Abstract
The superior longitudinal fasciculus (SLF) II and cingulum are two white matter tracts important for attention and other frontal lobe functions. These functions are often disturbed in children with drug-resistant (DR) partial epilepsy even when no abnormalities are seen on conventional MRI. We set out to determine whether abnormalities in these structures might be depicted on diffusion tensor imaging (DTI) studies in the absence of abnormalities on conventional MRI. We compared the DTI findings of 12 children with DR-partial epilepsy to those of 12 age- and gender-matched controls. We found that the fractional anisotropy (FA) values in the SLF II of the patients were significantly lower than those of the controls (mean: 0.398±0.057, 0.443±0.059, p=0.002). Similarly apparent diffusion coefficient (ADC) and parallel diffusivity values of the SLF II were also significantly lower in the patients. There were no differences in the FA and ADC values of the cingulum. Our findings are consistent with abnormal structural connectivity of the frontal lobe in children with DR-partial epilepsy and provide a possible explanation for the previously reported functional abnormalities related to the SLF II in these patients.
Keywords: Diffusion tensor imaging, pediatrics, intractable, seizures, superior longitudinal fasciculus, cingulum, fractional anisotropy
Introduction
Diffusion tensor imaging (DTI) is a noninvasive magnetic resonance imaging (MRI) technique that has been used in many brain disorders including epilepsy, to study the structural connectivity [1]. The two primary measurements used in DTI are fractional anisotropy (FA), which is a measure of anisotropy (i.e., tendency of water to diffuse in one direction as opposed to randomly), and apparent diffusion co-efficient (ADC), which is a measurement of the water diffusion rate without reference to one direction. Drug-resistant (DR) partial epilepsy, irrespective of the location of seizure focus, is often associated with frontal lobe dysfunction such as deficits in executive function, attention, memory, verbal abilities, and motor co-ordination [2-7], raising the possibility that structures involved in frontal lobe functions likely have impaired structural connectivity. The superior longitudinal fasciculus II (SLF II) and cingulum are two key white matter tracts that modulate many of the above functions and for that reason, were the focus of this study.
The SLF is a major fiber tract interconnecting parietal association areas with the pre-frontal lobe and also connects key regions of the frontoparietal dorsal attention system. It is divided into three components which have unique functions [8]. SLF II is involved with spatial attention and perception of visual space, and the SLF I and III modulate other functions. The cingulum connects the anterior cingulate cortex to other frontal lobe sites as well as to the amygdala, nucleus accumbens, and medial dorsal thalamus. It, thus, interconnects key regions of the default mode network, a network which allows individuals to respond to salient stimuli and to emotionally process and monitor their mental state [9-12].
In this study, we were specifically interested in SLF II and cingulum for a number of reasons. First, the SLF II and cingulum are respectively parts of the frontoparietal and default mode networks, two systems which are reciprocally activated and deactivated during tasks related to frontal lobe functions such as attention, memory and emotions [13, 14]. Second, DTI studies have shown that these two tracts manifest abnormal FA values in children with otherwise normal MRIs who have frontal lobe dysfunction, particularly attention problems such as attention deficit hyperactivity disorder (ADHD) [15, 16]. Finally, only limited DTI studies of the cingulum and, to our knowledge, no such studies of the SLF in pediatric epilepsy exist [17, 18].
Our specific hypothesis was that children with DR-partial epilepsy and normal frontal lobes on conventional MRI have DTI findings consistent with impaired connectivity of SLF II and cingulum.
Methods
This study was approved by the Institutional Review Board of Duke University Medical Center. Informed consent from the parents and when appropriate, assent from the subjects were obtained before scanning.
Subject Population
Patients
Consecutive patients undergoing MRI for evaluation of DR-partial epilepsy were invited to participate by their pediatric neurologist at our tertiary care center. Patients were enrolled over a time period of 9 months. Localization of epilepsy was determined based on a combination of clinical semiology reported by a reliable observer, interictal electroencephalogram (EEG) and, ictal video EEG recordings (when the nature of the spells was unclear). Patients with questionable types of spells or diagnosis were not included. Inclusion criteria for the patients consisted of: (1) a definitive prior diagnosis of DR-partial epilepsy with an interictal EEG or video EEG recording and clinical findings consistent with that diagnosis, and (2) age between 4 and 18 years. Exclusion criteria for the patients included: (1) EEG findings indicative of primary generalized epilepsy, (2) frontal lobe abnormality identified on conventional MRI, (3) contraindications to undergo MRI (e.g., vagal nerve stimulators or other implantable medical devices), (4) claustrophobia or previous problems with undergoing MRI or body weight > 250lbs, and (5) known degenerative or storage disease.
