Abstract
Background
Men and women differ in their ability to extinguish fear. Fear extinction requires the activation of brain regions including the ventromedial prefrontal cortex (vmPFC) and amygdala. Could estradiol modulate the activity of these brain regions during fear extinction?
Methods
All rat experiments were conducted in naturally cycling females. Rats underwent fear conditioning on day 1. On day 2, they underwent extinction training during the metestrus phase of the cycle (low estrogen and progesterone). Extinction recall was assessed on day 3. Systemic injections of estrogen-receptor beta and alpha agonists, and estradiol were administered at different time points to assess their influence on extinction consolidation and c-fos expression in the vmPFC and amygdala. In parallel, healthy naturally cycling women underwent an analogous fear conditioning extinction training while in a 3T fMRI scanner. Measurement of their estradiol levels and skin conductance responses were obtained throughout the experiment.
Results
In female rats, administration of the estrogen-receptor beta (but not alpha) agonist facilitated extinction recall. Immediate (but not delayed) post-extinction training administration of estradiol facilitated extinction memory consolidation and increased c-fos expression in the vmPFC while reducing it in the amygdala. In parallel, natural variance in estradiol in pre-menopausal cycling women modulated vmPFC and amygdala reactivity and facilitated extinction recall.
Conclusion
We provide translational evidence that demonstrates the influence of endogenous and exogenous estradiol on the fear extinction network. Our data suggest that women’s endogenous hormonal status should be considered in future neurobiological research related to anxiety and mood disorders.
Keywords: Fear inhibition, fMRI, classical conditioning, menstrual cycle, estrogen, progesterone
Introduction
In recent years, fear extinction research has taught us much about the neural correlates of learning not to fear(1, 2). Studies have identified a brain network involved in fear extinction including the ventromedial prefrontal cortex (vmPFC) and amygdala(3–5). In rodents, pharmacological and electrophysiological data indicate that the infralimbic cortex (IL), a region believed to be homologous to the human vmPFC, and the amygdala both play a critical role in extinction learning and recall(6–9). Human brain-imaging studies show consistent results; vmPFC structure and function is associated with extinction learning and recall(10–12). The amygdala, on the other hand, increases activation to conditioned cues during both fear acquisition and extinction learning--supporting its involvement in both processes(12–15).
In both human and animal research, most of what we have learned about fear extinction is acquired from studies on the male brain. We remain far less informed about sex differences in fear extinction, and the influence of sex hormones on fear extinction in the female brain. This is rather surprising given that 1) the prevalence of anxiety disorders is twice as high in women(16) and 2) fear extinction is impaired in PTSD(17). Could sex hormones influence the functional reactivity of the amygdala and the vmPFC during fear extinction in females? Indeed, sex differences in learning and memory across species and across different behavioral paradigms have been previously documented(18–22). The vmPFC and amygdala are sexually dimorphic structures and contain relatively high levels of estrogen receptors compared with other brain regions(23, 24). Neuroimaging studies have shown that functional activation of these brain regions in response to emotional stimuli varies across the menstrual cycle(25, 26). Furthermore, numerous studies have shown that the effects of stress on fear acquisition varies between male and female rats and that these differences are influenced by estradiol(27). These data suggest that variance in estrogen levels may modulate the functional activation of these brain regions during fear extinction. In support of this, in recent behavioral studies, we showed that elevated estradiol is associated with enhanced fear extinction in female rats(28) and in women(29).
Here, we use across-species multimodal approach to investigated whether estrogen enhances fear extinction recall through its interaction with the vmPFC and amygdala. Female rats underwent a three-day fear conditioning and extinction paradigm. To increase our ability to translate our data from rats to humans, we used naturally cycling instead of ovariectomized females; all female rats underwent fear extinction while in the metestrus phase of the cycle (low estrogen/progesterone). In rodents, we examined: 1) the effects of systemic administrations of estrogen-receptor beta (ER-β) or alpha (ER-α) agonists on fear extinction, 2) the effects of post-extinction systemic administration of estradiol on extinction consolidation and recall, and 3) the effects of estradiol on c-fos expression during extinction recall in IL and amygdala. In parallel, pre-menopausal cycling women who had abstained from oral or intrauterine contraceptives for a minimum of three months underwent a two-day fear conditioning and extinction paradigm while in an fMRI scanner; skin conductance response (SCR) and sex hormone levels were measured. We hypothesized that estradiol-enhanced fear extinction recall in both rats and humans would be associated with increased IL/vmPFC and decreased amygdala activation during extinction recall.
Materials and Methods
Animal Subjects
A total of 81 female Sprague-Dawley rats (approximately 300g) were housed individually housed at the Massachusetts General Hospital Center for Comparative Medicine in Charlestown, MA. They were maintained on 12h light/dark cycle, handled for five min/day for two days following their arrival, and restricted to ∼15g/day of laboratory rat chow with free access to water. Experiments were run with all rats at approximately 80% of their free-feeding weights to facilitate motivation to bar-press for food. All procedures were approved by the Subcommittee on Research Animal Care of the Massachusetts General Hospital in compliance with National Institutes of Health guidelines.
