Abstract
Improving vector control remains a key goal in reducing the world’s burden of infectious diseases. More cost-effective approaches to vector control are urgently needed, particularly as vaccines are unavailable and treatment is prohibitively expensive. The causative agent of AVL, Leishmania chagasi, Cunha and Chagas (Kinetoplastida: Trypanosomatidae) is transmitted between animal and human hosts by blood-feeding female sand flies, attracted to mating aggregations formed on or above host animals by male-produced sex pheromones. Our results demonstrate the potential of using synthetic pheromones to control populations of Lutzomyia longipalpis Lutz and Neiva (Diptera: Psychodidae), the sand fly vector of one of the world’s most important neglected diseases, American visceral leishmaniasis (AVL). We showed that a synthetic pheromone, (±)-9-methylgermacrene-B, produced from a low-cost plant intermediate, attracted females in the laboratory. Then by formulating dispensers that released this pheromone at a rate similar to that released by aggregating males, we were able to attract flies of both sexes to traps in the field. These dispensers worked equally well when deployed with mechanical light traps and inexpensive sticky traps. If deployed effectively, pheromone-based traps could be used to decrease AVL transmission rates through specific targeting and reduction of L. longipalpis populations. This is the first study to show attraction of a human disease-transmitting insect to a synthetic pheromone in the field, demonstrating the general applicability of this novel approach for developing new tools for use in vector control.
Keywords: sand flies, vector control, (S)-9-methylgermacrene-B, AVL
Effective vector control remains a key goal in reducing the world’s burden of infectious human and animal diseases. While new therapeutic drugs, vaccines, or techniques involving transgenic insects may one day offer long-term solutions for disease control (Knols et al. 2007), new techniques are urgently needed now to reduce disease incidence over a much shorter timeframe (World Health Organisation 2003). Recent advances in vector control, which include insecticide-treated bed nets (Curtis 2008, Kitua et al. 2008) and dog collars (Gavgani et al. 2002, Murray et al. 2005) offer considerable advantages over more traditional techniques such as blanket spraying of insecticides and destruction of reservoir habitats. These new, targeted, approaches are highly specific for the vector, and are therefore more cost effective. By reducing the amount of insecticide used, there is also less risk of damage to human health and the wider environment.
This study demonstrated the potential of sex pheromones as useful tools for controlling insect vectors and determined a synthetic sex pheromone, produced in the laboratory, could be used to lure the Leishmania vector Lutzomyia longipalpis to traps in the field.
American visceral leishmaniasis (AVL) is a potentially fatal zoonosis. The causative agent Leishmania infantum chagasi is transmitted between dogs and humans by blood-feeding female sand flies of the L. longipalpis (Diptera: Psychodidae) species complex (Grimaldi et al. 1989). AVL is a significant health problem in the Americas, but remains both neglected and underreported (Desjeux 2004). As no human or effective canine vaccine is available, current efforts to reduce AVL rely on treatment of human patients, culling of infected dogs and control of sand fly populations through insecticide spraying (Lainson and Rangel 2005). Although this combination of approaches has been partially successful, there are significant difficulties associated with each of these techniques. Therapeutic treatment is both expensive and unpleasant for the patient (Davies et al. 2003), and culling infected dogs is unpopular and difficult to implement effectively (Courtenay et al. 2002). The residual spraying of pyrethroid insecticides has largely replaced the widespread use of DDT, but is very expensive and therefore difficult to sustain long enough to achieve effective control (Lainson and Rangel 2005).
Male produced sex pheromones play a major role in the mating behaviour of L. longipalpis (Lane et al. 1985; Morton and Ward, 1989; Hamilton et al. 1994; Jones and Hamilton, 1998). These terpene chemicals are produced in glandular tissue located in the abdomen (Lane and Ward, 1984), and are biosynthesised from three or four five-carbon (C5) isoprene units (Hamilton, 2008). The exact structure of the pheromone differs between members of the L. longipalpis species complex (Hamilton et al, 2005), suggesting a possible role in reproductive isolation (Ward et al, 1983), but each functions to attract females to mating aggregations (leks) formed on or above host animals (Quinnell and Dye 1994, Morrison et al. 1995). Why particular sites are chosen is unclear, but these leks can remain over the course of several consecutive nights, and are the foci of nocturnal activity for blood-feeding sand flies (Kelly and Dye 1997). Residual spraying of lekking sites may displace sand flies towards human habitation, potentially increasing biting rates, infection risk and disease incidence (Kelly et al. 1997). However, if pesticides or traps were employed in conjunction with a synthetic pheromone, which would mimic the presence of a male lek, females could continue to be attracted and potentially killed in large numbers (Kelly and Dye 1997). Targeting females would be an efficient strategy for decreasing transmission risk, both in the short term by killing vector-competent individuals, and in the future by controlling sand fly population growth through reduction in total offspring production.
