Abstract
Production of reactive oxygen species (ROS) resulting from phagocytic NADPH oxidase (NOX2) activity has been reported to contribute to host defense against numerous microbial pathogens. In this study we explored the role of NOX2 production in experimental coccidioidomycosis, a human respiratory disease caused by a soil-borne fungal pathogen. Activated and non-activated macrophages isolated from either NOX2-/- knock-out or wild type (WT) mice showed comparable ROS production and killing efficiency in vitro when infected with parasitic cells of Coccidioides. Both mouse strains also revealed similar fungal burden in their lungs and spleen at 7 and 11 days after intranasal challenge with Coccidioides spores, although the NOX2-/- mice died earlier than the WT strain. Immunization of the NOX2-/- and WT mice with a live, attenuated vaccine strain of Coccidioides also resulted in comparable reduction of the fungal burden in both lungs and spleen. These combined results initially suggested that NOX2 activity and ROS production are not essential for protection against Coccidioides infection. However, the reduced survival of non-vaccinated NOX2-/- mice correlated with high, sustained numbers of lung-infiltrated neutrophils on days 7 and 11 postchallenge, an expansion of the regulatory T cell population in infected lungs in the knock-out mice, and elevated concentrations of pro-inflammatory cytokines and chemokines in lung homogenates compared to infected WT mice. Although NOX2-derived ROS appeared to be dispensable for both innate and acquired immunity to pulmonary Coccidioides infection, evidence is presented that NOX2 production plays a role in limiting pathogenic inflammation in this murine model of coccidioidomycosis.
Keywords: NADPH oxidase 2, ROS, NOX2-/- knock-out mice, Coccidioides, coccidioidomycosis
1. Introduction
Reactive oxygen species (ROS), such as superoxide anion (O2-), represent important constituents of the arsenal of chemical defenses produced by the mammalian host to combat microbial pathogens [1,2]. Phagocytic leukocytes generate ROS by activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX2), which consists of two membrane-associated components (gp91phox and p22phox) and four cytosolic regulatory subunits (p40phox, p47phox, p67phox, and Rac2), where phox represents phagocyte oxidase [3]. Rac2 is a member of a family of GTPases involved in regulating the activation of protein kinases. Assembly of the NADPH complex following phosphorylation of the cytosolic subunits is essential for respiratory burst oxidase activity in neutrophils and macrophages. Chronic granulomatous disease (CGD) is an inherited condition in which a deficiency of NOX2 results in the inability of phagocytes to generate microbicidal superoxide anion and its metabolites. Patients with CGD have recurrent, life-threatening bacterial and fungal infections as well as chronic inflammatory diseases due to dysregulated inflammatory pathways [4]. Introduction of a mutation in the gp91phox gene in mice has been shown to result in loss of function of the 91 kilodalton membrane glycoprotein and inability of phagocytes to generate superoxide by the respiratory burst oxidase complex [5]. This was further shown to result in a significant increase in susceptibility to microbial infection [6] and onset of chronic granulomatous disease [7]. Loss of NOX2 activity in this murine model of CGD has been shown to be associated with exceptional vulnerability to experimental infection with Mycobacterium tuberculosis [8], Acinetobacter baumanii [9], Chlamydia trachomatis [10], Candida albicans [11], and Aspergillus fumigatus [12]. Coccidioidomycosis, also referred to as San Joaquin Valley fever, is a systemic fungal disease typically contracted by inhalation of airborne spores of Coccidioides immitis or C. posadasii. In spite the genetic diversity revealed by comparative genomic sequence analyses of the two species, laboratory studies have shown no significant difference between their virulence in mice [13]. A peculiarity of this fungal pathogen is that the mature parasitic cells (spherules) are too large to be engulfed by phagocytes (typically >100 μm diam.), which poses a potential problem for host defense. Coccidioidomycosis is endemic to regions of Southwestern United States, Northern Mexico, and certain areas of Central and South America [14]. It is estimated that more than 100,000 new infections of Coccidioides occur annually in the United States alone, but the majority of these are asymptomatic or result in self-limited pneumonia. On the other hand, approximately 40% of infected individuals develop symptomatic disease which can progress to involve extrapulmonary organs and become life-threatening [15]. Vaccine immunity against coccidioidomycosis has been reported to be primarily mediated by CD4+ and CD8+ T cell activation [16] with concomitant stimulation of T helper (Th)1, Th2 and Th17 signal pathways [17].
Macrophages are believed to be the pivotal effector cells in the host innate response to infection. The general paradigm for granulomatous diseases applies to coccidioidomycosis: activated T-lymphocytes secrete cytokines, which activate macrophages, inducing the formation of a granuloma that kills or contains the organism [18]. However, the nature of the interactions between macrophages and Coccidioides is still not well defined. We recently demonstrated that primary macrophages isolated from genetically-engineered mice lacking inducible nitric oxide synthase (iNOS) activity and nitric oxide (NO) production revealed phagocytic and fungicidal activities which were comparable to macrophages isolated from wild type mice. We concluded that the observed in vitro fungicidal response of host phagocytes was not dependent on NO production [19]. Although phagocyte secretion of ROS has been implicated in host defense against certain fungal infections [4, 11], a recent study has shown that NOX2 is dispensable in murine defense against Coccidioides [20]. In this study, we have further confirmed that NOX2 activity is not essential for macrophage killing of Coccidioides parasitic cells, but plays a potentially important role in limiting pathogenic inflammation of infected lung tissue.
2. Results
2.1. Primary macrophages isolated from WT and NOX2-/- mice showed comparable phagocytic and fungicidal activities against spherule initials in vitro
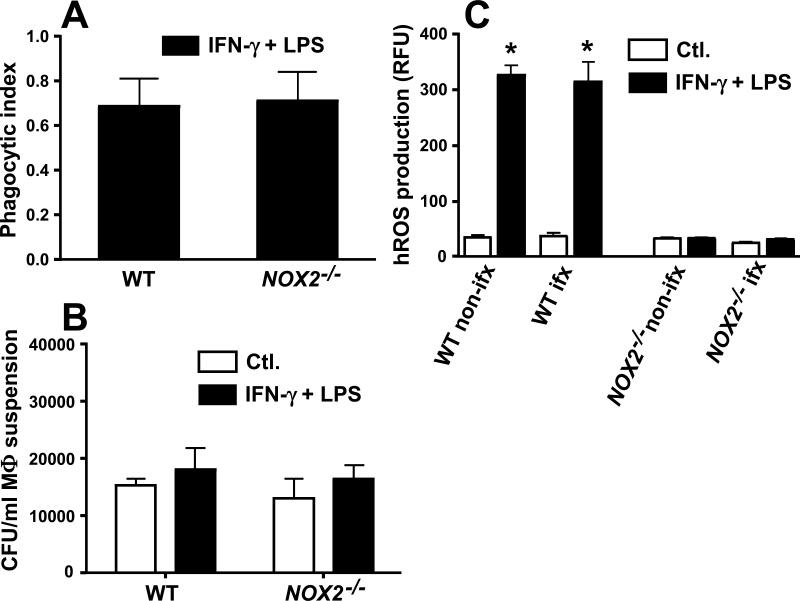
Primary macrophages isolated from peritoneal fluids of WT or NOX2-/- mice were incubated for 3 h in the presence of live spherule initials of the virulent strain of C. posadasii. In each case the macrophages were either activated with IFN-γ plus LPS or untreated (controls). A near-identical phagocytic index was recorded for both activated and non-activated peritoneal macrophages isolated from the two mouse strains (Fig. 1A; data for non-activated macrophages not shown). Intracellular killing was evaluated in co-cultures of the same host and fungal cells after incubation for 12 h. The residual numbers of viable fungal cells (CFUs) engulfed by activated or non-activated macrophages derived from the WT and NOX2-/- mouse strains showed no statistically significant difference when results of three separate experiments were compared (Fig. 1B). These data suggest that macrophage NOX2 activity in vitro is not essential for intracellular killing of Coccidioides spherule initials.
Fig. 1.
