Abstract
Chronic intestinal inflammation leads to increased risk of colorectal and small intestinal cancers, and is also associated with extraintestinal manifestations such as lymphomas, other solid cancers, and autoimmune disorders. We have previously found that acute and chronic intestinal inflammation causes DNA damage to circulating peripheral leukocytes, manifesting a systemic effect in genetic and chemically-induced models of intestinal inflammation. This study addresses the scope of tissue targets and genotoxic damage induced by inflammation-associated genotoxicity. Using several experimental models of intestinal inflammation, we analyzed various types of DNA damage in leukocyte subpopulations of the blood, spleen, mesenteric and peripheral lymph nodes; and, in intestinal epithelial cells, hepatocytes, and the brain. Genotoxicity in the form of DNA single and double stranded breaks accompanied by oxidative base damage was found in leukocyte subpopulations of the blood, diverse lymphoid organs, intestinal epithelial cells, and hepatocytes. The brain did not demonstrate significant levels of DNA double strand breaks as measured by γ-H2AX immunostaining. CD4+ and CD8+ T-cells were most sensitive to DNA damage versus other cell types in the peripheral blood. In vivo measurements and in vitro modeling suggested that genotoxicity was induced by increased levels of systemically circulating proinflammatory cytokines. Moreover, genotoxicity involved increased damage rather than reduced repair, since it not associated with decreased expression of the DNA double-strand break recognition and repair protein, ataxia telangiectasia mutated (ATM). These findings suggest that levels of intestinal inflammation contribute to the remote tissue burden of genotoxicity, with potential effects on non-intestinal diseases and cancer.
Keywords: intestinal inflammation, genotoxicity, ulcerative colitis, Crohn’s disease
Introduction
Inflammatory bowel disease (IBD), encompassing both ulcerative colitis and Crohn’s disease, affects millions of people worldwide and is characterized by recurring episodes of intestinal inflammation. Chronic intestinal inflammation is well known to increase the risk of colorectal and small intestinal cancers 1–3. Mechanisms of intestinal inflammation as well as its progression to colorectal cancer have been extensively studied, centering on a dysregulated immune response to commensal flora caused by transient breaks in the mucosal barrier 4. In addition to intestinal/colorectal cancer, long standing intestinal inflammation is associated with the development of various non-Hodgkin’s T-cell lymphomas; including colonic, rectal, hepatic, gut-associated lymphoid tissue (GALT), and enteropathy-associated T-cell lymphomas, as well as hepatobiliary and rectal carcinoma 2, 5. In addition to cancers, positive associations have been found with the development of autoimmune disorders such as systemic lupus erythromatosus (SLE), psoriasis, insulin dependent diabetes, and rheumatoid arthritis 6. Mechanisms involved in the development of these extraintestinal manifestations, however, have not been well studied.
Several animal models have been developed to study the pathogenesis of IBDs, including G-protein alpha-i2 subunit (Gαi2)−/− and interleukin (IL)-10−/− mice, both susceptible to immune-mediated colitis and inflammation-associated adenocarcinoma. Gαi2−/− mice develop a severe T helper (Th) 1/Th17 driven colitis as soon as a few weeks after birth and colorectal cancer within 3–4 months due to aberrant T-cell ontogeny, decreased IL-10 production, and defective chemotactic migration of thymic and colonic T-cells 7–9. On the other hand, IL-10−/− mice have a slower disease progression with mild intestinal inflammation initiating after 3–4 months 10, exemplified by a CD4+ Th1- and Th17- driven colitis involving various other innate cell mechanisms. As the mice age, clinical severity of inflammation progresses, eventually leading to development of adenocarcinoma between 6–12 months of age 11, 12.
Recently, we have found that intestinal inflammation in experimentally-induced and genetically susceptible models of spontaneous immune colitis induce genotoxicity to peripheral leukocytes, away from the local site of inflammation, correlating to clinical severity of inflammation 13. In an effort to further characterize this phenomenon and identify sensitive cell types, subpopulations of leukocytes in the peripheral blood as well as cells from various distant lymphoid and non-lymphoid tissues were analyzed for DNA single- and double- stranded breaks. Illuminating sensitive cell types to inflammation-associated genotoxicity will serve as a basis for delineating mechanisms of the development of extraintestinal manifestations including lymphomas, as well as allowing for utilization of DNA damage as cancer risk marker.
Materials and methods
Animals
Gαi2−/− and Gαi2+/− (B6/129Sv background, 3 months) 7, IL-10−/− (C3H/HeJBir background, 8 weeks or 6 months) and wildtype mice (C3H/HeJ and C57BL6/J) were housed in the UCLA Department of Laboratory and Animal Medicine under specific pathogen free conditions, autoclaved bedding and food, with standard rodent chow diet, acidified drinking water, and 12:12 light:dark cycle. All mice were bred at UCLA except IL-10−/− and C3H/HeJ which were purchased from Jackson Laboratory (Bar Harbor, ME).
Induction of experimental colitis
Chronic experimental colitis was induced in wildtype mice (C57BL6/J) by administering 3% (w/v) DSS (MP Biomedicals, MW 40,000) dissolved in sterile acidified drinking water ad libitum for 2 cycles, each cycle consisting of 7 days treated water followed by 14 days of normal water.
Blood and tissue collection
Peripheral blood was collected via the facial/mandibular vein with a 5 mm lancet (Braintree Scientific, Braintree, MA) into EDTA coated collection tubes (Braintree Scientific). For magnetic bead separations, a terminal bleed utilizing 500μL was used. For the comet assay, blood was immediately diluted 1:1 in 10% DMSO/RPMI media and immediately frozen at −80°C until further analysis. Freshly collected blood was immediately processed for all other assays. As a positive control for the DNA damage assays, an aliquot of peripheral blood was incubated with 80μM H2O2 for 15minutes. Spleens, peripheral lymph nodes (PLN) including both axillary and inguinal lymph nodes (at least 5/mouse) and mesenteric lymph nodes (MLN) (at least 5/mouse), and the liver were harvested and processed into single cell suspensions in 10% FBS, 10% DMSO, 25mM EDTA in RPMI for further analysis 14, 15. Lymphocytes were excluded from liver preparations by centrifugation, and by negative selection with magnetic beads and only hepatocytes were analyzed for DNA damage after visual confirmation. Isolation of intestinal epithelial/intraepithelial cells was done as described previously 16. Briefly, the small and large intestine were cut open longitudinally and washed extensively with DMEM media to remove lumen contents. Tissues were then cut into 5 mm pieces and incubated with 15 ml DMEM media containing 1 mM DTT for 30 min at 37 °C to release epithelial cells and intraepithelial lymphocytes. Resultant supernatants were filtered through 100 mm strainer (BD) to yield a single cell suspension which was harvested by centrifugation.