In this study it was shown that the racemate of the sex pheromone, (±)-9-methylgermacrene-B, which is a mixture of R and S isomers, prepared in bulk from a low cost plant derived intermediate, has similar biological activity in both the laboratory and the field to the natural pheromone (S)-9-methylgermacrene-B, which is produced by the most geographically widespread member of the L. longipalpis species complex. The results demonstrate that this synthetic pheromone can be readily formulated in inexpensive lures, which release the pheromone at a similar rate to a group of lekking males, and can be used to attract both female and male L. longipalpis to a lethal trap.
Materials and Methods
Pheromone Synthesis
Racemic 9-methylgermacrene-B (C16; mw 218) was synthesized in four steps from the plant derived intermediate, germacrone, using the synthetic pathway described previously (Hamilton and Bandi 2004). Germacrone was isolated from the aerial parts of the plant Geranium macrorrhizum, in methanol. The residue contained germacrone as the major component (80%) by gas chromatography-mass spectrometry (GC-MS). The germacrone was methylated at the α-position to the ketone group, by first deprotonating and then methylating. (±)-9-methylgermacrone was obtained on chromatography and then reduced to give the alcohol, (±)-9-methylgermacrol. After purification on silica gel, (±)-9-methylgermacrol was subsequently converted to (±)-9-methylgermacryl acetate and then purified on silica gel. Deoxygenation of the unsaturated acetate yielded the final compound (±)-9-methylgermacrene-B, which was purified on a silica gel column.
Laboratory bioassays
The racemate of the synthetic sex pheromone has the same biological activity as the natural sex pheromone (Hamilton et al. 1999b) and its attractiveness was confirmed using adult females from a laboratory colony established from our field site in Campo Grande, Mato Grosso do Sul, Brazil. Flies were collected using Centers for Disease Control (CDC) Miniature Light Traps (JW Hock, Gainesville, FL) left overnight in chicken sheds. Females were bloodfed and allowed to oviposit, and the eggs transported to the UK where a colony was raised and maintained at Keele University.
Attraction of female sand flies to natural and synthetic pheromone was measured using a Y-tube olfactometer. This apparatus consists of a Y-shaped glass tube (10mm internal diameter (ID)), comprising a 10cm-long stem branching into two-10cm long arms separated by an angle of 65°. The olfactometer was placed horizontally on a vibration-damped bench, with cotton netting placed around the opening of each arm to prevent flies from escaping. The two arms were attached to two lengths of Teflon tubing (40cm × 3mm ID; Supelco, Gillingham, UK) using brass connectors (Swagelok Company, Solon, OH), with the other end of each length attached to a shorter Teflon tube (4cm × 7mm ID) containing a rolled filter paper disk (2cm diameter, Whatman International Ltd., Maidstone, UK) onto which test or control samples could be injected. Air flow was provided by mixing outputs from zero grade air and 1% CO2 cylinders (BOC gases, Guildford, UK) at a ratio of 10:1 to give air containing 0.1% CO2. This mixture was cleaned using an activated charcoal filter (Supelpure HC, Gillingham, UK) and passed to each arm of the olfactometer through a T-junction in the Teflon tubing. Air speed at the outlet end of the stem of the olfactometer was measured and maintained at 5ml/s using a bubble flow meter.
Natural pheromone for testing was extracted from groups of 40-90 six- to seven-day-old-male sand flies maintained in mixed sex colony cages. Males were killed in a −20°C freezer before being transferred to glass vials containing hexane. Extracts from each group were reduced to a concentration of one male equivalent (ME) per μl by evaporating excess hexane.