Phagocytosis, killing efficiency, and production of highly reactive oxygen species (hROS) by primary peritoneal macrophages isolated from WT mice versus NOX2-/- mice infected with spherule initials of C. posadasii. (A) Phagocytic indices were determined for IFN-γ+LPS-activated macrophages isolated from WT mice (open bars) versus NOX2-/- mice (black bars) and co-cultured with spherule initials using a MOI of 2:1 (macrophages:fungal cells). No statistically significant difference was determined. (B) Fungicidal assays were conducted using monolayers of non-activated, control (Ctl.) macrophages (open bars) versus activated macrophages (black bars) derived from WT or NOX2-/- mice. Estimates of killing efficiency by macrophages were based upon recovered numbers of viable spherule initials which had interacted with (adhered to or engulfed by) the phagocytes. No statistically significant difference was evident between CFUs. (C) hROS production by non-activated (open bars) versus activated macophages (black bars) isolated from non-infected or Coccidioides-infected WT or NOX2-/-mouse strains was measured using a fluorescent probe (APF) and expressed as relative fluorescence units (RFU). A statistically significant difference between hROS production by activated, non-infected or infected WT macrophages versus non-activated, non-infected or infected WT macrophages (*p< 0.001), and between activated WT macrophages versus NOX2-/--derived macrophages (*p<0.0001) were determined. Error bars represent standard errors of the mean (SEM) for three independent determinations. Non-ifx., non-infected macrophages; ifx, infected macrophages.
2.2. ROS production by activated peritoneal macrophages was not altered by Coccidioides infection
To test whether levels of macrophages production of highly reactive oxygen species were altered upon Coccidioides infection, relative amounts of hROS detected in co-cultures of host and fungal cells were measured after 12 h of incubation (Fig. 1C). Activated WT macrophages produced significantly higher and comparable concentrations of hROS in the presence or absence of spherule initials compared to non-activated macrophages. The basal levels of hROS produced by non-activated NOX2-/- macrophages were comparable to that of IFN-γ + LPS-activated NOX2-/- macrophages, and showed no difference in hROS production in the presence or absence of spherule initials. These results indicate that Coccidioides spherule initials do not induce ROS production by primary murine macrophages even when the phagocytes are activated prior to exposure to the live fungal cells.
2.3. NOX2-/- mice challenged intranasally with Coccidioides died earlier than WT mice but showed comparable fungal burden
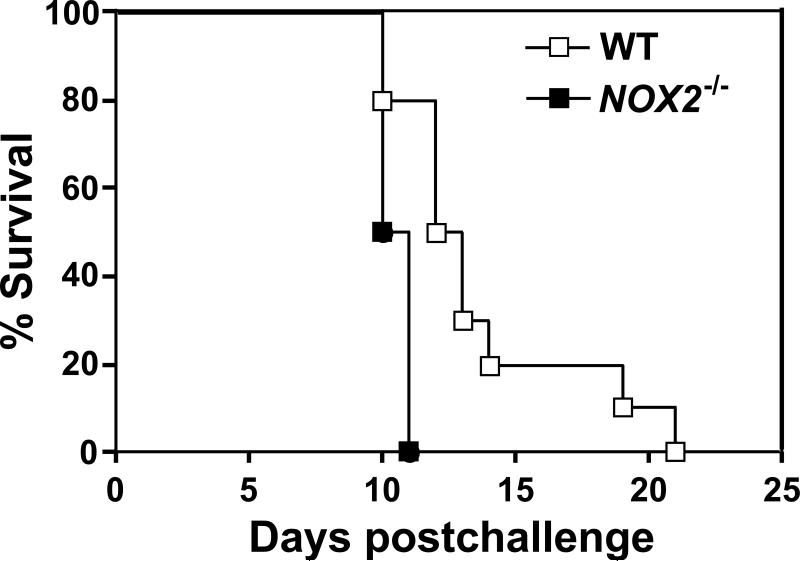
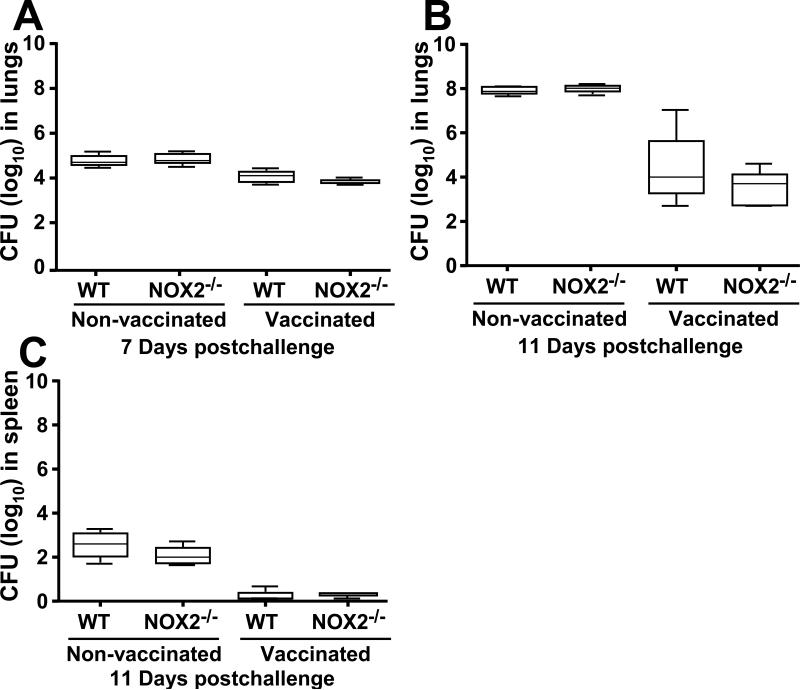
To assess the effect of phagocytic NOX2 activity on the outcome of pulmonary coccidioidomycosis, we compared the survival plots of vaccinated or nonvaccinated WT and NOX2-/- mice challenged intranasally with a potentially lethal inoculum of C. posadasii spores. Vaccinated WT and NOX2-/- mice showed 100% survival after 25 days postchallenge (data not shown). The mean survival times for the non-vaccinated WT and NOX2-/- mice was 12.5 and 10.5 days, respectively (Fig. 2), which was determined to be a statistically significant difference (p= 0.0012). On the other hand, comparison of the fungal burden between lungs of separate, non-vaccinated groups of WT and NOX2-/- mice, or vaccinated groups of the same two mouse strains after 7 and 11 postchallenge revealed no significant difference (Fig. 3A,B). Viable fungal cells were not detected in the spleen at 7 days postchallenge, but comparable CFUs in spleen homogenates were recorded between non-vaccinated WT and NOX2-/- mice, and between vaccinated mice of the same two strains at 11 days after intranasal challenge (Fig. 3C). NOX2 activity appeared to have no effect on the fungal burden, irrespective of whether the mice were vaccinated or non-vaccinated.
Fig. 2.
Survival plots for C57BL/6 wild-type (WT; n = 10) and NOX2-deficient mice (NOX2-/-; n = 10) after i.n. challenge with 80 viable spores of C. posadasii. These data are representative of three independent experiments. A statistically significant difference between the survival plots of the two groups of Coccidioides-infected mice was determined (p= 0.0012)
Fig. 3.
Comparison of fungal burden in the lungs (A, B) and spleen (C) of wild-type and NOX2-/- mice at days 7 and 11 postchallenge. These studies were conducted using non-vaccinated and vaccinated mice. The median CFUs (log10) are indicated by horizontal lines inside each box plot. No statistically significant differences were observed between the fungal burden in lungs and spleen of non-vaccinated WT and NOX2-/- mice at days 7 or 11 postchallenge. Both vaccinated WT and NOX2-/- mice showed reduced fungal burden in their lungs and spleen compared to the non-vaccinated mice, but no significant difference in CFUs was evident between these two mouse strains. The results are representative of two independent experiments.
2.4. Absence of NOX2 resulted in increased numbers of pulmonary leukocytes after intranasal challenge with Coccidioides spores compared to WT mice
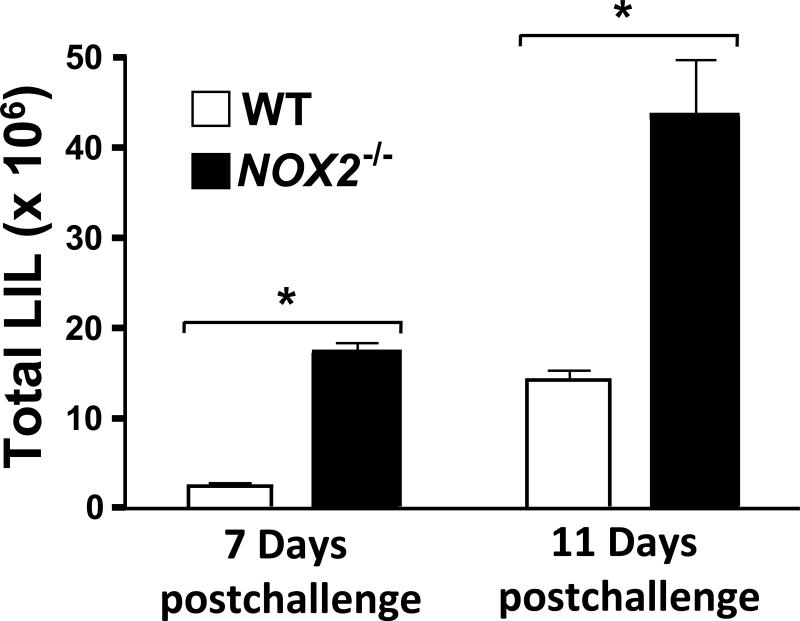
Leukocyte recruitment into the lungs of Coccidioides-infected WT and NOX2-/- mice was compared by flow cytometry. An increase in total number of lung-infiltrated leukocytes (LIL; CD45+ cells) was observed in NOX2-/- mice compared to WT mice at both 7 and 11 days postchallenge (Fig.4). The actual numbers of LIL detected in the lungs of NOX2-/- and WT mice at day 7 were 17.4 ± 0.93 × 106 vs 2.46 ± 0.21 × 106 (p< 0.05) and at day 11 postchallenge were 43.65 ± 6.0 × 106 vs 14.27 ± 1.13 × 106 cells (p< 0.001), respectively.
