Abstract
In vertebrate embryos, the dorsal aorta and the posterior cardinal vein form in the trunk to comprise the original circulatory loop. Previous studies implicate Hedgehog (Hh) signaling in the development of the dorsal aorta. However, the mechanism controlling specification of artery versus vein remains unclear. Here, we investigated the cell-autonomous mechanism of Hh signaling in angioblasts (endothelial progenitor cells) during arterial-venous specification utilizing zebrafish mutations in Smoothened (Smo), a G protein-coupled receptor essential for Hh signalling. smo mutants exhibit an absence of the dorsal aorta accompanied by a reciprocal expansion of the posterior cardinal vein. The increased number of venous cells is equivalent to the loss of arterial cells in embryos with loss of Smo function. Activation of Hh signaling expands the arterial cell population at the expense of venous cell fate. Time-lapse imaging reveals two sequential waves of migrating progenitor cells that contribute to the dorsal aorta and the posterior cardinal vein, respectively. Angioblasts deficient in Hh signaling fail to contribute to the arterial wave; instead, they all migrate medially as a single population to form the venous wave. Cell transplantation analyses demonstrate that Smo plays a cell-autonomous role in specifying angioblasts to become arterial cells, and Hh-signaling depleted angioblasts differentiate into venous cells instead. Collectively, these studies suggest that arterial endothelial cells are specified and formed via repressing venous cell fate at the lateral plate mesoderm by Hh signaling during vasculogenesis.
Keywords: Hedgehog, Endothelial Progenitor Cells, Artery, Vein, Specification
Introduction
In zebrafish embryos, progenitor cells of the dorsal aorta and the posterior cardinal vein migrate sequentially to the midline and coalesce to form the vascular cord. Subsequently, this endothelial cord undergoes differentiation and morphogenesis to form the dorsal aorta and the posterior cardinal vein (Jin et al., 2005; Torres-Vazquez et al., 2003; Zhong, 2005; Herbert et al., 2009). Despite recent progress in dissecting the regulation of arterial and venous endothelial cell differentiation (Lamont and Childs, 2006; Lawson et al., 2001), the mechanisms that control specification and formation of arterial and venous endothelial cells are not well understood.
Hedgehog (Hh) signaling plays important roles in specification and formation of a variety of cell types and organs during development (Ingham and McMahon, 2001). It has been shown to be involved in neovascularization and angiogenesis. In mouse, loss of sonic hedgehog (shh) causes lack of proper vascularization of the developing lungs (Rowitch et al., 1999), whereas shh overexpression in the dorsal neural tube results in hypervascularization (Pepicelli et al., 1998). In the developing heart, a wave of Hh activation is required for coronary vascular development (Lavine et al., 2008; Lavine et al., 2006). shh expression upregulates vegf and angiopoietins to enhance myocardial neovascularization in ischemic hearts (Kusano et al., 2005; Pola et al., 2001). Inactivation of the smoothened (smo) gene, encoding the co-receptor for Shh, Indian hedgehog (Ihh) and Tiggy-winkle hedgehog (Twhh), causes severe angiogenesis defects in the yolk sac of mouse embryos (Byrd et al., 2002).
Hh signaling has also been implicated in formation and differentiation of the dorsal aorta in zebrafish. Zebrafish smoothened mutants lack the dorsal aorta resulting in absence of all arterial gene expression (Gering and Patient, 2005). Mutations in sonic-you (syu) and you-too (yot), which encode zebrafish shh ortholog and its downstream effector gli2, respectively, display defects in arterial endothelial differentiation (Brown et al., 2000; Chen et al., 1996; Lawson et al., 2002). These data suggest that Hh signaling is required for formation of the dorsal aorta at early stages as well as for arterial endothelial differentiation during later developmental stages. Hh signaling has been implicated to regulate arterial endothelial differentiation via modulating Vegf (Lawson et al., 2002). Studies in avian and murine embryos, however, indicate that Hh signaling acts independently of Vegf to mediate vascular tubulogenesis and arterial assembly (Vokes et al., 2004). Although these studies reveal early roles for Hh signaling in artery development, it remains unclear when, where or how Hh signaling controls formation of the dorsal aorta. In addition, little is known about whether the absence of the dorsal aorta has any effect on development of the cardinal vein in Hh-deficient embryos.
In this study, we have analyzed early roles of Hh signaling in arterial-venous specification and identified the cell-autonomous requirement for reception of Hh signaling by endothelial progenitor cells to form the dorsal aorta. In Hh signaling-deficient embryos, we observed a reciprocal expansion of the cardinal vein that is accompanied by an absence of the dorsal aorta, whereas activated Hh signaling by Smo agonist causes arterial cell expansion that is proportional to reduction of venous cells. These data suggest that arterial endothelial cells develop at the expense of venous cell fate. In vivo time-lapse imaging has revealed two waves of migrating endothelial progenitor cells. The first wave contributes, mainly, to the formation of the dorsal aorta, and the second wave gives rise to the posterior cardinal vein. Angioblasts deficient in Hh signaling all migrate in the second (venous) wave to form an expanded cardinal vein. Our cell transplantation analyses demonstrated that reception of Hh signaling by angioblasts is required for them to adopt an arterial cell fate and contribute to formation of the dorsal aorta, whereas angioblasts with impaired Hh signaling differentiate into venous cells instead.
Methods and Materials
Zebrafish maintenance and staging
Embryos were produced by pairwise matings and raised at 28.5°C, then staged according to hours post fertilization (hpf) and days post fertilization (dpf) (Kimmel et al., 1995). Smohi640 null allele (Chen et al., 2001) and transgenic fish included Tg(flk:EGFP) (Jin et al., 2005), Tg(fli:EGFP-nuc)(Roman et al., 2002) and Tg(flk:DsRed)(Huang et al., 2005) were used in our studies.
Cyclopamine and purmorphamine treatment
Embryos were incubated in Embryo Medium (EM) containing 50 uM and 100 uM of cyclopamine or purmorphamine respectively. Treatments were carried out at 50% epiboly unless stated otherwise.
In situ hybridization and Immunostaining
RNA in situ hybridization was carried out using ephrinb2 (Zhong et al., 2001), dab2 (Song et al., 2004), flk1(Zhong et al., 2001), flt4 (Zhong et al., 2001), grl (Zhong et al., 2001), notch1b, notch 5 (Lawson et al., 2001), col2a (Appel et al., 1999) markers. Immunohistochemistry was performed as previously described (Trinh and Stainier, 2004). Briefly, embryos were fixed overnight with 4% paraformaldehyde in sucrose buffer. Embryos were processed in PBDT (1% BSA, 1%DMSO, 1% TritionX-100 in PBS). The following antibodies were used at the following dilutions: goat anti-EphrinB2 (R&D System) at 1:100, rabbit anti-goat IgG-Cy3 (Sigma Aldrich) at 1:200. Processed samples were mounted in 2% agarose and the images were acquired using a Leica dissecting microscope and a Zeiss LSM5 confocal microscope.