Controls
Normal controls were enrolled through recruitment from the community as part of another NIH-funded and IRB-approved study in which, scans were performed using the same scanning parameters, under HIPAA regulations, and in which informed consents were similarly obtained [19]. The data from that study were anonymized before being used in the current study. Inclusion criteria for the controls consisted of: (1) parental consent, and when applicable, subject assent, (2) age between 4 years and 18 years. Exclusion criteria for the controls included: (1) significant medical or neurological illness, (2) morbid obesity or failure to thrive, (3) full scale intelligence below 80 by the 2 subtest (Vocabulary, Block Design) short form of the WISC-III (Wechsler Intelligence Scale for Children –III) or WAISIII (Wechsler Adult Intelligence Scale –III), (4) major Axis 1 disorder in the last 6 months prior to MRI, (5) history of substance use disorder, (6) pregnancy, and (7) birth weight <5 lbs or severe birth complications.
Imaging Parameters
All subjects were scanned on a Siemens 3.0 Tesla scanner using our routine structural MRI clinical seizure protocol which consisted of axial T1-weighted, axial T2-weighted, axial FLAIR and axial diffusion-weighted sequences. A 5 minute DTI sequence was added for the purposes of this research. The DTI sequence was performed using the following parameters: single-shot echo-planar imaging; TE: 80 ms; TR: 8800 ms (TR = 196 ms for 45 slices); FOV 220×220 mm; Slice thickness: 3 mm; Matrix 128×50%; 1 Average; 1 Concacts. Images were acquired with diffusion weighting in each of 6 directions, all with b-values of 0, 1000. The choice of 6 diffusion directions was based on the fact that this parameter had previously been used in our normal control group and we wished to maintain continuity of imaging parameters across the two groups. In addition, an image with no diffusion weighting (b-value of 0) was acquired as reference. The set of seven diffusion weighted images were acquired with a total of 4 excitations and the four data sets were co-registered to improve image quality and signal-to-noise ratio. DTI data sets were transferred in DICOM format to a computer workstation. 3 Tesla routine MRIs of the candidate patients were reviewed by two neuroradiologists and any patient with definite or suspected frontal lobe abnormality was excluded.
Image Analysis
DTI data were analyzed using the software program Dti Studio, version 3.0.1 (H. Jiang, S. Mori, Dept. of Radiology, Johns Hopkins University, Baltimore, MD). The sets of diffusion weighted images were co-registered using automatic image registration. All images were visually inspected and images with visually apparent artifacts were removed. We studied two regions of interest (ROIs) of 60 mm2 each for the SLF II and cingulum which were placed using methods based on Bonekamp et al. 2007. For SLF II, the ROI was placed in the mid-portion of SLF II on an axial slice, lateral to the corona radiata and corpus callosum and medial to the middle frontal gyrus at a level immediately superior to corpus callosum in the coronal plane. The ROIs were placed in such a way that the mid-point of the ROI coincided with that of SLF II (Figure 1) [20, 21]. The ROI for cingulum was placed in the mid-portion of cingulum just superior to the corpus callosum on a sagittal slice at a level in which a full-cross section of the superior part of the cingulum could be seen in the axial slice. The ROIs were placed consistently in such a way that a vertical line drawn through the mid-point of the ROI intersected a perpendicular line connecting the most anterior and the most posterior part of the corpus callosum at its mid-point (Figure 1) [20]. In addition, the locations of these ROIs were further confirmed by us using tractography (FA threshold > 0.2, angle > 60 degrees) [22-25]. The intra-rater intra-class correlation coefficients of above methods of placing ROIs in SLF II and cingulum were 0.85 and 0.81 respectively. Both ROIs were placed on the non-diffusion weighted b = 0 image in both hemispheres.
Figure 1. DTI color maps showing ROIs for SLF (1) and cingulum (2) and normal appearing T2 weighted MRI (3) for subject No. 5.
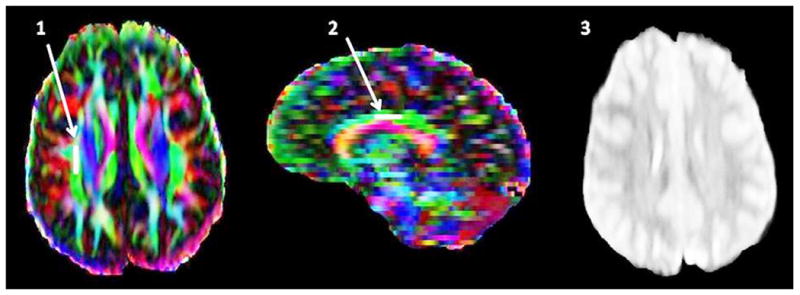
FA for right SLF = 0.35 (matched control FA = 0.44), FA for right cingulum = 0.47 (matched control FA = 0.52).
