Abstract
Background & Aims
Hepatitis C virus (HCV) infection affects 3% of the world population and is the leading cause of chronic liver disease worldwide. Current standard of care is effective in only 50% of the patients, poorly tolerated, and associated with significant side effects and viral resistance. Recently, our group and others demonstrated that the HCV lifecycle is critically dependent on host lipid metabolism and that its production is metabolically modulated.
Methods
The JFH1/Huh7.5.1 full lifecycle model of HCV was used to study the antiviral effects of naringenin on viral replication, assembly, and production. Activation of PPARα was elucidated using GAL4-PPARα fusion reporters, PPRE reporters, qRT-PCR, and metabolic studies. Metabolic results were confirmed in primary human hepatocytes.
Results
We demonstrate that the grapefruit flavonoid naringenin dose-dependently inhibits HCV production without affecting intracellular levels of the viral RNA or protein. We show that naringenin blocks the assembly of intracellular infectious viral particles, upstream of viral egress. This antiviral effect is mediated in part by the activation of PPARα, leading to a decrease in VLDL production without causing hepatic lipid accumulation in Huh7.5.1 cells and primary human hepatocytes. Long-term treatment with naringenin leads to a rapid 1.4 log reduction in HCV, similar to 1000 U of interferon. During the washout period, HCV levels returned to normal, consistent with our proposed mechanism of action.
Conclusions
The data demonstrates that naringenin is a non-toxic assembly inhibitor of HCV and that other PPARα agonists play a similar role in blocking viral production. The combination of naringenin with STAT-C agents could potentially bring a rapid reduction in HCV levels during the early treatment phase, an outcome associated with sustained virological response.
Keywords: HCV, Naringenin, Lipid metabolism, PPARα
Introduction
Hepatitis C virus (HCV) infection is a global public health concern, affecting an estimated 170 million individuals [1]. HCV infection develops into a chronic condition in over 70% of patients and is the leading cause of chronic liver disease world-wide. A simulation of US infections predicts nearly 200,000 deaths associated with HCV infection and direct medical expenditures in excess of $10 billion [2,3]. Current standards of care consist of peg-interferon-α (IFNα) and ribavirin, although this regimen is effective in only 50% of cases. In addition, this antiviral treatment is poorly tolerated and is associated with significant side effects and acquired resistance [4]. The recent release of HCV specific antivirals, such as Teleprevir (Vertex) and Boce-previr (Merck) offers to increase clearance rates to 70–80%, although incidents of HCV resistance are emerging. Therefore, there is a pressing need for the development of complementary and alternative treatment strategies to combat HCV infection, possibly through the targeting of host pathways on which the virus relies.
Recent work suggests a critical role for fatty acid and cholesterol metabolism in the HCV lifecycle. While the interaction between HCV infection and lipid metabolism initially received attention due to the development of steatosis [5], the lack of an efficient cell culture model of HCV limited research in the field. The development of a JFH1/Huh7.5 full lifecycle model of HCV infection enabled several groups to show that HCV replication is inhibited by statins [6,7]. This was shown to be in part due to the viral requirement for the geranylgeranylation of FBL2, a host factor which binds NS5A facilitating viral replication [6,8]. The replication of HCV on ER-associated lipid droplets has also been extensively characterized [9]. More recently, Gastaminza et al. demonstrated the existence of high density intracellular HCV precursors suggesting the virus binds to low density particles prior to egress [10]. Using a similar system, Huang et al. demonstrated that HCV assembled in vesicles enriched in ApoB and ApoE, structural proteins of very low-density lipoprotein (VLDL); and in microsomal triglyceride transfer protein (MTP), the rate-limiting enzyme in VLDL assembly [11]. Concomitantly, our group demonstrated that HCV is actively secreted while bound to VLDL [12,13], and that its production can be metabolically modulated by the addition of insulin and fatty acids. A comprehensive study of HCV effect on host metabolism was recently carried out [14].
As a consequence, modulators of hepatic lipid metabolism originally developed for atherosclerosis, could potentially interfere with HCV. Importantly, targeting a host pathway on which HCV relies, rather than a viral protein, may prove to be less susceptible to the emergence of resistant viral strains [15,16]. Despite the promise of such an approach, pilot in vivo trials using atorvastatin or bezafibrate failed to show significant effects on viral titer [17,18]. One compound that may prove efficient against HCV is the grapefruit flavonoid naringenin. Naringenin is a dietary supplement demonstrated to possess anti-oxidant, anti-inflammatory, and anti-carcinogenic properties both in vitro and in vivo [19]. A recent clinical trial in hypercholesterolemic patients demonstrated that naringenin’s precursor, naringin, significantly lowered plasma LDL levels [20]. Similar cholesterol-lowering effects of naringenin were demonstrated in rabbits [21,22] and rats [23]. More recently, Huff and coworkers have shown that naringenin helps correct metabolic disturbances associated with diabetes in LDL receptor (LDLR) deficient mice [24]. These effects have been attributed to MTP inhibition and thus potentially cause lipid accumulation and liver toxicity [25,26]. Interestingly, naringenin has also been shown to inhibit HMGR, the rate-limiting enzyme in cholesterol synthesis, while activating enzymes important in fatty acid oxidation such as acyl-CoA oxidase (ACOX) and CYP450 4A1 [27], suggesting a transcriptional regulation of lipid metabolism, possibly on the nuclear receptor level.
In this work, we demonstrate that naringenin dose-dependently inhibits the secretion of ApoB and HCV particles, without affecting intracellular levels of viral RNA or protein. We show that naringenin prevents the accumulation of intracellular infectious particles, suggesting that the flavonoid blocks the assembly of infectious HCV particles. Importantly, PPAR inhibitor GW9662 reverses naringenin effects. We further show that in chronically infected cells, naringenin induces PPARα, causing a decrease in triglyceride secretion without leading to hepatic lipid accumulation. Finally, we demonstrate that long-term treatment with naringenin leads to a rapid 1.4 log reduction in secreted HCV in cell culture and that this effect is reversible. Together, the data suggests that naringenin block the assembly of infectious HCV particles in cell culture and supports further investigation of naringenin in the management and care of HCV infection.
Materials and methods
Reagents
Lipoprotein-free FBS was purchased from Biomedical Technologies (Stoughton, MA). Naringenin, WY14, 643, GW9662, and Brefeldin A (BFA) were purchased from Sigma–Aldrich Chemicals (St. Louis, MO). Ciglitazone was purchased from Cayman Chemical (Ann Arbor, MI). All other chemicals were purchased from Invitrogen Life Technologies (Carlsbad, CA) unless otherwise noted. BMS-200150, a small molecule inhibitor of MTP was provided by Pablo Gastaminza and Francis Chisari.