Animal Behavioral Apparatus and Procedure
All apparatus and procedures were identical to those previously described (28). Briefly, the conditioned stimulus (CS) was a 30s tone, and the unconditioned stimulus (US) was a 0.5s foot shock with an intensity of 0.6mA. Vaginal smears and cycle phase assessment were conducted as previously described(30). All females underwent extinction learning and drug administration during the metestrus phase, and they continued to be swabbed for three days following the experiment to confirm cycle phase (for further, see Methods & Materials in the Supplement).
Animal Experiment 1: influence of receptor-specific estrogen agonists on extinction recall
Thirty-five female rats were used for this experiment. On day 1, the rats underwent a habituation phase (5 tones alone) immediately followed by the conditioning phase (7 tone+shock trials). On day 2, they received subcutaneous (s.c.) administration of estrogen-receptor alpha (ER-α) agonist propyl pyrazone triol (PPT, Sigma Aldrich, n=12, 1mg/kg in sesame oil), estrogen-receptor beta (ER-β) agonist diarylpropionitrile (DPN, n=12, 1mg/kg in sesame oil) or vehicle (n=11, sesame oil) 30 minutes prior to the extinction learning session (20 tone trials). All female rats underwent extinction learning while in the metestrus phase of the estrus cycle. On day 3, extinction recall was assessed (15 tone alone presentations).
Animal Experiment 2: influence of immediate vs. delayed post-extinction administration of estradiol on extinction recall
A new group of 46 female rats participated while in the metestrus phase on day 2. The protocol was identical to that used in Experiment 1. All rats underwent a 20-trial extinction learning phase in a drug-free state. Thereafter, they received either estradiol administration (15μg/kg in sesame oil, s.c. n=13, EST) or vehicle (n=17, VEH, sesame oil) immediately after extinction training. A third and fourth groups received either EST or VEH, but 4h after extinction training (EST, n=8; VEH, n=8, respectively). All groups underwent extinction recall on day 3 as described above.
Animal Experiment 3: influence of estradiol on c-fos expression in the vmPFC and amygdala
Ten female rats from Experiment 2 (five from both the immediate EST and VEH groups) were sacrificed after three extinction recall trials (day 3) and expression of the immediate early gene, c-fos, was measured. Whole brains were extracted and frozen in 2-methylbutane and stored at −80°C within five minutes of decapitation. The areas of the brain comprising the IL/vmPFC (3.72 to 2.52 mm AP, relative to bregma) and amygdala (−0.36 to −2.62 mm, relative to bregma,31) were dissected on a freezing microtome. Analysis of c-fos expression was done as previously described (32, see also Methods and Materials in the Supplement).
Behavioral Data Analysis
In the rodent experiments, freezing was quantified as the dependent measure of conditioned fear. Freezing was recorded and analyzed using motion-sensing computer software (FreezeScan, Clever Systems, Reston, VA, USA). Time spent freezing was converted into a percentage of the tone duration (30s).
Human Participants
Thirty-four healthy, right-handed women (18–30 years old) were recruited through advertisement from the local community. All participants were without neurologic, endocrinologic or other medical conditions. Subjects were screened for Axis I mental disorders via the Structured Clinical Interview for DSM-IV(33). No participant was using psychoactive or other potentially confounding drugs or medications, and all had abstained from oral contraceptives for at least three months prior to participating in the study. Written informed consent was obtained from all participants in accordance with the Partners Healthcare Human Research Committee.
Women were recruited to participate across all stages of the menstrual cycle in order to achieve wide variance in estradiol levels. On day 1, nine were in the early follicular phase (days 3–5 of the menstrual cycle), eight in the late follicular (days 10–12), eight in the early luteal (days 18–20) and nine in the late luteal (days 23–25). Blood samples were obtained prior to extinction learning (day 1) to measure serum estradiol and progesterone levels. Subjects were then divided into high-estradiol (H-EST, n=17, mean ± 1SE estradiol levels = 222.6 ± 22.4 pg/mL) and low-estradiol (L-EST, n=17, 51.9 ± 8.3 pg/mL) groups. A level of 108 pg/mL was used to separate groups based on the median split of a cohort of 38 women we previously examined(29).
Human Conditioning and Extinction Procedure
The two-day fear conditioning and extinction procedures used were identical to those previously described (34, see also Methods and Materials in the Supplement). Briefly, conditioned stimuli (CS) were pictures of lamps, and the unconditioned stimulus (US) was a mild electric shock delivered to the second and third finger of the subjects’ right hands (Figure S1 in the Supplement). On day 1, subjects underwent habituation (CS alone) immediately followed by conditioning (CS+US). In the conditioning phase, the to-be extinguished light (CS+E, blue light in Figure S1 in the Supplement) and the to-be non-extinguished light (CS+NE, red light Figure S1 in the Supplement) were each presented eight times with 62.5% partial reinforcement (five shocks each), while the light that was never followed by a shock (CS-, not shown) was intermingled and presented in 16 tials. The extinction training phase immediately followed, in which the 16 CS+E and 16 CS- trials were presented in a new context (extinction context, Figure S1 in the Supplement). On day 2, the extinction recall phase showed the extinction context and included the presentation of the CS+NE (red light, 8 trials) along with the CS+E (blue light, 8 trials) and CS- (16 trials) with no US presented. In each trial, the context images were presented for a total of 9s: 3s with the light off followed by 6s with the CS on. The mean inter-trial interval was 15s.