Fig. 4.
Total number of lung-infiltrated leukocytes (LIL) in non-vaccinated, C. posadasii-infected wild-type (WT; open bars) and NOX2-/- mice (black bars; n=5 for each group) at 7 and 11 days postchallenge. Cytometric analyses of fluorescent anti-CD45 mAb-labeled cells were performed to determine total LIL numbers within pulmonary cell suspensions of each infected mouse strain. The total LIL was significantly higher at 11 days compared to 7 days postchallenge (p< 0.002). Total LIL of NOX2-/- compared to WT mice were significantly higher at both 7 days (*p< 0.05) and 11 days postchallenge (*p< 0.001). Error bars represent standard errors of the mean (SEM) for three independent determinations.
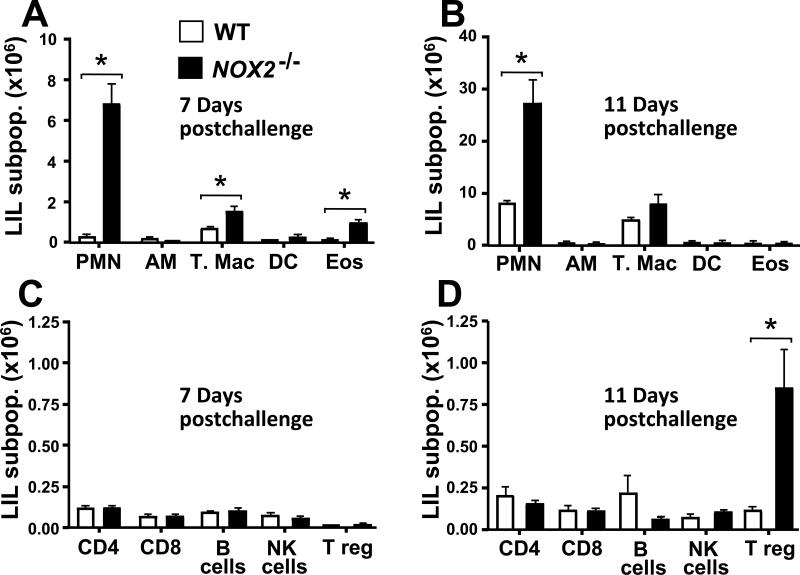
2.5. Increased infiltration of PMNs and expansion of the CD4+CD25+FoxP3+ Treg population were observed in lungs of Coccidioides-infected NOX2-/- mice compared to WT mice
We further characterized the subpopulations of leukocytes that had infiltrated the lungs of Coccidioides-infected WT and NOX2-/- mice on days 7 and 11 postchallenge by flow cytofluorometry. A striking difference and sustained high numbers of neutrophils (PMNs) were observed in the lungs of NOX2-/- mice compared to their wild type counterpart (Fig. 5A,B). The absolute numbers of PMNs in NOX2-/- compared to WT mice were 6.78 ± 1.02 versus 0.25 ± 0.04 (p< 0.001) and 27.0 ± 4.69 versus 8.0 ± 0.45 (p< 0.001) at day 7 and 11 postchallenge, respectively (all values ×106). Significantly higher numbers of tissue macrophages (T. Mac) and eosinophils (EOS) were also observed in NOX2-/- compared to WT mice at 7 days after challenge. The numbers of T. Mac in NOX2-/- versus WT mice on day 7 were 1.51 ± 0.22 versus 0.67 ± 0.06 (p< 0.01), while the EOS counts in the two mouse strains were 0.45 ± 0.12 versus 0.11 ± 0.01 (p< 0.01), respectively (Fig. 5A; all values ×106). Although the numbers of T. Mac were significantly higher on day 11 than on day 7 in both the WT mice (4.84 ± 0.56 × 106) and the NOX2-/- mice (7.98 ± 1.91 × 106), the difference in T. mac counts between the two groups of infected mice at the later time point was not statistically significant (Fig. 5A,B). A dramatic increase in the number of CD4+CD25+FoxP3+ Treg cells in Coccidioides-infected lungs of NOX2-/- mice was observed on day 11 compared to day 7 postchallenge (Fig. 5C,D). WT mice showed a slight increase in Treg counts over this same 4-day period, but significantly fewer cells were detected compared to the NOX2-/- mice. The number of Treg cells in NOX2-/- compared to WT mice was 0.84 ± 0.24 ×106 versus 0.11 ± 0.02 ×106), respectively (p< 0.001). The absolute numbers of alveolar macrophages (AM), dendritic cells (DC), CD4+, CD8+, total B cells, and natural killer (NK) cells in WT and NOX2-/- mice showed no significant difference between the two mouse strains at either 7 or 11 days postchallenge.
Fig. 5.
Absence of NOX2 activity in infected NOX-/- mice resulted in elevated numbers of certain subpopulations (sub-pop.) of lung-infiltrated leukocytes compared to infected WT mice. Determination of absolute numbers of selected granulocytes (i.e., polymorphonuclear neutrophils [PMN], alveolar macrophages [AM], tissue macrophages [T. Mac.], dendritic cells [DC], and eosinophils [Eos]) in pulmonary cell suspensions isolated from WT and NOX2-/- mice at days 7 (A) and 11 (B) postchallenge (n = 5 per time point) were conducted by flow cytofluorometry. Absolute numbers of subpopulation of selected lymphocytes (i.e., CD4+ T cells, CD8+ T cells , B cells, natural killer [NK] cells, and regulatory T cells [Treg]) at day 7 (C) and 11 (D) postchallenge were determined by the same methodology. Statistically significant differences between absolute numbers of PMNs and T. Mac at 7 and 11 days postchallenge (*p< 0.01), Eos at 7 days (*p< 0.05), and Treg at 11days (*p<0.001) between infected WT mice (white bars) versus NOX2-/- mice (black bars) were determined. Error bars represent standard errors of the mean (SEM) for three independent determinations.