Assessment of DNA damage
Alkaline comet assay
To detect DNA strand breaks, as well as alkali labile sites in DNA, the alkaline comet assay was performed as described elsewhere 13, 17, 18. Briefly, after lysis and electrophoresis, gels were stained with SYBR Gold (Molecular Probes) and visualized at 10x magnification. The olive tail moment, which represents both tail length and fraction of DNA in the tail, was used for data collection and analysis, in which apoptotic cells were excluded under previously proposed criteria 17.
Determination of oxidative DNA damage
For determination of oxidative DNA damage the enzyme hOgg1-modified comet assay was used as previously described elsewhere 13, 18, 19.
Immunofluorescence
Peripheral blood and splenocytes were incubated in Buffer EL (Qiagen, Valencia, CA) on ice to remove erythrocytes. Other single cell suspensions did not require erythrocyte lysis. Samples were then processed on coverslips as described elsewhere 13, 18, 20. Briefly, cells were incubated with mouse anti-phospho-Histone H2A.X S139(P) (Upstate, Temecula, CA) at 1:400 followed by FITC-conjugated anti-mouse IgG (Jackson ImmunoResearch, West Grove, PA) at 1:200. Coverslips were mounted with VECTASHIELD with 4,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA). At least 125 cells were counted and cells with more than four distinct foci in the nucleus were considered positive 20. Apoptotic cells are easily distinguishable due to presence of 10-fold higher number of nuclear foci than highly damaged cells 21, and were not included in analyses.
Paraffin sections (5 μm) of transverse sections of the brain from 12–14 week old Gαi2−/− and Gαi2+/− mice were microwaved in 10 mM citrate buffer with 0.05% Tween 20 (pH 6) for 10 min for antigen retrieval, blocked, then incubated with identical antibodies as described above at 1:120 for the primary antibody, and 1:200 for the secondary antibody. Images were captured with CytoVision® (Applied Imaging, UK) and at least 125 cells were counted per slide.
Magnetic bead isolation of cells
Individual subpopulations of cells in the peripheral blood were isolated by magnetic bead isolation. Leukocytes were separated from whole blood by density centrifugation with Histopaque-1119 (Sigma) according to manufacturer’s instructions. Cells were then labeled with MicroBeads conjugated to monoclonal mouse antibodies (anti-CD4, anti-CD8, anti-CD19, and anti-CD11b) and magnetically separated by positive selection on MS columns (Miltenyi Biotec, Bergisch Gladbach, Germany).
Gene and protein expression
Gene expression was measured as mRNA transcript levels of ataxia telangiectasia mutated (ATM), tumor necrosis factor-α (TNF-α), monocyte chemotactic protein-1 (Ccl2), interleukin-1β (IL1b), and interferon-γ (IFNg) standardized to TATA box binding protein (TBP), the internal control gene in peripheral leukocytes by quantitative real time PCR utilizing Taqman Gene Expression kits (Applied Biosystems) as previously described 13, 18. Protein expression was measured as mean fluorescence intensity of anti-ATM protein kinase (pSer1981) by flow cytometry. Briefly, cells were stained for cell surface markers (PE-anti-CD4 or PerCP anti-CD8), and then processed for intracellular staining. Cells were fixed with 1.5% paraformaldehyde for 10 min at room temperature, permeabilized, and then stained with FITC-anti-pATM (Rockland Immunochemicals, Inc) or the appropriate isotype control, and analyzed on a BD FACScan machine.
Statistical analyses
Results are expressed as mean ± standard error of the mean. Statistical significance was determined by nonparametric one-way/two-way ANOVAs with Dunn’s multiple comparison post test or paired Student’s t-tests, defined as p<0.05. Calculations were performed with the statistical analysis software GraphPad Instat version 3.00 (GraphPad Software, San Diego, CA) or R: A language and environment for statistical computing (R Development Core Team, Vienna, Austria).
Results
Differences in susceptibility to DNA damage in peripheral blood subpopulations
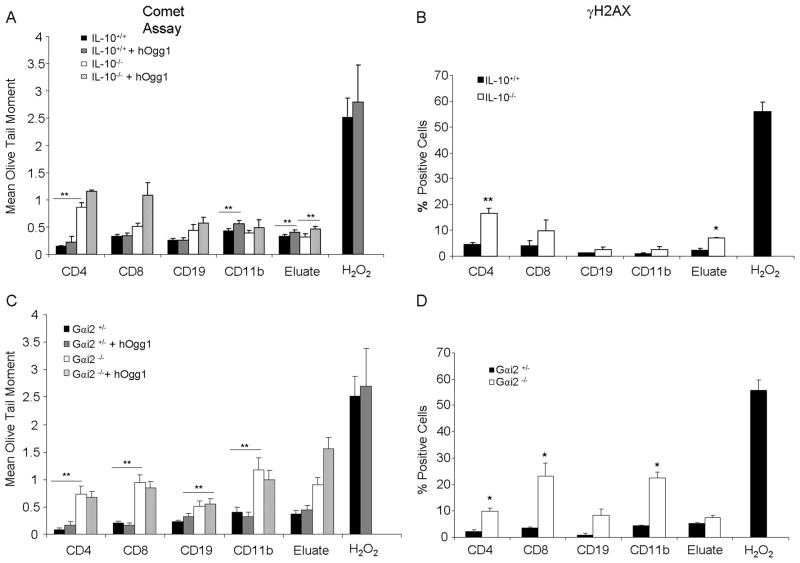
In order to determine leukocytes that may be sensitive to inflammation-associated genotoxicity, subpopulations in the peripheral blood were isolated via magnetic bead separation and analyzed for DNA damage in Gαi2−/− (3 months) and IL-10−/− mice (6 months) with colitis. CD4+ T-cells from IL-10−/− mice had significantly more DNA strand breaks as measured by the mean olive tail moment in the alkaline comet assay and as percent positive cells for γ-H2AX foci compared to wildtype littermates and to other cell types (Figures 1A and 1B). The eluate, containing cell types not positively selected by magnetic beads, from IL-10−/− mice also contained significantly more positive cells for γ-H2AX foci, indicative of DNA double-stranded breaks, compared to wildtype mice. Oxidative base damage, as measured with the modified alkaline comet assay with hOgg1 incubation, was also present in all cell types, though most differences were not statistically significant, except in cells from the eluate from both genotypes, and to CD11b+ cells from wildtype mice. As a positive control, cells were incubated with hydrogen peroxide, in which much higher levels of strand breaks were observed.