For each trial, 1μl of test chemical (one male equivalent of natural pheromone extract or 1μg synthetic pheromone in hexane) was injected onto the filter paper disk designated as test using a 10μl syringe (Sigma-Aldrich Chem. Co., Dorset, UK) inserted into a small hole drilled through the Teflon tubing. A further 1μl of hexane was similarly injected onto the control disk using an identical syringe. A female sand fly was then removed from a holding cage using a mouth aspirator and placed into a small Teflon container, the open end of which was inserted into the outlet end of the olfactometer stem. Sand flies released from the container entered the stem, and had a maximum of 2 min to choose either the test (pheromone) arm or the control (hexane) arm. Flies were considered to have chosen after moving more than halfway up an arm. Flies not choosing after 2 min were recorded as non-responders. To control for any effect of side bias, the position of the test and control arms were swapped every 10 replicates by rotating both the olfactometer and connecting tubing through 180° on the horizontal axis. Prior to each day’s trials, Teflon tubing was rinsed using hexane, and filter paper disks replaced. Glass apparatus was cleaned with a 5% solution of Teepol detergent (VWR International, Lutterworth, UK), distilled water and acetone, and baked in an oven at 200°C overnight. All experiments were carried out under white florescent light at 26-27°C between 0900-1300 hours.
Eighty replicates were performed to ascertain the response of flies to natural pheromone extract. A further 120 replicates were conducted to test attraction to the synthetic pheromone. Two-tailed binomial sign tests (Sokal and Rohlf 1995) examined whether flies showed a significant preference to pheromone (real or synthetic) versus hexane.
Pheromone Dispenser Preparation and Entrainment
In order that the pheromone might be successfully utilized in the field, we developed a dispenser that could release the synthetic sex pheromone at a rate similar to that released by a group of lekking males over the period of several hours at night during which sand flies are active. Pure synthetic pheromone was diluted in hexane, to give a 1μg/μl solution, 50μl of which was aliquotted into each dispenser (Hamilton et al. 2005). Control dispensers used in field experiments were prepared using 50μl of hexane containing no pheromone. Dispensers were stored at −20°C until use.
To measure the amount of pheromone released by a single dispenser, entrainment experiments were carried out. The experiments were conducted using an adaptation of the bioassay apparatus and air was passed over a single dispenser placed in a glass bulb connected to one of the Y-tube arms via Teflon tubing. A tube containing an adsorbent polymer (Tenax) (ORBO 402, Sigma-Aldrich Company Ltd, Dorset, UK) was inserted into the stem of the Y- tube through a hole in a machined Teflon end piece, placed over the stem outlet to create an airtight seal. Airflow at the outlet of the Tenax tube was measured and maintained at 5ml/s using a bubble meter.
After one hour, the Tenax tube was removed and the collected chemicals eluted using 2ml of hexane. The extract was reduced to 20μl, with 1μl used for GC-MS analysis. The amount of pheromone present in all the eluted solvent was determined from the peak area of the injected sample, compared to that obtained from aliquotting 10μg of synthetic pheromone directly onto a Tenax tube. For each dispenser, one hour entrainments were conducted at 0h, 3h and 8h from first use. Tenax tubes were cleaned between entrainments through thermal desorption: each Tenax tube was heated in an oven at 200°C for 4h while connected to a constant flow of nitrogen gas.
To compare the amount of pheromone released from dispensers to that produced by leks, entrainments were similarly conducted using groups of 50 male and 10 female virgin flies. Two replicates each were performed with dispensers and groups of flies.
Field Study Area
Field trials were conducted in Campo Grande (20° 28.06 S 54° 37. 21 W), the state capital of Mato Grosso do Sul, Brazil between February and July 2008. This city was chosen primarily for its high abundance of L. longipalpis, all of which belong to the same pheromone-producing sibling species. Campo Grande has more than 700,000 inhabitants, with an affluent center surrounded by poorer peri-urban areas. Climate is considered rainy tropical savannah, with definite rainy and dry seasons (Oliveira et al. 2006). Sand flies are most abundant during the wet summer, and the recent increase in VL prevalence has been linked to greater abundances of L. longipalpis (Oliveira et al. 2008). Pheromone extracts of sand flies collected in Campo Grande both prior to and during field experiments were examined by GC-MS (Hamilton et al. 1999a) to confirm that all males were of the (±)-9-methylgermacrene-B producing type. Male sand flies were also examined under a dissecting microscope to confirm their identification as L. longipalpis.
