Abstract
We previously showed that total sleep deprivation increased antioxidant responses in several rat brain regions. We also reported that chronic hypoxia enhanced antioxidant responses and increased oxidative stress in rat cerebellum and pons, relative to normoxic conditions. In the current study, we examined the interaction between these two parameters (sleep and hypoxia). We exposed rats to total sleep deprivation under sustained hypoxia (SDSH), and compared changes in antioxidant responses and oxidative stress markers, in the neocortex, hippocampus, brainstem and cerebellum, to those in control animals left undisturbed under either sustained hypoxia (UCSH) or normoxia (UCN). We measured changes in total nitrite levels, as an indicator of nitric oxide (NO) production, superoxide dismutase (SOD) activity and total glutathione (GSHt) levels as markers of antioxidant responses and levels of thiobarbituric acid reactive substances (TBARS) and protein carbonyls as signs of lipid and protein oxidation products, respectively. We found that acute (6h) SDSH increased NO production in the hippocampus and increased GSHt levels in the neocortex, brainstem and cerebellum, while decreasing hippocampal lipid oxidation. Additionally, we observed increased hexokinase (HK) activity in the neocortex of SDSH rats compared to UCSH rats, suggesting that elevated glucose metabolism may be one potential source of the enhanced free radicals produced in this brain region. We conclude that short term insomnia under hypoxia may serve as an adaptive response to prevent oxidative stress.
Keywords: antioxidant responses, glucose metabolism, sleep deprivation, sustained hypoxia, oxidative stress
Introduction
Sleep deprivation leads to cognitive slowing, memory impairment, decreased vigilance and diminished sustained attention [1]. It has been hypothesized that free radicals accumulate during waking as a result of enhanced metabolic activity and may be responsible for some of the effects of sleep deprivation [2].
People moving rapidly to high altitude, commonly experience acute mountain sickness, pulmonary edema, cerebral edema [3, 4], mental dysfunction, memory deficits [5–7], insomnia, dizziness, nausea [8], weight loss [9] and motor impairment [10]. Recent data suggest that humans exposed to high altitude hypoxia may be at increased risk of oxidative stress [3, 11–15]. Increased levels of oxidative stress and neuronal apoptosis have also been reported in animals subjected to hypobaric hypoxia [16–19].
Free radicals, which include reactive nitrogen and reactive oxygen species (RNS and ROS respectively), are difficult to detect and quantify directly due to their extreme reactivity. The amount of RNS, such as nitric oxide (NO) can be deduced from measurement of the level of its metabolites, nitrates/nitrites (NO3−/NO2−), while the involvement of ROS can be inferred from measurement of antioxidant responses. Antioxidant responses include changes in the activities of several antioxidative enzymes including superoxide dismutase (SOD), and in the levels of the endogenous antioxidant, glutathione (GSHt). If antioxidant responses are unable to successfully scavenge the free radicals, this will lead to oxidative damage to lipids (measured as thiobarbituric acid reactive substances, TBARS) and/or proteins (measured as protein carbonyls), resulting in oxidative stress [20].
We previously reported that long term (5–11 days) total sleep deprivation, by the disk-over-water method, decreased SOD activity in the rat hippocampus and brainstem [21]. The rat neocortex did not show any significant changes in SOD or glutathione peroxidase (GPx) activities with either short term (8h) or long term (5–11 days) total sleep deprivation [21,22]. We also previously showed that 6h of total sleep deprivation increased GPx activity in the rat hippocampus and cerebellum and increased GSHt levels in the neocortex, brainstem and basal forebrain [23]. On the other hand, D’Almeida et al. [24] reported that 96h of rapid eye movement (REM) sleep deprivation, by the platform technique, significantly decreased GSHt levels in the rat hypothalamus.
We previously also showed that chronic sustained hypoxia increased the activity of the antioxidative enzyme, glutathione reductase (GR) in the pons, and elevated the level of TBARS in the cerebellum, of experimental rats relative to control rats under normoxic conditions [25]. Maiti et al. [16] reported that hypoxia induced oxidative stress in the rat neocortex, hippocampus and striatum, while Jefferson et al. [13] showed that humans exposed to acute (48h) or chronic high altitude had increased levels of plasma TBARS and GSHt.
This study was carried out to determine the combined effects of total sleep deprivation and sustained/continuous hypoxia (10% O2) on antioxidant responses and oxidative stress. This condition is similar to, but not quite the same as, sleep apnea which is characterized by sleep deprivation/fragmentation under intermittent/cyclic hypoxia (alternating 21%O2 and 10% O2 [reviewed in 26, 27]). We analyzed changes in NO levels, SOD activity, GSHt levels and the levels of TBARS and protein carbonyls in rats subjected to 6h of total sleep deprivation under sustained hypoxia (SDSH), and compared them to rats left undisturbed under either sustained hypoxia (UCSH) or normoxia/room air (21% O2, UCN) for the same period of time. We hypothesized that any increase in free radical production could probably result from increased glucose metabolism. Hence we measured the activity of hexokinase (HK), which is the rate limiting enzyme in glucose metabolism.
Materials and Methods
Young adult male Sprague Dawley rats (400–500g) were used for all experiments. The experimental protocols were approved by our Institutional Animal Use and Care Committee and conform to the National Institutes of Health guide for the care and use of laboratory animals.
Hypoxic Exposures
Rats were individually housed in commercially designed chambers (30×20×20 inches; Oxycycler model A44XO; Biospheryx, Redfield, NY). The chambers were operated under a 12h light/dark cycle (8:00AM-8:00PM). On the day of the experiment, the O2 level was changed from 21% to 10% and the rats were subjected to 6 hours of total sleep deprivation beginning at lights on (8:00AM). Gas was circulated around each of the chambers at 60 l/min (i.e. one complete change every 10sec). The O2 concentration was continuously measured by an O2 analyzer and regulated throughout the 6h of experimental time by a computerized system controlling the gas valve outlets. Deviation from the desired concentration was automatically corrected by addition of N2 or O2 through solenoid valves. Ambient temperature was kept at 22–24°C.
