Abstract
A disposable pre-oxidation technique is reported that dramatically improves the detection and identification of volatile organic compounds (VOCs) by a colorimetric sensor array. By passing a vapor stream through a tube packed with chromic acid on silica immediately before the colorimetric sensor array, the sensitivity to less reactive VOCs is substantially increased and limits of detection (LODs) are improved ~300-fold, permitting the detection, identification, and discrimination of 20 commonly found indoor VOC pollutants at both their immediately dangerous to life or health (IDLH) and at permissible exposure limits (PEL) concentrations. LODs of these pollutants were on average 1.4% of their respective PELs.
Exposure to volatile organic compounds (VOCs) can have serious harmful effects, including a wide range of sensory irritation and acute and chronic diseases (e.g., nervous system impairment, asthma and cancer1–3). The detection and identification of VOCs therefore are particularly important for environmental, chemical workplace and even personal home monitoring.4 Currently, the concentration of VOCs is generally determined by standard analytical methods5 such as GC or GC-MS,6 which require expensive and non-portable instrumentation. Various other more mobile sensors for VOC detection exist, including the use of surface acoustic wave, semiconducting metal oxide-based, conductive polymer-based, and quartz crystal microbalances sensors,7–8 and new techniques continue to be reported.9 The performance of these sensors, and the electronic nose devices10–11 made from them, still fall short of the demands of many applications due to low sensitivity, low selectivity, lack of reproducible sensor production, interference from humidity, limited stability, or changing responses due to sensor aging.11c,f,12 There remains, therefore, a real need for the development of high-performance portable VOC sensors.
In recent years, our group has developed a disposable colorimetric sensor array methodology as an “optoelectronic nose”13 that has been applied successfully for the identification of a wide range both of gases/vapors14 and of analytes in aqueous liquids.15 This array utilizes strong interactions between the analyte and a diverse set of chemically responsive colorants whose colors depend on local polarity, Brønsted acidity/basicity, Lewis acid-base interactions, and redox reactions. The array utilizes nanoporous pigments created from the immobilization of dyes in organically modified siloxanes (ormosils).16–17 Porous sol-gel glasses provide excellent matrices for colorants due to high surface area, relative inertness, good stability, and optical transparency. The use of nanoporous pigments significantly improves the stability and shelf-life of the colorimetric sensor arrays and permits direct printing onto non-permeable polymer surfaces.14,17
While these colorimetric sensor arrays work exceedingly well for reactive volatiles, they have not had especially high sensitivity to less reactive vapors.14f For example, common VOC indoor air pollutants (e.g., aromatic hydrocarbons, chlorocarbons, other organic solvents) are generally not especially reactive and are not detected at low concentrations by our colorimetric sensor array. We report here a dramatic improvement in the sensitivity of colorimetric sensors for the detection and identification of such less-reactive VOCs.
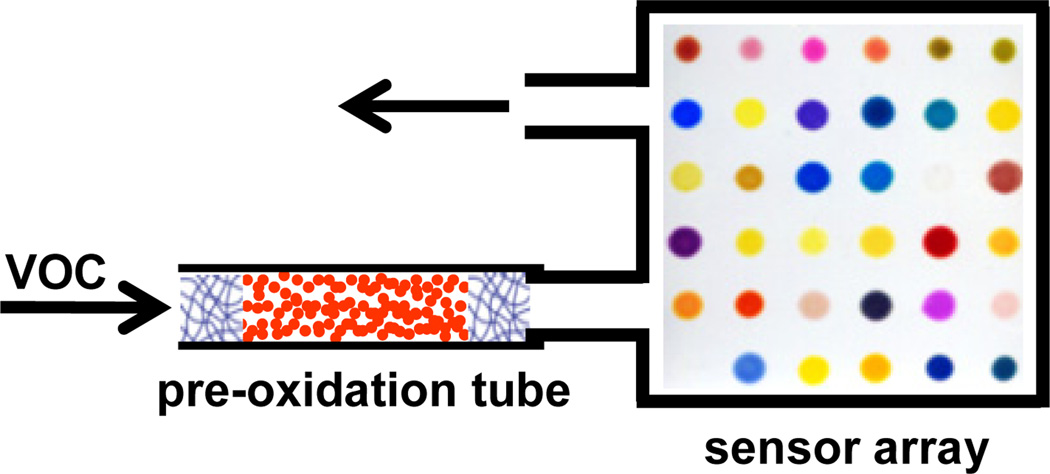
To improve array response, we have developed a disposable pre-oxidation technique in which a vapor-stream was passed through an oxidation tube before reaching the array (Figure 1). Though most indoor pollutant VOCs are relatively inert, their oxidation by-products (e.g., carboxylic acids, phenols, aldehydes, etc.) are reactive species and much more easily detected. Since each VOC produces a different mixture of oxidized derivatives, the array response to these more reactive volatile by-products provides a unique, but much more sensitive, signature for the initial VOC. We chose 20 commonly found VOC pollutants in indoor air1,18 as representative analytes (listed in Table 1); using this pre-oxidation method, we can discriminate among all of them both at their immediately dangerous to life or health (IDLH) and at their permissible exposure limit (PEL).