Fig. 1.
Genotoxicity to peripheral leukocyte subpopulations. A, B. DNA damage as measured by alkaline comet assay with or without hOgg1 incubation and γ-H2AX immunostaining, respectively, in IL-10−/− versus wildtype mice, or H2O2 control. C, D. DNA damage as measured by alkaline comet assay with or without hOgg1 incubation and γ-H2AX immunostaining, respectively, in Gαi2−/− versus Gαi2+/− mice or H2O2 control. *: p<0.05, **: p<0.01 by two way ANOVA with Dunn’s multiple comparison test. Error bars represent standard error of the mean (SEM) of 5–7 mice per group.
In Gαi2−/− mice, whose disease progression is faster and more severe than in IL-10−/− mice, DNA strand breaks were observed more frequently in multiple cell types including CD4+ and CD8+ T-cells, as well as CD11b+ monocytes including macrophages, and to CD19+ B-cells and cells in the eluate compared to heterozygous littermates which do not develop colitis (Figures 1C and 1D). Results from the alkaline comet assay and γ-H2AX immunostaining correlated with each other, demonstrating a wider array of cell types damaged in the peripheral blood compared to the IL-10−/− mice. Clinical severity of inflammation therefore may correlate to a wider array of cell types affected.
Genotoxicity in lymphoid organs
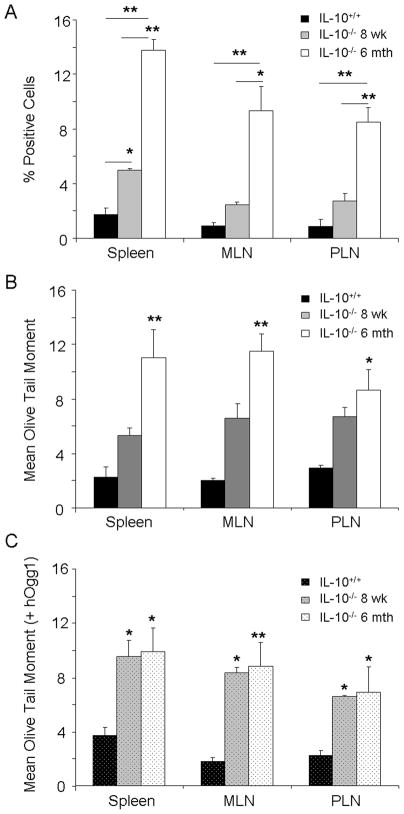
Lymphoid organs such as the spleen, mesenteric and peripheral lymph nodes were isolated into single cell suspensions from IL-10−/− (6 months) and wildtype mice, and analyzed for DNA damage. Surprisingly, all lymphoid tissues demonstrated significant genotoxicity compared to wildtype mice, characterized by DNA single- and double-stranded breaks with oxidative base damage, comparable to that seen in the peripheral leukocytes (Figures 2A – 2C). Mesenteric lymph nodes, though physically in closest contact with the site of inflammation in the colon, demonstrated similar levels of DNA damage found in the peripheral lymph nodes, collected at distant sites relative to the site of inflammation. The spleen also showed similar levels of DNA strand breaks, indicating systemic circulation of the cell types/immune mediators responsible for the observed genotoxicity.
Fig. 2.
DNA damage to peripheral lymphoid organs. A, B, C. DNA damage as determined by γ-H2AX immunostaining, alkaline comet assay without hOgg1 incubation, and alkaline comet assay with hOgg1 incubation, respectively, in IL-10−/− mice at 8 weeks of age and 6 months of age versus wildtype mice. MLN: mesenteric lymph node, PLN: peripheral lymph node.*: p<0.05, **: p<0.01 by two way ANOVA with Dunn’s multiple comparison test. Error bars represent SEM of 5–7 mice per group.
Previously, we demonstrated that DNA damage correlated with disease activity in the peripheral leukocytes of IL-10−/− mice 13. Similar to that of leukocytes, young IL-10−/−mice of 8 weeks of age with subclinical inflammation representing lower disease activity than those with severe colitis at 6 months of age, manifested lower DNA damage in all the lymphoid organs compared to the older mice (Figures 2A–2C). Levels of oxidative base damage, measured by the alkaline comet assay with hOgg1 incubation, however, were similar in the 8 week old mice as compared to the 6 month old mice, indicating a larger presence of these oxidative lesions in the 8 week old mice, though the levels of total DNA strand breaks were less than in the 6 month old mice (Figure 2C).
Large and small intestinal epithelial cell genotoxicity
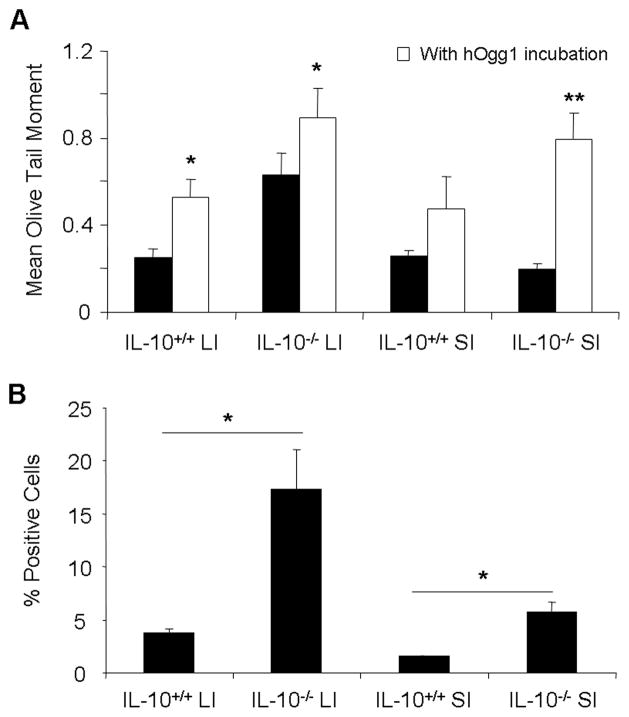
In addition to peripheral leukocytes and the lymphoid organs, intestinal epithelial cells and intraepithelial cells were isolated from the large and small intestine of IL-10−/− (6 months of age) and wildtype mice. As expected from the sites of major inflammatory activity, epithelial cells from the large intestine harbored greater genotoxicity than those from the small intestine, and IL-10−/− mice demonstrated significantly greater genotoxicity to intestinal epithelial cells from both the small intestine and large intestine than in wildtype mice (Figures 3A and 3B). Oxidative base damage was significantly elevated in the large intestine of both wildtype and knockout mice, and in the small intestine of the knockout mice (Figure 3A). Genotoxicity to these cell types was therefore characterized by the presence of DNA single- and double-stranded breaks with oxidative base damage.