Sleep Deprivation Habituation
Rats were housed individually in the commercially designed chambers as described above and handled for 1h each day for 1 week. This gentle handling procedure, as described in our earlier paper [23], included brushing their fur or gently touching them with a blunt-ended wire, introducing objects into their chambers (including paper towels and plastic weigh boats) and disturbing their chamber bedding. These procedures were performed through a small opening in the hypoxic chamber. The chambers were maintained at room air (21% O2) during the habituation period, and the animals were allowed food and water ad libitum.
Sleep Deprivation Under Sustained Hypoxia
After 1 week of habituation to the handling procedure, the animals were divided into three groups: Group 1: rats were left undisturbed under room air (21% O2, unhandled control under normoxia = UCN); Group 2: rats were subjected to sustained hypoxia (10% O2) beginning at lights on, on the day of the experiment (8:00 AM), and were left undisturbed (unhandled control under sustained hypoxia= UCSH); Group 3: rats were subjected to sustained hypoxia (10% O2) beginning at lights on, on the day of the experiment (8:00 AM), and were handled each time they showed physical signs of sleepiness (sleep deprived under sustained hypoxia= SDSH). Our sleep deprivation procedure is based on visual observation of the rat. Many investigators have used the gentle handling procedure, to induce short term sleep deprivation, based on behavioral signs of sleepiness [28–30] At the end of the experimental period (6h), the rats were sacrificed by decapitation after halothane anesthesia, and the neocortex, hippocampus, brainstem and cerebellum were dissected on ice and stored at −80°C until analyzed [23]. The level of NO, the activity of SOD, and the level of GSHt, as well as the levels of TBARS and protein carbonyls and the activity of HK were analyzed as described below.
Biochemical Analysis
Each brain region was divided into two portions, one portion was homogenized in a hand held homogenizer with 20 strokes in cold homogenizing buffer (50 mM Tris HCl, pH 7.5, 50mM MgCl2 and 5mM EDTA) containing protease inhibitors (Roche Diagnostics, Mannheim, Germany) to make a 10% homogenate (w/v). The homogenate was centrifuged in an Eppendorf micro-centrifuge (5415C) at 2,000 rpm (320×g) for 10 min. at 4°C. The pellet was discarded and approximately 300μl of the supernatant was used for determining NO levels and HK activity. The remaining supernatant was re-centrifuged at 13,500 rpm (14,000×g) for 30 min. at 4°C and used for determining SOD activity and GSHt levels. The remaining portion of each brain region was homogenized with 20 strokes in cold homogenizing buffer containing 20mM phosphate buffer (pH 7.4) to which 1% 0.5M butylated hydroxytoluene had been added to prevent oxidation during sample preparation. The homogenate (10%, w/v) was centrifuged in an Eppendorf micro-centrifuge at 6,000 rpm (2,900×g) for 10 min. at 4°C. The pellet was discarded and the supernatant was used for determining the levels of TBARS and protein carbonyls.
The protein content of the samples was determined with a protein assay kit (Bio-Rad Laboratories, Richmond, CA) using bovine plasma gamma globulin as the standard. The amount of protein in the standards and samples was determined on a microtitre plate reader (Molecular Devices Emax precision microplate reader) at a wavelength of 750nm.
Nitric oxide (NO) assay
NO production in situ is difficult to detect due to the rapid decay (within seconds) of this compound in physiological systems. The best index of total NO production is the measurement of the sum its metabolites, nitrates and nitrites (NOx). This is achieved by reduction of nitrates to nitrites by metallic cadmium followed by reaction with the Greiss reagent and spectrophotometric detection. [31]. In this study we measured the accumulation of total nitrites (NO2−) in the samples using the commercial kit from OXIS International (BIOXYTECH nitric oxide non-enzymatic assay, cat #. 22111N). After color development at RT, the absorbance of the samples was measured on a microplate reader at a wavelength of 540nm within 20 min. Sodium nitrite (NaNO2) was used as an external standard and the levels of NO in the samples were expressed as nmol nitrites/g tissue.
Superoxide Dismutase (SOD) activity
SOD activity was measured according to the method of Misra and Fridovich [32]. Tissue extract was added to carbonate buffer (50mM, pH 10.2 containing 0.1mM EDTA) and the reaction initiated with epinephrine (30mM in 0.05% acetic acid). The rate of autoxidation of epinephrine was measured at 480nm for 180s on a Hitachi U2000 spectrophotometer. SOD activity was expressed as units (U) SOD/mg protein, where one unit of SOD is defined as the amount of enzyme present that inhibits the autoxidation of epinephrine by 50%.
Total Glutathione (GSHt) levels
GSHt was measured by the enzymatic recycling procedure in which reduced glutathione (GSH) is sequentially oxidized by 5, 5′-dithiobis-(2-nitrobenzoic acid, DTNB) to oxidized glutathione (GSSG) which is then reduced by NADPH in the presence of GR back to GSH [33]. One hundred μl of either tissue extract or known amounts of GSH standard were added to 800μl of NADPH (0.3mM) and 100μl of DTNB (6mM). The reaction was initiated with 10μl of GR (50units/ml). All solutions were made up in stock buffer (pH 7.5) containing sodium phosphate (125mM) and sodium-EDTA (6.3mM). The rate of DTNB reduction was measured at 412nm continuously for 120s. GSH was used as an external standard, and the level of GSHt in the samples was expressed as nmol GSH/g tissue.
Lipid Oxidation (TBARS) assay
TBARS was measured by the method of Ohkawa et al. [34]. Tissue extract (100μl) was added to a mixture containing 50ul of sodium dodecyl sulphate (8.1%), 375ul of acetic acid (20%, pH 3.5) and 375ul of aqueous thiobarbituric acid (0.8%). The samples were heated in a boiling water bath for 60 min. The samples were allowed to cool at room temperature and then 1.25ml of n-butanol: pyridine (15:1, v/v) was added. The mixture was shaken vigorously and centrifuged at 4,000 rpm (1,300×g) for 10 min. The upper colored organic layer was removed and read at 532nm. The level of lipid peroxides in the samples was expressed as nmol malondialdehyde (MDA)/mg protein, using the molar extinction coefficient of MDA as 1.56×105 (Mcm)−1.