Cell culture and viruses
The Huh7.5.1 human hepatoma cell line and a plasmid containing the JFH-1 genome were kindly provided by Dr. Chisari (Scripps Research Institute, La Jolla, CA) and Dr. Wakita (National Institute of Infectious Diseases, Tokyo, Japan), respectively. Huh7.5.1 cells were cultured in DMEM supplemented with 10% FBS, 200 U/ml penicillin, and streptomycin in a 5% CO2-humidified incubator at 37 °C. In vitro transcribed genomic JFH-1 RNA was delivered to cells by liposome-mediated transfection [28]. Subsequent rounds of infection were carried out using viral stocks produced by the cells. Infected Huh7.5.1 cells were passaged every 3 days and used at passage <15.
Hepatocyte isolation and culture
Primary human hepatocytes were obtained from BD Biosciences (San Jose, CA) or were kindly provided by Dr. Stephen C. Strom, University of Pittsburgh. Cells were plated on collagen-coated dishes at a density of 100,000 cells/cm2 and cultured as previously described [29].
Cellular viability
Viability of primary human hepatocytes was quantified as previously described [30]. Briefly, medium samples were collected daily, frozen at −20 °C, and analyzed in bulk using Thermo Fisher Scientific (Waltham, MA) aspartate aminotransferase (AST) Infinity reagent. Values were normalized to the total amount of AST determined by total cell lysis [30]. Cell viability for all conditions was greater than 90%.
HCV Secretion
HCV-infected Huh7.5.1 cells were plated on a 6-well plate at a density of 2 × 105 cells/cm2 and cultured overnight in the standard medium. Prior to the beginning of the experiment, the cells were washed 3 times with PBS and cultured with DMEM containing 5% lipoprotein-free FBS. Naringenin was added at this time as described in the text. Following 24 h of incubation, the plate was gently agitated to release mechanically bound particles, and the medium was collected, filtered to remove cellular debris, and stored at −80 °C for further analysis. The attached cells were washed 3 times with PBS, harvested, pelleted, and stored at −80 °C for further analysis.
Intracellular and extracellular HCV infectivity
The infectivity of HCV particles was measured as previously described [12]. Briefly, naïve Huh7.5.1 cells were grown to 80% confluence and exposed to cell culture supernatants serially diluted 10-fold in the culture medium. Following 1 h of incubation at 37 °C, the medium was replaced, and cells were cultured for 3 additional days. Levels of HCV infection were determined by immunofluorescence staining for HCV core protein. The viral titer is expressed as focus forming units (FFU) per microliter of supernatant. To evaluate infectivity of intracellular particles, cells were scraped into PBS and lysed by freeze–thawing as previously described [13], diluted and used to assess infectivity as above.
GAL4-PPAR activation assays
PPAR activation was examined as previously described using the HGLN5 PPARα and PPARγ cell line [31]. Briefly, HGLN5 cells were seeded at a density of 100,000 cells/cm2, test compounds were added 8 h later and incubated for 16 h. Following treatment, cells were washed with PBS and lysed in 25 mM Tris buffer (pH 7.8). Protein concentration was calculated using the Bradford assay and used to normalize the luciferase activity. Finally, activation of PPARα and PPARγ reporters is presented as percent of maximal activation by the known agonists GW7647 and BRL49653, respectively.
PPRE activation in JFH1-infected Huh7.5.1 cells
The pAOx(X2)luc plasmid which contains two tandem repeats of the AOX PPAR response element (PPRE) upstream of the firefly luciferase reporter gene [32] was a kind gift of Dr. John P. Capone (McMaster University, Canada). The pRL-TK plasmid containing the Renilla luciferase reporter under the control of a constitutive thymidine kinase promoter was purchased from Promega Corporation (Madison, WI). JFH1-infected Huh7.5.1 cells were transiently transfected with the pAOx(X2)luc and pRL-TK plasmids using Lipofectamine 2000 according to manufacturer’s directions. Following 24 h incubation with naringenin, cells were lysed and assayed using a Dual-Luciferase Reporter Assay System (Promega, Madison, WI) according to the manufacturer’s instructions. All measurements were done in triplicate and data are shown as values normalized to Renilla luciferase internal controls.
Human ApoB enzyme-linked immunosorbent assay (ELISA)
Huh7.5.1-secreted ApoB-100 was detected in the medium with the ALerCHEK, Inc. (Portland, ME), total human ApoB-100 ELISA kit. The medium was diluted 1:10 with the specimen diluent and the assay was carried out according to the manufacturer’s directions.
HCV core ELISA
Huh7.5.1-secreted HCV core antigen was detected in the medium with the Wako Chemicals (Richmond, VA) ORTHO HCV antigen ELISA kit. The medium was used as is and the assay was carried out according to the manufacturer’s directions.
MTP activity assay
MTP activity was measured using a commercial kit (Roar Biomedical, New York, NY). After 24 h stimulation of Huh7.5.1, cells were scraped into PBS on ice, centrifuged at 600g for 3 min and resuspended in buffer supplemented with protease inhibitor cocktail (Thermo Scientific). Cell suspensions were sonicated on ice. 100 µg cell lysates were combined with 10 µl of donor and acceptor particles in 220 µl assay buffer, and incubated at 37 °C. Increase in fluorescence was measured using SpectraMAX Gemini plate reader with excitation and emission of 465/538 nm. MTP activity was normalized to sample protein content.
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
Virus samples were purified using a QIAamp Viral RNA Mini Kit (Qiagen, Valencia, CA). The reverse-transcription reaction step was performed on a Mastercycler epgradientS (Eppendorf) instrument using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Real-time PCR was performed on a MyiQ Real-Time PCR Detection System using iScript One-Step RT-PCR Kit with SYBR Green (Bio-Rad, Hercules, CA), according to the manufacturers’ instructions. HCV detection primers used in qRT-PCR were Forward 5′-GGGAAGACTGGGTCCTTTCTTGGAT-3′ and Reverse 5′-CGACGGTTGGTGTTTCTT TTGGTTT-3′ (Integrated DNA Technologies, Coralville, IA) [12].
Lipid measurements
Intracellular triglycerides were quantified in primary hepatocyte cell extracts using a commercial kit (Sigma Chemical, St. Louis, MO) based on enzymatic hydrolysis of triglycerides by lipase to glycerol. Measurements were normalized to total protein content measured by the Bradford assay.
Long-term treatments
JFH1-infected Huh7.5.1 cells were grown over a course of 7 days in OptiMEM culture medium. Media was collected every 24 h and stored at −80 °C for analysis. On days 1–4 (‘‘treatment’’), media contained 200 µM naringenin, 1000 U/ml IFNα, or DMSO. On days 5–7 (‘‘washout’’), all wells were cultured in standard media without treatment. Primary human hepatocytes were similarly cultured for 9 days with media collected every 24 h and stored at −80 °C for analysis. Primary cells were treated days 1–7 with 200 µM naringenin, followed by days 8–9 washout period.