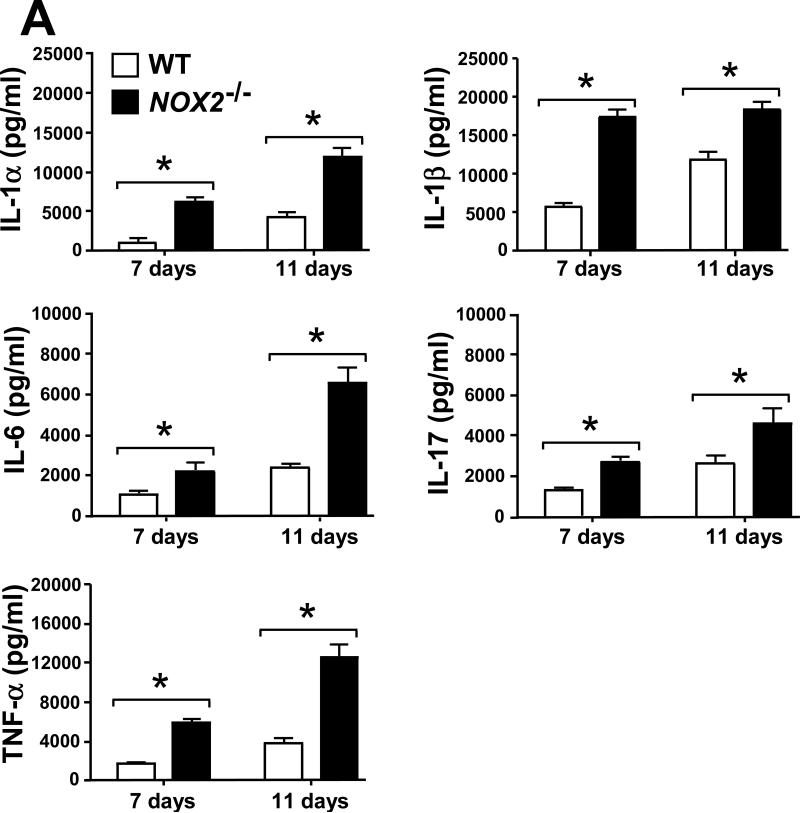
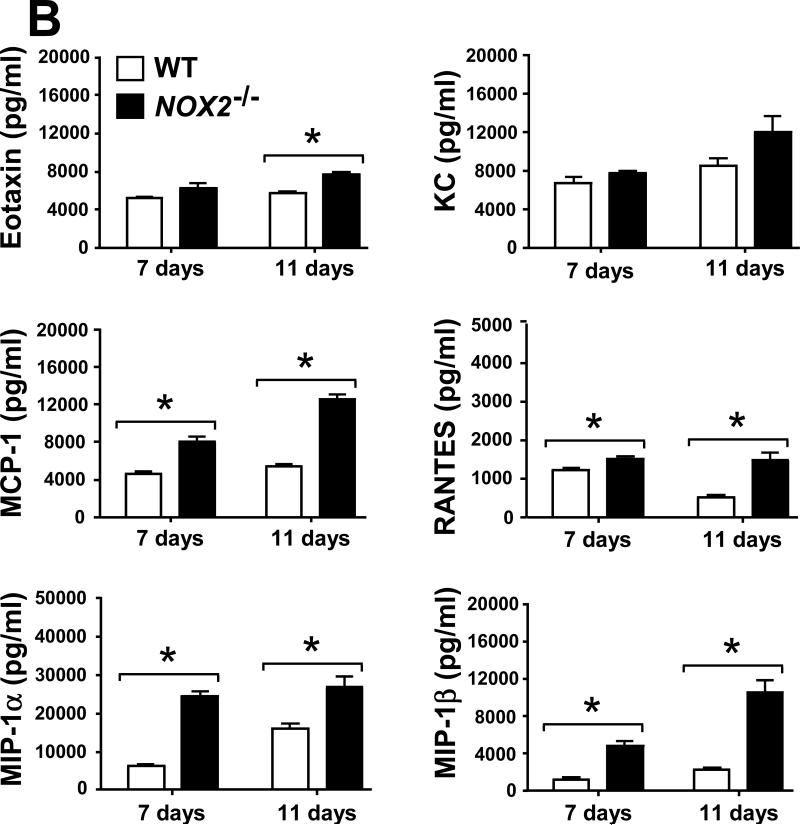
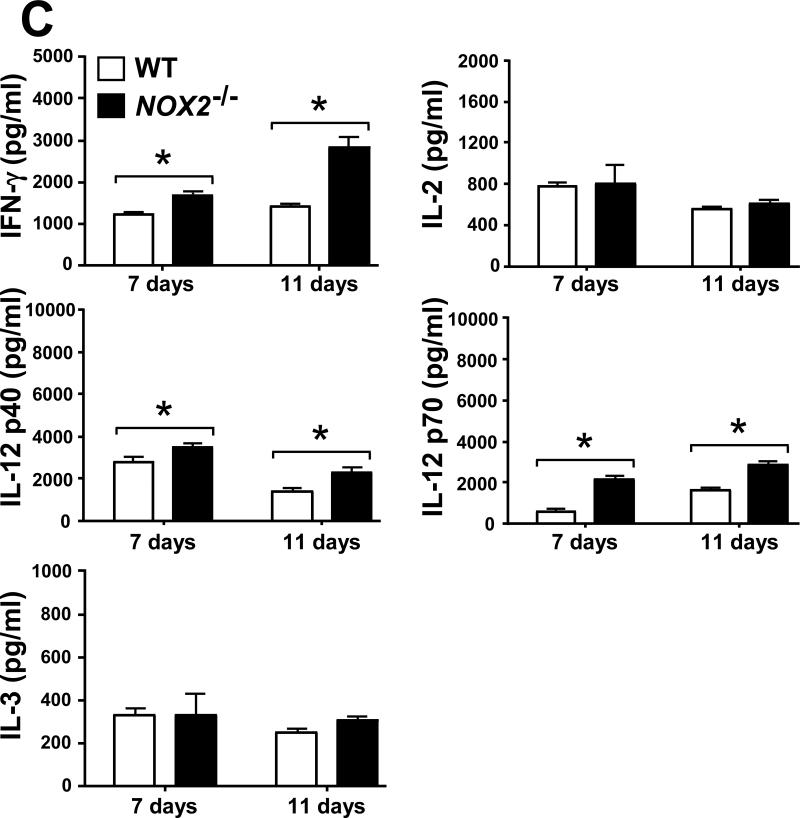
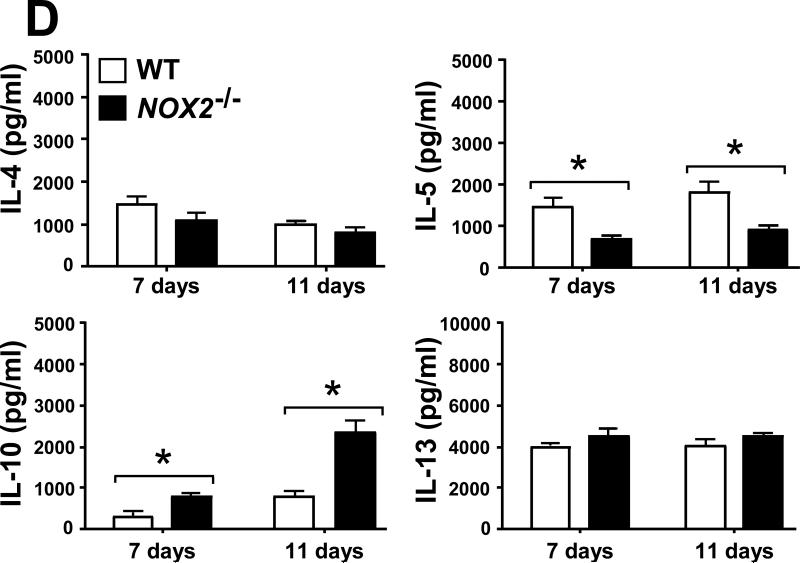
2.6. Selected leukocyte infiltration into lungs of Coccidioides-infected NOX2-/- mice correlates with an increase in levels of inflammatory cytokines and chemokines
Evidence has been presented that suggests a balance between pro-inflammatory and anti-inflammatory signals is essential for successful host protection against fungal pathogens [17, 21]. We measured the concentrations of pro-inflammatory cytokines (IL-1α, IL-1β, IL-6, IL-17 and TNF-α) and chemokines (Eotaxin, KC, MCP-1, RANTES, MIP-1α and MIP-1β), as well as cytokines associated with Th1 (IFN-γ, IL-2, IL-12p40, IL-12p70 and IL-3) and Th2 (IL-4, IL-5, IL-10 and IL-13) signal pathways in homogenates of lungs excised from Coccidioides-infected WT and NOX2-/- mice during progressive stages of infection. On days 7 and 11 postchallenge, Coccidoides-infected NOX2-/- mice showed significantly higher and sustained levels of all the above pro-inflammatory cytokines and most of the chemokines in their lungs compared to the WT mice (Fig. 6A,B). An exception was the KC chemokine, which is associated with PMN recruitment and revealed similar concentrations in the two mouse strains. At both 7 and 11 days postchallenge, IFN-γ, IL-12p40 and IL-12p70 were detected at higher concentrations in the lungs of NOX2-/- compared to WT mice. On the other hand, measured amounts of IL-2 and IL-3 in lung homogenates of the two groups of mice were similar (Fig. 6C). Significantly higher concentrations of IL-10 were detected in the lungs of NOX2-/- compared to WT mice, with an approx. 2.5-fold increase between days 7 and 11 postchallenge (Fig. 6D). Levels of IL-5 production were significantly higher in the infected lungs of WT mice at days 7 and 11 postchallenge, while concentrations of the other Th2-type cytokines (IL-4 and IL-13) were comparable in the two strains of mice. These data suggest that absence of NOX2 activity is associated with an overall increase in pulmonary production of pro-inflammatory cytokines, chemokines, and Th1/Th2 signals during the early phase of lung infection with Coccidioides.
Fig. 6.