Figure 1.
A schematic illustration of the pre-oxidation technique. A Teflon tube (2 cm × 3 mm ID) is packed with chromic acid to pre-treat the gas flow containing a VOC before passing over the colorimetric sensor array.
Table 1.
Twenty commonly found VOC pollutants in indoor air.
| VOCs | IDLH ppm |
PEL ppm |
LOD (without pre- oxidation) ppm |
LOD (with pre-oxidation) |
LOD(without pre-oxidation)/ LOD(with pre-oxidation) |
|
|---|---|---|---|---|---|---|
| ppm | %PEL | |||||
| Acetone | 2500 | 1000 | 1100 | 16 | 1.6% | 69 |
| Benzene | 500 | 1 | 5000 | 0.20 | 20% | 10000 |
| Camphene* | 225 | 45 | 140 | 1.1 | 2.4% | 130 |
| Chloroform | 500 | 50 | 290 | 0.60 | 1.2% | 480 |
| p-Dichlorobenzene | 150 | 75 | 100 | 1.6 | 2.1% | 63 |
| Ethanol | 3300 | 1000 | 130 | 0.50 | 0.05% | 260 |
| Ethyl acetate | 2000 | 400 | 760 | 7.4 | 1.9% | 100 |
| Ethylbenzene | 800 | 100 | 350 | 1.3 | 1.3% | 270 |
| Formaldehyde | 20 | 0.75 | 50 | 0.10 | 13% | 500 |
| d-Limonene* | 130 | 26 | 100 | 0.90 | 3.5% | 110 |
| Methylethylketone | 3000 | 200 | 1400 | 2.2 | 1.1% | 640 |
| Phenol | 250 | 5 | 0.50 | 0.60 | 12% | 0.80 |
| iso-Propanol | 2000 | 400 | 260 | 18 | 4.5% | 14 |
| Styrene | 700 | 100 | 100 | 0.50 | 0.5% | 200 |
| Toluene | 500 | 200 | 300 | 1.3 | 0.7% | 230 |
| 1,1,1-Trichloroethane | 700 | 350 | 8000 | 5.0 | 1.4% | 1600 |
| 1,2,4-Trimethylbenzene* | 135 | 27 | 300 | 0.60 | 2.2% | 500 |
| m-Xylene | 900 | 100 | 500 | 0.50 | 0.5% | 1000 |
| o-Xylene | 900 | 100 | 500 | 0.60 | 0.6% | 830 |
| p-Xylene | 900 | 100 | 550 | 0.60 | 0.6% | 920 |
| Average of 20 VOCs | 1006 | 214 | 1000 | 3.0 | 1.4% | 330 |
IDLH and PEL of these three VOCs are unknown; we have instead used 5% and 1% of their saturated vapor pressure to approximate their IDLH and PEL, respectively.
For vapor analysis, digital images of the array were acquired before and after exposure to a diluted vapor mixture using an ordinary flatbed scanner (see supporting information (SI) Figure S1), and the changes in the red, green, and blue (RGB) values of each spot were measured after exposure to the VOC (SI Tables 1 and 2). The vapor of each VOC was typically produced by bubbling nitrogen through the corresponding pure compound, followed by further dilution with dry and wet nitrogen to achieve the desired concentration in a gas stream at 50% relative humidity (RH) at a flow rate of 500 sccm; confer Supporting Information for further details. VOC concentrations were confirmed using inline FT-IR analysis in real time with a MKS 2030 multigas analyzer.