Psychiatric and Behavioral Measures
Each participant underwent a psychiatric assessment prior to participation in which the Beck Anxiety Inventory (BAI) and Beck Depression Inventory (BDI) were administered along with the Anxiety Sensitivity Index (ASI), STAI Trait (T) assessment and NEO Five Factor Inventory (NEO-FFI). After undergoing conditioning and extinction on day 1, participants completed the STAI State (S) assessment and the Mindful Attention Awareness Scale (MAAS).
Psychophysiological Data Analysis
For the human experiments, the skin conductance response (SCR) was the dependent measure of conditioned fear(17, 29, 34). Extinction memory expression during extinction recall was assessed by comparing the SCR during the CS+E to the CS+NE. We also calculated an “extinction retention index” that controls for the level of fear acquired during the conditioning phase (29). This index was calculated as follows: each subject’s SCR to the first four CS+ trials of the extinction recall phase was divided by their largest SCR to a CS+ trial during the conditioning phase. The prodcut was then multiplied by 100, yielding a percentage of maximal conditioned responding. This in turn was subtracted from 100% to yield the “extinction retention index.” Analysis of variance (ANOVA) with repeated measures was used to analyze the data across experimental phases, and analysis of covariance (ANCOVA) was used to adjust for potentially confounding variables such as baseline skin conductance level SCL, shock intensity and the unconditioned response UCR when performing between-group analyses. Statistical analysis used SPSS (Version 17.0, SPSS Inc., Chicago, IL 2008); post-hoc comparisons used the Tukey Honestly Significant Difference method. Student’s t-test was used when appropriate.
Image Acquisition and fMRI Data Analysis
Image acquisition parameters were identical to those previously described(10, 17, 34). Statistical parametric maps were calculated according to a general linear model for contrasts of interest across the time window. The contrasts used were: 1) fear conditioning: all 16 CS+ trials versus all 16 CS- trials; 2) extinction training: last 8 CS+E versus first 8 CS+E trials; and 3) extinction recall: first 4 CS+E versus first 4 CS+NE trials.
Group (H-EST vs. L-EST) x Stimulus (as described above) interactions were analyzed for each phase. Functional regions of interest (ROIs) were defined as clusters of contiguous voxels exceeding the a priori statistical threshold (p<0.005), which included the vmPFC and amygdala. The BOLD signal values were extracted from these ROIs to calculate percent signal change. These values were then used for regression analyses with estradiol levels. Coordinates for the peak voxels in each region were specified in terms of the Talairach atlas(35). A more stringent threshold of p<0.0001 was used for activations and deactivations in the remaining brain regions.
Results
Animal Studies
Experiment 1: Estrogen-receptor sub-types and fear extinction
Studies have suggested that activation of ER-β leads to anxiolytic effects while activation of ER-α leads to anxiogenic effects(36, 37). Experiment 1 tested whether the influence of estradiol on fear extinction is mediated via ER-β or ER-α. Freezing levels during conditioning (day 1) and extinction learning (day 2) did not differ between groups (see Results in the Supplement for statistical analyses of conditioning and extinction phases and subsequent rat experiments). Analysis of the extinction recall phase on day 3 showed a significant main effect of group (F(2,32)=6.55, p<0.01), but no effect of trial (F(2,64)=1.43, p=0.25), or interaction (F(4,64)=1.20, p=0.32). Post-hoc analyses revealed that the DPN (ER-β) group showed significantly lower freezing than the PPT (ER-α, p<0.01) and the VEH (p<0.01) groups (figure 1A shows block of first 5 trials).
Figure 1. Extinction recall is facilitated in female rats via activation of the ER-β receptors and immediate post-extinction administration of estradiol.
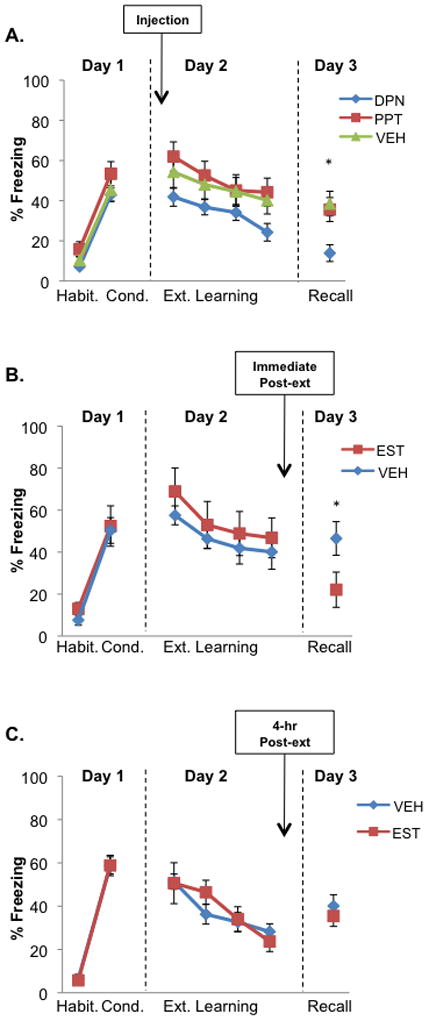
A) Freezing for female rats receiving β-agonist, diarylpropionitrile (DPN, n = 12), α-agonist, propyl pyrazone triol (PPT, n = 12), and vehicle (VEH, n = 11) injected subcutaneously 30 minutes prior to extinction learning. Females in this experiment (and in all subsequent experiments) underwent extinction learning and received injections while in the metestrus (low estrogen and progesterone) phase. B) Freezing for female rats receiving subcutaneous (s.c.) estradiol (EST, n = 13) or vehicle (VEH, n = 17) immediately following extinction learning. C) Freezing for metestrus female rats receiving estradiol (EST, n = 8) or vehicle (VEH, n = 8) four hours following extinction learning. Note that extinction recall was facilitated in rats receiving estradiol immediately following extinction, but this effect disappeared when administration was delayed 4 hours. Data points represent blocks of five trials ± standard error (S.E.) except during Recall in (B), where five animals in each group were sacrificed after three trials. Arrows indicate s.c. injections. Habit. = habituation, Cond. = conditioning, Ext. = extinction. *p < 0.05 compared to VEH.