Statistics
Data are expressed as the mean ± standard deviation. Unless noted differently, all experiments were carried out in triplicates. Statistical significance was determined by a one-tailed Student’s t test. A p value of 0.05 was used for statistical significance.
Results
Naringenin inhibits the production of ApoB and HCV
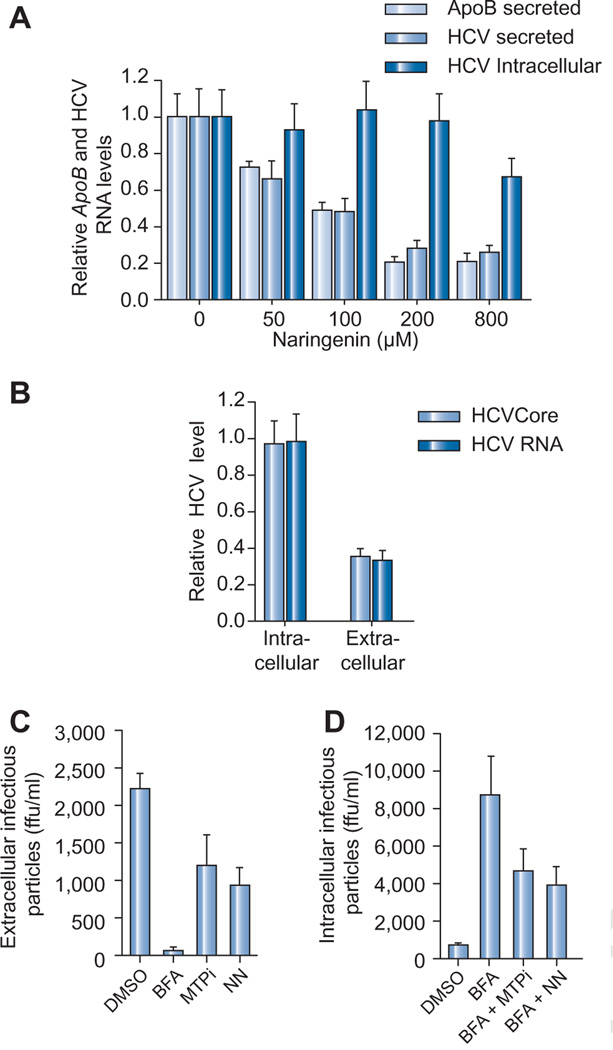
Recently, our group and others demonstrated that HCV production is dependent VLDL secretion, and that naringenin blocked HCV production in JFH1-infected Huh7.5.1 cells [12]. To further characterize naringenin’s antiviral activity we treated JFH1-infected Huh7.5.1 cells with increasing concentrations of naringenin for 24 h. Fig. 1A shows that naringenin led to a dose-dependent decrease in the secretion of ApoB and viral RNA, with an EC50 of 109 µM. Maximal inhibition of secretion of both ApoB and HCV RNA was 74 ± 4% at a concentration of 200 µM (p <0.001). The secretion of HCV core protein was similarly decreased by 64 ± 4% compared to untreated control (Fig. 1B). Naringenin causes a similar inhibition in ApoB secretion in primary human hepatocytes (Fig. 4A). Interestingly, no change in intracellular HCV RNA abundance was noted up to 200 µM (p = 0.43) suggesting the compound does not dramatically affect viral replication. Some decrease in intracellular HCV RNA was noted at a concentration of 800 µM. Fig. 1B shows changes in HCV RNA and core protein, both intracellular and extracellular levels, compared to untreated controls following 24 h stimulation. Treatment did not lead to an intracellular accumulation of viral core protein or viral RNA (Fig. 1B).
Fig. 1. Naringenin blocks the assembly of HCV-infectious particles.
(A) Naringenin dose-dependently inhibits ApoB-100 and HCV RNA secretion in JFH1-infected Huh7.5.1 cells. HCV RNA did not accumulate in cells. Maximal inhibition of HCV RNA secretion was 74 ± 4% at 200 µM naringenin at 24 h (p < 0.001). (B) 24 h of treatment with 200 µM naringenin decreased the secretion of virus into the media, but did not change the amount of viral RNA or core protein inside cells. Values are normalized to untreated controls. (C) Infected cells were treated for 5 h with 0.1 µg/ml BFA, an inhibitor of Golgi-dependent secretion, 10 µM of BMS-200150, a known MTP inhibitor (MTPi), or with 200 µM naringenin (NN). Infectivity of the secreted virus (extracellular) was quantified by colony titer. Extracellular infectivity was blocked by BFA treatment and inhibited by both BMS-200150 and naringenin, by 45% and 68%, respectively (p < 0.02). (D) To measure changes in the accumulation of intracellular infectious virus particles, the cells were lysed by freeze–thaw cycle and the virus-containing supernatant was used in an infectivity titer assay. BFA treatment led to accumulation of infectious virus in cells. Co-treatment of BFA with BMS-200150 blocked the accumulation of infectious virus in cells, leading to a 46% (p < 0.05) decrease in infectious particle accumulation. Naringenin had a similar effect to that of the MTP inhibitor, leading to a 55% (p < 0.05) decrease in infectious particle accumulation.
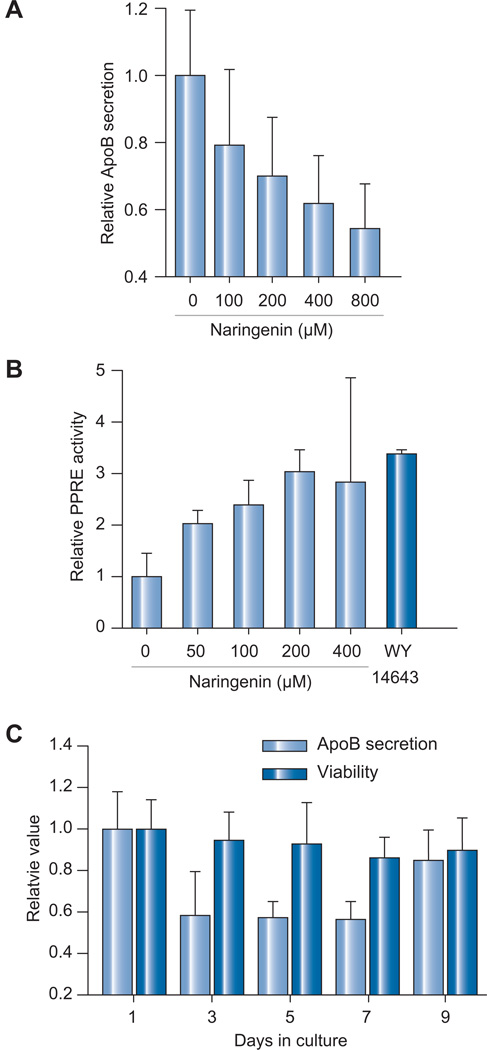
Fig. 4. Naringenin effects on primary human hepatocytes.