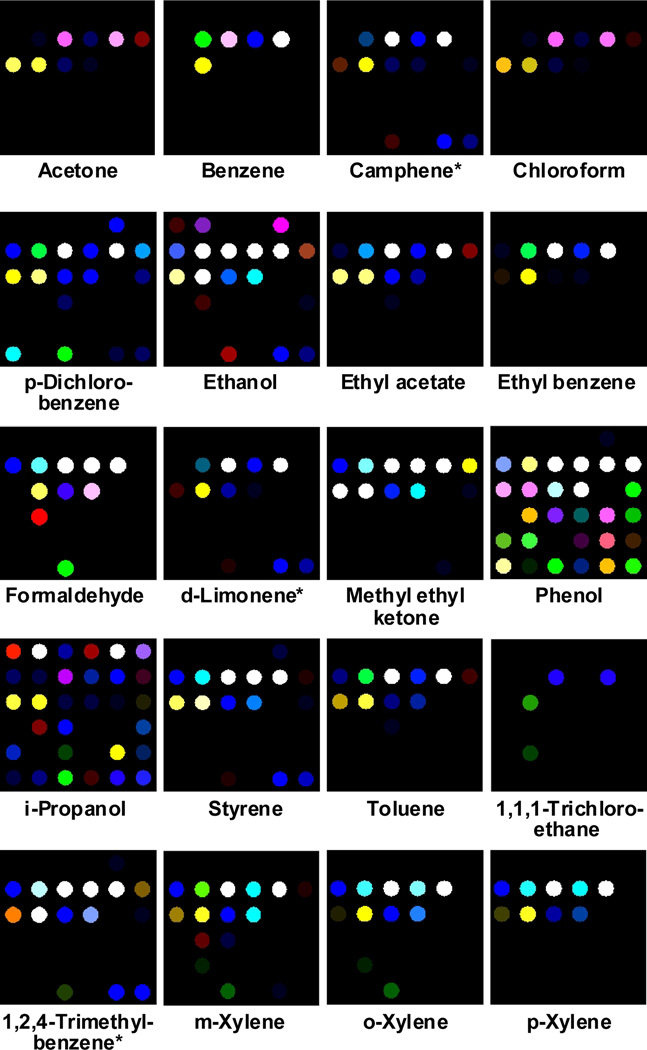
The influence of pre-oxidation on the array response was investigated by examining several different strongly oxidizing solid reagents in various amounts. The optimum array response occurred with pre-oxidation tubes (2 cm × 3 mm ID Teflon tubing) loaded with 30 mg of chromic acid (“sulfochromic acid”) on silica (250 µL H2SO4 and 400 mg Na2Cr2O7 per gram silica; see SI for details), so this formulation was used for all experiments. The color difference maps of the array response with pre-oxidation for the 20 VOCs at their IDLH are shown in Figure 2. The color difference maps of the array provided molecular fingerprints for each of the 20 VOCs, i.e. every VOC showed a different and specific color difference pattern. Note that the array responses do not arise only (or even primarily) from each VOC itself, but also from the oxidation products of the VOC. Monitoring the gas composition immediately exiting the pre-oxidation tube using the MKS multigas analyzer confirms that under our conditions, only partial oxidation occurs with different fractions of each VOC oxidized.
Figure 2.
Color change profiles of 20 representative indoor pollutant VOCs at their IDLH concentrations (given in Table 1) and a control after full equilibration (i.e., 5 min exposure) at 50% relative humidity and 298 K using a pre-oxidation tube. For purposes of visualization, the color range of these difference maps is expanded from 3 to 8 bits per color (RGB range of 3–10 expanded to 0–255); the full digital database is provided in SI Tables 1 and 2. All experiments were run in quintuplicate with 30 mg chromic acid on silica as the pre-oxidation reagent. *Camphene, d-limonene and 1,2,4-trimethylbenzene do not have defined IDLH concentrations, so 5% of their saturated vapor pressure was used instead.