Quantification of cell number
Heterozygous smo mutants [smohi640/+; Tg(fli:EGFP-nuc)] were crossed, where the nuclei of all endothelial cells were labelled with EGFP. The embryos were mounted in 1% agarose at 36 hpf or 72 hpf and subjected to confocal microscopy on a Zeiss LSM 510 confocal microscope using a 20x/0.75 NA objective. 30–40 confocal slices were acquired as a Z-stack for each embryo, focusing on four segment length of intersomitic vessels along the axial vessels above the yolk extension region in the middle trunk. Number of cells were counted in each confocal z-stack using Pickpointer embedded in Image J. Pickpointer permits a single user-defined mark to appear throughout z-stacks of images, allowing the tracking of a single cell in overlapped z sections to avoid double counting.
Time-lapse imaging
At 14 hpf, embryos carrying the transgene (fli:EGFP) were manually dechorionated and mounted in 1% low-melting agarose in 35 mm glass-bottom Petri dishes. Transgenic embryos were treated with 50μM cyclopamine from 50% epiboly for the duration of experiments. Time-lapse images were captured using a 20x dry (NA = 0.75) objective mounted on a motorized Zeiss Axiovert 200 microscope equipped with a heated chamber to maintain embryos at 28.5 1C using Ultraview ERS (Perkin-Elmer Inc). Z image stacks were collected every 10 min. Three-dimensional data sets were compiled using Sorenson 3 video compression and exported using QuickTime (Apple Inc.) to create movies. Z stacks were cropped and computationally rotated using 3D opacity function in Velocity Visualization Software (Improvision Inc.) to yield virtual transverse sections.
Transplantation
Blastula stage transplantations were conducted as previously described (Ho and Kane, 1990; Parker and Stainier, 1999). Donor embryos from crosses of smo mutant fish [smohi640 −/+; Tg(fli:EGFP-nuc)] or wild-type fish Tg(fli:EGFP-nuc) were used for mosaic analysis. Between 3 and 4 hpf, 50–100 donor blastomeres were transplanted from donor embryos and placed into the marginal region of wild-type Tg(flk:DsRed) hosts. After transplantation, the donor and mosaic embryos were subsequently grown at 28.5 °C in Ringer’s solution (116 mM NaCl, 2.9 mM KCl, 1.8 mM CaCl2, 5 mM HEPES, pH 7.2) in correlated wells until 72 hpf. Homozygous smo donors were distinguished from wild-type sibling donors based on their phenotypes. Endothelial cell contribution of transplanted cells was examined by EGFP expression in mosaic embryos using a Zeiss LSM 510 Meta Confocal microscope.
Results
smo mutant embryos display an expansion of venous cells with a reciprocal loss of arterial cells
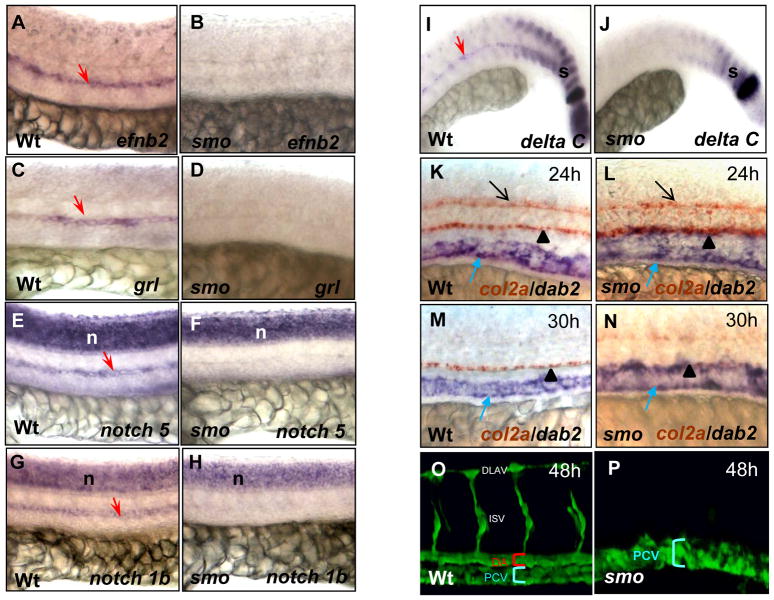
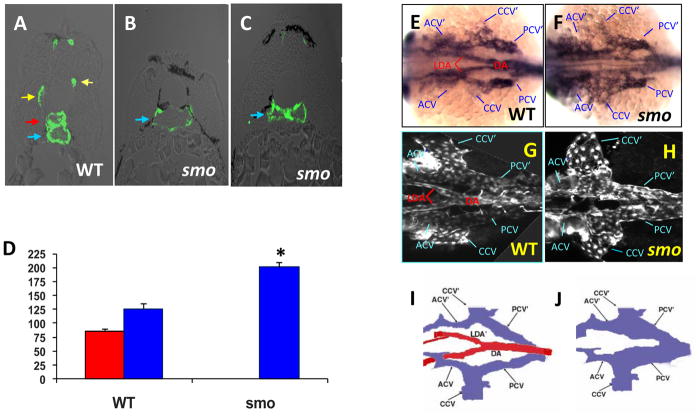
Previous studies have shown that embryos deficient in Hh signaling lack the dorsal aorta (Brown et al., 2000; Lawson et al., 2002; Gering and Patient, 2005). However, the developmental status of the cardinal vein has not been investigated. Thus, we examined venous development in smo mutant embryos using arterial and venous endothelial markers. In agreement with previous studies, expression of arterial markers efnb2, grl, notch5 and notch1b were completely absent in smo mutants (Fig. 1A–H) (Lawson et al., 2002). Absence of deltaC expression in the mutant aorta was observed, while deltaC expression in somites was reduced in the mutants (Fig. 1I, J). Notably, expression of the venous marker disabled homolog 2 (dab2) was expanded dorsally into the domain of the dorsal aorta and reached to the hypochord marked by col2a expression at 24 hpf and 30 hpf (Fig. 1K–N) (Appel et al., 1999). Confocal microscopy analysis revealed expansion of the posterior cardinal vein and absence of the dorsal aorta in smo mutants [smohi640/smohi640; Tg(flk:EGFP)] (Fig. 1P), when compared to the normal size of both the dorsal aorta and the posterior cardinal vein in wild-type embryos at 48 hpf (Fig. 1O). Transverse sections validated the enlarged lumen size of the posterior cardinal vein in smo mutants (Fig. 2B), which was collapsed in some places (Fig. 2C), as compared to normal size of the dorsal aorta and the posterior cardinal vein in wild-type embryos (Fig. 2A). Notably, the enlarged lumen size of the cardinal vein (201.2 ± 13.7 μm) in smo mutants is equal to the combined lumen size of the dorsal aorta (85.7 ± 4.3 μm) and the posterior cardinal vein (126.4 ± 5.5 μm) in wild-type embryos (Fig. 2D). Both in situ hybridization and confocal microscopy analyses revealed that, in the region of aortic bifurcation in wild-type embryos, bilateral cardinal veins flank the aortic bifurcation, where bilateral dorsal aortae merge into the single dorsal aorta at the midline in wild-type embryos (Fig. 2E;G;I). In smo mutants, bilateral dorsal aortae and the dorsal aorta at the aortic bifurcation were completely absent, whereas bilateral cardinal veins were significantly expanded (Fig. 2F;H;J). These data suggest that Hh signaling deficiency results in the expansion of cardinal veins at the expense of aortae.