Statistical Analysis
Data were analyzed using SAS 4.2 (SAS Institute, NC, USA). The statistical test used was the Wilcoxon signed-rank test for nonparametric data, due to the non-normal distribution of the data (Shapiro-Wilk test). Mean FA values of the SLF II and cingulum of the patients were compared with those of the controls. Similar comparisons were also done for mean ADC values. Power analysis was performed using G Power program version 2.0.
Results
Subject Population
The study population consisted of 12 patients (7 boys) between the ages of 4 and 17 years and 12 age- and gender- matched controls. The mean ± standard deviation (SD) age of the patient group was 10.1±1.2, and that of controls was 10.4±1.1 (p=0.85). All patients had a definitive diagnosis of DR-partial epilepsy as recently defined by the International League Against Epilepsy [26]. The mean duration of epilepsy was 2.53 ± 1.88 years. Of the 12 patients, 6 patients (3 girls) had frontal lobe epilepsy, and the remaining 6 (2 girls) had temporal lobe epilepsy. The clinical information of our 12 patients is illustrated in Table 1. Seven of the 12 (5, 6, 7, 8, 10, 11, 12) patients completed phase I pre-surgical evaluation, and had ictal recordings of their habitual seizures. Of these 7 patients, 3 (patients 6, 8, 11) also completed phase II pre-surgical evaluation and subsequently underwent resective epilepsy surgery. Of our 12 patients, four patients (the three who underwent epilepsy surgery and patient 12) had neuropsychological testing which showed impairments in attention and/or executive function in each as well as abnormalities in memory and other functions.
Table 1. Clinical information of our 12 patients with partial epilepsy.
| No. | Sex | Age | Duration of epilepsy | Handed-ness | Type of epilepsya | Seizure semiology (average frequency) | MRI | Interictal spike, sharp/focal waves | Ictal EEG | Current /previous AEDs |
|---|---|---|---|---|---|---|---|---|---|---|
| 1b | F | 7y | 2y | R | L frontal | Dialeptic seizures (4-5/day) | Normal | C3 | NA | Oxcarbazepine/phenobarbital |
| 2 | M | 17y | 3y | R | L temporal | Automotor with vocalization of non-sense words and post-ictal aphasia and paraphasias (1/week) | Bilateral temporal heterotopias | None | NA | Oxcarbazepine, levetiracetam, lamotrigine, lacosamide/same |
| 3 | F | 5y | 0.5y | L | R frontal | Hypomotor seizures (7/day) | Normal | C4 | NA | Levetiracetam/oxcarbazepine |
| 4 | M | 11y | 2.5y | R | R temporal | Automotor seizures (1-2/month) | Normal | T4, T6 | NA | Topiramate/levetiracetam, diazepam |
| 5 | F | 14y | 7.3y | R | R frontal | Adversive left body tonic seizures with secondary generalization (5/day) | Normal | FP2, F4 | R frontal onset electrographic seizures | Topiramate, lamotrigine, carbamazepine/levetiracetam |
| 6c | M | 11y | 1.5y | R | R temporal | Automotor seizures (2-3/week) | Normal | F8, T4 | T4-T6 onset electrographic seizures | Levetiracetam, pyridoxine, clorazepate/zonisamide, oxcarbazepine |
| 7 | M | 9y | 0.8y | R | Bilateral independent frontal foci | i) Right onset: hypermotor evolving into left body tonic seizure (3/month)ii) Left onset: hypermotor seizures (2-5/week) | Normal | Predominant independent R frontal, independent L frontal | i) R frontal onset electrographic seizureii) L frontal onset electrographic seizure | Oxcarbazepine, levetiracetam, topiramate/same |
| 8c | F | 6y | 3.5y | Ambi-dextrous | R temporal | Automotor with vocalization of english words (2-3/month) | R mesial temporal sclerosis | T4 | R temporal onset electrographic seizures | Topiramate/oxcarbazepine, lamotrigine, zonisamide, levetiracetam |
| 9 | M | 11y | 4y | L | L frontal | Adversive right body tonic seizures (3/month) | Normal | F3 | NA | Levetiracetam/topiramate |
| 10 | M | 14y | 1y | R | R frontal | Focal motor seizures (5-6/day) | Normal | C4 | Parasagittal predominantly P4 onset with subsequent build up, maximal over F4 | Levetiracetam/lamotrigine |
| 11c | F | 4y | 3y | R | L temporal | Automotor seizures (1-2/day) | Normal | L temporal | T3 onset electrographic seizures | Lamotrigine, clonazepam, gabapentin, carbamazepine/levetiracetam, pyridoxine, topiramate |
| 12 | M | 12y | 1.5y | Ambi-dextrous | R temporal | Automotor seizures (2-3/month) | Normal | T4 | T4-T6 onset electrographic seizures | Zonesamide, pregabalin/levetiracetam, valproate, oxcarbazepine |
NA-Not applicable (Patients 1 and 3 underwent video/EEG monitoring, but the typical seizures did not occur during their hospital stay); y-years, R-Right, L-Left Neurological examination was normal on all patients except patient 4 who had oromotor apraxia.