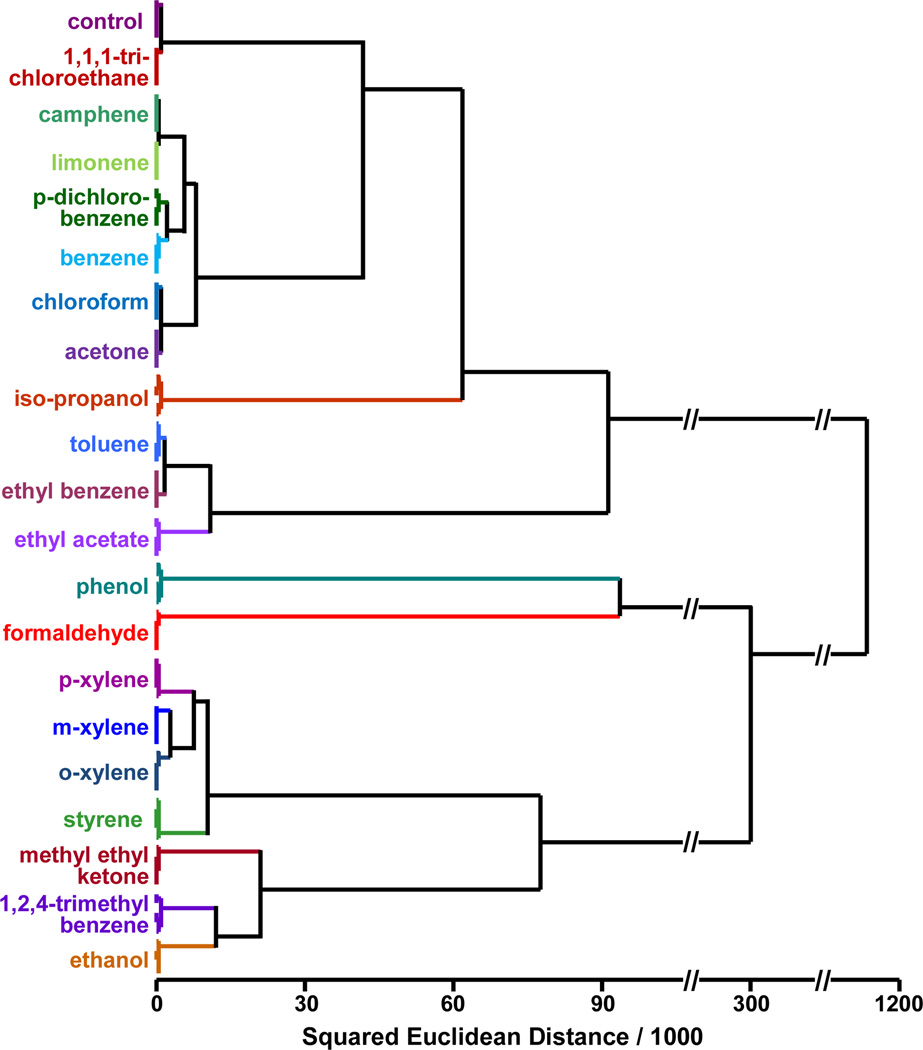
For quantitative comparisons of the color changes of the array, we can define a 108-dimensional vector (i.e, 36 changes in RGB values) for each trial, which can be compared and classified by standard chemometric techniques. We prefer the use of hierarchical cluster analysis (HCA), which is a model-free method based on the grouping of the analyte vectors according to their spatial distances in their full vector space.19 As shown in Figure 3, in quintuplicate trials, all 20 VOCs and a control were accurately classified with no errors out of 105 trials. We also collected array responses to the 20 VOCs after 5 min exposure at their PELs, as shown in SI Figure S2. Even at the PELs, HCA (SI Figure S3) indicates that all 20 VOCs and a control can be accurately identified with no error or misclassification out of 105 cases.
Figure 3.
Hierarchical cluster analysis for 20 commonly found VOCs at their IDLH concentrations and a control. All experiments were run in quintuplicate with 30 mg chromic acid on silica as the pre-oxidation reagent; no confusions or errors in classification were observed in 105 trials.
The ability of this array to discriminate among many analytes is due, in part, to the high dimensionality of the data. To probe the dimensionality of this data, we did a principal component analysis (PCA, which generates optimized linear combinations of the original 108 dimensions so as to maximize the amount of variance in as few dimensions as possible): based on the 105 trials of 20 VOCs and a control, the PCA results show that the array requires 13 dimensions to capture 90% of the total variance and 23 dimensions for 95% (see SI, Figure S4). This extremely high dispersion reflects the wide range of chemical-property space being probed by our choice of chemically responsive pigments.