Fig. 1. smo mutants display expansion of the posterior cardinal vein and absence of the dorsal aorta.
(A–J) Lateral views displaying absent expression of efnb2 (A,B), grl (C,D), notch5 (E,F), notch1b (G,H) and deltaC (I,J) in smo mutants, compared to wild-type embryos. (K–N) Double in situ hybridization exhibiting the expansion of dab2 venous domain and col2a expression in the neural tube and the hypocord in smo mutants (L,N) and wild-type embryos (K,M). (O,P) Confocal microscopy depicting the dorsal aorta and the posterior cardinal vein in wild-type embryos [Tg(flk:EGFP)] (O); and absence of the dorsal aorta and expansion of the posterior cardinal veins in smo mutants [smohi640/smohi640; Tg(flk:EGFP)] (P). Red arrow: dorsal aorta. Blue arrow: cardinal vein. Black arrow: floor plate. Black arrowhead: hypocord. s: somite. n: neural tube. DA: dorsal aorta. PCV: posterior cardinal vein. DLAV: dorsal longitudinal anastomotic vessel.
Fig. 2. smo mutation causes loss of the aortic bifurcation.
(A–C) Transverse section analyses showing normal lumen sizes of the dorsal aorta and the posterior cardinal vein in wild-type embryos (A); single enlarged cardinal vein (B) and collapsed cardinal vein in smo mutants (C). (D) Bar chart depicting perimeter measurements of lumen sizes of the dorsal aorta and the cardinal vein in wild-type embryos and smo mutants. Lumen sizes were measured in 15 transverse sections derived from three wild-type and three smo mutant embryos, respectively. Error bars indicate standard deviation and asterisks indicate statistical significance between wild-type and smo data (P<0.01). (E–H) In situ hybridization at 24 hpf (E,F) and confocal microscopy at 48 hpf (G,H) exhibiting loss of the aortic bifurcation and expansion of bilateral cardinal veins in smo mutants (F;H), compared to the normal aortic bifurcation and bilateral cardinal veins in wild-type embryos (E;G). (I, J) Schematics depicting the aortic bifurcation flanked by bilateral cardinal veins in wild-type embryos, compared to enlarged bilateral cardinal veins in smo mutants. Red arrow: aorta. Blue arrow: vein. Yellow arrow: intersomatic vessel. DA: dorsal aorta. LDA: lateral dorsal aorta. acv: anterior cardinal vein. ccv: common cardinal vein. pcv: posterior cardinal vein. LDA′, acv′,ccv′, pcv′: denote the corresponding vessel on the other side of the embryo
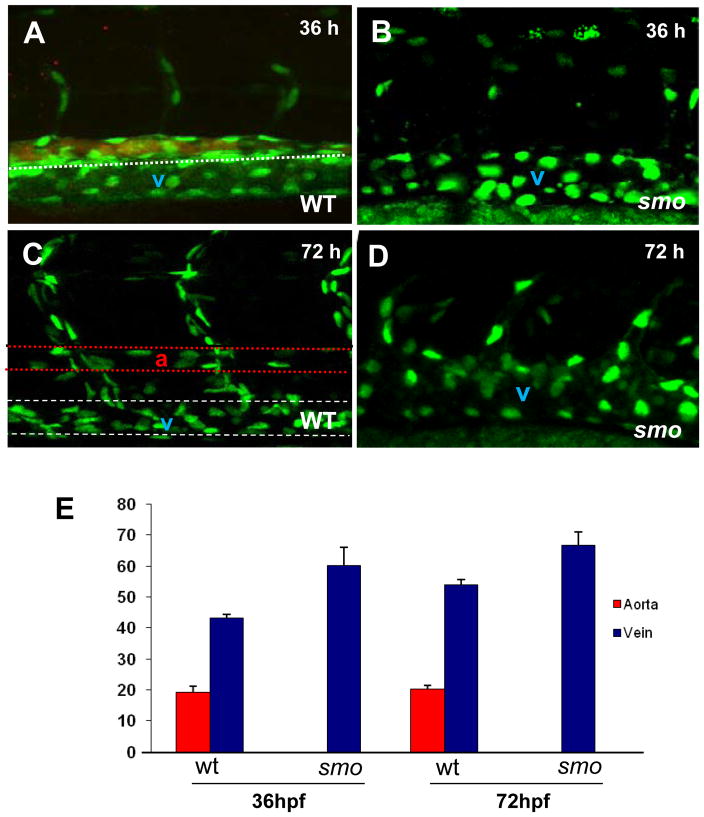
To determine whether the venous expansion is due to increased endothelial cell number, we quantified endothelial cells in the dorsal aorta and the posterior cardinal vein in transgenic embryos [Tg(fli:EGFP-nuc)], in which green fluorescent protein gene fused to a nuclear localization signal (EGFP-nuc) is under the control of the fli promoter (Roman et al. 2002). In these transgenic embryos, individual endothelial cell nuclei are marked by green fluorescence (Fig. 3), permitting quantitative assessment of endothelial cell number in the dorsal aorta and the posterior cardinal vein using confocal microscopy analysis. At 36 hpf, the number of cells in the expanded cardinal vein in smo mutants is equivalent to the combined cell number of the dorsal aorta labeled by efnb2 and the cardinal vein in wild-type embryos (Fig. 3A,B;E). The same arterial-venous specification defect persists in smo mutants at 72 hpf, when the dorsal aorta is clearly separated from the posterior cardinal vein (Fig. 3C,D;E). These data indicate that the increase in the venous cell number is directly proportional to the loss of arterial cells in smo mutants.
Fig. 3. The increase of venous cells is equavalent to loss of arterial cells in smo mutants.