(A) Naringenin caused a dose-dependent inhibition in ApoB-100 secretion following 24 h of treatment of primary human hepatocytes. Cell viability was >95%. (B) Primary human hepatocytes transfected with PPRE reporter plasmid were treated with naringenin. Naringenin dose-dependently enhanced PPRE activity following 24 h of treatment by 3-fold following 200 and 400 µM naringenin stimulation, not significantly different than 10 µM WY14643 (p = 0.129). (C) Primary human hepatocytes were treated for 7 days with 200 µM naringenin followed by a 2 day washout period. ApoB secretion decreased by 43 ± 8% (p < 0.01) while cell viability remained unchanged (p = 0.137). During the washout period, ApoB secretion returned to 86 ± 15% of basal level (p = 0.174).
Naringenin blocks the assembly of HCV infectious particles
We next wished to determine whether naringenin blocks the assembly of HCV prior to viral egress. To test this hypothesis, cells were treated with 0.1 µg/ml brefeldin A (BFA), a toxin known to disrupt Golgi-dependent export [33]. BFA treatment was previously shown to cause the accumulation of intracellular infectious HCV particles in Huh7.5.1 infected cells [12,13]. Co-treatment of cells with BFA and the MTP-inhibitor, BMS-200150, blocks the accumulation of intracellular infectious HCV particles, suggesting MTP is required for HCV assembly [13]. As naringenin was previously shown to inhibit MTP activity, we explored if the compound similarly blocks HCV assembly.
JFH1-infected Huh7.5.1 cells were treated with 0.1 µg/ml BFA and co-treated for 5 h with 10 µM BMS-200150 or 200 µM naringenin. The production of extracellular infectious particles (Fig. 1C) was significantly reduced when cells were treated with either BMS-200150 or naringenin (45% and 68%, respectively, p <0.02) and abolished entirely when cells were treated with BFA (p <0.001). Expectedly, BFA treatment which blocked the production of extracellular infectious particles, led to the accumulation of infectious HCV particles within the cells (Fig. 1D). Co-treatment with BMS-200150 decreased the accumulation of infectious particles by 46% (p <0.05) compared to BFA-only controls. Importantly, co-treatment with naringenin decreased the accumulation of infectious particles by 55% (p <0.05) compared to BFA-only controls (Fig. 1D) suggesting the flavonoid inhibit the assembly of HCV Lipo-viral particles [34].
Naringenin inhibits MTP activity while inducing PPAR activity
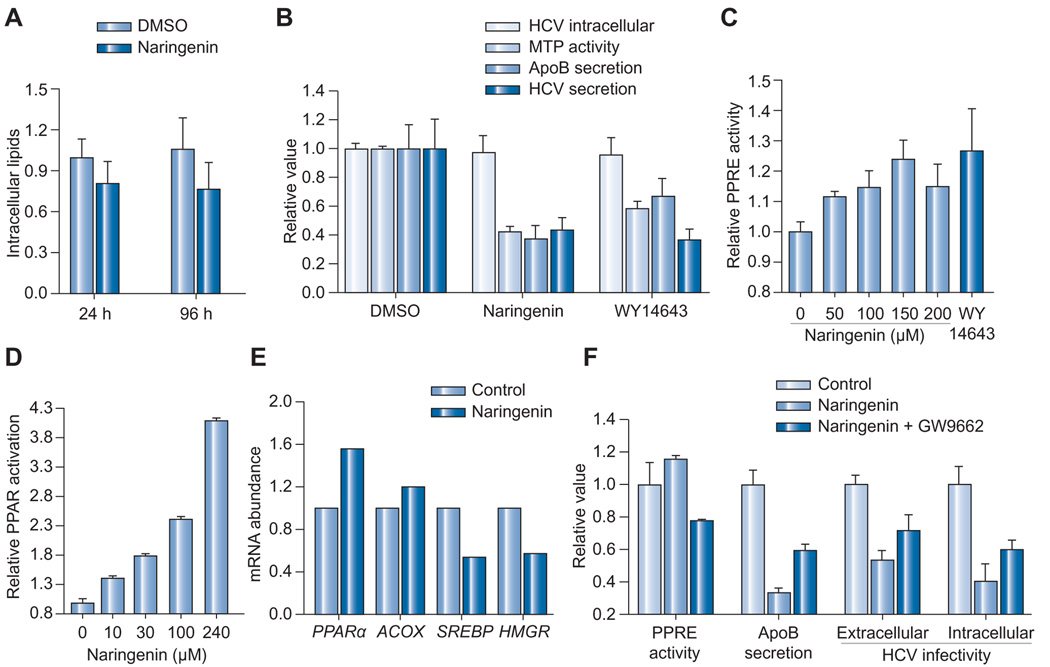
Naringenin has been previously shown to inhibit MTP, a critical enzyme in VLDL assembly, in HepG2 cells [25,26]. Previous reports have indicated that MTP inhibition can lead to lipid accumulation and steatosis. To test if naringenin leads to lipid accumulation, intracellular triglycerides were quantified in primary human hepatocytes following 24 or 96 h of treatment with 200 µM naringenin (Fig. 2A). Naringenin stimulation caused a decrease rather than an increase in intracellular triglyceride that was not judged to be statistically significant (p = 0.1 and 0.08, respectively).
Fig. 2. The activity of naringenin is mediated in part by PPARα.
(A) Continuous 24 or 96-h treatment with 200 µM naringenin does not cause triglyceride accumulation in primary human hepatocytes. (B) JFH1-infected Huh7.5.1 cells treated with 200 µM naringenin or 10 µM WY14643, a PPARα agonist, show a similar inhibition of MTP activity (p < 0.002), ApoB secretion (p < 0.02), and HCV RNA secretion (p < 0.012), without affecting intracellular levels of HCV core protein (p > 0.50). (C) Chronically infected Huh7.5.1 cells transfected with PPRE reporter plasmid were treated for 24 h with naringenin. Naringenin dose-dependently enhanced PPRE activity by up to 24 ± 7% (p = 0.015). Maximal induction was not different from WY14643 (p = 0.198). (D) HG5LN-PPARα reporter cells in which the PPARα LBD is fused to GAL4 were treated for 16 h with naringenin. Naringenin enhanced PPARα-LBD activity by 140 ± 5% (p < 0.001). (E) qRT-PCR analysis of JFH1-infected Huh7.5.1 cells treated with 200 µM naringenin for 24 h. Naringenin caused increased expression of PPARα and its target gene ACOX and a concomitant decrease in SREBP and its target HMGR. (F) 1 µM of PPAR antagonist GW9662 inhibited PPRE activity in the presence of 150 µM naringenin by 30% (p < 0.001) and increased ApoB secretion by 78% (p < 0.001). Extracellular HCV infectivity was increased by 34% (p < 0.05) while the accumulation of intracellular infectious HCV particles in the presence of 0.1 µg/ml BFA increased by 50% (p < 0.05).