Localization of epilepsy was based on clinical semiology reported by reliable observer, and interictal EEG on patients 1, 2, 3, 4, 9, and on clinical semiology, ictal video EEG recording, and interictal EEG on others.
Patient 1 had seizure during first month of life which was controlled with phenobarbital (given for 7 months and then discontinued). Seizures relapsed at age of 5 years-old.
Patients 6, 8, 11 underwent resective epilepsy surgery and were seizure free for 2, 1, and 6 months respectively.
Superior Longitudinal Fasciculus II
SLF FA values of patients were significantly lower than those of the control group (Table 2). Mean ± SD of the patient group: 0.398±0.057, control group: 0.443±0.059, and p=0.002. The power to detect the above observed difference (10%) with p<0.05 was 0.95. Similarly, ADC values of the patients were significantly lower than those of the controls. Mean ± SD of the patient group: 72.8±10.1 (×10-5 mm2/second), control group: 80.2±4.7 (×10-5 mm2/second), and p=0.02. The power to detect this difference (6%) with p<0.05 was 0.99. The FA values significantly correlated with the age both in controls (r=0.88, p=0.0003), and in patients (r=0.69, p=0.02).
Table 2. Fractional anisotropy and apparent diffusion coefficient of SLF II in the patients and controls.
| Subject Number | Mean Fractional Anisotropy (FA) | Mean Apparent diffusion coefficient (ADC) (×10-5 mm2/second) | ||
|---|---|---|---|---|
| Patients | Controls | Patients | Controls | |
| 1 | 0.33 | 0.385 | 75.7 | 82.6 |
| 2 | 0.445 | 0.425 | 72.9 | 70.8 |
| 3 | 0.32 | 0.4 | 86.7 | 83.4 |
| 4 | 0.395 | 0.42 | 74.0 | 77.2 |
| 5 | 0.36 | 0.42 | 71.8 | 74.5 |
| 6 | 0.38 | 0.42 | 76.7 | 77.2 |
| 7 | 0.405 | 0.48 | 69.8 | 86.6 |
| 8 | 0.365 | 0.415 | 44.5 | 81.2 |
| 9 | 0.465 | 0.515 | 74.3 | 85.9 |
| 10 | 0.48 | 0.525 | 70.7 | 78.8 |
| 11 | 0.35 | 0.365 | 81.5 | 83.2 |
| 12 | 0.475 | 0.545 | 74.9 | 80.9 |
| Mean±SD | 0.398±0.057 | 0.443±0.059 | 72.8±10.1 | 80.2±4.7 |
SD: Standard deviation
To better analyze the diffusion abnormalities in the SLF, we calculated parallel and perpendicular diffusivities, and the mean values of the patient and control groups were compared. Parallel diffusivity (also referred to by the term axial diffusivity) and perpendicular diffusivity (also referred to as radial diffusivity) are terms that provide information regarding the principle eigenvectors (i.e., axes) of the tensor matrix; the length of these axes is given by their eigenvalues. Parallel diffusivity is equivalent to the major eigenvalue (termed λ1) and perpendicular diffusivity refers to the average of the two minor eigenvalues (λ2 and λ3) that are perpendicular to λ1. FA values and ADC values can change based on changes in parallel diffusivity and perpendicular diffusivity; thus, knowledge of parallel diffusivity and perpendicular diffusivity can help one to explain what factors are responsible for FA and ADC changes.