Fig. 3.
Genotoxicity to intestinal epithelial cells. A, B. DNA damage by alkaline comet assay without (black bars) and with hOgg1 incubation (white bars), and by γ-H2AX immunostaining, respectively, in intestinal epithelial cells from small and large intestine of IL-10−/− versus wildtype mice. LI: large intestine, SI: small intestine, *: p<0.05, **: p<0.01 by one way ANOVA with Dunn’s multiple comparison test. Error bars represent SEM of 5–7 mice per group.
Genotoxicity to hepatocytes and the brain
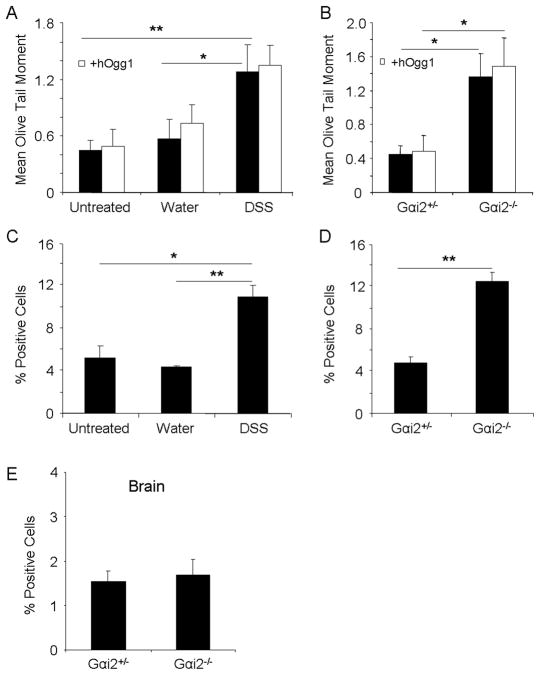
Hepatocytes from the liver were examined as a non-lymphoid cell type distant from the intestines for genotoxic effects of intestinal inflammation. Hepatocytes were isolated from infiltrating lymphoid cells in the liver via centrifugation and negative selection with magnetic beads. DNA strand breaks were analyzed before and after treatment with dextran sulfate sodium (DSS), a commonly used non-genotoxic chemical agent administered in the drinking water to induce acute and chronic intestinal inflammation in wildtype mice 22, 23. After two cycles of DSS treatment to induce chronic intestinal inflammation, with one cycle involving 7 days of treated water followed by 14 days of normal water, wildtype mice presented severe weight loss, diarrhea, and gross bleeding (data not shown), and as also described by others 22, 23. Hepatocytes from DSS treated wildtype mice demonstrated significantly more DNA single and double strand breaks versus mice prior to treatment or treated with water (Figures 4A and C). Oxidative base damage did not seem to make up a majority of the DNA damage, as levels were similar to those without hOgg1 incubation (Figure 4A). In addition to the DSS experimental model, hepatocytes from Gαi2−/− mice with severe colitis also demonstrated significant DNA strand breaks compared to age-matched Gαi2+/− mice without colitis (Figures 4B and D). Oxidative base damage also did not seem to constitute a majority of the damage indicated by the comet assay in this model (Figure 4B). Severe intestinal inflammation therefore leads to genotoxicity to hepatocytes.
Fig. 4.
DNA damage to hepatocytes and brain in colitic mice. A, C. DNA damage as measured by alkaline comet assay with and without hOgg1 incubation, and percent positive cells for γ-H2AX foci formation, respectively, in wildtype mice treated with DSS or water for two cycles. B., D. DNA damage as measured by alkaline comet assay with and without hOgg1 incubation, and percent positive cells for γ-H2AX foci formation, respectively, in Gαi2−/− or age-matched Gαi2−+ − mice. E. Percent positive cells for γ-H2AX foci formation in transverse sections of the brain in Gαi2+/− and Gαi2−/− mice. *: p<0.05, **: p<0.01 by one way ANOVA with Dunn’s multiple comparison test. Error bars represent SEM of 5–7 mice per group.
Genotoxicity of the brain was then explored as another distant organ from the site of inflammation, where manifestations of disease in patients with long standing chronic intestinal inflammation have not been commonly reported. Since detection of DNA double strand breaks via γ-H2AX foci formation in brain tissue has been previously reported24, 25, transverse sections of the brain including the ventricular zone and the neopallial cortex were stained for γ-H2AX foci formation in colitic 12–14 week old Gαi2−/− mice or age-matched Gαi2+/− mice. Neither demonstrated significant foci formation in the various sections of the brain, indicating a lack of genotoxic insult to this organ (Figure 4E). This may be due to additional protective mechanisms or decreased contact with the mediators involved in causing genotoxicity.