Cellular lipid metabolism is controlled by nuclear receptors, members of a family of ligand-activated transcription factors [35]. Activation of PPARα specifically, has been shown to induce β-oxidation and a reduction in lipogenesis and VLDL secretion [36]. Such activation could explain the lack of lipid accumulation. To examine if naringenin induces PPAR activation we used the HG5LN-PPAR3b1 reporter cell line [31]. In this cell line, the PPARα ligand-binding domain (LBD) is fused to a GAL4 DNA binding domain. Activation of PPARα-LBD allows GAL4 to bind its UAS response element transcribing luciferase. Fig. 2D shows that naringenin treatment enhanced the activity of the PPARα-LBD by 4-folds (p <0.001).
To examine whether the activation PPARα-LBD in reporter cells correlates with PPRE activity in JFH1-infected Huh7.5.1 cells, we transfected cells with the pACOX(X2)luc reporter plasmid which contains two tandem repeats of the ACOX, PPAR response element (PPRE) upstream of a firefly luciferase reporter [32]. Renilla luciferase expression was used as control. As shown in Fig. 2C, naringenin led to a dose-dependent increase in PPRE activity, reaching 23 ± 7% (p = 0.015) compared to DMSO-treated control. At 200 µM naringenin, PPRE induction was not statistically different from 10 µM of classical PPARα agonist WY14643 (p = 0.198). Predictably, analysis of PPARα and ACOX mRNA abundance by qRT-PCR demonstrated a 60% and 20% increase, respectively (Fig. 2E). Finally, PPARα induction is known to inhibit cholesterol synthesis through SREBP and its target gene, HMGR. Fig. 2E shows that both transcripts are downregulated by naringenin.
To verify that PPARα activation could indeed reduce ApoB and virus production in our system, we compared the treatment of 200 µM naringenin with 10 µM of WY14643 in Huh7.5.1 cells. Fig. 2B shows that both naringenin andWY14643 caused a significant 58 ± 4% and 42 ± 5% inhibition of MTP activity (p <0.002), and 62 ± 9% and 33 ± 13% inhibition in ApoB secretion (p <0.02). Similarly, both naringenin and WY14643 caused a significant 57 ± 9% and 63 ± 7% inhibition in HCV RNA secretion (p <0.012), without affecting intracellular levels of HCV core protein at 97 ± 11% (p = 0.70) and 95 ± 12% (p = 0.57). Finally, the co-administration of naringenin with WY14643 did not increase PPRE activation (p = 0.921, n = 3) nor decrease APOB100 secretion (p = 0.644, n = 3), compared to naringenin treatment alone.
These results suggest that naringenin effect is mediated by PPARα activation. We proceeded to explore if naringenin effects are reversed by PPARα inhibition. Fig. 2F shows that 1 µM of PPAR antagonist GW9662 inhibited PPRE activation in the presence of 200 µM naringenin by 33% (p <0.001) and increased ApoB secretion by 78% (p <0.001). GW9662 also increased extracellular infectivity by 34% (p <0.05) while the accumulation of intracellular infectious viral particles increased by 50% (p <0.05). These results strongly suggest that naringenin’s effects are at least partially mediated by PPARα activation.
Long-term inhibition of HCV production
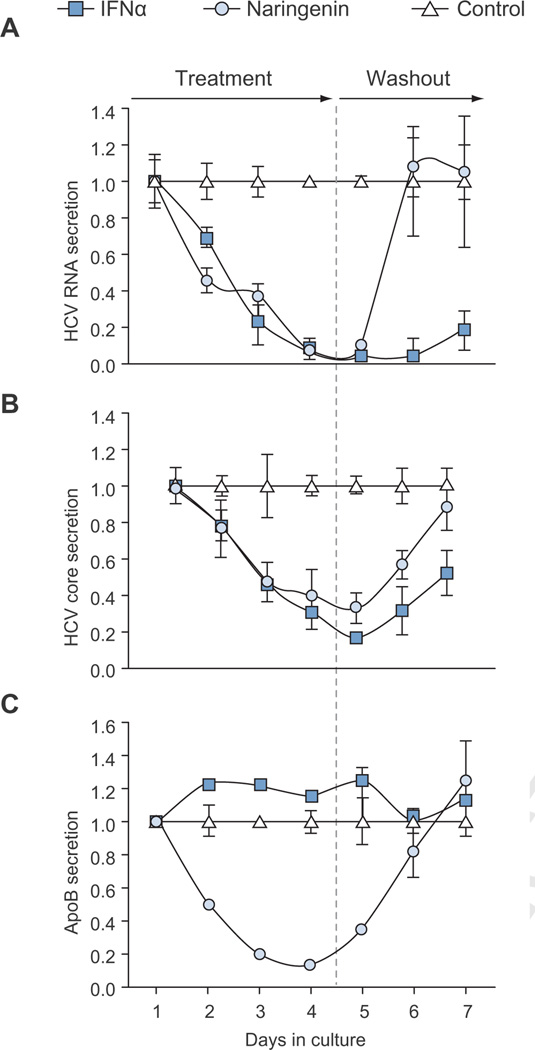
To explore the long-term effects of naringenin treatment on viral production we treated chronically infected Huh7.5.1 cells with 200 µM naringenin over a course of 4 days (treatment). As a positive control, cells were treated with 1000 i.u. of IFNα, a concentration previously shown to abolish viral production. On days 5–7, treatment was removed from all cells (washout).
As expected, our results showed that ApoB secretion was decreased by 86 ± 1% following naringenin treatment (p <0.01), but not by IFNα that showed a 16 ± 3% increase (Fig. 3). Importantly, naringenin treatment caused a rapid 1.4 log reduction in HCV RNA and a 67 ± 8% reduction in HCV core (p <0.05). This inhibition in HCV production was similar to IFNα, which showed a maximal 1.7 log reduction in HCV RNA and an 83% reduction in HCV Core (Fig. 3). Intracellular levels of HCV RNA remained unchanged through the course of naringenin treatment (Supplementary Fig. 1). Not surprisingly, during the washout period, HCV levels in naringenin-treated cells returned to normal suggesting that the compound did not affect viral replication.
Fig. 3. Naringenin causes a rapid inhibition of HCV production during long-term treatment.
Cells were treated daily for 4 days with 200 µM naringenin or 1000 i.u. IFNα. During the 3 days washout period, all wells were untreated. (A) Viral RNA release into media decreased during treatment, down to 4% with naringenin and 2% with IFNα compared to controls. During washout phase, naringenin-treated samples contained RNA levels similar to controls, suggesting viral replication was unchanged. (B) HCV core levels in media decreased during treatment for naringenin and IFNα. For naringenin-treated cells on days 4, core in the media was reduced to 33 ± 5%. Core accumulation was partly restored during the last day of washout in both naringenin and IFNα-treated samples 87 ± 12%, 52 ± 13%, respectively. (C) ApoB secretion declined during treatment with naringenin. On days 4, ApoB secretion in naringenin-treated cells decreased to 13 ± 1%.
