SUMMARY
Purpose
Radiation-induced inflammation and production of reactive oxygen species (ROS) play a critical role in normal tissue response. In this study we have examined some aspects of these effects in lung and skin.
Methods
The superoxide dismutase (SOD) catalase mimetic, EUK-207, and genistein, an isoflavone with anti-inflammatory properties, were given post irradiation and micronuclei (MN) formation was determined in cells derived from irradiated lung and skin. Changes in breathing rate were measured using a plethysmograph following irradiation of C57Bl6 mice knocked out for tumour necrosis factor (TNF)-alpha or its receptors, TNFR1/2, or treated with endotoxin (lipopolysaccharide - LPS).
Results
Both EUK-207 and genistein given after irradiation caused a large reduction in MN levels observed in lung cells during 14 weeks post-irradiation but ceasing treatment resulted in a rebound in MN levels at 28 weeks post-irradiation. In contrast, treatment with EUK-207 was largely ineffective in reducing MN observed in skin cells post-irradiation. Knock-out of TNF-alpha resulted in a reduced increase in breathing rate (peak at 12 weeks post-irradiation) relative to wild-type and TNFR1/2 knockout. Treatment with LPS 1 hour post-irradiation also reduced the increase in breathing rate.
Conclusions
The generation of MN in lung cells after treatment with EUK-207 or genistein was stopped suggests that continuing ROS production contributes to DNA damage in lung cells over prolonged periods. That this effect was not seen in skin suggests this mechanism is less prominent in this tissue. The reduced level of radiation pneumonitis (as monitored by breathing rate changes) in animals knocked out for TNF-alpha suggests that this cytokine plays a significant role in inducing inflammation in lung following irradiation. The similar effect observed following LPS given post-irradiation suggests the possibility that such treatment modifies the long-term cyclic inflammatory response following irradiation in lung.
INTRODUCTION
Normal tissue injury involves complex interactions between direct damage to parenchymal cells, damage to vasculature, particularly endothelial cells, damage to the stroma and interactions with and response of the tissue micro-environment, particularly the effects of inflammatory cytokines [1, 2]. Radiation-induced inflammation and the resulting generation of reactive oxygen and nitrogen species (ROS or RNOS) is increasingly recognized as playing an important role in normal tissue response to radiation damage [3–6]. Macrophages are regarded as the primary source of inflammatory cytokines and can help to drive a persistent cytokine cascade and chronic inflammatory state [7, 8]. However, the exact role of the pro- and anti-inflammatory cytokines involved and their interactions are often unclear and may vary from tissue to tissue.
Damage to cellular DNA is one of the primary targets for the biological and lethal effects of ionizing radiation and is generally thought to occur largely at the time of irradiation as a direct result of short-lived radiation-produced radicals. We have observed the prolonged presence of DNA damage in both lung and skin following irradiation by examining MN formation in fibroblasts in their first division after removal from the tissue [9–16]. In lung we have observed that treatment of the animals post-irradiation with ROS scavengers can reduce the high level of MN found in fibroblasts derived from lung and, importantly, that DNA damage appears to be regenerated if treatment with ROS scavengers is stopped weeks after the irradiation exposure [11, 14, 17]. This suggests that much of the DNA damage we observed at late times in lung fibroblasts was actually due to ROS produced in the tissue post-irradiation.
In the current study we have further examined the effects of ROS scavengers given post-irradiation on prolonged DNA damage in fibroblasts from the lung and skin of rodents. We have also investigated the potential role of TNF-alpha, one of the main mediators of inflammatory responses, by studying radiation-induced pneumonitis in mice bearing mutations in the TNF-alpha pathway. In addition we examined the effect of exacerbating the inflammation by intra-tracheal exposure to endotoxin (lipopolysaccharide - LPS) immediately post-irradiation. Our results demonstrate that ROS scavengers are highly effective in reducing MN observed in fibroblasts from lung but much less so in fibroblasts derived from skin. Mice knocked out for either of the TNF receptors (TNFR1 or TNFR2 K/O) showed similar radiation-induced pneumonitis as wild type mice but mice knocked out for TNF alpha (TNF alpha K/O) showed a reduced level of pneumonitis. In this context it is somewhat surprising that an intra-tracheal injection of LPS, which activates inflammatory pathways also results in some mitigation of pneumonitis in the mice.