(A–D) Confocal optics displaying individual nuclei of endothelial cells in the dorsal aorta (A,B stained with efnb2 antibody, the posterior cardinal vein and intersomitic vessels in wild-type embryos [Tg(fli:EGFP-nuc)] at 36 hpf (A) and 72 hpf (C),as compared to the increased endothelial nuclei in enlarged cardinal veins in smo mutants [smohi1640/smohi1640;Tg(fli:EGFP-nuc)] at 36 hpf (B) and 72 hpf (D). (E) Bar chart depicting number of arterial and venous cells in wild-type embryos compared to smo mutants. At 36 hpf, arterial cell number: 19.2± 1.7 (WT), 0 (smo); Venous cell number: 43.2±1.3 (WT), 60.3±2.4 (smo). At 72 hpf, arterial cell number: 20.2±1.3 (WT), 0 (smo); Venous cell number: 54±1.6 (WT), 66.8±3.4 (smo). Endothelial cells were counted from 12 lateral sections derived from 6 wild-type embryos and 6 smo mutants. Each section covers four segment lengths of intersomitic vessels along the dorsal aorta and the postertior cardinal vein in the middle trunk above the yolk extension region. a: aorta. v: vein.
Hh signaling regulates arterial-venous specification at the lateral plate mesoderm
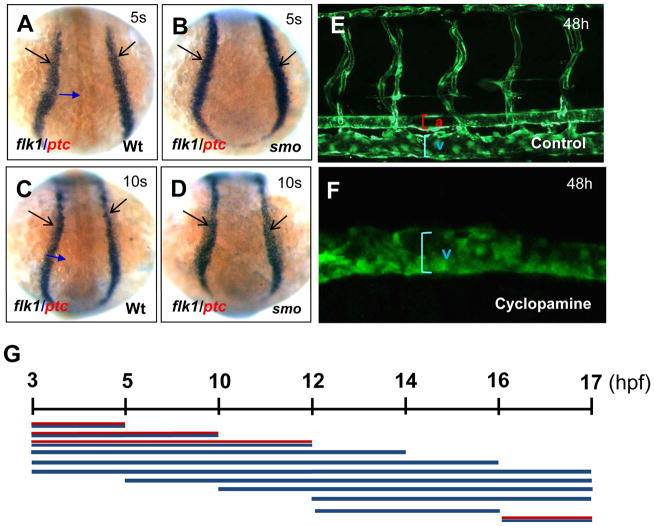
To determine whether angioblast generation is affected in smo mutants, we performed flk1 and ptc1 double in situ hybridization. We used expression of ptc1, a seven transmembrane receptor for Hh, to distinguish smo mutant embryos from sibling wild-type embryos during early development. Previous studies indicate that Hh positively regulates ptc1 expression; and in smo mutant embryos, ptc1 expression is down regulated (Hooper and Scott, 2005; Ingham and McMahon, 2001). At the 5- and 10-somite stages, formation of angioblasts visualized by flk1 expression at the lateral plate mesoderm was not altered in smo mutants compared to wild-type embryos, while ptc1 expression was drastically reduced (Fig. 4A–D). We next determined the time window when defective Hh signaling results in the absence of the dorsal aorta and expansion of the cardinal vein. We used cyclopamine to inhibit the Smo receptor and block Hh signaling in flk1 transgenic embryos [Tg(flk1:EGFP)] during a series of developmental time stages. In the first set of pulse experiments, cyclopamine was added at 3 hpf (1000-cell stage) and washed away at progressively later stages of development, including 5 hpf (50% epiboly), 10 hpf (tailbud stage), 12 hpf (6-somite stage), 14 hpf (10-somite stage), 16 hpf (14-somite stage) and 17 hpf (16-somite stage) (Fig. 4G). We examined the developmental status of the dorsal aorta and cardinal veins at 48 hpf using confocal microscope analysis (Fig. 3E, F). Cyclopamine treatment failed to cause expansion of the cardinal vein and absence of the dorsal aorta until 12 hpf (Fig. 4G), suggesting that cyclopamine treatment is effective in inhibiting artery specification only after the 6-somite stage. In a second set of experiments, cyclopamine was added at progressively later stages of development with treatments starting at 5 hpf, 10 hpf, 12 hpf, 16 hpf and washed away at 17 hpf (Fig. 4G). Cyclopamine treatment prior to 16 hpf resulted in the expansion of the veins and elimination of the aorta (Fig. 4G). Together, these data indicate that cyclopamine-mediated inhibition of Smo receptor causes expansion of the cardinal vein and elimination of the dorsal aorta between 12 hpf and 16 hpf at the lateral plate mesoderm. Indeed, cyclopamine-treatment of embryos during a short time period (12–16 hpf) caused venous expansion and arterial elimination (Fig. 4G). This developmental window correlates well with the stages when endothelial progenitor cells migrate at the posterior lateral mesoderm (Zhong, 2005; Zhong et al., 2001).
Fig. 4. Temporal development stages when Hh signaling affects artery-vein specification.
(A–D) Dorsal views displaying comparable levels of flk1-positive angioblasts in wild-type embryos (A, C) and smo mutants (B, D). (E, F) Confocal microscopy analysis revealing the normal size of the dorsal aorta, the posterior cardinal vein and intersomitic vessels in control embryos (E), and an expanded posterior cardinal vein in cyclopamine-treated embryos (F). (G) Schematic representation depicting the temporal activity of cyclopamine in artery-vein specification. Embryos were incubated in 50 μM cyclopamine during a series of developmental window. Red and blue line: artery and vein. Blue line: vein. 5s:5-somite stage. 10s:10-somite stage.
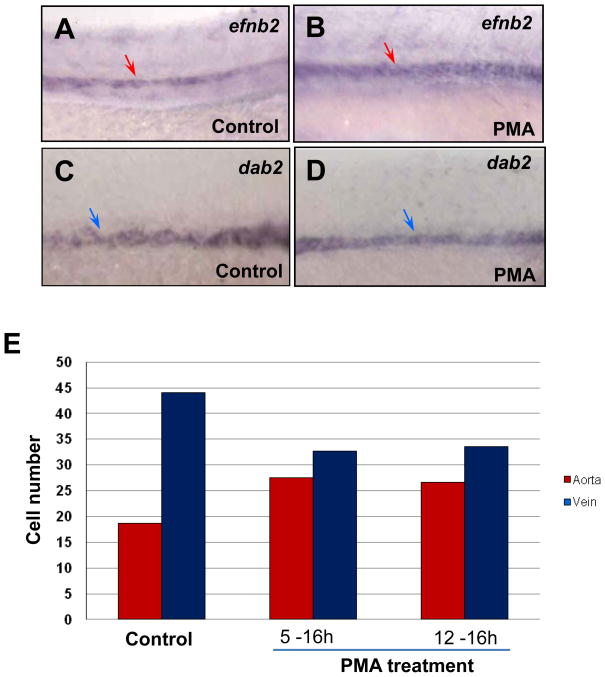
We next examined whether elevating Hh signaling causes an increase in arterial cells and a reduction of venous cells during the specific time window. We used purmorphamine (PMA) to activate the Hh signaling pathway. PMA directly targets and activates Smo receptor as an agonist (Sinha and Chen, 2006). When PMA was added to Tg(fli:EGFP-nuc) embryos during both time windows (5 – 16 hpf; 12 –16 hpf), efnb2 expression was increased in treated embryos compared to controls (Fig. 5A,B), whereas dab2 expression was reduced (Fig. 5C,D). We quantified endothelial cell number and found that PMA treatment caused an increase in arterial cell number and a reduction in venous cell number (Fig. 5E). Furthermore, the increase in arterial cells is proportional to the decrease of venous cells after treatment during both developmental time windows (Fig. 5E). Together, these findings suggest that activated Hh signaling causes expansion of arterial cell formation via repressing venous cell fate.