Absence of NOX2 activity resulted in statistically significant increases on concentrations of selected pro-inflammatory cytokines (A), chemokines (B), and Th1 (C) and Th2 (D) cytokines in lung homogenates of infected NOX2-/- mice compared to WT mice at 7 and 11 postchallenge (*p< 0.01). Error bars represent standard errors of the mean (SEM) for two independent determinations.
3. Discussion
The terms reactive oxygen species (ROS) and reactive oxygen intermediates (ROI) are sometimes used interchangeably. However, ROI refer to successive 1-electron reduction products of O2 in a reaction pathway that leads to the synthesis of water molecules, while ROS include ROI plus ozone (O3) and singlet oxygen (1O2) species [22]. Both ROS and ROI are known to play diverse roles in inflammation, host defense, and homeostasis. Reactive nitrogen intermediates (RNI) belong to a family of antimicrobial molecules derived from nitric oxide (NO) and are produced via the enzymatic activity of inducible nitric oxide synthase (iNOS) [23]. Intracellular concentrations of certain RNI, including NO, nitrogen dioxide (NO2), nitrite (NO2-) and peroxynitrite (ONOO-) are known to influence ROI levels [22]. Whether this interaction between production pathways of reactive oxygen and reactive nitrogen intermediates influences cytokine/chemokine production and host inflammatory cell response to microbial infection is not yet resolved. We have previously reported that parasitic cells (spherule initials) of Coccidioides are capable of in vitro suppression of the production of NO by activated, primary, murine macrophages [19]. The undefined, secreted fungal product responsible for this suppression was shown to be heat labile. To our surprise, however, macrophages obtained from iNOS gene-deficient mice revealed phagocytic activity and killing efficiency which were equal to that of macrophages isolated from wild type mice with the same genetic background. Moreover, we observed that the WT and iNOS knock-out mouse strains infected with Coccidioides by the natural, intranasal route showed similar percent survival over a 30-day period postchallenge, and the fungal burden in their lungs at 7 and 11 days was comparable [24]. On the other hand, the iNOS-deficient mice revealed higher fungal burden in their spleen at 11 days postchallenge compared to WT mice, indicating that the pathogen had disseminated and the disease had progressed more rapidly in the knock-out mice. We also observed that the infected lungs of the iNOS-deficient mice at 11 days contained significantly fewer neutrophils and tissue macrophages than the WT mice. We concluded from these studies that suppression of NO production by macrophages in the presence of Coccidioides parasitic cells may be advantageous to the pathogen by its apparent compromising effect on the host inflammatory response to pulmonary infection. On the other hand, it was evident that NO does not directly contribute to in vitro killing of Coccidiodes by activated macrophages. We proposed that other phagocytic mechanisms of defense must be involved. It was reasonable to speculate that production of reactive oxygen species resulting from phagocytic oxidase (NOX2) activity would be a good candidate for such an alternative mechanism of control of Coccidioides infection based on earlier reports of the involvement of ROS in macrophage defense against certain medically-important fungi [11, 12, 25].
Using the gp91phox knock-out mouse model of human chronic granulomatous disease, we essentially conducted the same series of investigations as reported above for iNOS-mediated nitric oxide production to explore the role of NOX2 activity in vitro and in vivo during Coccidioides infection. We initially confirmed that activated NOX2-deficient macrophages had only background evidence of ROS production in vitro compared to activated WT macrophages. As demonstrated by iNOS-deficient phagocytes, macrophages derived from NOX2-/-mice exhibited phagocytic and fungicidal activities against Coccidioides which were near-identical to that of macrophages isolated from wild type mice. Co-cultures of spherule initials with either non-activated or activated WT macrophages failed to enhance ROS production. Intranasal infection of WT and NOX2-/- mice with spores of Coccidioides was lethal for both groups of animals, although NOX2-/- mice died over a significantly shorter period than the WT mice. The fungal burden in the lungs and spleen of the two groups of mice at 7 and 11 days postchallenge was comparable, irrespective of whether the animals were immunized prior to infection with a live, attenuated vaccine strain [26] or not vaccinated. These results suggested that ROS is not essential for phagocytic defense against Coccidioides infection. On the other hand, the lungs of NOX2-/- mice revealed significantly enhanced inflammatory cell and cytokine/chemokine response to Coccidioides compared to WT mice. Similar observations were recently presented in a report of gp91phox knock-out mice infected with a potentially lethal inoculum of Coccidioides spores delivered either by the intraperitoneal or intranasal route [20]. Our observations support the conclusion of this earlier study that some mechanism other than NOX2 activity is responsible for protective immunity against coccidioidomycosis.
Both the absence of iNOS and NOX2 activity by macrophages resulted in dysregulation of the inflammatory cell response to lung infection with Coccidioides in the respective knock-out mouse model, but in an inverse fashion. Loss of NO production correlated in vivo with a significant reduction in the infiltration of neutrophils and macrophages to sites of infection in the lungs, resulting in less efficient containment of the pathogen and increased risk of dissemination to extrapulmonary organs [19, 24]. On the other hand, absence of NOX2 activity resulted in a major increase and sustained infiltration of neutrophils into infected lungs of the knock-out mice, enhanced production of a wide array of inflammatory cytokines and chemokines, and increased lethality to infection, apparently resulting from inflammatory pathology compared to WT mice. Our results are in agreement with other reports which have shown that elevated immune cell infiltration into the lungs of NADPH oxidase knockout mice occurred during fungal infection [1, 5]. We observed increased numbers of tissue macrophages and eosinophils on day 7 postchallenge but not on day 11, and an expanded subpopulation of regulatory T cells during the later stage of infection in NOX2-/- mice compared to the infected WT strain. The sharp increase in numbers of pulmonary Treg cells between 7 and 11 days in non-vaccinated NOX2-/- mice may be in response to host tissue damage and is an indicator of fungal persistence [21]. We also observed higher concentrations of Eotaxin, MCP-1, RANTES, MIP-1α and MIP-1β chemokines at both 7 and 11 days postchallenge in NOX2-/- mice compared to WT mice. In addition, higher concentrations of selected Th1 and Th2 cytokines were detected in Coccidioides infected lungs of NOX2-/- compared to the WT strain. Production of IL-10 was significantly higher in NOX2-/- compared to WT mice, suggesting that the mobilization of neutrophils and high cytokine/chemokine concentrations in the lungs of the knock-out mice was not due to an inability to generate an appropriate anti-inflammatory response. Instead the elevated amounts of both IFN-γ and IL-10 observed in the lungs of NOX2-/- mice at 11 days postchallenge suggested the onset of chronic infection dominated by non-resolving inflammation [27]. It has been reported that PMNs and macrophages derived from NOX2-/- mice and humans with CGD appear to be protected from apoptosis, which would result in their prolonged lifespan. Persistence of these viable phagocytes could lead to exaggerated inflammation [28, 29]. Romani and coworkers [30] have determined that absence of ROS leads to defective tryptophan catabolism accompanied by an overproduction of the proinflammatory cytokine IL-17 and defective regulatory T-cell activity. The combined effect in the NOX2-/- host could lead to severe tissue damage at infection sites due to intense and persistent inflammation. We have shown here that spherule initials in the presence of activated macrophages isolated from WT mice failed to trigger an increase in production of ROS. In an earlier investigation we demonstrated that a detergent extract of the cell wall of Coccidioides significantly inhibited the release of superoxide anion from activated rat alveolar macrophages in vitro [31]. The nature of the inhibitory product is still undefined. However, this observation of a potential mechanism of Coccidioides-induced suppression of ROS production in WT macrophages may be an important factor that supports its status as a primary pathogen with the ability to overcome host protective mechanisms and establish infection sites in the immunocompetent host.
Contradictory data have been reported on the effect of reactive oxygen and nitrogen intermediates on host defense against fungal infections. Henriet and coworkers [4] reported that A. nidulans, in contrast to A. fumigatus, is not susceptible to phagocytic NADPH oxidase. In vitro infection of human PMNs or peripheral blood mononuclear cells (PBMC) with live A. nidulans conidia did not enhance the release of ROS, as we have also shown in the case of Coccidiodes-infected, activated and non-activated murine macrophages. The authors also showed that pre-incubation of the WT phagocytes with diphenyleneiodonium chloride (DPI), an inhibitor of NADPH oxidase, resulted in significantly reduced damage of A. fumigatus but not A nidulans hyphae. Balish and coworkers [32] used mice defective in the production of both ROI and RNI to investigate the role of phagocytic cells during mucosal and systemic candidiasis in germfree mice. The authors showed that there was no significant difference in the ability of peritoneal macrophages from gp91phox-/-/iNOS-/- double knock-out, iNOS-/-, gp91phox-/-, or immunocompetent C57BL/6 mice to kill C. albicans in vitro. They proposed that anti-Candida factors other that ROI and RNI are responsible for the killing of phagocytized yeast in vitro, and that Candida-infected gp91phox-/-/iNOS-/- mice died due to the inability of the host to control its inflammatory response to the fungal pathogen. The authors further speculated that the defects in ROI and RNI enabled C. albicans to translocate and disseminate to internal organs, resulting in uncontrolled immune response, severe pathology, and death.