Protein Oxidation (protein carbonyl) assay
Protein carbonyls are formed through oxidation of proteins by a variety of mechanisms. They are sensitive markers of oxidative injury. The customary way of analyzing protein carbonyls is a colorimetric procedure that measures binding of dinitrophenylhydrazine (DNP). This test is an ELISA format using an anti-DNP antibody developed by Buss and Winterbourn [35]. In this study we used the protein carbonyl enzyme (immuno-assay) kit from Northwest Life Science Specialties (Biocell PC Test, cat # NWK-PCK-01). The quantity of protein carbonyls in each sample was determined by derivatizing with dinitrophenylhydrazine (DNP) and measuring bound DNP colorimetrically at 450nm. The amount of protein carbonyl in each sample was calculated from a standard curve, using the protein carbonyl standards provided in the kit, and expressed as nmol protein carbonyls/mg protein.
Hexokinase (HK) activity
HK was measured according to the procedure of Knull et al [36]. Tissue extract was added to the reaction mixture containing 100μl each of glucose (33mM), ATP (67mM), MgCl2 (67mM), potassium HEPES (400mM, pH 7.5), 1-thioglycerol (100mM), NADP+ (6.4mM) and 10ul of glucose 6-phosphate dehydrogenase (1 unit) in a total volume of 1.0 ml. NADPH formation was followed at 340nm for 3 min. HK activity was expressed as units (U) of HK/g tissue, where one unit of HK is defined as μmole NADPH formed/min. using the molar extinction coefficient of NADPH as 6.22×103 (Mcm)−1.
Statistical Analysis
The values from duplicate samples for each biochemical measure (NO, SOD, GSHt, TBARS, protein carbonyls and HK) were averaged to obtain one value point per brain region per animal. Four to six animals per biochemical measure were used. Each biochemical measure from the same brain region was analyzed on the same day. One way ANOVA with post-hoc Newman Keuls was used to determine significance differences between SDSH, UCSH and UCN rats for each biochemical measure for each brain region. Statistical significance was determined at the level of p<0.05.
Results
In this study we investigated whether (6h) sleep deprivation under sustained hypoxia (SDSH) results in free radical production and/or oxidative stress in the rat neocortex, hippocampus, brainstem and cerebellum. We did this by analyzing (i) NO levels, (ii) SOD activity (iii) GSHt levels, (iv) TBARS and (v) protein carbonyls as well as (vi) HK activity.
Indicators of free radical production
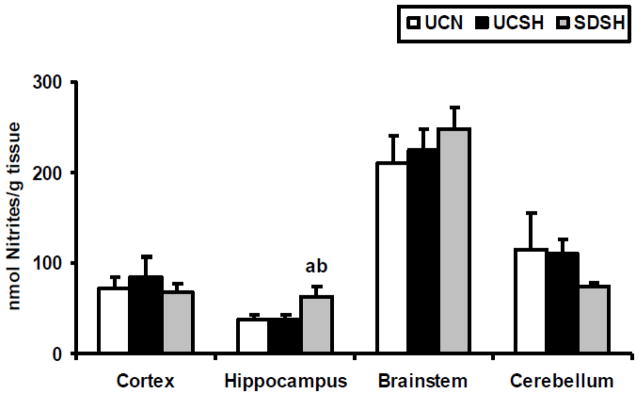
Changes in NO levels in the neocortex, hippocampus, brainstem and cerebellum of SDSH rats compared to UCSH and UCN rats are shown in Fig. 1. One way ANOVA revealed statistically significant differences in NO levels between groups in the hippocampus (F=4.57, P=0.03). Significant increases in NO levels were observed between SDSH and UCSH rats (70%, P=0.04) as well as between SDSH and UCN animals (69%, P=0.03). On the other hand, no significant differences in NO levels were observed in either the neocortex, the brainstem or the cerebellum between rats sleep deprived under hypoxia and sleeping controls under either hypoxia or normoxia (SDSH vs either UCSH or UCN, P>0.05). Furthermore, 6h of sustained hypoxia by itself did not significantly (UCN vs UCSH, P>0.05) change the level of NO in any of the brain regions studied here.
Fig. 1. Changes in Nitric Oxide Levels.
Changes in nitric oxide (NO) levels in the neocortex, hippocampus, brainstem and cerebellum of rats exposed to sleep deprivation under sustained hypoxia (SDSH) versus control animals left undisturbed under either sustained hypoxia (UCSH) or normoxia (UCN). Data are expressed as mean ± S.E.M. a= SDSH vs UCSH, p<0.05; b= SDSH vs UCN, p<0.05.
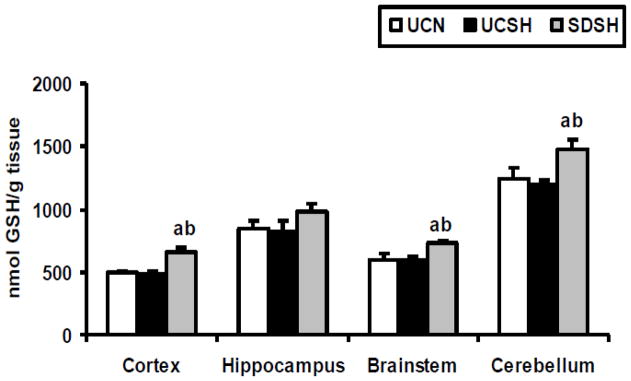
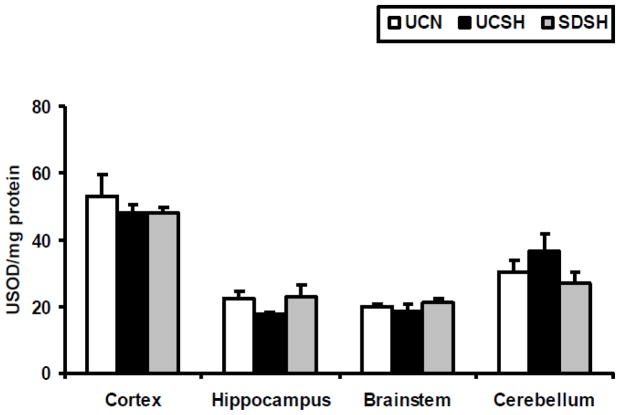
The level of oxygen free radicals was assayed in this study by measuring alterations in the activity of SOD and in the levels of GSHt. Changes in GSHt levels in different brain regions of SDSH rats compared to UCSH and UCN rats are shown in Fig 2. One way ANOVA revealed statistically significant differences between groups in the neocortex (F=10.03, P=0.002), the brainstem (F=9.72, P=0.002) and the cerebellum (F=5.78, P=0.01). Sleep deprivation under hypoxia significantly increased GSHt levels compared to sleeping controls under both hypoxia and normoxia, in the neocortex (SDSH vs UCSH= 35%, P=0.003 and SDSH vs UCN= 31%, P=0.005), the brainstem (SDSH vs UCSH= 21%, P=0.009, and SDSH vs UCN= 22%, P=0.002) and the cerebellum (SDSH vs UCSH= 24%, P=0.01 and SDSH vs UCN= 18%, P=0.04). On the other hand, sleep deprivation under hypoxia did not significantly alter SOD activity in any of the brain regions studied here, although SOD activity increased by 29% (SDSH vs UCSH, P>0.05) in the hippocampus and decreased by 26% (SDSH vs UCSH, P>0.05) in the cerebellum (Fig. 3) of sleep deprived rats versus sleeping controls under hypoxia. A similar effect was observed in the hippocampus (increase) and cerebellum (decrease) with regard to NO levels. Sustained hypoxia alone did not produce any significant changes in either GSHt levels or SOD activity (UCSH vs UCN, P>.0.05) in any of the brain regions studied here.