Gardens in which experiments were conducted often contained fruit trees and areas for growing food, as well as animal pens, all contained at least 10 chickens.
Field trials
All field experiments aimed to test whether dispensers loaded with synthetic pheromone presented at a test station attracted more females and males than simultaneously presented solvent-only controls. Stations consisted of boxes constructed from four panels of thick (10mm) plywood (55cm (width) by 105cm (height)), held together by plastic garden ties sewn through holes (1.5mm ID) drilled in the corner of each plywood panel. Each panel had three holes (10cm ID) drilled in a horizontal line across the middle to allow host odour to escape. Two opposing panels in each box had a groove across the top edge, onto which a wooden pole could be secured and a trap suspended.
At dusk (17:00-18:00), pairs of experimental stations (test and control) were placed 3m apart in residential gardens, previously identified by the local disease control authority (Centro de Controle de Zoonoses (CCZ)) as sites of sand fly aggregation in or around chicken coops. We began by using lures in conjunction with modified (Natal et al. 1991) CDC miniature light traps, recognized as an effective device for sampling sand fly populations. Traps, powered overnight by 6-volt rechargeable batteries, were fitted with 35cm-diameter metal lids, and suspended from the pole above each station. Captured flies were collected in pots (10cm diam × 8cm height) connected to each trap via a nylon sleeve (10cm diam). A chicken, selected at random from the garden, was placed in each box to provide a source of host odor.
A dispenser containing 50μg of synthetic pheromone was attached to the underside of the lid at each test station, and a dispenser containing solvent only was similarly positioned at each control station.
The next morning (07:00-08:00) nylon sleeves were tied with string to prevent collected flies from escaping, before pots and batteries were removed. Chickens were freed from boxes, dispensers removed from lids. The positions of test and control boxes was alternated between nights. Flies were identified as male or female L. longipalpis and counted in the laboratory under x40 magnification, and trap batteries recharged before reuse. This entire first trial was repeated a second time, with the light bulbs, which are attractive when lit, removed.
Under natural conditions, male sand flies only aggregate on or near host animals, and host odor has been shown to synergize female attraction to sex pheromone in the laboratory (Bray and Hamilton 2007). However, females can also be attracted to pheromone alone in the laboratory. To test the attractiveness of synthetic pheromone without host odor in the field, the first two experiments were repeated without chickens in test stations.
Finally, to test whether pheromone could be used to trap flies in conjunction with a cheaper alternative to mechanical light traps, the first trial (with chickens) was repeated, replacing CDCs with blue, 28cm wide agricultural sticky traps (Russell IPM, Deeside, UK) wrapped around the top of each box. Each morning, sticky traps were numbered and covered with clear film, before being removed to the laboratory for counting.
Each experiment was conducted over two to four nights, using six to seven pairs of boxes spread across two to three gardens. New dispensers were used for each night of experiments. Wilcoxon signed rank tests (Sokal and Rohlf 1995) compared numbers of flies captured at test and control boxes in each pair. Males and females were analyzed separately, and pairs excluded from analysis if at least one of the traps did not function correctly.
Results
Laboratory Bioassays
In Y-tube olfactometer tests, females showed significant attraction both to natural pheromone extracted from males (binomial test: flies to test = 57, flies to control = 15, P < 0.001) and the synthetic racemic mixture (test = 38 control = 15, P < 0.01).
Dispenser Entrainment
Dispensers released 1-3μg of pheromone in the first hour of use and 0.3-1μg in the third hour, with detectable release in the laboratory continuing for up to nine hours. By comparison, groups of 50 males produced approximately 0.3μg of pheromone in the first hour of entrainment.
Field trials
Using dispensers in conjunction with light traps and chicken odor, test traps with pheromone dispensers caught significantly more male and female sand flies than the solvent-only controls (females: P < 0.001, Fig. 1; males: P < 0.001 , Fig. 2). With light bulbs removed to test the attractiveness of the pheromone in the absence of a light source, test traps attracted more females and males than controls, although numbers of sand flies collected were generally lower than in the first experiment (females: test 0.7 ± 0.2 (mean ± SEM), control 0.03±0.03, P < 0.01. Males: test 3.7 ± 0.7 (mean ± SEM), control 0.0 ± 0.0, P< 0.001. 30 pairs).