Experiment 2: Estradiol and extinction memory consolidation
This set of experiments tested the effects of post-extinction systemic administration of estradiol (EST group) on extinction memory consolidation. Rats that received EST immediately following extinction learning showed significantly lower freezing during extinction recall compared to rats that received vehicle (21.9±4.86% for EST, and 43.2±5.87% for VEH, t(28)=2.63, p=0.01, figure 1B). When estradiol was administered 4 hours after extinction learning, freezing during recall between the EST and VEH groups did not significantly differ (35.5±4.82% for EST, and 40.1±5.20% for VEH; t(15)=−0.79, p=0.44, figure 1C).
Experiment 3: c-fos expression in the amygdala and vmPFC
Experiment 3 examined whether the effects of estradiol on extinction recall during Experiments 1 and 2 correlated with a change in activation levels in the IL/vmPFC and amygdala. Immediate–post-extinction administration of estradiol compared to vehicle resulted in significantly lower c-fos expression(38) (EST relative to VEH) in the amygdala (t(8)=−4.83, p<0.01), and significantly higher relative c-fos expression in the IL/vmPFC (t(8)=3.98, p<0.01, figure 2).
Figure 2. Extinction recall testing following estradiol administration led to decreased c-fos mRNA expression in amygdala and increased c-fos expression in the vmPFC.
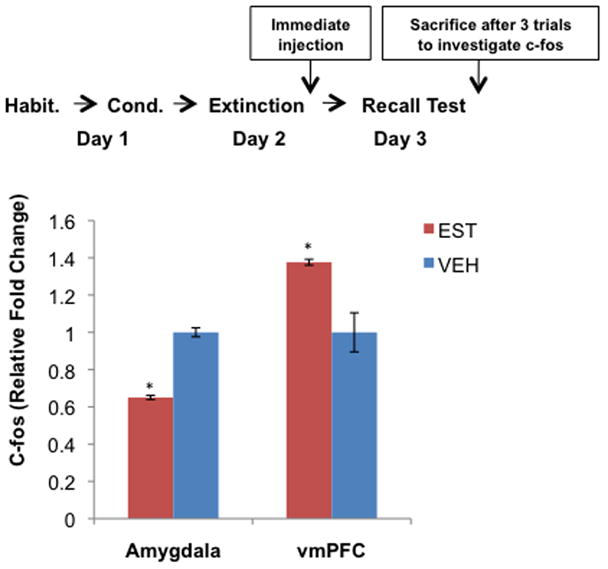
As shown in the diagram, these rats underwent the same testing procedure as those in experiments 1 and 2. Extinction learning took place and injections were administered, as in experiments 1 and 2, while rats were in the metestrus phase (bars show average relative gene expression +/− S.E., *p < 0.01).
Human studies
Progesterone and fear extinction
We have previously shown that progesterone does not significantly influence fear extinction or its recall in women(29). Re-examination of progesterone confirmed previous findings that progesterone levels do not correlate with the level of fear extinction (see Results in the Supplement).
Estradiol and fear extinction
Psychometric and behavioral tests
Psychophysiological and fMRI analyses were conducted between H-EST versus L-EST, and no significant differences in age, years of education, or ethnicity were observed. Similarly, no significant differences in personality traits (NEO-FFI, ASI, Beck Anxiety, MAAS, STAI Trait, and STAI State) were observed. H-EST women scored higher on the Beck Depression Inventory (BDI) than L-EST women, but both groups scored well below nine points—the threshold for minimally depressive symptoms. Moreover, there was no difference in UCR, SCL or shock level observed (Table S1 in the Supplement). We included BDI scores and SCL levels as covariates in all between-group analyses described below to ensure that these variables were not confounders, and all results remained the same.
Fear acquisition and functional activation during conditioning
On day 1, an ANOVA comparing maximum SCR (CS+) between the H-EST and L-EST groups revealed that both groups differentiated between CS+ and CS- (Figure S2A in the Supplement). Furthermore, comparison of average differential SCR across the conditioning phase (CS+ minus CS-) revealed no significant between-group differences (t(32)=0.65, p=0.52). No significant Group x Stimulus interaction was observed in the fMRI analysis in either the vmPFC or amygdala (contrast used: CS+ vs. CS-, H-EST vs. L-EST, Figure S2B in the Supplement).
