhesus monkeys. J Pharmacol Exp Ther. 1998;285:444–456. [PubMed] [Google Scholar]
- Mello NK, Negus SS, Lukas SE, Mendelson JH, Sholar JW, Drieze J. A primate model of polydrug abuse: cocaine and heroin combinations. J Pharmacol Exp Ther. 1995;274:1325–1337. [PubMed] [Google Scholar]
- Mori T, Ito S, Narita M, Suzuki T, Sawaguchi T. Combined effects of psychostimulants and morphine on locomotor activity in mice. J Pharmacol Sci. 2004;96:450–458. doi: 10.1254/jphs.fpj04039x. [DOI] [PubMed] [Google Scholar]
- Namiki M, Mori T, Sawaguchi T, Ito S, Suzuki T. Underlying mechanism of combined effect of methamphetamine and morphine on lethality in mice and therapeutic potential of cooling. J Pharmacol Sci. 2005;99:168–176. doi: 10.1254/jphs.fpj05004x. [DOI] [PubMed] [Google Scholar]
- Negus SS. Interactions between the reinforcing effects of cocaine and heroin in a drug-vs-food choice procedure in rhesus monkeys: a dose-addition analysis. Psychopharmacology (Berl) 2005;180:115–124. doi: 10.1007/s00213-004-2133-y. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Munn E. Polydrug self-administration in rats: cocaine-heroin is more rewarding than cocaine-alone. Neuroreport. 1998;9:2463–2466. doi: 10.1097/00001756-199808030-00007. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Wise RA. Intravenous self-administration of methamphetamine-heroin (speedball) combinations under a progressive-ratio schedule of reinforcement in rats. Neuroreport. 2000;11:2621–2623. doi: 10.1097/00001756-200008210-00003. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Polis IY, Gold LH. Intravenous self-administration of heroin, cocaine, and the combination in Balb/c mice. Eur J Pharmacol. 1997;326:119–125. doi: 10.1016/s0014-2999(97)85405-2. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Platt DM, Yao WD, Spealman RD. Modulation of heroin and cocaine self-administration by dopamine D1- and D2-like receptor agonists in rhesus monkeys. J Pharmacol Exp Ther. 2007;321:1135–1143. doi: 10.1124/jpet.107.120766. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Rodefer JS, Spealman RD. Self-Administration of cocaine-opioid combinations by rhesus monkeys: evaluation of the role of mu receptor efficacy using labor supply analysis. J Pharmacol Exp Ther. 2005;312:1289–1297. doi: 10.1124/jpet.104.076646. [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Woolverton WL. Evaluation of the effects of cocaine, heroin and naltrexone, alone and in combination, on milk drinking in rats. Behav Pharmacol. 1995;6:821–829. [PubMed] [Google Scholar]
- Rowlett JK, Woolverton WL. Self-administration of cocaine and heroin combinations by rhesus monkeys responding under a progressive-ratio schedule. Psychopharmacology (Berl) 1997;133:363–371. doi: 10.1007/s002130050415. [DOI] [PubMed] [Google Scholar]
- Sharp T, Zetterstrom T, Ljungberg T, Ungerstedt U. A direct comparison of amphetamine-induced behaviours and regional brain dopamine release in the rat using intracerebral dialysis. Brain Res. 1987;401:322–330. doi: 10.1016/0006-8993(87)91416-8. [DOI] [PubMed] [Google Scholar]
- Shimada A, Yamaguchi K, Yanagita T. Neurochemical analysis of the psychotoxicity of methamphetamine and cocaine by microdialysis in the rat brain. Ann N Y Acad Sci. 1996;801:361–370. doi: 10.1111/j.1749-6632.1996.tb17456.x. [DOI] [PubMed] [Google Scholar]
- Smith JE, Co C, Coller MD, Hemby SE, Martin TJ. Self-administered heroin and cocaine combinations in the rat: additive reinforcing effects-supra-additive effects on nucleus accumbens extracellular dopamine. Neuropsychopharmacology. 2006;31:139–150. doi: 10.1038/sj.npp.1300786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Drug Abuse Warning Network, 2007: National Estimates of Drug-Related Emergency Department Visits. 2010 Available online: https://dawninfo.samhsa.gov/files/ED2007/DAWN2k7ED.pdf. [PubMed]
- Substance Abuse and Mental Health Services Administration. The DASIS Report: Treatment Admissions for Injection of Mulitple Drugs: 2000. 2003 Available online: http://oas.samhsa.gov/2k3/IVmultipleTX/IVmultipleTX.pdf.
- Swerdlow NR, Vaccarino FJ, Amalric M, Koob GF. The neural substrates for the motor-activating properties of psychostimulants: a review of recent findings. Pharmacol Biochem Behav. 1986;25:233–248. doi: 10.1016/0091-3057(86)90261-3. [DOI] [PubMed] [Google Scholar]
- Torrens M, San L, Peri JM, Olle JM. Cocaine abuse among heroin addicts in Spain. Drug Alcohol Depend. 1991;27:29–34. doi: 10.1016/0376-8716(91)90083-b. [DOI] [PubMed] [Google Scholar]
- Trujillo KA, Akil H. The NMDA receptor antagonist MK-801 increases morphine catalepsy and lethality. Pharmacol Biochem Behav. 1991;38:673–675. doi: 10.1016/0091-3057(91)90032-w. [DOI] [PubMed] [Google Scholar]
- Trujillo KA, Herman JP, Schäfer MK-H, Mansour A, Meador-Woodruff J, Watson SJ, Akil H. Drug reward and brain circuitry: recent advances and future directions. In: Korenman SG, Barchas JD, editors. Biological Basis of Substance Abuse. New York: Oxford University Press; 1993. pp. 119–142. [Google Scholar]
- Uemura K, Sorimachi Y, Yashiki M, Yoshida K. Two fatal cases involving concurrent use of methamphetamine and morphine. J Forensic Sci. 2003;48:1179–1181. [PubMed] [Google Scholar]
- Watson SJ, Trujillo KA, Herman JP, Akil H. Neuroanatomical and neurochemical substrates of drug-seeking behavior: overview and future directions. In: Goldstein A, editor. Molecular and Cellular Aspects of the Drug Addictions. New York: Springer Verlag; 1989. pp. 29–91. [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Zhang Y, Loonam TM, Noailles PA, Angulo JA. Comparison of cocaine- and methamphetamine-evoked dopamine and glutamate overflow in somatodendritic and terminal field regions of the rat brain during acute, chronic, and early withdrawal conditions. Ann N Y Acad Sci. 2001;937:93–120. doi: 10.1111/j.1749-6632.2001.tb03560.x. [DOI] [PubMed] [Google Scholar]