Fig. 1.
Mean ± SEM female L. longipalpis captured on test traps (gray bars) with synthetic pheromone dispensers and control traps (white bars) with solvent -only dispensers.
Fig. 2.
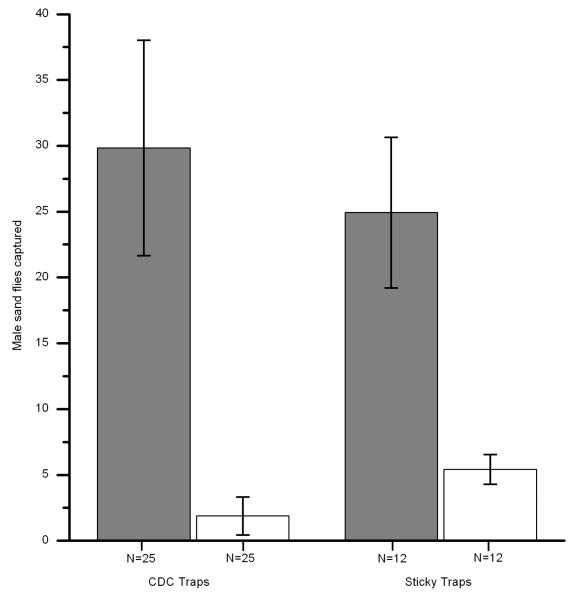
Mean ± SEM male L. longipalpis captured on test traps (gray bars) with synthetic pheromone dispensers and control traps (white bars) with solvent-only dispensers.
With chickens removed, more males were caught in test traps than solvent-only controls, demonstrating that the synthetic pheromone was capable of attracting males without host odor (with lights: test 2.0 ± 0.7, control 0.1 ± 0.1, P<0.01, 20 pairs, without lights: test 1.8 ± 0.3, control 0.0 ± 0.0, P<0.001, 26 pairs). However, no significant difference was found in the number of females caught in either experiment without chickens, though very few females were captured (with lights, seven flies captured, 20 pairs, without lights four flies captured, 26 pairs).
When chickens were replaced and sticky traps used instead of CDC traps, more female and male sand flies were again captured at test stations than controls (females: Fig. 1, P < 0.05, males: Fig. 2, P < 0.01). This demonstrates the attractiveness of synthetic pheromone without the need for a mechanical, air-circulating trap. Both test CDC and blue sticky traps caught approximately six times more females than the controls, with the same approximate proportional increase for males (Figs. 1 and 2).
Discussion
The results of this study demonstrate the potential of synthetic pheromones for use in L. longipalpis control. We were able to synthesize the L. longipalpis sex pheromone in bulk, and formulate it in inexpensive dispensers that were attractive to female and male sand flies in the field. To our knowledge, this is the first study to demonstrate attraction of a human disease vector to a synthetic pheromone outside of the laboratory. Considerable research effort will be needed to determine the most efficient way of applying these pheromones in sand fly control programmes, and measure their long-term effect on both sand fly numbers and AVL transmission. Nevertheless, our results demonstrate the feasibility of using pheromones for control, with implications for management of L. longipalpis and other pheromone-producing vector species.
While our dispensers were designed to target blood-feeding females, approximately six times more males than females were attracted to test stations, although a similar sex ratio was also observed in controls. This points to a skewed sex ratio in the trappable population at our field site, rather than a bias in response towards the synthetic pheromone. Previous field studies have demonstrated that sand flies of both sexes are attracted to live males in the presence of host odor (Dye et al. 1991, Kelly and Dye 1997), and it seems that males and females respond to pheromone in order to locate established leks. Attracting males to traps would aid control by reducing the chances of lek formation elsewhere, and the possibility of sand fly displacement towards human habitation.
Laboratory studies have demonstrated attraction of female sand flies to pheromone alone, but also that host odor both activates female sand flies and synergizes attraction towards pheromone (Bray and Hamilton 2007). Here, very few flies were caught in the absence of chickens in test stations, indicating that host odor is likely be an important component of any semiochemical-based control measure effective against L. longipalpis. Host odor could be provided by placing pheromone dispensers in the vicinity of host animals (as done here) or by formulating dispensers with both pheromone and attractive host odor components (Dougherty et al. 1999).