Fig. 2. Changes in Total Glutathione (GSHt) Levels.
Changes in total glutathione (GSHt) levels in the neocortex, hippocampus, brainstem and cerebellum of rats exposed to sleep deprivation under sustained hypoxia (SDSH) versus control animals left undisturbed under either sustained hypoxia (UCSH) or normoxia (UCN). Data are expressed as mean ± S.E.M. a= SDSH vs UCSH, p<0.05; b= SDSH vs UCN, p<0.05
Fig. 3. Changes in Superoxide Dismutase (SOD) Activity.
Changes in superoxide dismutase (SOD) activity in the neocortex, hippocampus, brainstem and cerebellum of rats exposed to sleep deprivation under sustained hypoxia (SDSH) versus control animals left undisturbed under either sustained hypoxia (UCSH) or normoxia (UCN). Data are expressed as mean ± S.E.M.
Markers of Oxidative stress
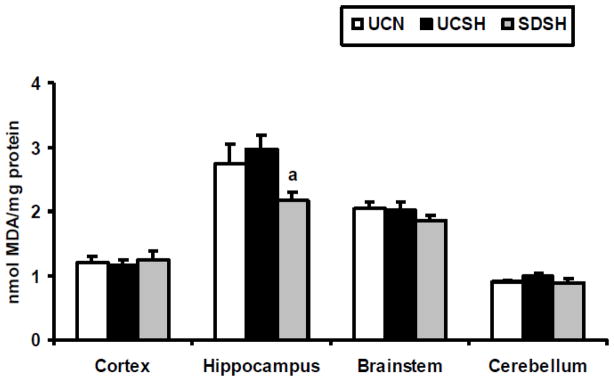
Oxidative stress ensues when antioxidative mechanisms (such as SOD and GSHt) are unable to successfully scavenge the free radicals produced. In this study we measured oxidative stress by analyzing the levels of TBARS (as an indicator of oxidized lipids) and protein carbonyls (as an indicator of oxidized proteins). The levels of TBARS in the neocortex, hippocampus, brainstem and cerebellum of SDSH rats compared to controls (UCSH and UCN) are shown in Fig. 4. Significant differences in the level of TBARS, between sleep deprived under hypoxia and sleeping controls, were observed only in the hippocampus (one way ANOVA, F=5.41, P=0.02) where TBARS was significantly decreased by 27% in sleep deprived hypoxic animals compared to hypoxic controls (SDSH vs UCSH, P=0.02) and insignificantly decreased by 21% compared to normoxic controls (SDSH vs UCN, P=0.06). The levels of protein carbonyls in the brain regions studied here are shown in Fig. 5. Large but non-significant decreases in protein carbonyl levels, between sleep deprived under hypoxia and sleeping control rats, were observed in the hippocampus (SDSH vs UCSH = −28% and SDSH vs UCN= −23%, P>0.05). On the other hand, large but non-significant increases in protein carbonyls, between sleep deprived under hypoxia and sleeping controls, were observed in the brainstem (SDSH vs UCSH= 35% and SDSH vs UCN= 23%, P>0.05). Sustained hypoxia by itself did not produce any significant changes in the levels of TBARS or protein carbonyls (UCSH vs UCN, p>0.05) in any of the brain regions studied here.
Fig. 4. Changes in Levels of TBARS.
Changes in levels of thiobarbituric acid reactive substances (TBARS) in the neocortex, hippocampus, brainstem and cerebellum of rats exposed to sleep deprivation under sustained hypoxia (SDSH) versus control animals left undisturbed under either sustained hypoxia (UCSH) or normoxia (UCN). Data are expressed as mean ± S.E.M. a= SDSH vs UCSH, p<0.05.
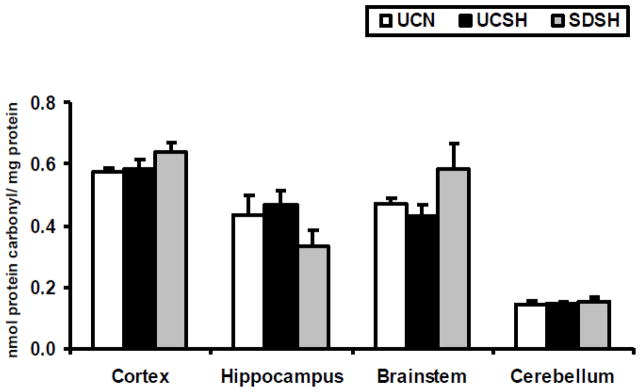
Fig. 5. Changes in Levels of Protein Carbonyls.
Changes in levels of protein carbonyls in the neocortex, hippocampus, brainstem and cerebellum of rats exposed to sleep deprivation under sustained hypoxia (SDSH) versus control animals left undisturbed under either sustained hypoxia (UCSH) or normoxia (UCN). Data are expressed as mean ± S.E.M.