Blood cytokine levels correlate with DNA damage in models of intestinal inflammation
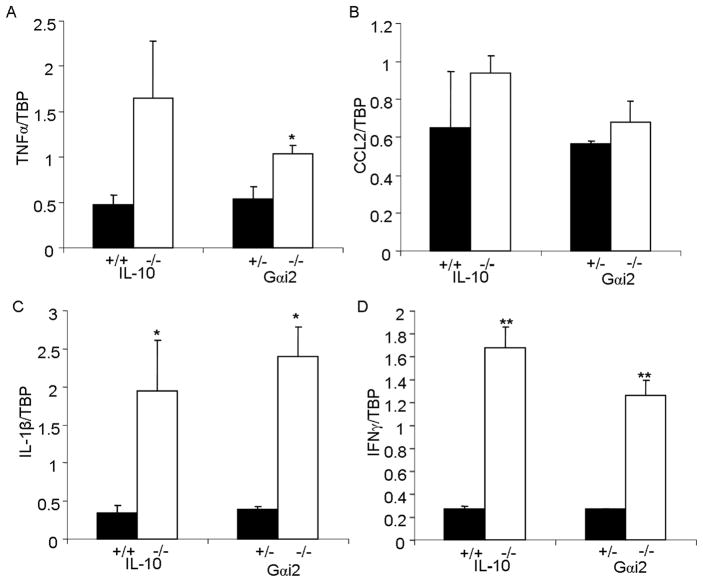
Increased levels of systemically circulating proinflammatory cytokines in intestinal inflammation may play a role in induction of systemic genotoxicity. Gene expression of cytokines in peripheral leukocytes, from which DNA damage was assayed in identical samples, was quantified in IL-10−/− and Gαi2−/− mice with colitis by quantitative real-time PCR. Transcript levels of TNFα, Ccl2, IL1b, and IFNg were upregulated in IL-10−/−compared to wild-type mice, and in Gαi2−/− compared to Gαi2+/− mice (Figures 5A–D). Mice with intestinal inflammation manifesting increased transcript levels of proinflammatory cytokines therefore also demonstrate higher levels of systemic DNA damage to various cell types. Increased blood and stool cytokine levels of proinflammatory cytokines have similarly been reported in IL-10−/− mice with colitis26–28, in Gαi2−/− mice 29–31, and in DSS-induced colitis in wildtype mice 13, 18, supporting our observations.
Fig. 5.
Transcript levels of proinflammatory cytokines in the blood. A, B, C, D. Levels of TNFα, CCL2, IL1b, and IFNg, respectively, relative to the internal control TBP in IL-10−/−versus wildtype mice, and Gαi2−/− versus Gαi2−+ − mice. *: p<0.05, **: p<0.01 by unpaired Student’s t-test. Error bars represent SEM of 5 mice per group.
Increased genotoxicity is not due to decreased expression of ATM
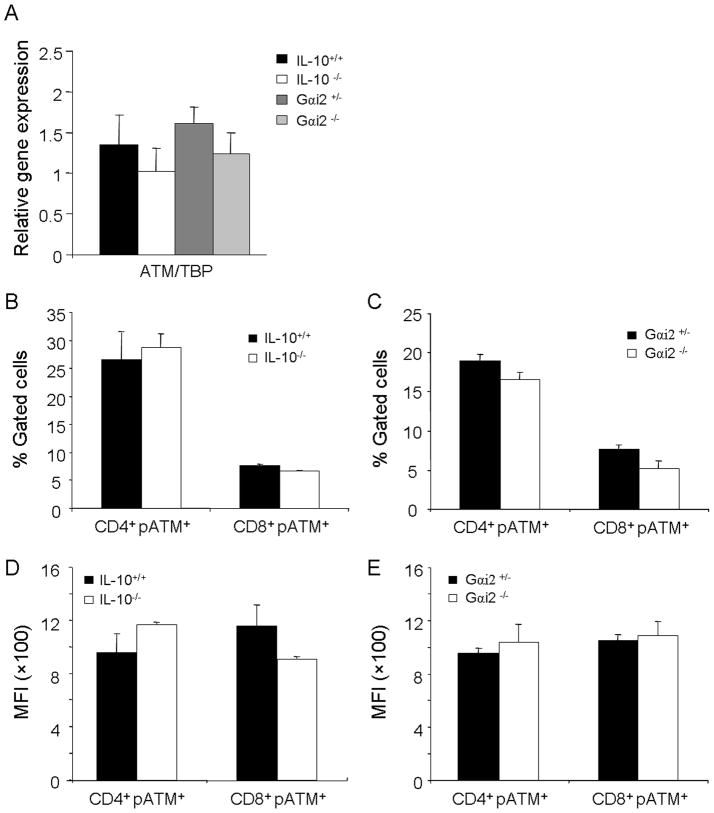
We have recently demonstrated increased susceptibility to chemically induced colitis in ATM deficient mice 18, and a recent study demonstrated that CD4+ T-cells from rheumatoid arthritis patients selectively were deficient in transcript and protein levels of ATM, causing increased DNA strand breaks and rendering them sensitive to apoptosis and premature immunosenescence 32. Levels of expression in terms of transcript levels and protein levels of ATM were therefore analyzed in order to see whether or not similar decreased DNA repair capabilities were a potential explanation for sustained systemic genotoxicity in the peripheral leukocytes of IL-10−/− and Gαi2−/− mice with colitis. Transcript levels of ATM, as well as protein expression of activated phospho-ATM measured by flow cytometry in CD4+ and CD8+ T-cell subsets in wildtype mice, however, were not significantly different from IL-10−/− and Gαi2−/− mice (Figures 6A–E).
Fig. 6.
ATM expression in genetic models of intestinal inflammation. A. Transcript levels of ATM relative to the internal control TBP in IL-10−/− versus wildtype mice, and Gαi2−/−versus Gαi2−+ − mice in peripheral leukocytes. B., D. Protein expression of pATM in CD4+ and CD8+ T-cells in IL-10−/− versus wildtype mice, expressed as % gated cells and mean fluorescent intensity (MFI), respectively, as determined by flow cytometry. C., E. Protein expression of pATM in CD4+ and CD8+ T-cells in Gαi2−/− versus Gαi2−+ − mice expressed as % gated cells and mean fluorescent intensity (MFI), respectively, as determined by flow cytometry.*: p<0.05, **: p<0.01 by unpaired Student’s t-test. Error bars represent SEM of 5–7 mice per group.
Discussion
Different levels of subclinical to clinical intestinal inflammation are induced by different commensal microbial communities, and host genetic variants for microbial control and immunoregulation 6, 10. Several studies have shown that such inflammation is associated with systemic genotoxicity, which may be a mechanistic factor linking such inflammation with known associations of chronic inflammation to a variety of systemic diseases 13, 32–35. In this study, we characterized the systemic effects of intestinal inflammation by determining genotoxic sensitivity of multiple cell types in different anatomical locations to gain insights into the consequences of chronic mucosal inflammation on extra-intestinal tissues.