Naringenin effects in primary human hepatocytes
The metabolic activity and gene expression of hepatoma cell lines, like Huh7.5.1, are significantly lower than those of primary hepatocytes. To test whether naringenin effects translate to a more relevant model of liver metabolism, we examined its activity in stabilized cultures of primary human hepatocytes as previously described [30]. Fig. 4A shows that naringenin caused a dose-dependent inhibition of ApoB secretion in primary human hepatocytes, reaching 38 ± 14% inhibition at 400 µM concentration. Similarly, Fig. 4B shows that PPRE activation increased by 3-fold following 200 and 400 µM naringenin stimulation, not significantly different than 10 µM WY14643 (p = 0.129). Finally, Fig. 4C demonstrates the long-term transient inhibition of ApoB secretion in primary human hepatocytes. Following 7 days of continuous treatment with 200 µM naringenin, ApoB secretion decreased by 43 ± 8% (p <0.01) while cell viability remained unchanged (p = 0.137). During the washout period, ApoB secretion returned to 86 ± 15% of basal level (p = 0.174). Intracellular triglyceride levels remained unchanged following 1 or 4 days of continuous treatment (Fig. 2A).
Discussion
HCV is a global public health problem affecting close to 3% of the world population. The current standard of care consists of peg-IFNα and ribavirin, effective in approximately half of the patients, and associated with the emergence of resistant strains [4,37]. While protease and polymerase inhibitors developed the context of STAT-C are showing remarkable potential, indications of resistant strains have already been reported in clinical trials [16]. A complimentary approach is to target a host pathway, such as VLDL assembly, on which the virus depends. HCV would require numerous mutations and a significant alteration of its lifecycle to escape, essentially becoming a different virus.
Targeting host metabolic pathways on which HCV relies could deprive the virus from critical resources needed to complete its lifecycle. Such strategy may prove to be less susceptible to the emergence of resistant viral strains [15,16]. Despite the promise of such an approach, pilot in vivo trials using atorvastatin or bezafibrate failed to show significant effects on viral titer [17,18]. The concentration of atorvastatin needed to effect viral replication was judged to be too close to its toxic dose, while 8 weeks of treatment with PPARα agonist bezafibrate lowered viral titer by only 30% [17,18]. It is hoped that another agent would be more successful is blocking the relevant pathways.
Our group has previously suggested that the grapefruit flavonoid naringenin could be effective in inhibiting HCV production, although its mechanism of action was unclear. At the time, naringenin was considered an MTP inhibitor, thus likely to cause lipid accumulation and liver toxicity [12]. Interestingly, the compound demonstrates anti-inflammatory and normolipidemic effects in vitro and in vivo [19]. The ability of naringenin to significantly reduce plasma cholesterol levels has been demonstrated in a recent clinical trial in hypercholesterolemic patients in which the compound lowered LDL levels by 17% [20]. Similar cholesterol lowering effects were demonstrated in rabbits [21,22] and rats [23]. Another appealing aspect of using naringenin, is that the compound has demonstrated anti-cancer properties, blocking in vivo replication of HepG2, Hep3B, and Huh7 cells [38]. As HCV dramatically increases the risk of liver cancer, inhibiting tumor formation is especially important.
In this study, we first showed that naringenin induced a dose-dependent inhibition of HCV RNA and core protein secretion, down to 28% of untreated controls (Fig. 1). The reduction in viral RNA and protein production correlated with inhibition of HCV infectivity. On the other hand, intracellular HCV RNA and protein levels showed no change in response to naringenin treatment, suggesting naringenin was inhibiting a step downstream of viral replication. Nonetheless, given the reduction in viral secretion, the lack of intracellular viral RNA and protein accumulation may suggest a concurrent inhibition of viral replication or enhanced viral degradation.
Assembly of infectious HCV particles has been previously demonstrated to be dependent on MTP activity, an enzyme critical to VLDL assembly. The accumulation of infectious viral particles in cells treated with BFA, a toxin that disrupts Golgi-mediated transport, is consistent with the observations of Chisari and coworkers [12,13]. Here we showed that naringenin, like MTP inhibitor BMS-200150, blocks the accumulation of infectious particles in BFA-treated cells up to 55% (p <0.05) suggesting that the flavonoid blocks the assembly of infectious viral particles. Consistent with this observation, we showed that naringenin inhibited MTP activity, consistent with prior observations [25]. In a recent clinical study, Cuchel and colleagues showed that while the MTP inhibitor BMS-201038 (AEGR-733) lowered LDL values it was associated with steatosis and elevated ALT levels, leading to an early study termination [39]. To test if naringenin leads to a similar lipid accumulation, intracellular triglycerides were quantified in primary human hepatocytes following 24 or 96 h of treatment with 200 µM naringenin. Remarkably, there was no lipid accumulation in naringenin treated cells.
The ability of naringenin to modulate the expression of genes important in fatty acid oxidation, led us to examine its effects on PPARα. We demonstrate that naringenin specifically activates the ligand-binding domain of PPARα (Fig. 2). Consistently, naringenin showed a dose-dependent increase in PPRE activity in Huh7.5.1 cells and primary human hepatocytes, similar to PPARα classical agonist WY16463. Gene expression studies also correlate with a PPARα-induced shift from cholesterol synthesis to fatty acid oxidation. The inhibition of HMGR could deprive the virus from associating with geranylgeranylated FBL-2 inhibiting its replication, potentially explaining the decrease in HCV RNA at high naringenin concentration (Fig. 1A). However, this does not appear to be naringenin’s main mechanism of action. Importantly, JFH1-infected Huh7.5.1 cells treated with WY16463, show a similar inhibition of MTP activity, ApoB and HCV RNA secretion to naringenin. Like naringenin, WY 16463, did not affect intracellular levels of HCV core protein (Fig. 2B). Finally, to demonstrate that naringenin effects are mediated by PPARα activation, we explored its activity in the presence of PPARα and PPARγ irreversible antagonist GW9662. We showed that in the presence of GW9662, naringenin effects were reversed with PPRE activity reduced, while ApoB secretion, HCV secretion, and HCV assembly increased by 78%, 34%, and 50% (Fig. 2F). These results strongly suggest that naringenin’s effects are at least partially mediated by PPARα activation. Interestingly, chronic HCV infection has long been associated with steatosis [40,41], possibly through the inhibition of PPARα by viral proteins [42,43]. One intriguing possibility is that PPARα activation by compounds such as naringenin could reduce steatosis and its effects by augmenting hepatic fatty acid oxidation.