To quantify the improvement in sensitivity provided by our pre-oxidation technique, we determined limits of detection (LODs) for these 20 VOCs both with and without pre-oxidation using our previously described sensor array (cf. SI for experimental details).14a We have defined a conservative LOD for our array response as the VOC concentration needed to give three times the S/N (signal to noise) vs. background for the sum of the three largest responses among the 108 color changes. The array response to the 20 VOCs in the absence of pre-oxidation is not very strong (with the exception of phenol, which is acidic); the color difference maps at their IDLH concentrations with a 5 min exposure without pre-oxidation are compared to those with pre-oxidation in SI Figure S5. For array data collected with the use of pre-oxidation, LODs were extrapolated from the 5 min response at PEL. The extrapolated LODs in the absence and presence of pre-oxidation are provided in Table 1. The ratios of LODs without pre-oxidation vs. with pre-oxidation, also listed in Table 1, indicate an average sensitivity enhancement using pre-oxidation of more than 300-fold. Indeed, on average the LODs were just 1.4% of the PEL. In comparison, limits of recognition (LOR) will be higher, but it is important to realize that LORs are library dependent, unlike LODs, which are absolute.
The color changes of the array are dependent upon the concentration of each vapor, which provides an easy method for semi-quantitative analysis of VOC concentration. For example, by combining the data sets of the 20 VOCs at IDLH and PEL, we can clearly differentiate the array responses to the same analytes at different concentrations (with the exception of ethanol, where the extremely high concentrations required for the IDLH and PEL of ethanol saturate the array response). Cluster analysis for the full set of IDLH and PEL databases can be seen in SI Figure S6. For any given VOC, semi-quantitative interpolation of its concentration can be easily accomplished using the total Euclidean distance (i.e., square root of the sum of the squares of the color differences) of the VOC compared to a set of known concentrations in the library.
In real world situations, changes in humidity are highly problematic for prior electronic nose technologies. The sol-gel formulations used in our optoelectronic nose are essentially impervious to changes in relative humidity. Using 50% RH as a control, arrays were exposed to various humidity concentrations without any additional analyte; no significant response to humidity was observed from 10% to 90% RH, either with or without the pre-oxidation tube (SI Figure S7). The effect of humidity on the array response to VOCs with peroxidation was also evaluated. One example, o-xylene under different relative humidity, is shown in SI Figure S8, compared to other closely related aromatic hydrocarbons (i.e., BTEX components) all at their IDLH concentrations. As shown, changes in relative humidity do not lead to confusion between o-xylene and even m- or p-xylenes. Thus, changes in humidity do not generally affect the response of our optoelectronic nose to analytes, even at low analyte concentrations.
Finally, we investigated the reproducibility and shelf life of the pre-oxidation reagent (i.e., chromic acid on silica) for VOCs detection. Because we are detecting the partial oxidation products of the primary VOCs, one might have legitimate concerns as to the reproducibility of the resulting oxidized mixture. Fortunately, we find excellent reproducibility from batch to batch of the pre-oxidation reagent, as shown in SI Figure S9: three separately preparing batches of chromic acid on silica gave nearly identical results in separate tests of toluene and p-xylene (done in triplicate), which were also easily differentiated from benzene, ethylbenzene, o-xylene, and m-xylene (all at their IDLH). In addition, we observed very little change in the array response as the pre-oxidation reagent ages over a two month period (SI Figure S10).
In conclusion, we have developed a pre-oxidation technique for substantially improved colorimetric sensor array detection of VOCs. Twenty commonly found VOCs in indoor air can be identified at both their IDLH and PEL concentrations. Classification analysis reveals that this array with the pre-oxidation tube has an extremely high dimensionality with the consequent ability to discriminate among a large number of VOCs over a wide range of concentrations. The comparison of the limits of detection of the array to VOCs with and without pre-oxidation indicates an average sensitivity enhancement of ~300-fold, with LODs that are only 1.4% of the PEL. Such high sensitivity is particularly relevant to future epidemiological studies of the health effects of long-term low-level exposures. While the laboratory studies reported here made use of inexpensive flat-bed scanners for imaging, we have a prototype handheld array reader under development,14a and we are working on a wearable device to provide rapid, inexpensive, and highly sensitive personal monitoring of VOC vapors.