DNA damage to CD4+ and CD8+ T-cells predominantly versus other cell types in the peripheral blood in IL-10−/− mice demonstrates that inflammation-induced DNA damage is not completely random, given that T-cells only represent 30–40% of total circulating leukocytes. In the Gαi2−/− model with more severe inflammation, though the DNA of CD4+ and CD8+ T-cells may have been damaged first due to their relative sensitivity, other cell types including macrophages and B-cells to a lesser extent, were also damaged. This may indicate a correlation between severity of pathology and DNA damage to multiple cell types. In accordance with our data, others have also found relatively more damage to T-cells versus other cell types in the blood, such as in rheumatoid arthritis patients 32, and both basally and after treatment with hydrogen peroxide in isolated peripheral blood 36, 37. Further basal differences have also been observed between naive and memory T-cells in which the latter has been found to have greater DNA damage and thus a shorter lifespan than the naïve T-cells, which have a half life of 150–160 days 38. We have demonstrated that decreased gene and protein expression of ATM is not responsible for the sensitivity of CD4+ and CD8+ T-cells to genotoxicity, as proposed to be the cause in sensitivity of these cell types in rheumatoid arthritis patients 32. However, expression of other players in the DNA damage response pathway may demonstrate differences between cell types and between models of intestinal inflammation, contributing to the increased sensitivity of T-cells.
Long lifespan can also lead to accumulation of DNA damage over time, despite having intact DNA repair capabilities, such as when comparing generally long-lived T-cells to short-lived B-cells, depending on many factors including antigenic stimulation 36. The sensitivity of the CD4+ and CD8+ T-cell populations during inflammation may indicate excessive cellular stress, in which repair and cellular defense mechanisms cannot keep up with the oxidative environment of an inflammatory response. This is further supported by the fact that activated T-cells or T-cells infected with human immunodeficiency virus (HIV) contain elevated levels of mitochondrial superoxide and thus carry a reduction in the mitochondrial membrane potential 39. Excessive oxidative stress in the extracellular milieu also impacts T-cell signaling function and activation status, whereas B-cell receptor signaling has been shown to be enhanced by oxidants via synergy with calcium signaling, demonstrating different functional consequences in cell types 40. Macrophages are selectively known to have defense mechanisms, such as the constitutive expression of heme-oxygenase 1 (HO-1), acting as an “anti-inflammatory” agent which is further upregulated during acute inflammation 41, as well as a large number of strong antioxidant enzymes including superoxide dismutases, catalases, and glutathione peroxidases. Therefore, differing reactive oxygen species scavenging and levels of antioxidant response enzymes, DNA repair capabilities, life span, and cellular permeability may explain the differences observed in sensitivity of cell types and in the context of the clinical severity of disease. Importantly, as the results of our study indicate, increased overall sensitivity and DNA damage to CD4+ and CD8+ T-cells may lead to genetic instability and chromosome translocations over time, leading to the development of various T-cell lymphomas, as commonly reported in patients with intestinal inflammation.
Genotoxicity to lymphoid organs including the mesenteric and peripheral lymph nodes and spleen, as well as to the intestinal epithelial cells in the IL-10−/− mice indicate both local as well as systemic damage that either may be indicative of circulating damaged leukocytes into and out of the peripheral lymphoid organs, or leukocytes that are damaged at these distant sites due to a systemic inflammatory response. Epithelial cells from the colon demonstrated more severe genotoxicity than in the small intestine, indicating the major site of inflammatory activity and tissue atrophy, as well as presence of bacterial colonization, may be factors promoting DNA damage at this site. Importantly, IL-10−/− mice demonstrated increasing levels of DNA damage with age in peripheral lymphoid organs. Levels of DNA damage may be therefore heavily impacted by duration and clinical activity of inflammation, which increases with age in these mice. Chemically-induced colitis in wildtype mice with DSS administration was also able to induce DNA damage to hepatocytes, distant from the intestinal epithelial cells and a cell type not of lymphoid origin. However, it is imaginable that damage to these cells may occur via proximity to infiltrating activated lymphocytes and monocytes in the liver. Though S-phase cells have been reported to manifest more DNA double strand breaks than cells in other cell cycle phases via γH2AX immunostaining, lymphocytes have a relatively long half life, and hepatocytes are mostly quiescent cells residing in G0 phase. The observed DNA strand breaks were therefore most likely not due to DNA replication, excluding intestinal epithelial cells during intestinal inflammation, in which there is significant proliferation. The lack of significant difference in DNA damage to the brain of colitic Gαi2−/− versus the age-matched Gαi2+/− mice may be due to decreased exposure or decreased bioavailability to these inflammatory cells or mediators. Though cytokines are known to be capable of crossing the blood brain barrier, transport rates differ among cytokines, among brain regions, and among various physiological circumstances, leading to potentially different bioavailability versus other tissues42. However, the brain is still very susceptible to DNA damage, as evidenced by ageing due to endogenously derived oxidative stress 43.
As we have demonstrated with blood cytokines, Gαi2−/−, IL-10−/−, and DSS-treated mice are characterized by the presence of activated T-cells and increased cytokine production such as TNF-α and IFN-γ, in not only the colon and lamina propria, but also in splenocytes and peripheral lymph nodes 28, 44. Systemic inflammatory activation and immune hyper-responsiveness in these models observed in distant immune cells may therefore serve as a mechanism for the observed systemic genotoxicity. In support of this, cytokines including TNF-α, IL-2, interferons, and others have been shown to be genotoxic in vitro, to multiple human cell lines 45, 46. The persistently elevated levels of large networks of cytokines/chemokines as well as their interactions, which better represent actual active intestinal inflammation, may therefore explain the chronically elevated levels of DNA damage observed systemically in multiple cell types.
These findings may illuminate the origin of DNA damage to peripheral leukocytes and other tissue cell subpopulations observed in various human inflammatory diseases, including type 1 and 2 diabetes, rheumatoid arthritis, systemic lupus erythromatosus, liver cirrhosis, and pre-neoplastic conditions such as myelodysplastic syndrome 32–34, 47. Further studies are required to define the mechanisms linking intestinal inflammation and systemic genotoxicity, although recent evidence has already begun to implicate certain processes, such as the cellular response to systemically-elevated levels of certain inflammation-associated cytokines 48. Further investigation utilizing specific enzymatic inhibitors or anti-proliferative agents will be necessary to identify specific pathways and drivers of intestinal inflammation-induced genotoxicity. Clarification of these mechanisms may offer new targets for monitoring and reducing the effects of subclinical or clinical inflammation in a variety of extraintestinal diseases.