Mean parallel diffusivity values of the patients were significantly lower than those of controls. Mean ± SD of the patient group: 0.00111±0.00008, control group: 0.00119±0.00009, and p=0.02. There were no differences in the perpendicular diffusivity values between two groups.
Cingulum
No significant difference of the cingulum FA values between the patient and control groups was found (Table 3). Mean ± SD: 0.463±0.079 for the patients, 0.482±0.052 for the controls and p=0.34. Power analysis revealed that with the given sample size and the observed mean and SD, a difference of 6% could have been detected at a power of 0.75 and a difference of 10% with a power of 0.993. Similarly, no differences in the mean ADC values between the patient and control groups were found. Mean ± SD: 81.9±7.4 (×10-5 mm2/second) for the patients, 86.4±4.1 (×10-5 mm2/second) for the controls, p=0.06. With the observed mean and SD of ADC, a difference of 6% could have been detected at a power of 1. The FA values significantly correlated with the age in controls (r=0.85, p=0.0009), but not in patients (r=0.07, p=0.8).
Table 3. Fractional anisotropy and apparent diffusion coefficient of cingulum in the patients and controls.
| Subject Number | Mean Fractional Anisotropy (FA) | Mean Apparent diffusion coefficient (ADC) (×10-5 mm2/second) | ||
|---|---|---|---|---|
| Patients | Controls | Patients | Controls | |
| 1 | 0.34 | 0.48 | 103.0 | 90.3 |
| 2 | 0.44 | 0.54 | 80.7 | 91.2 |
| 3 | 0.485 | 0.44 | 87.1 | 83.6 |
| 4 | 0.375 | 0.465 | 78.7 | 87.0 |
| 5 | 0.465 | 0.53 | 79.1 | 87.0 |
| 6 | 0.48 | 0.465 | 76.0 | 84.9 |
| 7 | 0.445 | 0.425 | 76.7 | 81.7 |
| 8 | 0.40 | 0.435 | 84.0 | 86.7 |
| 9 | 0.655 | 0.495 | 80.8 | 95.4 |
| 10 | 0.525 | 0.555 | 75.3 | 84.3 |
| 11 | 0.455 | 0.40 | 79.6 | 83.8 |
| 12 | 0.495 | 0.55 | 81.7 | 81.5 |
| Mean±SD | 0.463±0.079 | 0.482±0.052 | 81.9±7.4 | 86.4±4.1 |
SD: Standard deviation
Discussion
DTI is increasingly being utilized to assess brain abnormalities in various disease states; at times, DTI can depict abnormalities that are not evident on conventional MRI. We set out to study whether abnormalities could be detected in the SLF II and cingulum of children with DR-partial epilepsy in whom no abnormalities were detected on conventional MRIs. We did indeed find statistically significant FA and ADC abnormalities of SLF II in such children. To our knowledge, this is the first study to investigate the presence of such abnormalities in SLF in this patient population.
The SLF II is important for spatial attention and visual space perception and thus, abnormalities in these functions seen in children with DR-partial epilepsy may be at least in part related to abnormal connectivity in the SLF II. Decreased FA values in the SLF have been reported in patients with ADHD and thus, suggest the possibility of similarities in the patterns of abnormal frontal lobe connectivity in these two disorders [15]. Reductions in FA values are generally considered to represent evidence of loss of integrity of a white matter region. This presumption has recently been validated in a recent in vivo DTI and histopathological study in adults with temporal lobe epilepsy, in which, reduced FA values in the fimbria-fornix correlated with histological findings of decreased myelination and reduced numbers of axons [27].
In addition to lower FA, ADC and parallel diffusivity of SLF II was also significantly lower in children with DR-partial epilepsy; however, there was no change in the perpendicular diffusivity. In general, decreased parallel diffusivity is thought to reflect axonal injury whereas increased perpendicular diffusivity is considered to indicate evidence of diminished myelination [28]. These findings have been validated by subsequent studies [29, 30]. For instance, studies in a mouse model of optic nerve ischemia showed that axonal loss was accompanied solely by a decrease in parallel diffusivity without change in perpendicular diffusivity [29]. Thus, our findings raise the possibility that the diffusion abnormalities seen in SLF II in children with DR-partial epilepsy could be due to axonal injury, rather than due to abnormalities in myelination. Further studies are needed to elucidate this.