Source of free radicals
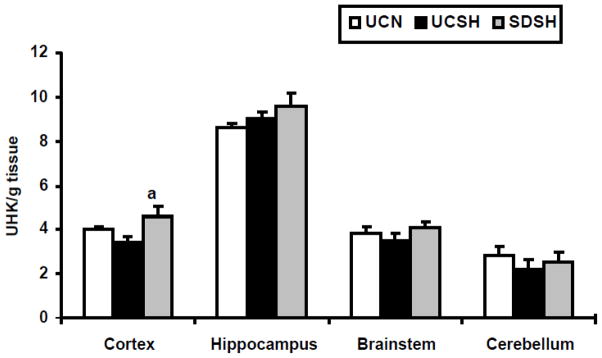
We hypothesize that glucose metabolism may be one potential source of free radicals. Hexokinase (HK) activity was used as a measure of alteration in glucose metabolism. Changes in HK activity in the neocortex, hippocampus, brainstem and cerebellum of SDSH rats compared to UCSH and UCN rats are shown in Fig. 6. The neocortex was the only brain region studied here that showed a significant increase in HK activity with sleep deprivation under sustained hypoxia compared to hypoxic controls (SDSH vs UCSH= 34%, P=0.03). Sustained hypoxia alone did not increase the activity of HK in any of the brain regions studied here.
Fig. 6. Changes in Hexokinase(HK) Activity.
Changes in hexokinase (HK) activity in the neocortex, hippocampus, brainstem and cerebellum of rats exposed to sleep deprivation under sustained hypoxia (SDSH) versus control animals left undisturbed under either sustained hypoxia (UCSH) or normoxia (UCN). Data are expressed as mean ± S.E.M. a= SDSH vs UCSH, p<0.05.
Discussion
This study is the first to demonstrate that sleep deprivation under sustained hypoxia increased antioxidant responses (NO production in the hippocampus, and GSHt levels in the neocortex, brainstem and cerebellum) while decreasing lipid oxidation (TBARS) in the hippocampus, compared to both unhandled hypoxic and normoxic controls. Table 1 summarizes the findings of this study. We also showed increased HK activity in the neocortex of rats subjected to sleep deprivation under sustained hypoxia compared to sleeping controls under hypoxia, indicating that increased glucose metabolism may be one potential source of the elevated free radicals produced in this brain region.
Table 1.
Changes in different markers with normoxia (UCN), sustained hypoxia (UCSH) and sleep deprivation under sustained hypoxia (SDSH) in various rat brain regions.
| Cortex | Hippocampus | Brainstem | Cerebellum | |
|---|---|---|---|---|
| NO Levels | NS | SDSH vs UCSH (p<0.05) SDSH vs UCN (P<0.05) |
NS | NS |
| GSHt Levels | SDSH vs UCSH (p<0.05) SDSH vs UCN (p<0.05) |
NS | SDSH vs UCSH (p<0.05) SDSH vs UCN (p<0.05) |
SDSH vs UCSH (p<0.05) SDSH vs UCN (p<0.05) |
| SOD Activity | NS | NS | NS | NS |
| TBARS Levels | NS | SDSH VS UCSH (p<0.05) SDSH vs UCN (p=0.06) |
NS | NS |
| Protein Carbonyl Levels | NS | NS | NS | NS |
| HK Activity | SDSH vs UCSH (p<0.05) | NS | NS | NS |
GSHt = total glutathione, HK= hexokinase, NO = nitric oxide, NS = not significant, SDSH = sleep deprivation under sustained hypoxia, SOD = superoxide dismutase, TBARS = thiobarbhuric acid reactive substances, UCN = unhandled controls under normoxia, UCSH = unhandled controls under sustained hypoxia
We previously reported that under normoxia/room air, sleep deprivation increased GSHt levels and GPx activity in several rat brain regions, compared to control rats left undisturbed [23]. In this study we report that sleep deprivation under sustained hypoxia increased NO and GSHt levels, compared to sleeping controls under either hypoxia or normoxia. Taken together our findings suggest that 6h of SD under either normoxia [23] or hypoxia (current study) results in increased antioxidative responses, indicative of increased free radical production.
This study demonstrates increased NO levels in the hippocampus of rats subjected to sleep deprivation under sustained hypoxia, compared to sleeping controls under either sustained hypoxia or normoxia. NO is very unstable and rapidly oxidizes to its more stable metabolites, nitrates and nitrites (NOx). In this study, NO was measured by changes in total nitrite levels, after the reduction of nitrates to nitrites. NO is the only endogenous source of brain nitrates and nitrites, since dietary nitrates and nitrites do not cross the blood brain barrier [37]. Increased NO levels, assessed by changes in total nitrite levels, have been observed in the perifornical lateral hypothalamus (PFLH) [38] the basal forebrain (BF), the frontal cortex (CC), but not the cingulate cortex [39] with sleep deprivation (SD). NO levels in the BF gradually increased during the first 3h of SD, while NO levels in the FC increased only after 5h of SD [39]. These authors also reported a similar time course of increase in inducible nitric oxide synthase (iNOS) mRNA and protein levels, mimicking the changes in NO levels in the BF and FC. On the other hand, Hsu et al. [40] reported that 5 days of total SD decreased nicotinamide adenine dinucleotide phosphate-diaphorase (NADPH-d) reactivity in the rat hippocampal CA1, CA2 and CA3 regions as well as in the dentate gyrus, suggesting a suppression of NO production in these regions. These authors also showed a concomitant decrease in neuronal nitric oxide synthase (nNOS) protein levels. Khadrawy et al. [41] on the other hand, showed that 72h of REM sleep deprivation increased hippocampal NO production without affecting cortical NO production. The neuronal phenotype within the hippocampus as well as the source of NO in our study remains to be determined.