Functional activation during extinction learning
During extinction learning, only one CS+ was presented (i.e. extinguished). Thus, the contrasts and comparisons during extinction learning were made between the extinguished CS+ (CS+E) and the CS-. Regarding the SCR data, we conducted a repeated measures ANOVA comparing trial block (last 8 CS+E vs. first 8 CS+E trials) during extinction learning in the two groups (H-EST vs. L-EST). This analysis revealed no main effect of group (F(1,32)=0.72, p=0.40), no main effect of trial block (F(1,32)=0.58, p=0.45), and no interaction (F(1,32)=1.90, p=0.18). The inclusion of large number of trials in the early block of extinction was done to match the block of extinction to be analyzed for the fMRI data (see below), and therefore limited our ability to examine extinction learning between groups. Additional between group analyses were conducted showing that extinction did take place, and that there was no between group differences during extinction learning (see Results in the Supplement).
Despite the lack of between-group differences in the SCR during extinction learning, analysis of fMRI data (last 8 CS+E vs. first 8 CS+E trials) revealed a significantly higher activation in the vmPFC in the H-EST group relative to the L-EST group (Talairach coordinates: x=4, y=23, z=−20, t(32)=3.20, p=0.003, figure 3A,B). Furthermore, a positive correlation between estradiol levels and vmPFC activation was observed in all women (r = 0.48, p < 0.01, figure 3C).
Figure 3. Estradiol is associated with increased vmPFC activation during extinction learning.
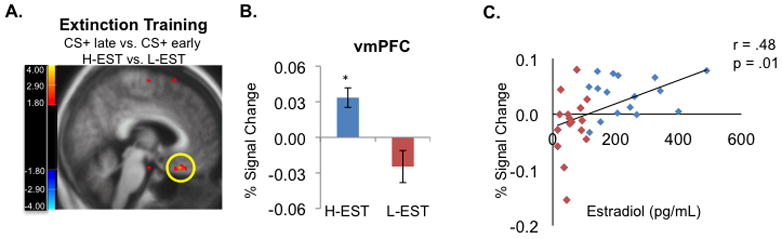
Women in the high estradiol group (H-EST, n = 17), show increased vmPFC activation during extinction learning relative to the low estradiol group (L-EST, n = 17) A) Group x Stimulus interaction in the vmPFC (Talairach coordinates: x = 4, y = 23, z = -20), contrast compares H-EST vs. L-EST, functional activation during last eight CS+ trials of Extinction minus first eight CS+ trials. The H-EST group showed higher activity relative to the L-EST group. Threshold for display image is set at p = 0.01. B) Percent BOLD signal change extracted from the vmPFC functional region of interest shown in (A) (36 voxels, 288 mm3). C) Linear regression contrasting percent BOLD signal change (extracted from the vmPFC functional region of interest shown) in the vmPFC versus estradiol levels across all subjects (n = 34) shows a significant positive correlation between estradiol levels and vmPFC activation across all women. *p < 0.001.
Extinction recall and functional activation of the vmPFC and amygdala
The analysis of the extinction recall phase was focused on differences between the two conditioned stimuli; one was extinguished the previous day (CS+E) while the other was not (CS+NE). In line with our previous psychophysiological results(29), the H-EST group displayed significantly lower SCRs relative to the L-EST group during extinction recall, suggesting facilitated extinction memory expression in the H-EST group. A two-way ANOVA revealed no main effect of Stimulus (CS+E early vs. CS+NE early; F(1,29)=0.08, p=0.78) or Group (H-EST versus L-EST, F(1,29)=0.06, p=0.81), whereas a significant Group x Stimulus interaction was found (F(1,30)=7.48, p=0.01, figure 4). The H-EST group exhibited facilitated extinction retention index relative to the L-EST group (105.1%±13.4% and 51.5%±13.1% respectively, t(29)=2.78, p=0.01). We further observed a positive correlation trend between estradiol and extinction retention (r=0.35, p=0.07, figure 4).
Figure 4. Women in the high estradiol group (H-EST) show increased extinction retention relative to the low estradiol group (L-EST).
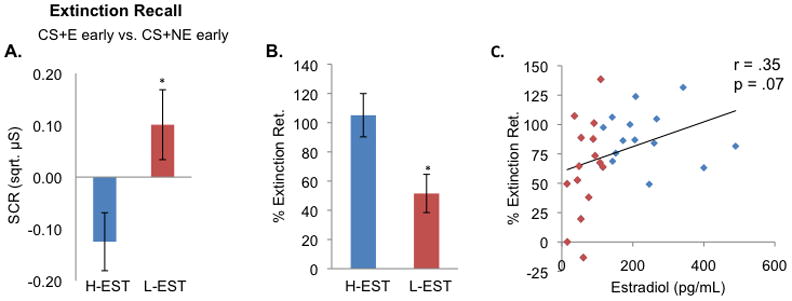
A) Skin conductance response (SCR) during extinction recall for women in the high estradiol group relative to the low estradiol group (H-EST vs. L-EST). Contrast compares first four trials of CS+E (extinguished cue) minus first four trials of CS+NE (non-extinguished cue). The H-EST group displayed significantly lower SCRs relative to the L-EST group during extinction recall, suggesting facilitated extinction memory expression in the H-EST group. B) Percent extinction retention in H-EST group versus L-EST group comparing CS+E to CS+NE. C) Regression plot between percent extinction retention and estradiol levels across all women (n = 32). Two subjects were excluded from these analyses as an outlier due to SCR responses that were three standard deviations beyond the mean. *p < 0.05.