In summary, our results agree with observations of earlier investigators that factors other than direct exposure of the fungal pathogen to oxidative oxygen and nitrogen intermediates are responsible for the ability of phagocytes to kill the organism in vitro. Our data also suggest that neutrophils play a prominent role in the inflammatory response to coccidoidal infection, and dysregulation of their infiltration into the lungs during the early course of disease can result in metastasis of the pathogen. Myeloperoxidase (MPO) is released from azurophilic granules of neutrophils upon appropriate stimulation and plays a key role in the formation of neutrophil extracellular traps (NETs) [33]. The latter are composed of activated neutrophils that have died and released decondensed chromation and antimicrobial proteins that trap and inhibit the growth of a broad range of microbes, including certain fungal pathogens [34, 35]. CGD patients and gp91phox-/- knock-out mice fail to make NETs because NOX2 activity is essential for production of hydrogen peroxide, the substrate of MPO. NETs have been suggested to be particularly important in host defense against certain fungal pathogens, such as Coccidioides, that are difficult to phagocytose due to their large size [33]. NET formation during coccidioidomycosis has not yet been explored.
4. Materials and Methods
4.1. Mice
Wild-type C57BL/6 mice were obtained from the National Cancer Institute/Charles River Laboratory (Wilmington, MA, USA). Breeding pairs of the homozygous C57BL/6 gp91phox/NADPH oxidase-deficient mice (NOX2-/- strain, B6.129S-Cybbtm1), were kindly supplied by Dr. Joshua Fierer (University of California at San Diego, School of Medicine, San Diego, CA). NOX2-/- mice were given water containing an oral suspension of sulfamethoxazole and trimethoprim to prevent opportunistic microbial infections. Mice were maintained in a specific pathogen free facility at The University of Texas at San Antonio and accommodated according to the guidelines approved by the University Institutional Animal Care and Use Committee. Mice were transferred to a CDC-certified biological safety level 3 (BSL3) laboratory prior to immunization with the live, attenuated (ΔT) vaccine strain of Coccidioides and infected with viable spores of a virulent strain of the fungal pathogen. Infected animals were housed in a Thoren caging system (Thoren, Hazelton, PA) equipped with high efficiency particulate air (HEPA) filters and housed in the BSL3 laboratory.
4.2. Fungal strains and culture conditions
Coccidioides posadasii (isolate C735) was used to prepare the virulent inocula for all experimental procedures reported in this study. The saprobic phase of this fungus was grown on glucose-yeast-extract agar (GYE; 1 % glucose, 0.5 % yeast extract, 2 % agar) at 30°C for 3 to 4 weeks to induce sporulation. Culture plates were flooded with 10 ml of sterile, endotoxin-free saline and the suspension of spores (arthroconidia) was transferred to a plastic centrifuge tube containing glass beads (5 mm diam.). The tubes were hand-shaken to disrupt the spore chains. The cell suspension was filtered through a layer of nylon fiber to remove hyphal fragments, washed twice and resuspended in saline. The spore suspensions were used to inoculate a defined glucose-salts medium for growth of the parasitic cells at 39°C in the presence of 10% CO2 [36]. In vitro growth and differentiation of successive stages of the first generation of the parasitic cycle of Coccidioides are fairly well synchronized [37]. This permitted the isolation of specific cell types of the parasitic phase produced during progressive stages of incubation. In this project we focused on the isolation of spherule initials, which are isotropically germinated spores (10-15 μm diam.). The cells were isolated from parasitic phase, liquid cultures after 24 h of incubation. Spherule initials are encountered by host phagocytes during early pulmonary infection [38], and were used to evaluate macrophage response to Coccidioides in this study.
4.3. Isolation of murine peritoneal macrophages
WT and NOX2-/- mice (8-12 weeks-old) were injected intraperitoneally (i.p.) with 2 ml of sterile PBS containing 3% thioglycolate to stimulate in vivo recruitment of peritoneal macrophages. The mice were euthanized 72 h later and the peritoneal fluid was recovered and transferred to 10 ml of ice-cold RPMI 1640 (Mediatech Inc. Manassas, VA) supplemented with 1% penicillin–streptomycin, 10 mM HEPES buffer and 1 mM sodium pyruvate. The peritoneal macrophages were washed once with the same supplemented medium and aliquots were transferred to either 96-well tissue culture plates (OptipluxTM, Falcon/Becton Dickinson; Franklin Lakes, NJ) at a concentration of 2 × 105 cells per well for measuring reactive oxygen intermediates (ROI), or 48-well tissue culture plates (Corning Inc., Corning, NY) at 3 × 105 cells per well for phagocytosis and killing assays.
Peritoneal macrophages were allowed to adhere to the surface of the plates for 2 h. Non-adherent cells were removed by washing and adherent cells were incubated for 48 h in the presence of 5% CO2 at 37°C in RPMI supplemented as above plus the addition of 10% heat-inactivated fetal bovine serum (FBS; HyClone/Fisher Scientific, Logan, UT).
4.4. Phagocytosis and killing assays
Peritoneal macrophages were cultured in the presence or absence of recombinant murine interferon-gamma (IFN-γ, 10 ng/ml; BD Biosciences/Pharmingen, San Jose, CA) plus 100 ng/ml lipopolysaccharide (LPS; Invitrogen, San Diego, CA) for 16-18 h to yield activated and non-activated macrophages, respectively. The peritoneal macrophage growth medium described above was replaced in each well of the 48-well plates with 0.3 ml of RPMI to which 0.01 ml of a suspension of viable Coccidioides spherule initials was added to achieve a multiplicity of infection (MOI) of 2:1 (macrophages:spherule initials). The macrophages and fungal cells were co-cultured for 3 or 12 h in the presence of 5% CO2 at 37°C. This infection ratio and incubation time points were determined optimal for evaluation of macrophage response to infection as previously reported [19]. For phagocytosis assays, spherule initials (5 × 106) were stained with a fluorescent probe (FUN® 1; Invitrogen) as previously reported [19]. The stained fungal cells were added to either activated or non-activated macrophages at a MOI of 2:1 as described above and incubated for 3 h. Adherent monolayers of infected macrophage were then washed and fixed with 1 % paraformaldehyde (Sigma Chemical Company, St. Louis, MO) for 1h at 4°C [19]. Phagocytosis was quantified by phase-contrast and fluorescence microscopy, and the phagocytic index was calculated on the basis of the total number of ingested fungal cells per 200 macrophages observed microscopically. Killing assays were conducted as previously reported [19]. Briefly, the activated or non-activated peritoneal macrophages were infected with Coccidioides spherule initials at a MOI of 2:1 and cultured for 12 h. The adherent macrophages were then washed twice with culture media without FBS. The macrophages were lysed by incubation with 0.5 ml of sterile, deionized water for 30 min at 37°C and the cell suspension was mixed vigorously by vortexing. The lysate was collected and wells containing residual intact macrophages were again washed with 0.5 ml of sterile water. The two lysates were combined and an aliquot of 0.1 ml was plated on a GYE agar and incubated for 72 h at 37°C to determinate the colony-forming units (CFUs) of viable Coccidioides cells. Assays of phagocytic activity and killing efficiency were repeated in three separate experiments.
4.5. Measurement of ROS production
Detection of highly reactive oxygen species (hROS) was conducted using a specific indicator (aminophenyl fluorescein [APF]; Cell Technology, Inc., Mountain View, CA) [39]. Briefly, after macrophage activation with IFN-γ plus LPS added to the cell culture medium as above, the phagocytes were washed with warm (30°C) Hank's balanced salt solution (HBSS). A working solution of APF was diluted in HBSS (pH 7.0-7.5) according to the manufacturer's instructions, added to each well of the 96-well tissue culture plate containing adherent macrophages, and incubated for 1 h to pre-load the dye into the host cells. Macrophages were then washed with HBSS to remove excess dye and incubated in non-supplemented RPMI containing the desired concentration of spherule initials for 12 h at 37 °C in the presence of 5% CO2 as above. The fluorescence intensity, which is indicative of the amount of hROI production, was determined using a SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA) with excitation at 488 nm and emission at 515 nm. The amount of hROS production was expressed as relative fluorescence units (RFU). Endotoxin-free water was used for preparation of HBSS and the culture medium.