Finally, we demonstrate that naringenin inhibits long-term virus production and that its effects are reversible both in chronically infected Huh7.5.1 cells and primary human hepatocytes. As expected, naringenin reduced ApoB secretion by 86%. This effect was not observed in IFNα-treated cells. When media was examined for viral core and RNA content, both naringenin and IFNα significantly reduced the secretion of HCV RNA by 1.4 and 1.7 log, respectively. Importantly, naringenin’s effect on viral secretion was reversible, as viral secretion rebounded during washout, consistent with our proposed mechanism of action. We note that the decrease in core protein secretion on day 5, was not statistically significant (p = 0.214), and considered to be an artifact of normalization.
Our results suggest that naringenin blocks the assembly of infectious HCV particles. The tight link between HCV and VLDL assembly, in which infectious virus takes the form of lipo-viral particles, suggests a new approach for combating the virus. We expect that targeting host metabolism rather than viral proteins will decrease the development of resistant strains. As HCV does not integrate into the host DNA and relies on continuous infection of fresh hepatocytes to persist, blocking its ability to produce infectious particles could dramatically affect its persistence. Moreover, application of naringenin or other PPARα agonists with STAT-C agents could bring a rapid reduction in HCV levels [44], especially during the early treatment phase. Such early rapid reduction in HCV levels has been associated with sustained virological response [4]. Our work strongly supports further investigation of naringenin in the management of HCV infections.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (K01DK080241) and the Harvard Clinical Nutrition Research Center (P30-DK040561). Resources were provided by European Research Council Starting Grant (TMIHCV 242699), the BioMEMS Resource Center (P41EB-002503), and the Alexander Silberman Institute of Life Sciences.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.jhep.2011.02.011.
References
- 1.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 2.Wong JB, McQuillan GM, McHutchison JG, Poynard T. Estimating future hepatitis C morbidity, mortality, and costs in the United States. Am J Public Health. 2000;90:1562–1569. doi: 10.2105/ajph.90.10.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stribling R, Sussman N, Vierling JM. Treatment of hepatitis C infection. Gastroenterol Clin North Am. 2006;35:463–486. doi: 10.1016/j.gtc.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diseases AAftSoL, diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serfaty L, Andreani T, Giral P, Carbonell N, Chazouilléres O, Poupon R. Hepatitis C virus induced hypobetalipoproteinemia: a possible mechanism for steatosis in chronic hepatitis C. J Hepatol. 2001;34:428–434. doi: 10.1016/s0168-8278(00)00036-2. [DOI] [PubMed] [Google Scholar]
- 6.Chisari Kapadia. Hepatitis C virus RNA replication is regulated by host geranylgeranylation and fatty acids. Proc Natl Acad Sci USA. 2005;102:2561–2566. doi: 10.1073/pnas.0409834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng Kim, Choe Lin, Kato Sakamoto, et al. A cell-based, high-throughput screen for small molecule regulators of hepatitis C virus replication. Gastroenterology. 2007;132:311–320. doi: 10.1053/j.gastro.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 8.Gale Wang, Huang Keller, Goldstein Brown, et al. Identification of FBL2 as a geranylgeranylated cellular protein required for hepatitis C virus RNA replication. Molecular Cell. 2005;18:425–434. doi: 10.1016/j.molcel.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Miyanari Y, Atsuzawa K, Usuda N, Watashi K, Hishiki T, Zayas M, et al. The lipid droplet is an important organelle for hepatitis C virus production. Nat Cell Biol. 2007;9:1089–1097. doi: 10.1038/ncb1631. [DOI] [PubMed] [Google Scholar]
- 10.Gastaminza Kapadia, Chisari F. Differential biophysical properties of infectious intracellular and secreted hepatitis C virus particles. J Virol. 2006;80:11074–11081. doi: 10.1128/JVI.01150-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Sun, Li Owen, Gale Chen, et al. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc Natl Acad Sci USA. 2007;104:5848–5853. doi: 10.1073/pnas.0700760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldwasser Nahmias, Casali Poll V, Wakita Chung, et al. Apolipoprotein B-dependent hepatitis C virus secretion is inhibited by the grapefruit flavonoid naringenin. Hepatology. 2008;47:1437–1445. doi: 10.1002/hep.22197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng Gastaminza, Zhong Wieland, Chisari Liao. Cellular determinants of hepatitis C virus assembly, maturation, degradation, and secretion. J Virol. 2008;82:2120–2129. doi: 10.1128/JVI.02053-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diamond DL, Syder AJ, Jacobs JM, Sorensen CM, Walters K-A, Proll SC, et al. Temporal proteome and lipidome profiles reveal hepatitis C virus-associated reprogramming of hepatocellular metabolism and bioenergetics. PLoS Pathogens. 2010;6 doi: 10.1371/journal.ppat.1000719. e1000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manns MP, Foster GR, Zeuzem S, Zoulim F. The way forward in HCV treatment – finding the right path. Nat Rev Drug Discov. 2007 doi: 10.1038/nrd2411. [DOI] [PubMed] [Google Scholar]
- 16.Kuntzen T, Timm J, Berical A, Lennon N, Berlin AM, Young SK, et al. Naturally occurring dominant resistance mutations to hepatitis C virus protease and polymerase inhibitors in treatment-naïve patients. Hepatology. 2008;48:1769–1778. doi: 10.1002/hep.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Leary JG, Chan JL, McMahon CM, Chung RT. Atorvastatin does not exhibit antiviral activity against HCV at conventional doses: a pilot clinical trial. Hepatology. 2007;45:895–898. doi: 10.1002/hep.21554. [DOI] [PubMed] [Google Scholar]
- 18.Fujita N, Kaito M, Kai M, Sugimoto R, Tanaka H, Horiike S, et al. Effects of bezafibrate in patients with chronic hepatitis C virus infection: combination with interferon and ribavirin. J Viral Hepatitis. 2006;13:441–448. doi: 10.1111/j.1365-2893.2005.00718.x. [DOI] [PubMed] [Google Scholar]
- 19.Borradaile Wilcox, Huff MW. Antiatherogenic properties of naringenin, a citrus flavonoid. Cardiovasc Drug Rev. 1999 [Google Scholar]