Analysis of the fMRI data during the same phase showed that H-EST women exhibited significantly higher activation in the vmPFC (x=−3, y=49, z=−14, t(29)=3.38, p=0.002) and left amygdala (x=−27, y=−6, z=−14, t(29)=3.57, p=0.001) when compared to L-EST women (figure 5). Moreover, we observed a significant positive correlation between percent signal change extracted from each region and estradiol levels across all participants (vmPFC: r=0.56, p=0.002, amygdala: r=0.47, p=0.01, figure 5). We also observed increased hippocampal and dorsal anterior cingulate cortex (dACC) activation in the H-EST group during recall (CS+E early vs. CS+NE early, hippocampus, x=32, y=−17, z=−16, t(29)=3.86, p=0.001, and dACC: x=−3, y=22, z=23, t(29)=4.08 p<0.001).
Figure 5. Women in the H-EST group show increased vmPFC and amygdala activation during extinction recall relative to L-EST group.
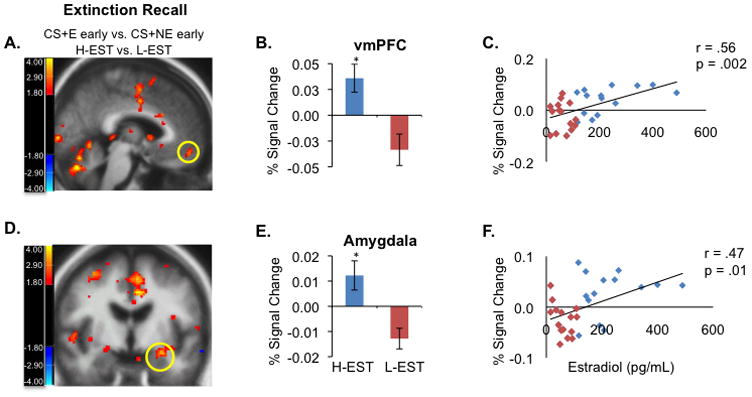
(A) Group × Stimulus interaction in the vmPFC (contrast compares H-EST vs. L-EST, functional activation during first four CS+E trials of extinction recall minus first four CS+NE trials (Talairach coordinates: x = −3, y = 49, z = −14) shows greater activation of the vmPFC in the H-EST vs. L-EST group. (B) Percent signal change extracted from vmPFC functional ROI shown in (A)(C) Linear regression contrasting BOLD signal change extracted from the vmPFC functional region of interest (27 voxels, 216 mm3) shown in (A) in the vmPFC versus estradiol levels shows positive correlation between estradiol and vmPFC reactivity. (D) Group × Stimulus interaction in the amygdala shows greater activation of the vmPFC in the H-EST vs L-EST group. (E) Percent BOLD signal change extracted from the amygdala functional region of interest shown in (D). (F) Linear regression contrasting percent BOLD signal change in the amygdala (extracted from the amygdala functional region of interest shown, 11 voxels, 88 mm3) versus estradiol levels across all subjects (n = 32) shows positive correlation between estradiol and amygdala reactivity. Threshold for functional display images is set at p = 0.01. *p < 0.001.
Discussion
The present study used a multi-modal, translational approach to show that estradiol facilitates extinction memory retention in female humans and rodents by modulating the functional reactivity of the vmPFC and amygdala—regions involved in fear expression and extinction in both species. In rodents, we show: a) estradiol administration facilitates extinction memory consolidation via ER-β activation in a time-dependent manner, and b) estradiol administration increases and decreases c-fos expression in the IL/vmPFC and amygdala, respectively, during extinction recall. In parallel to human studies, we show that natural variation in estradiol levels in women are associated with both the degree to which women retain extinction memory and the functional activation of the vmPFC and amygdala during extinction recall. Together, these data suggest that estradiol might improve the ability to retain extinguished fear memories by modulating neuronal activity and synaptic plasticity in the IL/vmPFC and amygdala.
The rodent experiments begin to illustrate the mechanisms by which estradiol can facilitate fear extinction recall. Consistent with the finding that ER-β agonists enhances extinction recall, a recent study showed that estradiol facilitates extinction of contextual fear conditioning via activation of ER-β in the hippocampus(36), and additional studies have shown that activation of ER-β induces anxiolytic behavior(39, 40). The time-dependent effect of estradiol on extinction consolidation and the change in c-fos expression in both the vmPFC and amygdala suggest that estradiol might enhance extinction-induced synaptic plasticity in these brain regions. Given that estradiol has been shown to increase NMDA receptor transmission as well as long-term potentiation(41) and extinction consolidation involves NMDA receptors(42, 43), we hypothesize that the changes in c-fos expression observed here might be due to the influence of estradiol on NMDA receptors. Future studies are needed to elucidate the interplay between estradiol and NMDA receptors (as well as other molecular indices of extinction learning) during fear extinction.