In our study we also showed that sleep deprivation under sustained hypoxia significantly increased GSHt levels in the rat neocortex, brainstem and cerebellum compared to sleeping controls under either sustained hypoxia or normoxia. We previously reported that sleep deprivation under normoxia increased GSHt levels in the rat neocortex, brainstem and basal forebrain and we speculated that this may account for the ability of sleep-deprived rats to compensate for any deficit in working memory [23]. D’Almeida et al. [24] reported that 96h of REM sleep deprivation, on the other hand, significantly decreased GSHt levels in the rat hypothalamus. Glutathione is one of the most important physiological antioxidant involved in the detoxification of hydrogen peroxide and lipid hydroperoxide [42]. In the presence of transition metal ions (Cu 2+ and Fe 2+) these peroxides can form the highly reactive hydroxyl radical which can cause the oxidation of lipids, proteins and nucleic acids, resulting in oxidative stress. Glutathione is found in the cytosol of cells in the range of 1–10mM [43]. Glutathione exists in either its reduced (GSH) or oxidized (GSSG) states. GSH is able to donate a reducing equivalent to other unstable molecules, such as reactive oxygen species, and in the process becomes GSSG. GSH can then be regenerated from GSSG by the enzyme glutathione reductase. In normal cells, more than 90% of the total glutathione pool is in the GSH form with less than 10% in the GSSG form. However, even during oxidative stress, the ratio of GSH/GSSG remains very high [42], indicating that most of the glutathione pool exists as reduced glutathione, which is a very potent antioxidant.
In the present study we also report that sleep deprivation under sustained hypoxia decreased the level of TBARS in the rat hippocampus compared to sleeping controls under either sustained hypoxia or normoxia. On the other hand, Suer et al. [44] showed that 21 days of intermittent REM sleep deprivation (with 6h of recovery sleep every day) increased lipid oxidation, while decreasing SOD and GPx activities in the rat hippocampus. They further suggested that this alteration in the antioxidant defense system may account for the impaired maintenance of long term potentiation observed in these rats. Khadrawy et al. [41] also reported that 72h of REM sleep deprivation increased lipid oxidation in both the hippocampus and the cortex. The levels of TBARS in the neocortex, brainstem and cerebellum were not altered in our study. The increased GSHt levels in these brain regions suggest an increase in free radical production. The lack of change in the levels of TBARS indicate that the free radicals were successfully removed by the endogenous antioxidant (GSH), thus preventing oxidative stress.
We did not find any significant changes in the level of carbonyl proteins in any of the brain regions studied here under any of the three treatment conditions. Carbonyl protein levels were decreased in the hippocampus and increased in the brainstem. These changes although large, were insignificant due to the high degree of variation in the samples.
We speculated that one potential source of free radicals is from elevated glucose metabolism. Reimund et al. [2] hypothesized that free radicals accumulate during waking as a result of enhanced metabolic activity. In this study we showed that sleep deprived rats under hypoxia had higher neocortical HK activity compared to control rats allowed to sleep under hypoxia. This increase in HK activity indicates enhanced glucose metabolism, which could result in elevated free radical production. We previously also reported that sleep deprivation under normoxia significantly increased HK activity in the rat neocortex and hypothalamus compared to sleeping controls [23]. The mitochondrial electron transport chain is a main source of free radicals and the mitochondrial enzyme, Mn-SOD is a potent antioxidant [reviewed in 45, 46]. We did not measure mitochondrial activity in our current study, but we will do so in future experiments.
Bailey et al. [47] reported increased cerebral oxidative and nitrosative stress in healthy men exposed to 9 hours of hypoxia (12.9% oxygen) compared to normoxia. They showed increased arterial and venous levels of lipid hydroperoxides and alkoxyl-alkyl free radicals that correlated with increased acute mountain sickness/headache scores, as well as increased levels of 3-nitrotyrosine and decreased levels of plasma NO metabolites that correlated against acute mountain sickness/headache scores. Maiti et al. [48] reported that high altitude (hypobaric hypoxia) exposure increased the formation of reactive oxygen and nitrogen species in several rat brain regions. These authors showed that rats subjected to simulated altitude of 6100 m for 3 or 7 days exhibited decreased antioxidant responses and increased oxidative stress in the cortex, hippocampus and striatum, compared to rats kept at sea level. These authors suggested that the hippocampus was the most susceptible brain region to the effects of hypoxia. We previously also reported that sustained/continuous hypoxia enhanced GR activity in the rat pons (after 6h) and elevated TBARS levels in the rat cerebellum (after 1 day) [25], indicating that sustained hypoxia increases antioxidant responses and results in oxidative stress. In our current study, however, we did not observe any changes in the measured antioxidant responses (SOD activity and GSHt levels), nitrogen free radical production or oxidative stress markers (TBARS and carbonyl proteins) due to hypoxia itself. This could be due to the short term exposure to sustained hypoxia, only 6 hours. In future studies we will prolong the sleep deprivation procedure as well as include a sleep recovery group under sustained hypoxia, in order to see the effects on antioxidant responses and oxidative stress markers.
In conclusion, this study is the first to demonstrate that sleep loss under sustained hypoxia, leads to increased nitric oxide production in the rat hippocampus and increased total glutathione levels in the rat neocortex, brainstem and cerebellum, while decreasing hippocampal levels of TBARS. Hence, short term insomnia under sustained hypoxia may serve as an adaptive response to prevent oxidative stress.
Highlights.
Rats were exposed to total sleep deprivation under sustained hypoxia (SDSH),
SDSH increased nitric oxide (NO) production in the hippocampus,
Increased total glutathione (GSHt) levels in the neocortex, brainstem and cerebellum,
Decreased hippocampal lipid oxidation,
Insomnia under hypoxia could be an adaptive response in preventing oxidative stress
Acknowledgments
This research was supported by grants NS14610, MH64109 and the Medical Research Service of the Department of Veterans Affairs.