Fig. 5. Activated Hh signaling causes an increase of arterial cells and loss of venous cells.
(A–D) In situ hybridization analysis displaying the increased efnb2 expression in purmorphamine (PMA)-treated embryos (A) compared to controls (B); and the reduced dab2 expression in embryos treated with PMA (C) compared to controls (0.1% DMSO) (D). (E) Bar chart depicting numbers of arterial and venous cells in PMA-treated embryos from 5–16 hpf and 12–16 hpf, compared to controls (0.1% DMSO). Arterial cell number: 19.3±0.6 (control); 27.5± 0.9 (5–16 hpf; PMA treated); 26.7± 0.6 (12–16 hpf; PMA treated). Venous cell number: 41.6±1.5 (control); 32.7±1.2 (5–16 hpf; PMA treated); 33.5± 1.8 (12–16 hpf; PMA treated). Endothelial cells were counted from 6 sections derived from 3 wild-type embryos or 3 PMA-treated embryos. Each section covers four segment lengths of intersomitic vessels along the dorsal aorta and the posterior cardinal vein in the middle trunk above the yolk extension region. a: aorta. v: vein.
Hh signaling deficiency causes loss of the first wave of angioblast migration
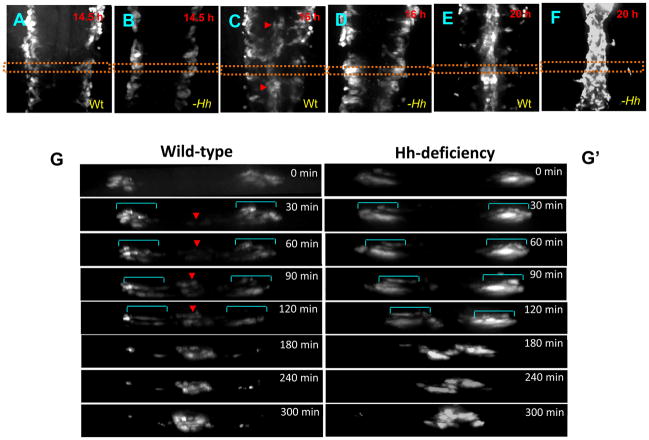
Angioblasts migrate to the midline to form the dorsal aorta and the posterior cardinal vein. Previous immunostaining and histochemical studies suggest that there are two distinct waves of angioblast migration: The first migratory wave of angioblasts, the arterial wave, contributes largely to the formation of the dorsal aorta, while the second wave of migration, the venous wave, gives rise to the posterior cardinal vein (Jin et al., 2005; Isogai et al., 2003). To determine whether angioblast migration is affected in smo mutants, we performed time-lapse experiments in fli1 transgenic embryos [Tg(fli1:EGFP)] using spinning disk confocal microscopy from 14 hpf, when angioblasts start to migrate, to 24 hpf, when the dorsal aorta and the posterior cardinal vein form. We observed that in wild-type embryos, angioblasts migrate in streams towards the midline (Supplementary movie 1). Moreover, the initial vascular cord was observed at 16 hpf (Fig. 6C), most cells of which is largely associated with formation of the dorsal aorta (Jin et al., 2005). In silico cross-sections, derived from the z-stack time sequence of a single embryo, reveal sequential migration of angioblasts (Fig. 6G; 0 to 300 min; Supplementary movie 1). The first migratory wave formed the initial vascular cord during 60-minute interval (Fig. 6G; 0 to 60 min). The second venous wave followed the first wave to coalesce into the formed vascular cord (Fig. 6G; 0 to 300 min). These data suggest that the arterial wave moves much faster than the venous wave. In Hh-deficient embryos treated with cyclopamine, the initial vascular cord failed to form (Fig. 6D; G′). Time-lapse sequence from 30 min to 120 min displayed the absence of the first arterial wave of angioblasts (Fig. 6G′; Supplementary movie 2). Notably, in Hh signaling-deficient embryos, angioblasts moved in a concerted fashion (Fig. 6G′ vs. G; 30 min to 120 min; Supplementary movie 2). These angioblasts migrated towards the midline to form the vascular cord that is destined to be the posterior cardinal vein (Fig. 6G′; Fig. 1L;N;P). Thus, Hh-deficient progenitor cells fail to contribute to the first arterial wave; instead, they all migrate medially in the venous wave to contribute to the posterior cardinal vein. These data suggest that migratory ability of Hh-depleted angioblasts is not affected.
Fig. 6. The first wave of angioblast migration is diminished in embryos with Hh signaling deficiency.
(A–F) Time-lapse imaging revealing sequential migration of angioblasts toward to the midline from 14.5 hpf to 20 hpf in wild-type embryos (A;C;E), and cyclopamine-treated embryos (B;D;F). (G and G′) Crossover optical sections derived from the time-lapse sequence viewing angioblast migration in wild-type embryos (G), and cyclopamine-treated embryos (G′). Red arrowhead: arterial cells. Blue bracket: venous cells. Frames were captured from 300-min time-lapse sequence. Images were obtained from the dorsal side of transgenic embryos Tg(fli1:EGFP) (A–F). The 0 min time point is from the approximately 14.5 hpf stage (G;G′). Times shown in right corners represent time elapsed after the start of the sequence (G;G′). Yellow dot lines (A–F) indicate where the optical crossover sections in G and G′ were derived from.