4.6. Mouse immunization and infection protocols
Live spores isolated from cultures of a genetically-engineered, attenuated vaccine strain (ΔT) of C. posadasii were used to immunize mice against coccidioidomycosis as previously described [26]. Separate groups of WT or NOX2-/- mice (n=10) were immunized either with the ΔT vaccine or PBS by the subcutaneous route as reported [26]. Mice were challenged by the intranasal (i.n.) route at 4 weeks after completion of the vaccination protocol. The challenge dose contained 60 to 80 viable spores obtained from a culture of the C. posadasii (isolate C735) parental strain as previously described [26]. The mice were sacrificed at 7 or 11 days postchallenge to determine the residual fungal burden of Coccidioides (colony-forming units [CFUs]) in their lungs and spleen. CFU determinations per organ were expressed on a log scale. Vaccinated and nonvaccinated WT and NOX2-/- mice were also used to compare the percent survival over a 25-day period after challenge.
4.7. Evaluation of lung-infiltrated leukocytes by flow cytometry
Five mice per group of each strain (WT and NOX2-/-) were used to evaluate the total numbers of lung-infiltrating leukocytes (LIL) and leukocyte subpopulations at days 7 and 11 postchallenge. The procedures employed for isolation of LIL and cytometric assays were the same as previously reported [24]. In brief, absolute numbers of pulmonary leukocytes in the lung tissue preparations were enumerated by flow cytofluorometry using a FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ). Data were acquired with CellQuest Pro software (BD Biosciences) and analyzed using a FlowJo software package (Tree Star, Inc., Ashland, OR). Pulmonary cells were suspended in FACS buffer (PBS containing 0.5% FBS) and incubated with unlabeled anti-CD16/CD32 (clone 2.4G2) monoclonal antibodies (mAb; BD Biosciences) for 15 min at 4 °C to block non-specific binding of cell surface antigen-specific mAb to Fc receptors. Cocktails containing appropriate fluorochrome-conjugated mAb were added to the Fc-blocked pulmonary cells and incubated for 30 min at 4 °C for analyses of selected phenotypes of LIL as reported [24]. All labeled mAb were obtained from BD Biosciences. Anti-CD45 (clone 30-F11) was employed to determine the total number of leukocytes in each preparation. The mAb in the cocktail preparations included anti-Ly6G (clone IA8), anti-CD11b (clone M1/70), anti-CD11c (clone HL3), anti-Mac3 (clone M3/84), and anti-SiglecF (clone E50-2440), which were used to determine the absolute numbers of neutrophils (PMN; CD45+/Ly6G+), alveolar mcrophages (AM; CD45+/CD11bMedium/CD11c+) tissue macrophages (T. Mac; CD45+/Mac3+/CD11cLow), dendritic cells (DC; CD45+/CD11bHigh/CD11c+), and eosinophils (Eos; CD45+/CD11c-/SiglecF+). Anti-CD3 (clone 17A2), anti-CD4 (clone RM4-5), anti-CD8α (clone 53-6,7) and anti-NK1.1 (clone PK136) mAb were used to determine the absolute numbers of CD4+ T cells (CD3+/CD4+), CD8+ T cells (CD3+/CD8+) and natural killer cells (NK1.1+), respectively. A cocktail which contained anti-CD19 (clone ID3), anti-CD23 (clone B3B4), and anti-sIgM (clone II/41) mAb (BD Biosciences) was used to determine the absolute number of B cells present in the total leukocyte population. Regulatory T cells (Tregs) were determined by intracellular staining of Foxp3 using a Treg staining kit from BD Biosciences. The detected fluorescence levels of labelled CD25 at the cell surface and intracellular FoxP3 were back-gated on the CD4+ T cell population. The absolute number of each subpopulation of leukocytes was quantified by multiplying the total number of CD45+ cells by the percentage of the individual mAb cocktail-labeled cell types determined by flow cytofluorometry. Following incubation with the selected, labeled mAb the cells were washed twice with FACS buffer, fixed with 200 μl of 1 % paraformaldehyde (Polysciences Inc., Warrington, PA), and stored in the dark at 4°C unti l ready for quantitative analysis.
4.8. Pulmonary chemokine and cytokine assays
At days 7 and 11 postchallenge, non-vaccinated, Coccidioides-infected WT and NOX2-/- mice (n=5 per group) were sacrificed, their lungs excised and homogenized in 0.5 ml of ice-cold sterile PBS, and mixed with a cytokine extraction buffer (0.5 ml) containing 0.05 % Triton X-100 and a EDTA-free protease inhibitor cocktail (Roche, Indianapolis, IN) as previously described [24]. Each lung homogenate sample was centrifuged at 8,000×g at 4°C for 10 min. The supernatants were recovered and stored at -80°C until assayed for selected chemokines and cytokines using a Bio-Plex suspension array system (Bio-Plex; Bio-Rad Laboratories, Hercules, CA) as reported [24].
4.9. Statistical analyses
Comparative analysis of the results of in vitro experiments were conducted using ANOVA software followed by the Bonferroni's test or Student's t-test for determination of statistically significant difference between sets of data. Comparison of the results of in vivo experiments was conducted by the Mann-Whitney U test or ANOVA followed by the Student's t-test. Survival data were compared by the Kaplan-Maier method (percentage of surviving animals) using log rank analysis. All data were expressed as mean values ± standard error of the mean (SEM). Statistical calculations were performed using GraphPad Prism version 4.0 software for Macintosh (GraphPad, San Diego, CA). Values of p<0.05 were considered statistically significant.
I am sending the highlights requested.
We demonstrated that NOX2 does not exert an antifungal effect against Coccidioides.
Absence of NOX2 leads to a severe and uncontrolled inflammatory process in lungs.
Absence of NOX2 is associated with increased infiltration of PMNs and Treg cells.
NOX2 appears to play a role as a master regulator key in inflammatory processes.
Acknowledgements
Support for this study was provided by Public Health Service grants AI071118 and AI070891 from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health, awarded to GTC. Additional support has been provided by the Margaret Batts Tobin Foundation, San Antonio, TX. The authors are grateful for the technical assistance provided by Ms. Natalia Castro-Lopez and Mr. Michael Bellecourt.