Acknowledgments
This work was supported by National Institutes of Health [grant numbers DK46763 (J.B.), and AI078885 (JB and RS)], the Crohn’s and Colitis Foundation of America grant (B.W.), UCLA-NIEHS training grant in Molecular Toxicology (A.W.).
Footnotes
Conflict of interest: Patent filed, “Genotoxicity as a biomarker for inflammation” US Patent Office Application # 61/169,528 AW, BW, JB, RS
References
- 1.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–35. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001;91:854–62. doi: 10.1002/1097-0142(20010215)91:4<854::aid-cncr1073>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN. Ulcerative colitis and Crohn’s disease: a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35:1590–2. doi: 10.1136/gut.35.11.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sartor RB. Mechanisms of Disease: pathogenesis of Crohn’s disease and ulcerative colitis. Na Clin Pract Gastroenterol Hepatol. 2006;3:390. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 5.Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008;14:378–89. doi: 10.3748/wjg.14.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westbrook AM, Szakmary A, Schiestl RH. Mechanisms of intestinal inflammation and development of associated cancers: Lessons learned from mouse models. Mutat Res. 2010;705:40–59. doi: 10.1016/j.mrrev.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rudolph U, Finegold MJ, Rich SS, Harriman GR, Srinivasan Y, Brabet P, Boulay G, Bradley A, Birnbaumer L. Ulcerative colitis and adenocarcinoma of the colon in G alpha i2-deficient mice. Nat Genet. 1995;10:143–50. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- 8.Huang TT, Zong Y, Dalwadi H, Chung C, Miceli MC, Spicher K, Birnbaumer L, Braun J, Aranda R. TCR-mediated hyper-responsiveness of autoimmune Galphai2 (−/−) mice is an intrinsic naive CD4 (+) T cell disorder selective for the Galphai2 subunit. Int Immunol. 2003;15:1359–67. doi: 10.1093/intimm/dxg135. [DOI] [PubMed] [Google Scholar]
- 9.Elgbratt K, Bjursten M, Willen R, Bland PW, Hörnquist EH. Aberrant T-cell ontogeny and defective thymocyte and colonic T-cell chemotactic migration in colitis-prone Gai2-deficient mice. Immunology. 2007;122:199–209. doi: 10.1111/j.1365-2567.2007.02629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asquith MJ, Boulard O, Powrie F, Maloy KJ. Pathogenic and protective roles of MyD88 in leukocytes and epithelial cells in mouse models of inflammatory bowel disease. Gastroenterology. 139:519–29e2. doi: 10.1053/j.gastro.2010.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rennick DM, Fort MM. Lessons from genetically engineered animal models: XII. IL-10-deficient (IL-10−/−) mice and intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2000;278:G829–33. doi: 10.1152/ajpgi.2000.278.6.G829. [DOI] [PubMed] [Google Scholar]
- 12.Asquith M, Powrie F. An innately dangerous balancing act: intestinal homeostasis, inflammation, and colitis-associated cancer. J Exp Med. 207:1573–7. doi: 10.1084/jem.20101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westbrook AM, Wei B, Braun J, Schiestl RH. Intestinal mucosal inflammation leads to systemic genotoxicity in mice. Cancer Res. 2009;69:4827–34. doi: 10.1158/0008-5472.CAN-08-4416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartmann A, Agurell E, Beevers C, Brendler-Schwaab S, Burlinson B, Clay P, Collins A, Smith A, Speit G, Thybaud V, Tice RR. Recommendations for conducting the in vivo alkaline Comet assay. Mutagenesis. 2003;18:45–51. doi: 10.1093/mutage/18.1.45. [DOI] [PubMed] [Google Scholar]
- 15.Hartmann A, Schumacher M, Plappert-Helbig U, Lowe P, Suter W, Mueller L. Use of the alkaline in vivo Comet assay for mechanistic genotoxicity investigations. Mutagenesis. 2004;19:51–9. doi: 10.1093/mutage/geg038. [DOI] [PubMed] [Google Scholar]
- 16.Van der Heijden PJ, Stok W. Improved procedure for the isolation of functionally active lymphoid cells from the murine intestine. J Immunol Methods. 1987;103:161–7. doi: 10.1016/0022-1759(87)90285-7. [DOI] [PubMed] [Google Scholar]
- 17.Olive PL, Banath JP. The comet assay: a method to measure DNA damage in individual cells. Nat Protocols. 2006;1:23–9. doi: 10.1038/nprot.2006.5. [DOI] [PubMed] [Google Scholar]
- 18.Westbrook AM, Schiestl RH. Atm-deficient mice exhibit increased sensitivity to dextran sulfate sodium-induced colitis characterized by elevated DNA damage and persistent immune activation. Cancer Res. 2010;70:1875–84. doi: 10.1158/0008-5472.CAN-09-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith CC, O’Donovan MR, Martin EA. hOGG1 recognizes oxidative damage using the comet assay with greater specificity than FPG or ENDOIII. Mutagenesis. 2006;21:185–90. doi: 10.1093/mutage/gel019. [DOI] [PubMed] [Google Scholar]
- 20.Goldstine JV, Nahas S, Gamo K, Gartler SM, Hansen RS, Roelfsema JH, Gatti RA, Marahrens Y. Constitutive phosphorylation of ATM in lymphoblastoid cell lines from patients with ICF syndrome without downstream kinase activity. DNA Repair. 2006;5:432–43. doi: 10.1016/j.dnarep.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Muslimovic A, Ismail IH, Gao Y, Hammarsten O. An optimized method for measurement of gamma-H2AX in blood mononuclear and cultured cells. Nat Protocols. 2008;3:1187–93. doi: 10.1038/nprot.2008.93. [DOI] [PubMed] [Google Scholar]
- 22.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 23.Cooper HS, Murthy S, Kido K, Yoshitake H, Flanigan A. Dysplasia and cancer in the dextran sulfate sodium mouse colitis model. Relevance to colitis-associated neoplasia in the human: a study of histopathology, B-catenin and p53 expression and the role of inflammation. Carcinogenesis. 2000;21:757–68. doi: 10.1093/carcin/21.4.757. [DOI] [PubMed] [Google Scholar]