We did not find FA abnormalities in the cingulum in children with DR-partial epilepsy, which reflects, as reported by other investigators, absence of abnormalities in the cingulum in such patients [18]. Comparison of our findings regarding the cingulum with those in the previous two studies of this structure in children with epilepsy is informative. Nilsson and colleagues compared 8 children (age: 9.5-17.2 years) with complex partial seizures (6-left temporal, 1-right temporal, 1-fronto temporal, and based on a mean duration of epilepsy of 5.2 years, likely intractable epilepsy) and 10 age-matched controls and did not find FA abnormalities in the cingulum. [18]. Similarly, Hutchinson and colleagues, who studied 11 children (age: 8-18 years) with new-onset idiopathic localization related epilepsy (4 benign rolandic, 2 temporal lobe, 1 frontal lobe and 4 other focal) observed, solely a trend for lower FA in patients that was not statistically significant, compared to controls. Power analysis of their data revealed that approximately a difference of 2.8% could have been detected with a power of 0.85. However, when they combined this group with a group of 8 patients with new-onset idiopathic generalized epilepsy, a significantly reduced cingulum FA was found as compared to controls [17]. All the above including the data from Hutchinson et al, Nilsson et al and our study suggest that any differences that may exist in the cingulum in children with partial epilepsy are likely to be relatively small (i.e <5%) because the power available in each of these studies would have allowed detection of larger differences. Power to detect a 6% difference in the mean cingulum FA in Hutchinson et al study is 1, in our study, 0.75, and in the Nilsson et al study, 0.79.
Prior studies in adults with temporal lobe epilepsy have reported apparently smaller decreases in FA values in SLF (approximately 2-3% difference) and larger decreases in cingulum FA values (approximately 8-12% difference) [27, 31-33]. Whether there are age or etiology related vulnerabilities or progressive decreases in FA values the longer the epilepsy lasts is yet to be determined. Concha et al. [33] also reported that the diffusion abnormalities they observed in adults did not revert even after one year of seizure freedom post temporal lobectomy, raising the possibility that DTI abnormalities reported in other studies, such as ours, may also be persistent.
As in any study, our study has a number of limitations: i) It is possible that there may have been small differences in the FA of the cingulum that could not be detected due to insufficient power in our study as discussed above; ii) We studied only two white matter tracts out of several ones that connect frontal lobe to other regions of the brain; iii) We did not study the potential neuropsychological correlates of the DTI abnormalities that we observed, however, as stated before, our goal was to examine the structural connectivity of the frontal lobe rather than to correlate DTI parameters with neuropsychological performance; and iv) we studied patients with frontal lobe and temporal lobe epilepsy and our numbers were not high enough to study each type separately. However, we chose to combine these two types based on the fact that there is extensive literature available showing that both types have common manifestations with respect to frontal lobe dysfunction [2, 6, 7, 34], and several previous studies of DTI in epilepsy have used a similar approach [17, 35, 36].
The etiology of the diffusion abnormalities we observed is likely to be multi-factorial. This could include the underlying cause of the epilepsy in addition to the acute and sub-acute post ictal as well as long-term cumulative effects of seizures [37, 38]. Of note, even though none of our patients had experienced seizures within 24 hours prior to scanning, 8/12 patients had seizures within 36-72 hours raising the possibility that the diffusion abnormalities observed could have been in part due to reversible effects of acute seizures. Other causes could include the effects of interictal discharges, anticonvulsant drugs, psychosocial deprivation, the location of the epileptogenic zone, or the presence of frontal lobe malformations not detected by 3T MRI [39]. For example, patients with mesial temporal sclerosis could still potentially have structural frontal lobe lesions that are not detectable by MRI. This is also true with patients with completely normal MRIs. However, we did rule out, as much as possible, frontal lobe malformations in our patient population by performing 3T MRI studies. An additional consideration is that the abnormalities we have detected potentially could represent epiphenomena without any specific pathophysiological significance. Longitudinal studies in children with DR-partial epilepsy, using larger numbers of patients and incorporating functional correlations, would be beneficial in understanding the potential clinical and pathophysiological correlates of such DTI abnormalities. We conclude that the findings of decreased FA, ADC and parallel diffusivity of the SLF II in our study indicate abnormal frontal lobe structural connectivity in the above patient population and suggest that detection of such abnormal connectivity could lead to a better understanding of the pathophysiological processes in childhood DR-epilepsy.
Acknowledgments
This work was supported by funding from R01 AA12479, RO1DA020989, K24 MH071434 and 4530526.