Reception of Hh signaling is required cell-autonomously for the formation of arterial endothelial cells
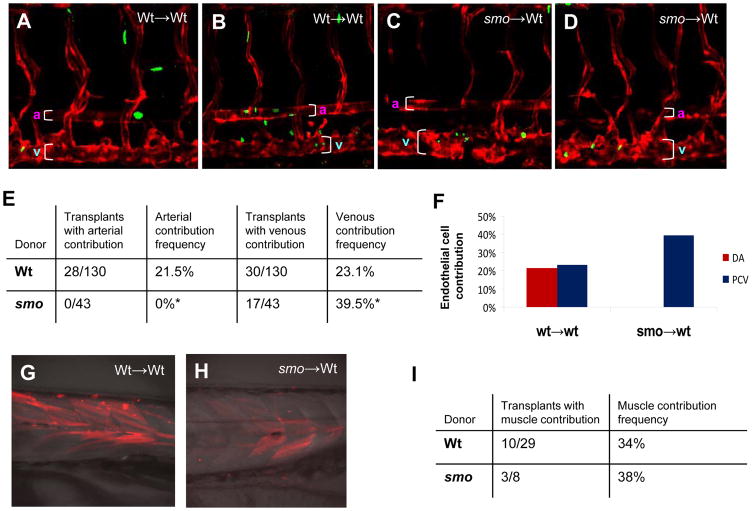
We hypothesized that angioblasts that receive Hh signaling at early stages adopt an arterial cell fate and contribute to formation of the dorsal aorta, while angioblasts that are deficient in Hh signaling adopt a venous cell fate and give rise to the posterior cardinal vein. Hence, we conducted transplantation experiments in zebrafish to test whether receipt of Hh signaling is required for arterial versus venous cell specification. Blastomeres were transplanted at blastula stages, using donor embryos from heterozygous cross carrying smo mutation and transgene Tg(fli:EGFP-nuc) to assess arterial or venous contribution. The recipients were wild-type embryos carrying the transgene Tg(flk:DsRed). Analyses of the resulting chimeric embryos at 72 hpf revealed that wild-type donor cells were able to contribute to both arterial and venous cells at approximately equal frequency (Fig. 7A, B; E,F). In contrast, none of the transplanted cells from smo mutant donors contributed to the arterial endothelial system; instead, all donor cells from smo embryos contributed to the venous system, including the posterior cardinal vein (Fig. 7C, D; E, F). One explanation for the lack of arterial contribution is that smo donor cells might not survive in the arterial endothelium, a general problem of cell transplantation. As a control, we performed transplantations using donor cells labeled with a fluorescein-dextran lineage and found that smo donor cells were present in many cell lineages in wild-type hosts. For example, smo cells were capable of contributing to fast muscle as the same frequency as wild-type cells (Fig. 7G,H;I). Thus, angioblasts that receive Hh signaling contribute to both arterial and venous endothelia, whereas Hh-depleted angioblasts only give rise to venous cells. These data suggest that reception of Hh signaling is required cell-autonomously in angioblasts to be specified into arterial endothelial cells at the expense of venous cell fate.
Fig. 7. Hh signaling acts cell autonomously to induce arterial cell formation by inhibiting venous cell development.
(A, B) Confocal microscopic analysis revealing the presence of EGFP-positive donor cells derived from wild-type Tg(fli:EGFP-nuc) embryos in the dorsal aorta and the posterior cardinal vein of wild-type host embryos Tg(flk:DsRed) at 72 hpf, when the dorsal aorta is completely separated from the cardinal vein. (C, D) Confocal optics showing the presence of EGFP-positive smo-null cells derived from heterozygous crosses of smo mutants [smo −/+; Tg(fli:EGFP-nuc)] in the posterior cardinal vein in wild-type host embryos Tg(flk:DsRed) at 72 hpf. (E) Data table quantitating transplantation results. A ratio of hosts with donor-derived arterial (or venous) cells to all host embryos with donor cells in any tissue was used to determine arterial (or venous) contribution frequency. (F) Bar chart depicting arterial or venous contribution frequency. (G,H) Fluorescent optics showing transplantation of fluorescein-dextran injected donor cells into wild-type hosts. Both wild-type and smo donors give rise to skeletal muscle tissue. (I) Data table quantitating transplantation results of fluorescein-dextran injected hosts. A ratio of hosts with donor-derived muscle cells to all host embryos was used to determine muscle contribution frequency.
Discussion
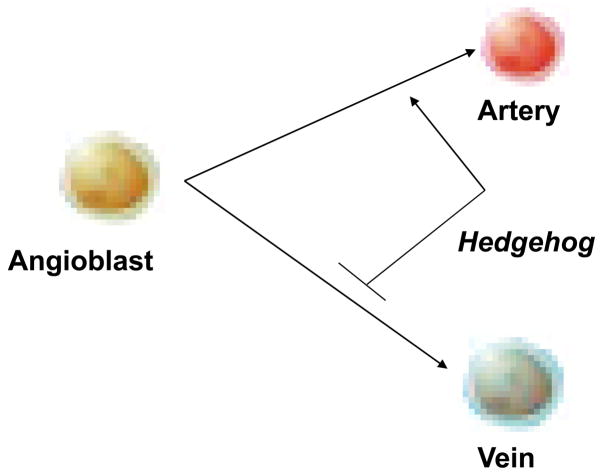
In this study, we show that smo mutants create a surplus of venous cells as an alternative fate to the lost arterial cells. Activation of Hh signaling by Smo agonist causes an increase in the arterial cell number and a reduction in venous cells. The arterial and venous cell specification occurs at the lateral plate mesoderm during migration before the formation of the vascular cord. Hh signaling deficiency causes the loss of arterial migration wave that is accompanied by reciprocal expansion of the venous wave. We thus propose a model that reception of Hh signaling is required cell-autonomously for angioblasts to differentiate into arterial cells at the expense of venous cell fate (Fig. 8).
Fig. 8.
Shh signal instructs angioblasts to differentiate into arterial cells rather than venous cells.
Previous studies revealed absence of the dorsal aorta in smo mutants and cyclopamine-treated embryos, concluding that this might be due to defects in the medial migration of angioblasts contributing to the dorsal aorta (Gering and Patient, 2005). We have extended these studies to show that loss of the dorsal aorta is associated with the corresponding expansion of the posterior cardinal vein in Hh signaling-deficient embryos. We have further used time-lapse imaging to directly visualize two distinct waves of angioblast migration in wild-type embryos, with the first wave contributing to the arterial and the second wave to the venous system. Previous studies (Gering and Patient, 2005) had only compared the static flk1 expression at different stages using in situ hybridization, and thus failed to observe dynamic migration of venous angioblasts to the midline. Moreover, we show that Hh signaling deficiency causes all angioblasts to reach to the midline in the later venous wave of migration. Importantly, our studies provided evidence that loss of the dorsal aorta in Hh-depleted embryos is due to adoption of a venous cell fate by all angioblasts, leading to a surplus of venous cells. Our transplantation experiments reveal that smo null angioblasts fail to contribute to the dorsal aorta but rather contribute to the posterior cardinal vein in wild-type hosts. Thus, lack of the arterial migration wave in Hh signaling-depleted embryos could be an indirect consequence of the venous cell fate assignment. Hh signaling has been shown to be involved in cell fate assignment in several other cell lineages. For example, activation of Hh pathway specifies neuroblasts in Drosophila (Bhat, 1996; McDonald and Doe, 1997), determines ventral cell fate in the neural tube (Chiang et al., 1996; Gunhaga et al., 2000; Jessell, 2000) and promotes cardiomyocyte formation in the developing heart (Thomas et al., 2008). Interestingly, mouse embryos lacking function of smo fail to form the anterior paired dorsal aortae and some segments of the descending dorsal aorta. However, flk1-positive endothelial cells were accumulated in these arterial regions (Vokes et al., 2004). Although this is attributed to tubulogenesis defects, it remains unknown whether these accumulated endothelial cells have a venous cell identity.