This work was conducted in the Department of Biology, University of Texas, San Antonio, TX while the senior author (AG) was a postdoctoral fellow supported by a National Institutes of Health grant (AI071118) awarded to GTC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morgenstern DE, Gifford MA, Li LL, Doerschuk CM, Dinauer MC. Absence of respiratory burst in X-linked chronic granulomatous disease mice leads to abnormalities in both host defense and inflammatory response to Aspergillus fumigatus. J Exp Med. 1997;185:207–18. doi: 10.1084/jem.185.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao XP, Standiford TJ, Rahman A, Newstead M, Holland SM, Dinauer MC, et al. Role of NADPH oxidase in the mechanism of lung neutrophil sequestration and microvessel injury induced by gram-negative sepsis: Studies in p47phox–/– and gp91phox–/– mice. J Immunol. 2002;168:3974–82. doi: 10.4049/jimmunol.168.8.3974. [DOI] [PubMed] [Google Scholar]
- 3.Li XJ, Marchal CC, Stull ND, Stahalin RV, Dinauer MC. p47phoxPhox homology domain regulates plasma membrane but not phagosome neutrophil NADPH oxidase activation. J Biol Chem. 2010;35:169–79. doi: 10.1074/jbc.M110.164475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henriet SS, Hermans PW, Verweij PE, Simonetti E, Holland SM, Sugui JA, et al. Human leukocytes kill Aspergillus nidulans by reactive oxygen species-independent mechanisms. Infect Immun. 2011;79:767–73. doi: 10.1128/IAI.00921-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–9. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 6.Leto TL, Geiszt M. Role of Nox family NADPH oxidases in host defense. Antioxd Redox Signal. 2006;8:1549–61. doi: 10.1089/ars.2006.8.1549. [DOI] [PubMed] [Google Scholar]
- 7.Segal BH, Leto TL, Gallin JI, Malech HL, Holland SM. Genetic, biochemical, and clinicla features of chronic granulomatous disease. Medicine (Baltimore) 2000;79:170–200. doi: 10.1097/00005792-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Yang CS, Shin DM, Kim KH, Lee ZW, Lee CH, Park SG, et al. NADPH oxidase 2 interaction with TLR2 is required for efficient innate immune responses to Mycobacteria via cathelicidin expresión. J Immunol. 2009;182:3696–3705. doi: 10.4049/jimmunol.0802217. [DOI] [PubMed] [Google Scholar]
- 9.Qiu H, KuoLee R, Harris G, Chen W. Role of NADPH oxidase in host defense against acute respiratory Acinetobacter baumanii infection in mice. Infec Immun. 2009;77:1015–21. doi: 10.1128/IAI.01029-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boncompain G, Schneider B, Delevoye C, Kellerman O, Dautry-Varsat A, Subtil A. Production of reactive oxygen species is turne on and rapidly shut down in epithelial cells infected with Chlamydia trachomatis. Infect. Immun. 2010;78:80–87. doi: 10.1128/IAI.00725-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aratani Y, Kura F, Watanabe H, Akagawa H, Takano Y, Suzuki MC, et al. Critical role of myeloperoxidase and nicotinamide adenine dinucleotide phosphate-oxidase in high-burden systemic infection of mice with Candida albicans. J Infect Dis. 2002;185:1833–7. doi: 10.1086/340635. [DOI] [PubMed] [Google Scholar]
- 12.Segal BH, Romani R. Invasive aspergillosis in chronic granulomatous disease. Med Mycol. 2009;47:S282–90. doi: 10.1080/13693780902736620. [DOI] [PubMed] [Google Scholar]
- 13.Sharpton TJ, Stajich JE, Rounsley SD, Gardner MJ, Wortman JR, Jordar VS, et al. Comparative genomic analyses of the human fungal pathogens Coccidioides and their relatives. Genome Res. 2009;19:1722–31. doi: 10.1101/gr.087551.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galgiani JN. Coccidioidomycosis: a regional disease of national importance: rethinking approaches for control. Ann Inter Med. 1999;130:293–300. doi: 10.7326/0003-4819-130-4-199902160-00015. [DOI] [PubMed] [Google Scholar]
- 15.Cole GT, Xue J, Okeke C, Tarcha EJ, Basrur V, Schaller RA, et al. A vaccine against coccidioidomycosis is justified and attainable. Med Mycol. 2004;42:189–216. doi: 10.1080/13693780410001687349. [DOI] [PubMed] [Google Scholar]
- 16.Fierer J, Waters C, Walls L. Both CD4+ and CD8+ T cells can mediate vaccine-induced protection against Coccidioides immitis infection in mice. J Infect Dis. 2006;193:1323–31. doi: 10.1086/502972. [DOI] [PubMed] [Google Scholar]
- 17.Wüthrich M, Gern B, Hung CY, Ersland K, Rocco N, Pick-Jacobs J, et al. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J Clin Inv. 2011;121:554–68. doi: 10.1172/JCI43984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirkland TN, Cole GT. Coccidioidomycosis: pathogenesis, immune response and vaccine development. In: Calderone R, Chilar R, editors. Fungal pathogenesis: principles and clinical applications. Marcel Dekker; New York: 2001. pp. 365–99. [Google Scholar]
- 19.Gonzalez A, Hung CY, Cole GT. Coccidioides releases a soluble factor that suppresses nitric oxide production by murine primary macrophages. Microb Pathogen. 2011;50:100–108. doi: 10.1016/j.micpath.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Margolis DA, Viriyakosol S, Fierer J, Kirkland TN. The role of reactive oxygen intermediates in experimental coccidioidomycosis in mice. BMC Microbiology. 2011;11:71e. doi: 10.1186/1471-2180-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romani L, Puccetti P. Protective tolerance to fungi: the role of IL-10 and tryptophan catabolism. Trends Microbiol. 2006;14:183–9. doi: 10.1016/j.tim.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Nathan C, Ding A. Snapshot: reactive oxygen intermediates (ROI). Cell. 2010;140:95e. doi: 10.1016/j.cell.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Iovine NM, Pursnani S, Voldman A, Wasserman G, Blaser MJ, Weinrach Y. Reactive nitrogen species contribute to innate defense against Campylobacter jejuni. Infect Immun. 2008;76:986–93. doi: 10.1128/IAI.01063-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez A, Hung CY, Cole GT. Nitric oxide synthase activity has limited influence on the control of Coccidioides infection in mice. Microb Pathogen. 2011 doi: 10.1016/j.micpath.2011.03.013. doi:10.1016/j.micpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonnett CR, Cornish EJ, Harmsen AG, Burrit JM. Early neutrophil recruitment and aggregation in the murine lung inhibit germination of Aspergillus fumigatus conidia. Infect Immun. 2006;74:6528–39. doi: 10.1128/IAI.00909-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue J, Chen X, Selby D, Hung CY, Yu JJ, Cole GT. A genetically engineered live attenuated vaccine of Coccidioides posadasii protects BALB/c mice against coccidioidomycosis. Infect Immun. 2009;77:3196–208. doi: 10.1128/IAI.00459-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romani L. Immunity to fungal infections. Nat Rev Immunol. 2011;11:275–88. doi: 10.1038/nri2939. [DOI] [PubMed] [Google Scholar]
- 28.Petersen JE, Hiran TS, Goebel WS, Johnson C, Murphy RC, Azmi FH, et al. Enhanced cutaneous inflammatory reactions to Aspergillus fumigatus in a murine model of chronic granulomatous disease. J Invest Dermatol. 2002;118:424–9. doi: 10.1046/j.0022-202x.2001.01691.x. [DOI] [PubMed] [Google Scholar]
- 29.Sanmun D, Witasp E, Jitkaew S, Tyurina YY, Kagan VE, Ahlin A, et al. Involvement of a functional NADPH oxidase in neutrophils and macrophages during programmed cell clearance: implications for chronic granulomatous disease. Am J Physiol Cell Physiol. 2009;297:621–31. doi: 10.1152/ajpcell.00651.2008. [DOI] [PubMed] [Google Scholar]
- 30.Romani L, Fallarino F, De Luca A, Montagnoli C, D'Angelo C, Zelante T, Vacca C, Bistoni F, Fioretti MC, Grohmann U, Segal BH, Puccetti P. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–5. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 31.Cole GT, Kirkland TN. Conidia of Coccidioides immitis. Their significance in disease initiation. In: Cole GT, Hoch HC, editors. The Fungal Spore and Disease Initiation in Plants and Animals. Plenum Press; New York: 1991. pp. 403–43. [Google Scholar]
- 32.Balish E, Warner TF, Nicholas PJ, Paulling EE, Westwater C, Schofield DA. Susceptibility of germfree phagocyte oxidase- and nitric oxide synthase 2-deficient mice, defective in the production of reactive metabolites of both oxygen and nitrogen, to mucosal and systemic candidiasis of endogenous origin. Infect Immun. 2005;73:1313–20. doi: 10.1128/IAI.73.3.1313-1320.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Metzler KD, Fuchs TA, Nauseef WM, Reumaux D, Roesler J, Schulze I, et al. Myeloperoxidase is requires for neutrophil extracellular trap formation: implication for innate immunity. Blood. 2011;117:953–9. doi: 10.1182/blood-2010-06-290171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8:668–76. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 35.Bianchi M, Niemic MJ, Siler U, Urban CF, Reichenbach J. Restoration of anti-Aspergillus defense by neutrophil extracellular traps in human chronic granulomatous disease after gene therapy is calprotectin-dependent. J Allergy Clin Immunol. 2011;127:1243–52. doi: 10.1016/j.jaci.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 36.Levine HB. Purification of the spherule-endospore phase of Coccidioides immitis. Sabouraudia. 1961;1:112–5. doi: 10.1080/00362176285190231. [DOI] [PubMed] [Google Scholar]
- 37.Hung CY, Yu JJ, Seshan KR, Reichard U, Cole GT. A parasitic phase-specific adhesin of Coccidioides immitis contributes to the virulence of this respiratory fungal pathogen. Infect Immun. 2002;70:3443–56. doi: 10.1128/IAI.70.7.3443-3456.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cole GT, Xue J, Seshan K, Borra P, Borra R, Tarcha E, et al. Virulence mechanisms of Coccidioides. In: Heitman J, Filler SG, Mitchell AP, editors. Molecular Principles of Fungal Pathogenesis. American Society for Microbiology Press; Washington DC: 2006. pp. 363–91. [Google Scholar]
- 39.Setsukinai K, Urano Y, Kakinuma K, Majima HJ, Nagano T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J Biol Chem. 2003;278:3170–5. doi: 10.1074/jbc.M209264200. [DOI] [PubMed] [Google Scholar]