Supplementary Material
ACKNOWLEDGMENT
We thank Dr. Sung Lim (iSense Medical Corp.) for many useful discussions. This work was supported through the NIH Genes, Environment and Health Initiative through award U01ES016011.
Footnotes
Supporting Information: Experimental details, Figures S1–S10 and Table S1 and S2. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Bloeman HJ, Th., Burn J. Chemistry and Analysis of Volatile Organic Compounds in the Environment. Glasgow, UK: Blackie Academic & Professional, An Imprint of Chapman &Hall; 1993. [Google Scholar]
- 2.(a) Caprino L, Tonga G. Environ. Health Perspect. 1998;106:115–125. doi: 10.1289/ehp.98106115. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Molhave L. Indoor Air. 1991;1:357–376. [Google Scholar]
- 3.USEPA: Health effects notebook for hazardous air pollutants. [accessed Jan. 2011]; www.epa.gov/ttnatw01/hlthef/hapindex.html.
- 4.Wallace LA. Annu. Rev. Energ. Environ. 2001;26:269–301. [Google Scholar]
- 5.Salthammer T, Bahadir M. Clean-Soil Air Water. 2009;37:417–435. [Google Scholar]
- 6.(a) Hübschmann H-J. Handbook of GC/MS: Fundamentals and Applications. Weinheim: Wiley-VCH; 2008. [Google Scholar]; (b) Eiceman GA, Gardea-Torresdey J, Dorman F, Overton E, Bhushan A, Dharmasena HP. Analytical Chemistry. 2006;78:3985–3996. doi: 10.1021/ac060638e. [DOI] [PubMed] [Google Scholar]
- 7.Janata J. Principles of Chemical Sensors. 2nd Ed. New York: Springer; 2009. [Google Scholar]
- 8.(a) Kida T, Seo M-H, Kishi S, Kanmura Y, Yamazoe N, Shimanoe K. Anal. Chem. 2010;82:3315–3319. doi: 10.1021/ac100123u. [DOI] [PubMed] [Google Scholar]; (b) Kida T, Harano H, Minami T, Kishi S, Morinaga N, Yamazoe N, Shimanoe K. J. Phys. Chem. C. 2010;114:15141–15148. [Google Scholar]; (c) Elosua C, Matias IR, Bariain C, Arregui FJ. Sensors. 2006;6:1440–1465. [Google Scholar]; (d) Tamaki J. Sens. Lett. 2005;3:89–98. [Google Scholar]
- 9.(a) Truax SB, Demirci KS, Beardslee LA, Luzinova Y, Hierlemann A, Mizaikoff B, Brand O. Anal. Chem. 2011;83:3305–3311. doi: 10.1021/ac1029902. [DOI] [PubMed] [Google Scholar]; (b) Samain F, Ghosh S, Teo YN, Kool ET. Angew. Chem. Int. Ed. 2010;49:7025–7029. doi: 10.1002/anie.201002701. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Samain F, Dai N, Kool ET. Chem. Eur. J. 2011;17:174–183. doi: 10.1002/chem.201002836. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Chen KJ, Lu CJ. Talanta. 2010;81:1670–1675. doi: 10.1016/j.talanta.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 10.Gardner JW, Bartlett PN. Electronic Noses: Principles and Applications. New York: Oxford University Press; 1999. [Google Scholar]
- 11.(a) Borisov SM, Wolfbeis OS. Chem. Rev. 2008;108:423–461. doi: 10.1021/cr068105t. [DOI] [PubMed] [Google Scholar]; (b) Röck F, Barsan N, Weimar U. Chem. Rev. 2008;108:705–725. doi: 10.1021/cr068121q. [DOI] [PubMed] [Google Scholar]; (c) Hierlemann A, Gutierrez-Osuna R. Chem. Rev. 2008;108:563–613. doi: 10.1021/cr068116m. [DOI] [PubMed] [Google Scholar]; (d) Walt DR, Aernecke MJ, Guo J, Sonkusale S. Anal. Chem. 2009;81:5281–5290. doi: 10.1021/ac900505p. [DOI] [PubMed] [Google Scholar]; (e) Wilson AD, Baietto M. Sensors. 2009;9:5099–5148. doi: 10.3390/s90705099. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Meier DC, Raman B, Semancik S. Annu. Rev. Anal. Chem. 2009;2:463–484. doi: 10.1146/annurev-anchem-060908-155127. [DOI] [PubMed] [Google Scholar]; (g) Berna A. Sensors. 2010;10:3882–3910. doi: 10.3390/s100403882. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Stich MIJ, Fischer LH, Wolfbeis OS. Chem. Soc. Rev. 2010;39:3102–3114. doi: 10.1039/b909635n. [DOI] [PubMed] [Google Scholar]