List of Abbreviations
- HK
hexokinase
- NO
nitric oxide
- SDSH
sleep deprived under sustained hypoxia
- GSHt
total glutathione
- SOD
superoxide dismutase
- TBARS
thiobarbituric acid reactive substances
- UCN
undisturbed control under normoxia
- UCSH
undisturbed control under sustained hypoxia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huck NO, McBride SA, Kendall AP, Grugle NL, Killgore WD. The effects of modafinil, caffeine and dextroamphetamine on judgments of simple versus complex emotional expressions following sleep deprivation. Int J Neurosci. 2008;118:487–502. doi: 10.1080/00207450601125907. [DOI] [PubMed] [Google Scholar]
- 2.Reimund E. The free radical flux theory of sleep. Med Hypotheses. 1994;43:231–233. doi: 10.1016/0306-9877(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 3.Bailey DM, Davies B, Young IS, Hullin DA, Seddon PS. A potential role for free radical-mediated skeletal muscle soreness in the pathophysiology of acute mountain sickness. Aviat Space Environ Med. 2001;72:513–521. [PubMed] [Google Scholar]
- 4.Baumgartner RW, Eichenberger U, Bartsch P. Postural ataxia at high altitude is not related to mild to moderate acute mountain sickness. Eur J Appl Physiol. 2002;86:322–326. doi: 10.1007/s00421-001-0534-8. [DOI] [PubMed] [Google Scholar]
- 5.Regard M, Landis T, Casey J, Maggiorini M, Bärtsch P, Oelz O. Cognitive changes at high altitude in healthy climbers and in climbers developing acute mountain sickness. Aviat Space Environ Med. 1991;62:291–295. [PubMed] [Google Scholar]
- 6.Kramer A, Coyne JT, Strayer DL. Cognitive function at high altitude. Hum Factors. 1993;35:329–344. doi: 10.1177/001872089303500208. [DOI] [PubMed] [Google Scholar]
- 7.Bartholomew C, Jensen W, Petros TV, Ferraro FR, Fire KM, Biberdorf D, Fraley E, Schalk J, Blumkin D. The effect of moderate levels of simulated altitude on sustained cognitive performance. Int J Aviat Psychol. 1999;9:351–359. doi: 10.1207/s15327108ijap0904_3. [DOI] [PubMed] [Google Scholar]
- 8.Bahrke MS, Hale SB. Effects of altitude on mood, behavior and cognitive functioning. Sports Med. 1993;16:97–125. doi: 10.2165/00007256-199316020-00003. [DOI] [PubMed] [Google Scholar]
- 9.Lippi FJ, Neubauer S, Schipfer S, Lichter N, Tufman A, Otto B, Fischer R. Hypobaric hypoxia causes body weight reduction in obese subjects. Obesity. 2010;18:675–681. doi: 10.1038/oby.2009.509. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton AJ, Trad LA, Cymerman A. Alterations in human upper extremity motor function during acute exposure to simulated altitude. Aviat Space Environ Med. 1991;62:759–764. [PubMed] [Google Scholar]
- 11.Joanny P, Steinberg J, Robach P, Richalet JP, Gortan C, Gardette B, Jammes Y. Operation Everest III (Comex’97): the effect of simulated sever hypobaric hypoxia on lipid peroxidation and antioxidant defence systems in human blood at rest and after maximal exercise. Resuscitation. 2001;49:307–314. doi: 10.1016/s0300-9572(00)00373-7. [DOI] [PubMed] [Google Scholar]
- 12.Moller P, Loft S, Lundby C, Olsen NV. Acute hypoxia and hypoxic exercise induce DNA strand breaks and oxidative DNA damage in humans. FASEB J. 2001;15:1181–1186. doi: 10.1096/fj.00-0703com. [DOI] [PubMed] [Google Scholar]
- 13.Jefferson JA, Simoni J, Escudero E, Hurtado ME, Swenson ER, Wesson DE, Schreiner GF, Schoene RB, Johnson RJ, Hurtado A. Increased oxidative stress following acute and chronic high altitude exposure. High Alt Med Biol. 2004;5:61–69. doi: 10.1089/152702904322963690. [DOI] [PubMed] [Google Scholar]
- 14.Magalhaes J, Ascensão A, Viscor G, Soares J, Oliveira J, Marques F, Duarte J. Oxidative stress in humans during and after 4 hours of hypoxia at a simulated altitude of 5500 m. Aviat Space Environ Med. 2004;75:16–22. [PubMed] [Google Scholar]
- 15.Pialoux V, Mounier R, Brown AD, Steinback CD, Rawling JM, Poulin MJ. Relationship between oxidative stress and HIF-1 alpha mRNA during sustained hypoxia in humans. Free Radic Biol Med. 2009;46:321–326. doi: 10.1016/j.freeradbiomed.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 16.Maiti P, Singh SB, Sharma AK, Muthuraju S, Banerjee PK, Ilavazhagan G. Hypobaric hypoxia induces oxidative stress in rat brain. Neurochem Int. 2006;49:709–716. doi: 10.1016/j.neuint.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Maiti P, Singh SB, Mallick B, Muthuraju S, Ilavazhagan G. High altitude memory impairment is due to neuronal apoptosis in hippocampus, cortex and striatum. J Chem Neuroanat. 2008;36:227–238. doi: 10.1016/j.jchemneu.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Maiti P, Singh SB, Ilavazhagan G. Nitric oxide system is involved in hypobaric hypoxia-induced oxidative stress in rat brain. Acta histochem. 2010;112:222–232. doi: 10.1016/j.acthis.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Swamy M, Salleh MJ, Sirajudeen KN, Yusof WR, Chandran G. Nitric oxide (no), citrulline - no cycle enzymes, glutamine synthetase and oxidative stress in anoxia (hypobaric hypoxia) and reperfusion in rat brain. Int J Med Sci. 2010;7:147–154. doi: 10.7150/ijms.7.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramanathan L, Gulyani S, Nienhuis R, Siegel JM. Sleep deprivation decreases superoxide dismutase activity in rat hippocampus and brainstem. Neuroreport. 2002;13:1387–1390. doi: 10.1097/00001756-200208070-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gopalakrishnan A, Ji LL, Cirelli C. Sleep deprivation and cellular responses to oxidative stress. Sleep. 2004;27:27–35. doi: 10.1093/sleep/27.1.27. [DOI] [PubMed] [Google Scholar]