In our experiments, inexpensive sticky traps caught similar numbers of sand flies as mechanical light traps. This indicates that pheromones could be applied successfully with a range of capture devices or killing agents. In agriculture, pheromones are used for widespread pest monitoring, in order that existing control measures can be targeted more effectively, to lure and kill insects using traps and insecticides, and to disrupt mating through persistent long-term release (Shani 2000). Potentially, L. longipalpis pheromones could be applied in a number of similar ways for control. However, in order to have the most immediate impact of sand fly populations, priority should be given to incorporating pheromones into those activities already undertaken by local disease control agencies, such as spraying of residual insecticides. Longitudinal studies, or controlled comparisons between cities employing different methodologies for control (similar to those undertaken to test efficacy of dog collars (Maroli et al. 2001, Gavgani et al. 2002)) will be needed to test and refine pheromone-based integrated control strategies.
In order to develop similar tools for use in controlling other major diseases, it will first be necessary to identify and synthesize pheromones produced by the appropriate insect vectors. Priority targets include the sand flies responsible for transmitting Leishmania in the Old World, where new control measures are needed urgently (Dalton 2008). We already have some behavioural evidence for the existence of pheromones in Old World species and intend to be able to develop a similar lure system to that tested here (Bray et al. 2009).
The adoption of pheromones for use in controlling crop pests has been a major success worldwide, and has helped growers reduce their overall reliance on insecticides (Shani 2000). We expect that what has been learnt in developing and applying these techniques in agriculture will quickly be adapted to vector control and lead to novel, integrated strategies to combating the world’s most important diseases.
Acknowledgements
The authors would like to thank G. Borges and E. Dorval for assistance and support in carrying out the field work and Dr. P. Taylor for maintaining the sand fly colony at Keele University. D. Bray, K. Bandi and J.G.C. Hamilton are supported by the Wellcome Trust. R. P. Brazil is supported by Fundação de Apoio à Pesquisa do Estado do Rio de Janeiro (FAPERJ). The Leverhulme Trust supported earlier aspects of the work. Sand fly colony maintenance was performed under U.K. Home Office Licence.
References Cited
- Bray DP, Hamilton JGC. Host odor synergizes attraction of virgin female Lutzomyia longipalpis (Diptera: Psychodidae) J. Med. Entomol. 2007;44:779–787. doi: 10.1603/0022-2585(2007)44[779:hosaov]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Bray DP, Ward RD, Hamilton JGC. The Chemical Ecology of Sandflies (Diptera: Psychodidae) In: Takken W, Knols BGJ, editors. Ecology and Control of Vector-Borne Diseases Vol. 2: Olfaction In Vector-Host Interactions. Wageningen Academic Publishers; Wageningen, The Netherlands: 2009. (in press) [Google Scholar]
- Courtenay O, Quinnell RJ, Garcez LM, Shaw JJ, Dye C. Infectiousness in a cohort of Brazilian dogs: Why culling fails to control visceral leishmaniasis in areas of high transmission. J. Infect. Dis. 2002;186:1314–1320. doi: 10.1086/344312. [DOI] [PubMed] [Google Scholar]
- Curtis CF. Insecticide-treated bednets and child survival in rural Kenya. Lancet. 2008;371:115–116. doi: 10.1016/S0140-6736(08)60100-2. [DOI] [PubMed] [Google Scholar]
- Dalton R. Entomology: battlefield insectica. Nature. 2008;454:18–9. doi: 10.1038/454018a. [DOI] [PubMed] [Google Scholar]
- Davies CR, Kaye P, Croft SL, Sundar S. Leishmaniasis: new approaches to disease control. BMJ. 2003;326:377–382. doi: 10.1136/bmj.326.7385.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjeux P. Leishmaniasis: current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Dougherty MJ, Guerin PM, Ward RD, Hamilton JGC. Behavioural and electrophysiological responses of the phlebotomine sandfly Lutzomyia longipalpis (Diptera: Psychodidae) when exposed to canid host odour kairomones. Physiol Entomol. 1999;24:251–262. [Google Scholar]
- Dye C, Davies CR, Lainson R. Communication among phlebotomine sandflies - A field study of domesticated Lutzomyia longipalpis populations in Amazonian Brazil. Anim. Behav. 1991;42:183–192. [Google Scholar]
- Gavgani ASM, Hodjati MH, Mohite H, Davies CR. Effect of insecticide-impregnated dog collars on incidence of zoonotic visceral leishmaniasis in Iranian children: a matched-cluster randomised trial. Lancet. 2002;360:374–379. doi: 10.1016/s0140-6736(02)09609-5. [DOI] [PubMed] [Google Scholar]
- Grimaldi G, Tesh RB, McMahon-Pratt D. A review of the geographic distribution and epidemiology of leishmaniasis in the New World. Am. J. Trop. Med. Hyg. 1989;41:687–725. doi: 10.4269/ajtmh.1989.41.687. [DOI] [PubMed] [Google Scholar]
- Hamilton JGC, Bandi KK. No. I0500799 Patent. 2004 Brazil.