- 24.Rübe CE, Grudzenski S, Kühne M, Dong X, Rief N, Löbrich M, Rübe C. DNA double-strand break repair of blood lymphocytes and normal tissues analysed in a preclinical mouse model: implications for radiosensitivity testing. Clin Cancer Res. 2008;14:6546–55. doi: 10.1158/1078-0432.CCR-07-5147. [DOI] [PubMed] [Google Scholar]
- 25.Kim D, Frank CL, Dobbin MM, Tsunemoto RK, Tu W, Peng PL, Guan JS, Lee BH, Moy LY, Giusti P. Deregulation of HDAC1 by p25/Cdk5 in neurotoxicity. Neuron. 2008;60:803–17. doi: 10.1016/j.neuron.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–74. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 27.Berg DJ, Davidson N, Kühn R, Müller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4 (+) TH1–like responses. J Clin Invest. 1996;98:1010. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rennick DM, Fort MM, Davidson NJ. Studies with IL-10−/− mice: an overview. J Leukoc Biol. 1997;61:389–96. doi: 10.1002/jlb.61.4.389. [DOI] [PubMed] [Google Scholar]
- 29.Bjursten M, Hultgren OH, Hultgren Hörnquist E. Enhanced pro-inflammatory cytokine production in Gai2-deficient mice on colitis prone and colitis resistant 129Sv genetic backgrounds. Cellular Immunol. 2004;228:77–80. doi: 10.1016/j.cellimm.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Hornquist C, Lu X, Rogers-Fani P, Rudolph U, Shappell S, Birnbaumer L, Harriman G. G (alpha) i2-deficient mice with colitis exhibit a local increase in memory CD4+ T cells and proinflammatory Th1-type cytokines. J Immunol. 1997;158:1068–77. [PubMed] [Google Scholar]
- 31.Scheinin T, Butler DM, Salway F, Scallon B, Feldmann M. Validation of the interleukin 10 knockout mouse model of colitis: antitumour necrosis factor antibodies suppress the progression of colitis. Clin & Exp Immunol. 2003;133:38–43. doi: 10.1046/j.1365-2249.2003.02193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shao L, Fujii H, Colmegna I, Oishi H, Goronzy JJ, Weyand CM. Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J Exp Med. 2009;206:1435–49. doi: 10.1084/jem.20082251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pitozzi V, Giovannelli L, Bardini G, Rotella CM, Dolara P. Oxidative DNA damage in peripheral blood cells in type 2 diabetes mellitus: higher vulnerability of polymorphonuclear leukocytes. Mutat Res. 2003;529:129–33. doi: 10.1016/s0027-5107(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 34.Grossi S, Sumberaz A, Gosmar M, Mattioli F, Testino G, Martelli A. DNA damage in peripheral blood lymphocytes of patients with cirrhosis related to alcohol abuse or to hepatitis B and C viruses. Eur J Gastroenterol Hepatol. 2008;20:22–5. doi: 10.1097/MEG.0b013e3282f163fe. [DOI] [PubMed] [Google Scholar]
- 35.Ruzena T. Systemic inflammation in chronic obstructive pulmonary disease: May adipose tissue play a role? Review of the literature and future perspectives. Mediators Inflamm. 2010;2010:11. doi: 10.1155/2010/585989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weng H, Lu Y, Weng Z, Morimoto K. Differential DNA damage induced by H2O2 and bleomycin in subpopulations of human white blood cells. Mutat Res. 2008;652:46–53. doi: 10.1016/j.mrgentox.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Morillas MJ, Guillamet E, Surralles J, Creus A, Marcos R. Spontaneous and induced genetic damage in T lymphocyte subsets evaluated by the Comet assay. Mutat Res. 2002;514:39–48. doi: 10.1016/s1383-5718(01)00319-9. [DOI] [PubMed] [Google Scholar]
- 38.Scarpaci S, Frasca D, Barattini P, Guidi L, Doria G. DNA damage recognition and repair capacities in human na ve and memory T cells from peripheral blood of young and elderly subjects. Mech Ageing Dev. 2003;124:517–24. doi: 10.1016/s0047-6374(03)00030-7. [DOI] [PubMed] [Google Scholar]
- 39.Castedo M, Macho A, Zamzami N, Hirsch T, Marchetti P, Uriel J, Kroemer G. Mitochondrial perturbations define lymphocytes undergoing apoptotic depletion in vivo. Eur J Immunol. 1995;25:3277–84. doi: 10.1002/eji.1830251212. [DOI] [PubMed] [Google Scholar]
- 40.Larbi A, Kempf J, Pawelec G. Oxidative stress modulation and T cell activation. Exp Gerontol. 2007;42:852–8. doi: 10.1016/j.exger.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Yachie A, Toma T, Mizuno K, Okamoto H, Shimura S, Ohta K, Kasahara Y, Koizumi S. Heme oxygenase-1 production by peripheral blood monocytes during acute inflammatory illnesses of children. Exp Biology Med. 2003;228:550–6. doi: 10.1177/15353702-0322805-26. [DOI] [PubMed] [Google Scholar]
- 42.Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241–8. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- 43.Lu T, Pan Y, Kao S-Y, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–91. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- 44.Dieleman LA, Akol H, Bloemena E, PeÑA AS, Meuwissen SGM, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–91. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lazutka J. Genetic toxicity of cytokines. Mutat Res. 1996;361:95–105. doi: 10.1016/s0165-1161(96)00027-1. [DOI] [PubMed] [Google Scholar]
- 46.Zhuang JC, Lin D, Lin C, Jethwaney D, Wogan GN. Genotoxicity associated with NO production in macrophages and co-cultured target cells. Free Radic Biol Med. 2002;33:94–102. doi: 10.1016/s0891-5849(02)00866-3. [DOI] [PubMed] [Google Scholar]
- 47.McConnell JR, Crockard AD, Cairns AP, Bell AL. Neutrophils from systemic lupus erythematosus patients demonstrate increased nuclear DNA damage. Clin Exp Rheumatol. 20:653–60. [PubMed] [Google Scholar]
- 48.Redon CE, Dickey JS, Nakamura AJ, Kareva IG, Naf D, Nowsheen S, Kryston TB, Bonner WM, Georgakilas AG, Sedelnikova OA. Tumors induce complex DNA damage in distant proliferative tissues in vivo. Proc Nat Acad Sci. 2010;107:17992–7. doi: 10.1073/pnas.1008260107. [DOI] [PMC free article] [PubMed] [Google Scholar]