Hh signaling has been shown to be important in arterial endothelium differentiation and maturation after formation of the dorsal aorta. This primarily reflects absent expression of arterial gene ephrinb2 in the formed aorta in syu mutants as well as cyclopamine-treated embryos at late stages from the 15-somite stages (Gering and Patient, 2005; Lawson et al., 2002; Byrd et al., 2002). This Hh-dependent arterial differentiation and maturation is thought to act via Vegf, which acts upstream of Notch pathway (Lawson et al., 2002; Byrd and Grabel, 2004). Likewise, interrupted Notch signaling in mib mutants or vegf knockdown in zebrafish reduces expression of the arterial marker efnb2 but fails to ablate the dorsal aorta in the midline (Nasevicius et al., 2000; Lawson et al., 2001; Lawson et al., 2002; Jin et al., 2005). This is not due to defective specification; rather, due to their roles in regulating arterial-venous segregation at later stages (Herbert et al., 2009). After the vascular cord formation, Vegf and Notch act upstream of Efnb2-EphB4 signaling to sort arterial and venous angioblasts into the separate trunk vessels (the dorsal aorta and the cardinal vein) from the single precursor vessel (Herbert et al., 2009). Thus, the Hh-dependent arterial differentiation and maturation could act at the levels of arterial-venous segregation through modulation of Vegf signaling. Our studies demonstrate that the early role of Hh signaling involved in arterial-venous specification is via a cell-autonomous manner. In avian embryos, endoderm-derived shh has been shown to directly act on angioblasts and regulate vascular assembly (Vokes et al., 2004). In zebrafish, three Hh ligands (shh, ihh and twhh) are expressed in the notochord and the floor plate but not the endoderm during the early segmentation stages (Roy et al. 2001). Most likely, the midline-derived Hh signals are required for action directly on migrating angioblasts (Byrd and Grabel, 2004). The diffusible range and concentration gradient of Hh encountered by angioblasts, or the timing and duration of Hh exposure to angioblasts may be critical for them to make an arterial versus a venous cell fate choice. Collectively, these findings suggest that Hh signaling plays multiple roles during vasculogenesis. Further elucidation of the Hh-dependent components and signaling mechanisms in regulating arterial-venous specification may lead to novel therapeutic strategies for diverse disorders such as coronary heart disease and cancer.
Supplementary Material
Moive 1. QuickTime movie displays two sequential waves of angioblast migration in wild-type embryos.
Movie 2. QuickTime movie displays a single wave of angioblast migration in Hh-signaling deficient embryos.
Acknowledgments
We acknowledge Sarah Kucenas, Robert Taylor and Wenbiao Chen for their invaluable assistance during our experimental procedures. We thank excellent fish care by John Quan. We are grateful to members of Solnica-Krezel and Zhong laboratories for comments on the manuscript and helpful discussions. This research was supported by NIH grants (R01HL073348 to TPZ; RO1GM055101 to LSK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary movies:
2D projection of a 3D confocal time-lapse that was performed on a Tg(fli1:EGFP) zebrafish embryo from 14 hpf to 24 hpf, capturing at 10-min intervals and playing at five frames per second. Anterior to the left.
References
- Appel B, Fritz A, Westerfield M, Grunwald DJ, Eisen JS, Riley BB. Delta-mediated specification of midline cell fates in zebrafish embryos. Curr Boil. 1999;9:247–56. doi: 10.1016/s0960-9822(99)80113-4. [DOI] [PubMed] [Google Scholar]
- Barresi MJ, Stickney HL, Devoto SH. The zebrafish slow-muscle-omitted gene product is required for Hedgehog signal transduction and the development of slow muscle identity. Development. 2000;127:2189–99. doi: 10.1242/dev.127.10.2189. [DOI] [PubMed] [Google Scholar]
- Bhat KM. The patched signaling pathway mediates repression of gooseberry allowing neuroblast specification by wingless during Drosophila neurogenesis. Development. 1996;122:2921–32. doi: 10.1242/dev.122.9.2921. [DOI] [PubMed] [Google Scholar]
- Brown L, Rodaway A, Schilling T, Jowett T, Ingham P, Patient R, Sharrocks A. Insights into vasculogenesis revealed by expression of ETS-domain transcription factor Fli-1 in wild type and mutant zebrafish embryos. Mechanisms of development. 2000:237–252. doi: 10.1016/s0925-4773(99)00256-7. [DOI] [PubMed] [Google Scholar]
- Byrd N, Beck S, Maye P, Narasimhaiah R, St-Jacques B, Zhang X, McMahon J, McMahon AP, Grabel L. Hedgehog is required fo murine yolk sac angiogenesis. Development. 2002;129:361–372. doi: 10.1242/dev.129.2.361. [DOI] [PubMed] [Google Scholar]
- Byrd N, Grabel L. Hedgehog signaling in murine vasculogenesis and angiogenesis. Trends Cardiovasc Med. 2004;14:308–13. doi: 10.1016/j.tcm.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Chen JN, Haffter P, Odenthal J, Vogelsang E, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Nusslein-Volhard C. Mutations affecting the cardiovascular system and other internal organs in zebrafish. Development. 1996;123:293–302. doi: 10.1242/dev.123.1.293. [DOI] [PubMed] [Google Scholar]
- Chen W, Burgess S, Hopkins N. Analysis of the zebrafish smoothened mutant reveals conserved and divergent functions of hedgehog activity. Development. 2001;128:2385–2396. doi: 10.1242/dev.128.12.2385. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–13. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Concordet JP, Lewis KE, Moore JW, Goodrich LV, Johnson RL, Scott MP, Ingham PW. Spatial regulation of a zebrafish patched homologue reflects the roles of sonic hedgehog and protein kinase A in neural tube and somite patterning. Development. 1996;122:2835–46. doi: 10.1242/dev.122.9.2835. [DOI] [PubMed] [Google Scholar]
- Gering M, Patient R. Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Dev Cell. 2005;8:389–400. doi: 10.1016/j.devcel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Gunhaga L, Jessell TM, Edlund T. Sonic hedgehog signaling at gastrula stages specifies ventral telencephalic cells in the chick embryo. Development. 2000;127:3283–93. doi: 10.1242/dev.127.15.3283. [DOI] [PubMed] [Google Scholar]
- Herbert SP, Huisken J, Kim TN, Feldman ME, Houseman BT, Wang RA, Shokat KM, Stainier DY. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science. 2009;326:294–298. doi: 10.1126/science.1178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho RK, Kane DA. Cell-autonomous action of zebrafish spt-1 mutation in specific mesodermal precursors. Nature. 1990;348:728–30. doi: 10.1038/348728a0. [DOI] [PubMed] [Google Scholar]
- Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol. 2005;6:306–17. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- Huang H, Zhang B, Hartenstein PA, Chen JN, Lin S. NXT2 is required for embryonic heart development in zebrafish. BMC Dev Biol. 2005;5:7. doi: 10.1186/1471-213X-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes & Development. 2001;15:3059–87. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- Isogai S, Lawson ND, Torrealday S, Horiguchi M, Weinstein BM. Angiogenic network formation in the developing vertebrate trunk. Development. 2003;130:5281–90. doi: 10.1242/dev.00733. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–9. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Jin SW, Bels D, Mitchell T, Chen JN, Stainier DYR. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 2005;132:5199–5209. doi: 10.1242/dev.02087. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Developmental Dynamics. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kusano KF, Pola R, Murayama T, Curry C, Kawamoto A, Iwakura A, Shintani S, Ii M, Asai J, Tkebuchava T, Thorne T, Takenaka H, Aikawa R, Goukassian D, von Samson P, Hamada H, Yoon YS, Silver M, Eaton E, Ma H, Heyd L, Kearney M, Munger W, Porter JA, Kishore R, Losordo DW. Sonic hedgehog myocardial gene therapy: tissue repair through transient reconstitution of embryonic signaling. Nature Medicine. 2005;11:1197–204. doi: 10.1038/nm1313. [DOI] [PubMed] [Google Scholar]
- Lamont R, Childs S. MAPping out arteries and veins. Science STKE. 2006:PE39, pe39. doi: 10.1126/stke.3552006pe39. [DOI] [PubMed] [Google Scholar]
- Lavine KJ, Long F, Choi K, Smith C, Ornitz DM. Hedgehog signaling to distinct cell types differentially regulates coronary artery and vein development. Development. 2008;135:3161–71. doi: 10.1242/dev.019919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine KJ, White AC, Park C, Smith CS, Choi K, Long F, Hui CC, Ornitz DM. Fibroblast growth factor signals regulate a wave of Hedgehog activation that is essential for coronary vascular development. Genes & Development. 2006;20:1651–66. doi: 10.1101/gad.1411406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–83. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Developmental Cell. 2002;3:127–36. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- McDonald JA, Doe CQ. Establishing neuroblast-specific gene expression in the Drosophila CNS: huckebein is activated by Wingless and Hedgehog and repressed by Engrailed and Gooseberry. Development. 1997;124:1079–87. doi: 10.1242/dev.124.5.1079. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Larson J, Ekker SC. Distinct requirements for zebrafish angiogenesis revealed by a VEGF-A morphant. Yeast. 2000;17:294–301. doi: 10.1002/1097-0061(200012)17:4<294::AID-YEA54>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker L, Stainier DY. Cell-autonomous and non-autonomous requirements for the zebrafish gene cloche in hematopoiesis. Development. 1999;126:2643–51. doi: 10.1242/dev.126.12.2643. [DOI] [PubMed] [Google Scholar]
- Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Current Biology. 1998;8:1083–6. doi: 10.1016/s0960-9822(98)70446-4. [DOI] [PubMed] [Google Scholar]
- Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, Shapiro R, Taylor FR, Baker DP, Asahara T, Isner JM. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nature Medicine. 2001;7:706–11. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- Roman BL, Pham VN, Lawson ND, Kulik M, Childs S, Lekven AC, Garrity DM, Moon RT, Fishman MC, Lechleider RJ, Weinstein BM. Disruption of acvrl1 increases endothelial cell number in zebrafish cranial vessels. Development. 2002;129:3009–19. doi: 10.1242/dev.129.12.3009. [DOI] [PubMed] [Google Scholar]
- Rowitch DH, BSJ, Lee SM, Flax JD, Snyder EY, McMahon AP. Sonic hedgehog regulates proliferation and inhibits differentiation of CNS precursor cells. Journal of Neuroscience. 1999;19:8954–65. doi: 10.1523/JNEUROSCI.19-20-08954.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Qiao T, Wolff C, Ingham PW. Hedgehog signaling pathway is essential for pancreas specification in the zebrafish embryo. Curr Biol. 2001;17:1358–63. doi: 10.1016/s0960-9822(01)00402-x. [DOI] [PubMed] [Google Scholar]
- Sinha S, Chen JK. Purmorphamine activates the Hedgehog pathway by targeting Smoothened. Nat Chem Biol. 2006;2:29–30. doi: 10.1038/nchembio753. [DOI] [PubMed] [Google Scholar]
- Song HD, Sun XJ, Deng M, Zhang GW, Zhou Y, Wu XY, Sheng Y, Chen Y, Ruan Z, Jiang CL, Fan HY, Zon LI, Kanki JP, Liu TX, Look AT, Chen Z. Hematopoietic gene expression profile in zebrafish kidney marrow. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16240–5. doi: 10.1073/pnas.0407241101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumoy L, Keasey JB, Dittman TD, Kimelman D. A role for notochord in axial vascular development revealed by analysis of phenotype and the expression of VEGR-2 in zebrafish flh and ntl mutant embryos. Mech Dev. 1997;63:15–27. doi: 10.1016/s0925-4773(97)00671-0. [DOI] [PubMed] [Google Scholar]
- Thomas NA, Koudijs M, van Eeden FJ, Joyner AL, Yelon D. Hedgehog signaling plays a cell-autonompus role in maximizing cardiac developmental potential. Development. 2008;135:3789–3799. doi: 10.1242/dev.024083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Vazquez J, Kamei M, Weinstein BM. Molecular distinction between arteries and veins. Cell & Tissue Research. 2003;314:43–59. doi: 10.1007/s00441-003-0771-8. [DOI] [PubMed] [Google Scholar]
- Trinh LA, Stainier DY. Fibronectin regulates epithelial organization during myocardial migration in zebrafish. Dev Cell. 2004;6:371–82. doi: 10.1016/s1534-5807(04)00063-2. [DOI] [PubMed] [Google Scholar]
- Vokes SA, Yatskievych TA, Heimark RL, McMahon J, McMahon AP, Antin PB, Krieg PA. Hedgehog signaling is essential for endothelial tube formation during vasculogenesis. Development. 2004;131:4371–80. doi: 10.1242/dev.01304. [DOI] [PubMed] [Google Scholar]
- Zhong TP. Zebrafish Genetics and Formation of Embryonic Vasculature. Current Topics in Developmental Biology. 2005;71:53–81. doi: 10.1016/S0070-2153(05)71002-4. [DOI] [PubMed] [Google Scholar]
- Zhong TP, Childs S, Leu JP, Fishman MC. Gridlock signalling pathway fashions the first embryonic artery. Nature. 2001;414:216–20. doi: 10.1038/35102599. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Moive 1. QuickTime movie displays two sequential waves of angioblast migration in wild-type embryos.
Movie 2. QuickTime movie displays a single wave of angioblast migration in Hh-signaling deficient embryos.