- Hamilton JGC, Dougherty MJ, Ward RD. Sex pheromone activity in a single component of tergal gland extract of Lutzomyia longipalpis (Diptera: Psychodidae) from Jacobina, Northeastern Brazil. J. Chem. Ecol. 1994;20:141–151. doi: 10.1007/BF02065997. [DOI] [PubMed] [Google Scholar]
- Hamilton JGC, Hall DR, Kirk WDJ. Identification of a male-produced aggregation pheromone in the western flower thrips Frankliniella occidentalis. J. Chem. Ecol. 2005;31:1369–1379. doi: 10.1007/s10886-005-1351-z. [DOI] [PubMed] [Google Scholar]
- Hamilton JGC, Maingon RDC, Alexander B, Ward RD, Brazil RP. Analysis of the sex pheromone extract of individual male Lutzomyia longipalpis sandflies from six regions in Brazil. Med. Vet. Ent. 2005;19:480–488. doi: 10.1111/j.1365-2915.2005.00594.x. [DOI] [PubMed] [Google Scholar]
- Hamilton JGC, Morgan ED, Brazil RP, Alexander B. Chemical analysis of oxygenated homosesquiterpenes: a putative sex pheromone from Lutzomyia lichyi (Floch and Abonnenc) (Diptera: Psychodidae) Bul. Entomol. Res. 1999a;89:139–145. [Google Scholar]
- Hamilton JGC, Hooper AM, Ibbotson HC, Kurosawa S, Mori K, Muto SE, Pickett JA. 9-Methylgermacrene-B is confirmed as the sex pheromone of the sandfly Lutzomyia longipalpis from Lapinha, Brazil, and the absolute stereochemistry defined as S. Chem. Commun. (Camb.) 1999b:2335–2336. [Google Scholar]
- Jones TM, Hamilton JGC. The role of pheromones in mate choice in a lekking sandfly Lutzomyia longipalpis. An. Behav. 1998;56:891–898. doi: 10.1006/anbe.1998.0857. [DOI] [PubMed] [Google Scholar]; Kelly DW, Dye C. Pheromones, kairomones and the aggregation dynamics of the sandfly Lutzomyia longipalpis. Anim. Behav. 1997;53:721–731. [Google Scholar]
- Kelly DW, Mustafa Z, Dye C. Differential application of lambda-cyhalothrin to control the sandfly Lutzomyia longipalpis. Med. Vet. Entomol. 1997;11:13–24. doi: 10.1111/j.1365-2915.1997.tb00285.x. [DOI] [PubMed] [Google Scholar]
- Kitua AY, Mboera L, Magesa SM, Maxwell CA, Curtis CF. The untapped potential of bed nets. Science. 2008;319:900–900. doi: 10.1126/science.319.5865.900c. [DOI] [PubMed] [Google Scholar]
- Knols BGJ, Bossin HC, Mukabana WR, Robinson AS. Transgenic mosquitoes and the fight against malaria: Managing technology push in a turbulent GMO world. Am. J. Trop. Med. Hyg. 2007;77:232–242. [PubMed] [Google Scholar]
- Lane R, Phillips A, Molyneux DH, Procter G, Ward RD. Chemical-analysis of the abdominal glands of two forms of Lutzomyia longipalpis - site of a possible sex-pheromone. Ann Trop Med Parasit. 1985;79:225–229. doi: 10.1080/00034983.1985.11811912. [DOI] [PubMed] [Google Scholar]
- Lane R, Ward RD. The morphology and possible function of abdominal patches in males of two forms of Lutzomyia longipalpis (Diptera:Phlebotominae). Cahiers d’Office de la Recherche Scientifique et Technique Outre- Mer. Ent Méd Parasit. 1984;22:245–249. [Google Scholar]