The current series of studies translate rodent data into humans by using neuroimaging techniques to demonstrate that estradiol affects vmPFC and amygdala functional activation when recalling a safety memory. Previous functional imaging studies have reported increased amygdala activation during fear extinction(10, 12) and increased vmPFC-amygdala connectivity during extinction recall(10). Interestingly, the present imaging data seem to contradict the observed reduction in c-fos expression in the amygdala during extinction recall in female rats. However it is challenging to interpret BOLD signal changes in the amygdala during fear extinction recall due to the well-documented role of the amygdala in both fear learning and fear extinction(2, 4, 44). Previous studies have shown that enhanced GABAergic neural activation is critical for fear extinction(8). In addition, Herry et al.(7) recently demonstrated that there are two neural populations within the amygdala: one signals fear learning, and the other signals fear extinction. The tools used in rodents versus those in humans might not be directly comparable since both inhibitory and excitatory processes could both lead to an increase the BOLD signal. Nonetheless, these data show that estradiol does indeed influence the function of the amygdala across species, although the direction of the effect needs further elaboration to compare the results between species.
Given that results from our previous work suggest that progesterone may not facilitate fear extinction in healthy women(29), we chose to focus primarily on the effects of estradiol in the present study. We did, however, find that a majority of women with high estradiol levels also had elevated progesterone levels, making it difficult to rule out the potential influence of progesterone on fear extinction. Previous studies(28) have shown that estrogen and progesterone independently facilitate extinction recall in female rats. In fact, when these hormones are injected together, the effect is more pronounced, which is consistent with other data in the field(45). Thus, further examination of progesterone independently as well as in interaction with estradiol on fear extinction in women is required to.
Though substantial effort in designing parallel rat and human experiments was made, there remain some differences between the two designs that could be viewed as potential limitations of the present study. One difference is that while women were conditioned and extinguished within the same phase of the menstrual cycle while female rats underwent fear conditioning and extinction training at two different phases of the estrus cycle (proestrus and metestrus, respectively). Though this limitation could have been mitigated by the use of ovariectomized female rats, we used naturally cycling female rats to allow for maximum translation between species. We understand that this may introduce effects of other hormones in relation to estradiol that may produce potential confounds. We have previously shown, however, that conducting conditioning or extinction recall at different phases of the estrus cycle had no effect on either fear conditioning or extinction recall(28). Nonetheless, future studies of ovariectomized rats are needed to identify the specific mechanisms of estrogen’s influence on the fear extinction circuit. For example, it will be important to detail how estrogen influences c-fos expression as well as other molecular indices of neural plasticity within different nuclei of the amygdala and the infralimbic and prelimbic subdivisions of the vmPFC in rats. Additional studies using cannula-guided estrogen administration into vmPFC subregions will be critical.
The translational evidence presented in this study begins to establish a role of estradiol in modulating vmPFC and amygdala activation when recalling a safety memory and highlights the importance of this understudied area in neuroscience(25). Our data suggest that estradiol may serve a protective function against elevated fear and anxiety and that transient periods of low estradiol levels may be associated with impaired retention of safety memory. The functional integrity of the fear extinction network in women using oral contraceptives and in menopausal women with and without hormone replacement therapy should be examined, especially given that the use of oral and intrauterine contraceptives is known to reduce endogenous cycling estradiol levels(46). Moreover, women appear to be vulnerable to developing mood and anxiety disorders during postpartum(47) and menopausal periods(48) when endogenous estradiol levels are low. Additional studies investigating the effects of naturally cycling and exogenous hormone manipulations on the function of the fear extinction circuitry could ultimately introduce ways to adapt, improve or produce therapies specifically tailored to women.
Supplementary Material
Acknowledgments
We thank the Massachusetts General Hospital Clinical Research Center for assistance in obtaining hormone measurements. This work was supported by a grant from the National Institute of Mental Health (K01MH080346) to M.R.M. The project described was also supported by Grant 1UL1 RR025758-01, Harvard Clinical and Translational Science Center from the National Center for Research Resources, and ORWH-NIMH P50 MH082679 (JMG, P.I.) for JMG’s time. JBL was supported by The John Henry Foundation, The Benson-Henry Institute for Mind Body Medicine, Massachusetts General Hospital, and by The Medical Research Programs of the Shriners Burns Hospital for Children, Boston. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. We thank Drs. Sharon Furtak and Bronwyn Graham for helpful comments on the manuscript.