References
- 1.Yogarajah M, Duncan JS. Diffusion-based magnetic resonance imaging and tractography in epilepsy. Epilepsia. 2008;49:189–200. doi: 10.1111/j.1528-1167.2007.01378.x. [DOI] [PubMed] [Google Scholar]
- 2.Auclair L, Jambaque I, Dulac O, LaBerge D, Sieroff E. Deficit of preparatory attention in children with frontal lobe epilepsy. Neuropsychologia. 2005;43:1701–12. doi: 10.1016/j.neuropsychologia.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Culhane-Shelburne K, Chapieski L, Hiscock M, Glaze D. Executive functions in children with frontal and temporal lobe epilepsy. J Int Neuropsychol Soc. 2002;8:623–32. doi: 10.1017/s1355617702801308. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez MT, Sauerwein HC, Jambaque I, De Guise E, Lussier F, Lortie A, Dulac O, Lassonde M. Deficits in executive functions and motor coordination in children with frontal lobe epilepsy. Neuropsychologia. 2002;40:384–400. doi: 10.1016/s0028-3932(01)00130-0. [DOI] [PubMed] [Google Scholar]
- 5.Hernandez MT, Sauerwein HC, Jambaque I, de Guise E, Lussier F, Lortie A, Dulac O, Lassonde M. Attention, memory, and behavioral adjustment in children with frontal lobe epilepsy. Epilepsy Behav. 2003;4:522–36. doi: 10.1016/j.yebeh.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Laurent A, Arzimanoglou A. Cognitive impairments in children with nonidiopathic temporal lobe epilepsy. Epilepsia. 2006;47 2:99–102. doi: 10.1111/j.1528-1167.2006.00703.x. [DOI] [PubMed] [Google Scholar]
- 7.Vingerhoets G. Cognitive effects of seizures. Seizure. 2006;15:221–6. doi: 10.1016/j.seizure.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Schmahmann JD, Pandya DN. Fiber pathways of the brain. New York: Oxford; 2006. [Google Scholar]
- 9.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–8. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teipel SJ, Bokde AL, Meindl T, Amaro E, Jr, Soldner J, Reiser MF, Herpertz SC, Moller HJ, Hampel H. White matter microstructure underlying default mode network connectivity in the human brain. Neuroimage. 2010;49:2021–32. doi: 10.1016/j.neuroimage.2009.10.067. [DOI] [PubMed] [Google Scholar]
- 11.van den Heuvel M, Mandl R, Luigjes J, Hulshoff Pol H. Microstructural organization of the cingulum tract and the level of default mode functional connectivity. J Neurosci. 2008;28:10844–51. doi: 10.1523/JNEUROSCI.2964-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp. 2009;30:3127–41. doi: 10.1002/hbm.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, Raichle ME, Petersen SE, Schlaggar BL. The maturing architecture of the brain's default network. Proc Natl Acad Sci U S A. 2008;105:4028–32. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–42. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamilton LS, Levitt JG, O'Neill J, Alger JR, Luders E, Phillips OR, Caplan R, Toga AW, McCracken J, Narr KL. Reduced white matter integrity in attention-deficit hyperactivity disorder. Neuroreport. 2008;19:1705–8. doi: 10.1097/WNR.0b013e3283174415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makris N, Buka SL, Biederman J, Papadimitriou GM, Hodge SM, Valera EM, Brown AB, Bush G, Monuteaux MC, Caviness VS, Kennedy DN, Seidman LJ. Attention and executive systems abnormalities in adults with childhood ADHD: A DT-MRI study of connections. Cereb Cortex. 2008;18:1210–20. doi: 10.1093/cercor/bhm156. [DOI] [PubMed] [Google Scholar]
- 17.Hutchinson E, Pulsipher D, Dabbs K, Myers y Gutierrez A, Sheth R, Jones J, Seidenberg M, Meyerand E, Hermann B. Children with new-onset epilepsy exhibit diffusion abnormalities in cerebral white matter in the absence of volumetric differences. Epilepsy Res. 2010;88:208–14. doi: 10.1016/j.eplepsyres.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nilsson D, Go C, Rutka JT, Rydenhag B, Mabbott DJ, Snead OC, 3rd, Raybaud CR, Widjaja E. Bilateral diffusion tensor abnormalities of temporal lobe and cingulate gyrus white matter in children with temporal lobe epilepsy. Epilepsy Res. 2008;81:128–35. doi: 10.1016/j.eplepsyres.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 19.De Bellis MD, Van Voorhees E, Hooper SR, Gibler N, Nelson L, Hege SG, Payne ME, MacFall J. Diffusion tensor measures of the corpus callosum in adolescents with adolescent onset alcohol use disorders. Alcohol Clin Exp Res. 2008;32:395–404. doi: 10.1111/j.1530-0277.