- 23.Ramanathan L, Hu S, Frautschy S, Siegel JM. Short-term total sleep deprivation in the rat increases antioxidant responses in multiple brain regions without impairing spontaneous alternation behavior. Behav Brain Res. 2010;207:305–309. doi: 10.1016/j.bbr.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Almeida V, Lobo LL, Hipolide DC, de Oliveira AC, Nobrega JN, Tufik S. Sleep deprivation induces brain region-specific decreases in glutathione levels. Neuroreport. 1998;9:2853–2856. doi: 10.1097/00001756-199808240-00031. [DOI] [PubMed] [Google Scholar]
- 25.Ramanathan L, Gozal D, Siegel JM. Antioxidant responses to chronic hypoxia in the rat cerebellum and pons. J Neurochem. 2005;93:47–52. doi: 10.1111/j.1471-4159.2004.02988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drager LF, Jun JC, Polotsky VY. Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best Pract Res Clin Endocrinol Metab. 2010;24:843–851. doi: 10.1016/j.beem.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Y, Zhang SX, Gozal D. Reactive oxygen species and the brain in sleep apnea. Respir Physiol Neurobiol. 2010;174:307–316. doi: 10.1016/j.resp.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalinchuk AV, Lu Y, Stenberg D, Rosenberg PA, Porkka-Heiskanen T. Nitric oxide production in the basal forebrain is required for recovery sleep. J Neurochem. 2006;99:483–498. doi: 10.1111/j.1471-4159.2006.04077.x. [DOI] [PubMed] [Google Scholar]; stroke severity in rats. Exp Neurol. 2010;222:135–143. doi: 10.1016/j.expneurol.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 29.Poirrier J-E, Guillonneau F, Renaut J, Sergeant K, Luxen A, Maquet P, Leprince P. Proteomic changes in rat hippocampus and adrenals following short-term sleep deprivation. Proteome Sci. 2008;22:6–14. doi: 10.1186/1477-5956-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moldovan M, Conctantinescu A-O, Balseanu A, Oprescu N, Zagrean L, Wagner A-P. Sleep deprivation attenuates experimental stroke severity in rats. Exp Neurol. 2010;222:135–143. doi: 10.1016/j.expneurol.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 31.Vodovotz Y. Modified microassay for serum nitrite and nitrate. Bio Techniques. 1996;20:390–394. doi: 10.2144/19962003390. [DOI] [PubMed] [Google Scholar]
- 32.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 33.Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- 34.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 35.Buss H, Chan TP, Sluis KB, Domigan NM, Winterbourn CC. Protein carbonyl measurement by a sensitive ELISA method. Free Radic Biol Med. 1997;23:361–366. doi: 10.1016/s0891-5849(97)00104-4. [DOI] [PubMed] [Google Scholar]
- 36.Knull HR, Taylor WF, Wells WW. Effects of energy metabolism on in vivo distribution of hexokinase in brain. J Biol Chem. 1973;248:5414–5417. [PubMed] [Google Scholar]
- 37.Clark RS, Kochanek PM, Obrist WD, Wong HR, Billiar TR, Wisniewski SR, Marion DW. Cerebrospinal fluid and plasma nitrite and nitrate concentrations after head injury in humans. Crit Care Med. 1996;24:1243–1251. doi: 10.1097/00003246-199607000-00030. [DOI] [PubMed] [Google Scholar]
- 38.Kostin A, Rai S, Kumar S, Szymusiak R, McGinty D, Alam MN. Nitric oxide production in the perifornical-lateral hypothalamic area and its influences on the modulation of perifornical lateral hypothalamic area neurons. Neuroscience. 2011;179:159–169. doi: 10.1016/j.neuroscience.2011.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalinchuk AV, McCarley RW, Porkka-Heiskanen T, Basheer R. The time course of adenosine, nitric oxide (NO) and inducible NO synthase changes in the brain with sleep loss and their role in the non-rapid eye movement sleep homeostatic cascade. J Neurochem. 2011;116:260–272. doi: 10.1111/j.1471-4159.2010.07100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu JC, Lee YS, Chang CN, Chuang HL, Ling EA, Lan CT. Sleep deprivation inhibits expression of NADPH-d and NOS while activating microglia and astroglia in the rat hippocampus. Cells Tissues Organs. 2003;173:242–254. doi: 10.1159/000070380. [DOI] [PubMed] [Google Scholar]
- 41.Khadrawy YA, Nour NA, Aboul Ezz HS. Effect of oxidative stress induced by paradoxical sleep deprivation on the activities of Na+, K+-ATPase and acetylcholinesterase in the cortex and hippocampus of rat. Transl Res. 2011;157:100–107. doi: 10.1016/j.trsl.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Forman HJ, Zhang H, Rinna A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meister A. Glutathione metabolism and its selective modification. J Biol Chem. 1988;263:17205–17208. [PubMed] [Google Scholar]
- 44.Suer C, Dolu N, Artis AS, Sahin L, Yilmaz A, Cetin A. The effects of long term sleep deprivation on the long-term potentiation in the dentate gyrus and brain oxidation status in rats. Neurosci Res. 2011;70:71–77. doi: 10.1016/j.neures.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Ozden O, Park SH, Kim HS, Jiang H, Coleman MC, Spitz DR, Gius D. Acetylation of MnSOD directs enzymatic activity responding to cellular nutrient status or oxidative stress. Aging (Albany NY) 2011;3:102–107. doi: 10.18632/aging.100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holley AK, Dhar SK, St Clair DK. Manganese superoxide dismutase versus p53: the mitochondrial center. Ann NY Acad Sci. 2010;1201:72–78. doi: 10.1111/j.1749-6632.2010.05612.x. [DOI] [PubMed] [Google Scholar]
- 47.Bailey DM, Taudorf S, Berg RM, Lundby C, McEneny J, Young IS, Evans KA, James PE, Shore A, Hullin DA, McCord JM, Pedersen BK, Möller K. Increased cerebral output of free radicals during hypoxia: implications for acute mountain sickness? Am J Physiol Regul Integr Comp Physiol. 2009;297:R1283–1292. doi: 10.1152/ajpregu.00366.2009. [DOI] [PubMed] [Google Scholar]
- 48.Maiti P, Singh SB, Sharma AK, Muthuraju S, Banerjee PK, Ilavazhagan G. Hypobaric hypoxia induces oxidative stress in rat brain. Neurochem Int. 2006;49:709–716. doi: 10.1016/j.neuint.2006.06.002. [DOI] [PubMed] [Google Scholar]