- 20.Jung UJ, Kim HJ, Lee JS, Lee MK, Kim HO, Park EJ, et al. Naringin supplementation lowers plasma lipids and enhances erythrocyte antioxidant enzyme activities in hypercholesterolemic subjects. Clin Nutr (Edinburgh, Scotland) 2003;22:561–568. doi: 10.1016/s0261-5614(03)00059-1. [DOI] [PubMed] [Google Scholar]
- 21.Kurowska EM, Borradaile, Spence JD. Hypocholesterolemic effects of dietary citrus juices in rabbits. Nutr Res. 2000 [Google Scholar]
- 22.Lee CH, Jeong TS, Choi YK, Hyun BH, Oh GT, Kim EH, et al. Anti-atherogenic effect of citrus flavonoids, naringin and naringenin, associated with hepatic ACAT and aortic VCAM-1 and MCP-1 in high cholesterol-fed rabbits. Biochem Biophys Res Commun. 2001;284:681–688. doi: 10.1006/bbrc.2001.5001. [DOI] [PubMed] [Google Scholar]
- 23.Kim SY, Kim HJ, Lee MK, Jeon SM, Do GM, Kwon EY, et al. Naringin time-dependently lowers hepatic cholesterol biosynthesis and plasma cholesterol in rats fed high-fat and high-cholesterol diet. J Med Food. 2007;9:582–586. doi: 10.1089/jmf.2006.9.582. [DOI] [PubMed] [Google Scholar]
- 24.Mulvihill EE, Allister EM, Sutherland BG, Telford DE, Sawyez CG, Edwards JY, et al. Naringenin prevents dyslipidemia, apoB overproduction and hyperinsulinemia in LDL-receptor null mice with diet-induced insulin resistance. Diabetes. 2009 doi: 10.2337/db09-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borradaile Wilcox, Dreu D, Huff MW. Secretion of hepatocyte apoB is inhibited by the flavonoids, naringenin and hesperetin, via reduced activity and expression of ACAT2 and MTP. J Lipid Res. 2001;42:725–734. [PubMed] [Google Scholar]
- 26.Borradaile Allister, Huff Edwards. Inhibition of microsomal triglyceride transfer protein expression and apolipoprotein B100 secretion by the citrus flavonoid naringenin and by insulin involves activation of the mitogen-activated protein kinase pathway in hepatocytes. Diabetes. 2005;54:1676–1683. doi: 10.2337/diabetes.54.6.1676. [DOI] [PubMed] [Google Scholar]
- 27.Huong DT, Takahashi Y, Ide T. Activity and mRNA levels of enzymes involved in hepatic fatty acid oxidation in mice fed citrus flavonoids. Nutrition (Burbank, Los Angeles County, Calif) 2006;22:546–552. doi: 10.1016/j.nut.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Gastaminza Zhong, Cheng Kapadia S, Kato T, Burton DR, et al. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci USA. 2005;102:9294–9299. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kidambi S, Yarmush R, Novik E, Chao PB, Yarmush ML, Nahmias Y. Oxygen-mediated enhancement of primary hepatocyte metabolism, functional polarization, gene expression, and drug clearance. PNAS. 2009;106:15714–15719. doi: 10.1073/pnas.0906820106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nahmias Y, Goldwasser J, Casali M, Poll DV, Wakita T, Chung RT, et al. Apolipoprotein B dependent Hepatitis C Virus Secretion is Inhibited by the Grapefruit Flavonoid Naringenin. Hepatology. 2008;47:1437–1445. doi: 10.1002/hep.22197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lemaire Seimandi, Perrin Pillon, Voegel Carlavan, et al. Differential responses of PPARalpha, PPARdelta, and PPARgamma reporter cell lines to selective PPAR synthetic ligands. Anal Biochem. 2005;344:8–15. doi: 10.1016/j.ab.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Miyata Marcus, Zhang Subramani, Rachubinski Capone. Diverse peroxisome proliferator-activated receptors bind to the peroxisome proliferator-responsive elements of the rat hydratase/dehydrogenase and fatty acyl-CoA oxidase genes but differentially induce expression. Proc Natl Acad Sci USA. 1993;90:5723–5727. doi: 10.1073/pnas.90.12.5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujiwara T, Oda K, Yokota S, Takatsuki A, Ikehara Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J Biol Chem. 1988;263:18545–18552. [PubMed] [Google Scholar]
- 34.André Komurian-Pradel F, Deforges S, Perret M, Berland JL, Sodoyer M, et al. Characterization of low- and very-low-density hepatitis C virus RNA-containing particles. J Virol. 2002;76:6919–6928. doi: 10.1128/JVI.76.14.6919-6928.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gustafsson Gronemeyer, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov. 2004;3:950–964. doi: 10.1038/nrd1551. [DOI] [PubMed] [Google Scholar]
- 36.Spann NJ, Kang S, Li AC, Chen AZ, Newberry EP, Davidson NO, et al. Coordinate transcriptional repression of liver fatty acid-binding protein and microsomal triglyceride transfer protein blocks hepatic very low density lipoprotein secretion without hepatosteatosis. J Biol Chem. 2006;281:33066–33077. doi: 10.1074/jbc.M607148200. [DOI] [PubMed] [Google Scholar]
- 37.Taylor DR, Shi ST, Lai MM. Hepatitis C virus and interferon resistance. Microbes Infect. 2000;2:1743–1756. doi: 10.1016/s1286-4579(00)01329-0. [DOI] [PubMed] [Google Scholar]
- 38.Kanno S-i, Tomizawa A, Hiura T, Osanai Y, Shouji A, Ujibe M, et al. Inhibitory effects of naringenin on tumor growth in human cancer cell lines and sarcoma S-180-Implanted Mice. Biol Pharm Bull. 2005;28:527–530. doi: 10.1248/bpb.28.527. [DOI] [PubMed] [Google Scholar]
- 39.Cuchel M, Bloedon LT, Szapary PO, Kolansky DM, Wolfe ML, Sarkis A, et al. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med. 2007;356:148–156. doi: 10.1056/NEJMoa061189. [DOI] [PubMed] [Google Scholar]
- 40.Pekow JR, Bhan AK, Zheng H, Chung RT. Hepatic steatosis is associated with increased frequency of hepatocellular carcinoma in patients with hepatitis C-related cirrhosis. Cancer. 2007 doi: 10.1002/cncr.22701. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 41.Guidotti LG, Chisari FV. Immunobiology and pathogenesis of viral hepatitis. Ann Rev Pathol: Mech of Dis. 2006;1:23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- 42.Cheng Y, Dharancy S, Malapel M, Desreumaux P. Hepatitis C virus infection down-regulates the expression of peroxisome proliferator-activated receptor alpha and carnitine palmitoyl acyl-CoA transferase 1A. World J Gastroenterol. 2005;11:7591–7596. doi: 10.3748/wjg.v11.i48.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dharancy S, Malapel M, Perlemuter G, Roskams T, Cheng Y, Dubuquoy L, et al. Impaired expression of the peroxisome proliferator-activated receptor alpha during hepatitis C virus infection. Gastroenterology. 2005;128:334–342. doi: 10.1053/j.gastro.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 44.Delang L, Paeshuyse J, Vliegen I, Leyssen P, Obeid S, Durantel D, et al. Statins potentiate the in vitro anti-hepatitis C virus activity of selective hepatitis C virus inhibitors and delay or prevent resistance development. Hepatology. 2009;50:6–16. doi: 10.1002/hep.22916. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.