Footnotes
Conflict of Interest: Dr. Mohammed R Milad received consulting fees from Microtranspondor for a project unrelated to that described in this manuscript. The remaining authors declare no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A. Neuronal circuits of fear extinction. Eur J Neurosci. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- 3.Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn Sci. 2010;14:268–276. doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- 7.Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- 8.Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 10.Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc Natl Acad Sci U S A. 2005;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phelps EA, Delgado MR, Nearing KI, Ledoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 13.Sehlmeyer C, Schoning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, et al. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaBar KS, Gatenby JC, Gore JC, Ledoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 15.Knight DC, Nguyen HT, Bandettini PA. The role of the human amygdala in the production of conditioned fear responses. Neuroimage. 2005;26:1193–1200. doi: 10.1016/j.neuroimage.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 16.Kinrys G, Wygant LE. Anxiety disorders in women: does gender matter to treatment? Rev Bras Psiquiatr. 2005;27(Suppl 2):S43–50. doi: 10.1590/s1516-44462005000600003. [DOI] [PubMed] [Google Scholar]
- 17.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frye CA, Edinger K, Sumida K. Androgen administration to aged male mice increases anti-anxiety behavior and enhances cognitive performance. Neuropsychopharmacology. 2008;33:1049–1061. doi: 10.1038/sj.npp.1301498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith CC, Vedder LC, McMahon LL. Estradiol and the relationship between dendritic spines, NR2B containing NMDA receptors, and the magnitude of long-term potentiation at hippocampal CA3-CA1 synapses. Psychoneuroendocrinology. 2009;34(Suppl 1):S130–S142. doi: 10.1016/j.psyneuen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milad MR, Goldstein JM, Orr SP, Wedig MM, Klibanski A, Pitman RK, et al. Fear conditioning and extinction: influence of sex and menstrual cycle in healthy humans. BehavNeurosci. 2006;120:1196–1203. doi: 10.1037/0735-7044.120.5.1196. [DOI] [PubMed] [Google Scholar]
- 21.Leuner B, Mendolia-Loffredo S, Shors TJ. High levels of estrogen enhance associative memory formation in ovariectomized females. Psychoneuroendocrinology. 2004;29:883–890. doi: 10.1016/j.psyneuen.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr, et al. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb Cortex. 2001;11:490–497. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- 24.Ostlund H, Keller E, Hurd YL. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Ann N Y Acad Sci. 2003;1007:54–63. doi: 10.1196/annals.1286.006. [DOI] [PubMed] [Google Scholar]
- 25.Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learn Mem. 2009;16:248–266. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, et al. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci. 2005;25:9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalla C, Shors TJ. Sex differences in learning processes of classical and operant conditioning. Physiol Behav. 2009;97:229–238. doi: 10.1016/j.physbeh.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164:887–895. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, et al. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–658. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, et al. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 31.Paxinos G, Watson C. The Rat Brain, in Stereotaxic Coordinates. San Diego: Academic Press; 2007. [Google Scholar]
- 32.Levine JB, Leeder AD, Parekkadan B, Berdichevsky Y, Rauch SL, Smoller JW, et al. Isolation rearing impairs wound healing and is associated with increased locomotion and decreased immediate early gene expression in the medial prefrontal cortex of juvenile rats. Neuroscience. 2008;151:589–603. doi: 10.1016/j.neuroscience.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. New York: Biometrics Research, New York Psychiatric Institute; 2002. Research Version, Patient Edition. ed. [Google Scholar]
- 34.Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry. 2007;62:1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 35.Talairach J, Tournoux P. 3-D Proportional System: an Approach to Cerebral Imaging. New York: Thieme Publishers; 1988. Co-planar Stereotaxic Atlas of the Human Brain. [Google Scholar]
- 36.Chang YJ, Yang CH, Liang YC, Yeh CM, Huang CC, Hsu KS. Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor beta. Hippocampus. 2009;19:1142–1150. doi: 10.1002/hipo.20581. [DOI] [PubMed] [Google Scholar]
- 37.Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor beta activation prevents glucocorticoid receptor-dependent effects of the central nucleus of the amygdala on behavior and neuroendocrine function. Brain Res. 2010;1336:78–88. doi: 10.1016/j.brainres.2010.03.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greer PL, Greenberg ME. From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59:846–860. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Osborne DM, Edinger K, Frye CA. Chronic administration of androgens with actions at estrogen receptor beta have anti-anxiety and cognitive-enhancing effects in male rats. Age (Dordr) 2009;31:191–198. doi: 10.1007/s11357-009-9114-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomihara K, Soga T, Nomura M, Korach KS, Gustafsson JA, Pfaff DW, et al. Effect of ER-beta gene disruption on estrogenic regulation of anxiety in female mice. Physiol Behav. 2009;96:300–306. doi: 10.1016/j.physbeh.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith CC, McMahon LL. Estrogen-induced increase in the magnitude of long-term potentiation occurs only when the ratio of NMDA transmission to AMPA transmission is increased. J Neurosci. 2005;25:7780–7791. doi: 10.1523/JNEUROSCI.0762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. J Neurosci. 2001;21:9009–9017. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santini E, Quirk GJ, Porter JT. Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J Neurosci. 2008;28:4028–4036. doi: 10.1523/JNEUROSCI.2623-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- 45.Llaneza DC, Frye CA. Progestogens and estrogen influence impulsive burying and avoidant freezing behavior of naturally cycling and ovariectomized rats. Pharmacol Biochem Behav. 2009;93:337–342. doi: 10.1016/j.pbb.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taneepanichskul S, Patrachai S. Effects of long-term treatment with depot medroxy progesterone acetate for contraception on estrogenic activity. J Med Assoc Thai. 1998;81:944–946. [PubMed] [Google Scholar]
- 47.Altshuler LL, Hendrick V, Cohen LS. Course of mood and anxiety disorders during pregnancy and the postpartum period. J Clin Psychiatry. 1998;59(Suppl 2):29–33. [PubMed] [Google Scholar]
- 48.Schnatz PF, Whitehurst SK, O’Sullivan DM. Sexual dysfunction, depression, and anxiety among patients of an inner-city menopause clinic. J Womens Health (Larchmt) 2010;19:1843–1849. doi: 10.1089/jwh.2009.1800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