- Lainson R, Rangel EF. Lutzomyia longipalpis and the eco-epidemiology of American visceral leishmaniasis, with particular reference to Brazil - A Review. Mem. Inst. Oswaldo Cruz. 2005;100:811–827. doi: 10.1590/s0074-02762005000800001. [DOI] [PubMed] [Google Scholar]
- Maroli M, Mizzon V, Siragusa C, D’Oorazi A, Gradoni L. Evidence for an impact on the incidence of canine leishmaniasis by the mass use of deltamethrin-impregnated dog collars in southern Italy. Med. Vet. Entomol. 2001;15:358–363. doi: 10.1046/j.0269-283x.2001.00321.x. [DOI] [PubMed] [Google Scholar]
- Morrison AC, Ferro C, Pardo R, Torres M, Wilson ML, Tesh RB. Nocturnal activity patterns of Lutzomyia longipalpis (Diptera, Psychodidae) at an endemic focus of visceral leishmaniasis in Colombia. J. Med. Entomol. 1995;32:605–617. doi: 10.1093/jmedent/32.5.605. [DOI] [PubMed] [Google Scholar]
- Morton IE, Ward RD. Laboratory response of female Lutzomyia longipalpis sandflies to a host and male pheromone source over distance. Med. Vet. Ent. 1989;3:219–223. doi: 10.1111/j.1365-2915.1989.tb00218.x. [DOI] [PubMed] [Google Scholar]
- Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet. 2005;366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- Natal D, Marucci D, Reis IM, Galati EAB. Modificação da armadilha CDC com testes para coletas de flebotomíneos (Diptera) Rev. Bras. Entomol. 1991;35:697–700. [Google Scholar]
- Oliveira AG, Galati EA, Fernandes CE, Dorval ME, Brazil RP. Seasonal variation of Lutzomyia longipalpis (Lutz & Neiva, 1912) (Diptera: Psychodidae: Phlebotominae) in endemic area of visceral leishmaniasis, Campo Grande, state of Mato Grosso do Sul, Brazil. Acta. Trop. 2008;105:55–61. doi: 10.1016/j.actatropica.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Oliveira AG, Galati EA, de Oliveira O, de Oliveira GR, Espindola IA, Dorval ME, Brazil RP. Abundance of Lutzomyia longipalpis (Diptera: Psychodidae: Phlebotominae) and urban transmission of visceral leishmaniasis in Campo Grande, state of Mato Grosso do Sul, Brazil. Mem. Inst. Oswaldo Cruz. 2006;101:869–74. doi: 10.1590/s0074-02762006000800008. [DOI] [PubMed] [Google Scholar]
- Quinnell RJ, Dye C. An experimental study of the peridomestic distribution of Lutzomyia longipalpis (Diptera, Psychodidae) Bull. Entomol. Res. 1994;84:379–382. [Google Scholar]
- Shani A. Chemical communication agents (pheromones) in integrated pest management. Drug. Dev. Res. 2000;50:400–405. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry: the Principles and Practice of Statistics in Biological Research. W.H. Freeman and Company; New York, NY: 1995. [Google Scholar]
- Ward RD, Ribeiro AL, Ready PD, Murtagh A. Reproductive isolation between different forms of Lutzomyia longipalpis (Lutz and Neiva), (Diptera: Psychodidae), the vector of Leishmania dovani chagasi Cunha & Chagas and its significance to kala-azar distribution in South America. Mem. Inst. Oswaldo Cruz. 1983;78:269–280. [Google Scholar]
- World Health Organisation . Report of the Scientific Working Group Meeting on Insect Vectors and Human Health. Scientific Working Group, WHO; Geneva: 2003. [Google Scholar]