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonekamp D, Nagae LM, Degaonkar M, Matson M, Abdalla WM, Barker PB, Mori S, Horska A. Diffusion tensor imaging in children and adolescents: reproducibility, hemispheric, and age-related differences. Neuroimage. 2007;34:733–42. doi: 10.1016/j.neuroimage.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr, Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15:854–69. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- 22.Bernal B, Altman N. The connectivity of the superior longitudinal fasciculus: a tractography DTI study. Magn Reson Imaging. 2010;28:217–25. doi: 10.1016/j.mri.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Concha L, Gross DW, Beaulieu C. Diffusion tensor tractography of the limbic system. AJNR Am J Neuroradiol. 2005;26:2267–74. [PMC free article] [PubMed] [Google Scholar]
- 24.Mori S, Kaufmann WE, Davatzikos C, Stieltjes B, Amodei L, Fredericksen K, Pearlson GD, Melhem ER, Solaiyappan M, Raymond GV, Moser HW, van Zijl PC. Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn Reson Med. 2002;47:215–23. doi: 10.1002/mrm.10074. [DOI] [PubMed] [Google Scholar]
- 25.Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 26.Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshe SL, Perucca E, Wiebe S, French J. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–77. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 27.Concha L, Livy DJ, Beaulieu C, Wheatley BM, Gross DW. In vivo diffusion tensor imaging and histopathology of the fimbria-fornix in temporal lobe epilepsy. J Neurosci. 2010;30:996–1002. doi: 10.1523/JNEUROSCI.1619-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–36. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 29.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–22. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Wu Q, Butzkueven H, Gresle M, Kirchhoff F, Friedhuber A, Yang Q, Wang H, Fang K, Lei H, Egan GF, Kilpatrick TJ. MR diffusion changes correlate with ultra-structurally defined axonal degeneration in murine optic nerve. Neuroimage. 2007;37:1138–47. doi: 10.1016/j.neuroimage.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 31.Ahmadi ME, Hagler DJ, Jr, McDonald CR, Tecoma ES, Iragui VJ, Dale AM, Halgren E. Side matters: diffusion tensor imaging tractography in left and right temporal lobe epilepsy. AJNR Am J Neuroradiol. 2009;30:1740–7. doi: 10.3174/ajnr.A1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Concha L, Beaulieu C, Gross DW. Bilateral limbic diffusion abnormalities in unilateral temporal lobe epilepsy. Ann Neurol. 2005;57:188–96. doi: 10.1002/ana.20334. [DOI] [PubMed] [Google Scholar]
- 33.Concha L, Beaulieu C, Wheatley BM, Gross DW. Bilateral white matter diffusion changes persist after epilepsy surgery. Epilepsia. 2007;48:931–40. doi: 10.1111/j.1528-1167.2007.01006.x. [DOI] [PubMed] [Google Scholar]
- 34.Lippe S, Lassonde M. Neuropsychological profile of intractable partial epilepsies. Rev Neurol (Paris) 2004;1605(Spec No 1):S144–53. [PubMed] [Google Scholar]
- 35.Chen Q, Lui S, Li CX, Jiang LJ, Ou-Yang L, Tang HH, Shang HF, Huang XQ, Gong QY, Zhou D. MRI-negative refractory partial epilepsy: role for diffusion tensor imaging in high field MRI. Epilepsy Res. 2008;80:83–9. doi: 10.1016/j.eplepsyres.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Rugg-Gunn FJ, Eriksson SH, Symms MR, Barker GJ, Duncan JS. Diffusion tensor imaging of cryptogenic and acquired partial epilepsies. Brain. 2001;124:627–36. doi: 10.1093/brain/124.3.627. [DOI] [PubMed] [Google Scholar]
- 37.Concha L, Gross DW, Wheatley BM, Beaulieu C. Diffusion tensor imaging of time-dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. Neuroimage. 2006;32:1090–9. doi: 10.1016/j.neuroimage.2006.04.187. [DOI] [PubMed] [Google Scholar]
- 38.Mac Donald CL, Dikranian K, Bayly P, Holtzman D, Brody D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci. 2007;27:11869–76. doi: 10.1523/JNEUROSCI.3647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anneken K, Evers S, Mohammadi S, Schwindt W, Deppe M. Transient lesion in the splenium related to antiepileptic drug: case report and new pathophysiological insights. Seizure. 2008;17:654–7. doi: 10.1016/j.seizure.2008.01.004. [DOI] [PubMed] [Google Scholar]