- 12.(a) Harper WJ. In: Headspace Analysis of Foods and Flavors: Theory and Practice. Rouseff RL, Cadwallader KR, editors. Vol. 488. New York: Kluwer Academic / Plenum Publ; 2001. pp. 59–71. [Google Scholar]; (b) Romain AC, Nicolas J. Sens. Actuator B-Chem. 2010;146:502–506. [Google Scholar]; (c) Munoz RMR, Sivret EC, Parcsi G, Lebrero R, Wang XG, Suffet IH, Stuetz RM. Water Res. 2010;44:5129–5149. doi: 10.1016/j.watres.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 13.(a) Rakow NA, Suslick KS. Nature. 2000;406:710–713. doi: 10.1038/35021028. [DOI] [PubMed] [Google Scholar]; (b) Suslick KS, Bailey DP, Ingison CK, Janzen M, Kosal ME, McNamara WB, III, Rakow NA, Sen A, Weaver JJ, Wilson JB, Zhang C, Nakagaki S. Quimica Nova. 2007;30:677–681. [Google Scholar]
- 14.(a) Lim SH, Feng L, Kemling JW, Musto CJ, Suslick KS. Nature Chem. 2009;1:562–567. doi: 10.1038/nchem.360. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Feng L, Musto CJ, Kemling JW, Lim SH, Suslick KS. Chem. Commun. 2010;46:2037–2039. doi: 10.1039/b926848k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Feng L, Musto CJ, Suslick KS. J. Am. Chem. Soc. 2010;132:4046–4047. doi: 10.1021/ja910366p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Feng L, Musto CJ, Kemling JW, Lim SH, Zhong W, Suslick KS. Anal. Chem. 2010;82:9433–9440. doi: 10.1021/ac1020886. [DOI] [PubMed] [Google Scholar]; (e) Lin H-W, Suslick KS. J. Am. Chem. Soc. 2010;132:15519–15521. doi: 10.1021/ja107419t. [DOI] [PubMed] [Google Scholar]; (f) Janzen MC, Ponder JB, Bailey DP, Ingison CK, Suslick KS. Anal. Chem. 2006;78:3591–3600. doi: 10.1021/ac052111s. [DOI] [PubMed] [Google Scholar]
- 15.(a) Zhang C, Suslick KS. J. Am. Chem. Soc. 2005;127:11548–11549. doi: 10.1021/ja052606z. [DOI] [PubMed] [Google Scholar]; (b) Musto CJ, Lim SH, Suslick KS. Anal. Chem. 2009;81:6526–6533. doi: 10.1021/ac901019g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(a) Baldini F, Chester AN, Homola J, Martellucci S. Optical Chemical Sensors. Erice, Italy: Springer; 2006. [Google Scholar]; (b) Jeronimo PCA, Araujo AN, Montenegro M. Talanta. 2007;72:13–27. doi: 10.1016/j.talanta.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 17.Lim SH, Kemling JW, Feng L, Suslick KS. The Analyst. 2009;134:2453–2457. doi: 10.1039/b916571a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Brown SK, Sim MR, Abramson MJ, Gray CN. Indoor Air. 1994;4:123–134. [Google Scholar]; (b) Girman JR, Hadwen GE, Burton LE, Womble SE, McCarthy JF. Proc. 8th Intl. Conf. Indoor Air Quality & Climate; Edinburgh. 1999. pp. 460–465. [Google Scholar]
- 19.(a) Johnson RA, Wichern DW. Applied Multivariate Statistical Analysis. 6th ed. Upper Saddle River, NJ: Prentice Hall; 2007. [Google Scholar]; (b) Hair JF, Black B, Babin B, Anderson RE, Tatham RL. Multivariate Data Analysis. 6th ed. Upper Saddle River, NJ: Prentice Hall; 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.