METHODS
Animals and drugs
Female Sprague-Dawley (SD) rats (Charles River Laboratories International, Inc., Wilmington, MA, USA) and C57Bl6 wild type (C57WT) or C3H/HeJ mice (Jax Labs, Bar Harbor, ME, USA) used in the studies were housed in animal facilities accredited by the Canadian Council on Animal Care and treated in accordance with approved protocols. C57Bl6 mice bearing mutations in the TNF alpha pathway were obtained from Drs Tak Mak and Rama Khokha at OCI and were bred in house. The genistein diet (750 mg/kg of genistein) was formulated as described previously [11, 14]. The salen-manganese(Mn) superoxide dismutase(SOD)-catalase mimetic, EUK-207 was custom-synthesized and characterized as described previously [18]. For long term treatment in the lung studies, the animals received doses of 8mg/kg body weight/day administered by Alzet 2ML4 infusion pump (delivery rate 2.5 µl/hr) (Alzet Osmotic Pumps, Durect Corporation, Cupertino, CA, USA) implanted subcutaneously and changed every 4 wks as described previously [14]. For short term treatment in the skin studies the animals received daily doses of 30 mg/kg injected intraperitoneally (i.p.).
Irradiation
The animals were anesthetised by isofluorane inhalation and placed in custom-designed Lucite holding containers. For lower lung irradiation the rats were treated with a Co-60 gamma ray source at 0.6 Gy/min with 10 cm thick lead collimators used to define the radiation field [11]. For whole lung irradiation the rats and the initial groups of mice (C57WT and TNFR1 and TNFR2 K/O) were irradiated with a double-headed 100 kVp X-ray unit [14, 17]. Two circular surface collimators made of lead were inserted on both sides of the jig to confine the radiation only to the lung volume. The animals were set-up individually by eye to ensure the whole lung was in the beam. The remaining groups of mice (C57WT and TNF alpha K/O) were irradiated with an image-guided micro-irradiator (CX-Rad-225, Precision X-ray Inc. North Branford, CT, USA) that allowed targeting and irradiating the desired volume in each animal. The details of this unit have been reported [19]. The mice received 10 Gy at 100 kVp to the whole lung given at approximately 3.5 Gy/min with anterior-posterior (a-p) and (p-a) beams. The mid-plane of the animal was located at the iso-center. For the skin studies the mice were irradiated locally on their back (3 × 4 cm field) with a single beam from the image guided small animal irradiator at 100 kVp at a dose rate of approximately 3.5Gy/min.
Breathing Rate Measurement
We measured the breathing frequency of mice using a respiration rate monitor (Columbus Instruments, Columbus, Ohio, USA). The animals were acclimatised to the method several times in the two weeks preceding the start of the experiment. At each time point the breathing rate of each animal was monitored for two minutes after an initial 45 second acclimatization period. Breathing rate was then determined by taking the mean of a maximum of five 6 second intervals of calm breathing within the two minutes measurement period. Due to movement it was not always possible to obtain data at each time point for every animal. The number of animals contributing to the various data points varied between four and ten.
Micronuclei Assay
Assay of micronuclei in lung or skin cells (largely fibroblasts) was performed as described previously [10, 12, 16].
PCR analysis
The RT-PCR analysis of TNF-alpha expression was performed essentially as described [12]. Briefly lungs of rats were removed and a strip measuring approximately 0.5cm around the centre of the lung (the expected position of the radiation field edge) was removed. The remaining tissue lung was minced, placed in 5ml of RNAlater (Qiagen, Mississauga, Canada) and held at 4°C for up to one month, prior to analysis. Total RNA was isolated from 60 mg of tissue, reverse transcribed and analysed by Real-Time RT-PCR (ABI Prism7700® Sequence Detection System). The housekeeping gene GAPDH was used to calculate the relative mRNA expression. The RT-PCR analysis was done twice on each sample and the mean Ct value was used for analysis.
Statistical analysis
Multiple linear regressions and Tukey’s method for the adjustment of least square means in multiple comparisons were used for analysis of the data sets. Mixed modeling was used to examine time trends in the breathing rate data. SAS (enterprise guide-4) software was used for the analysis. A p value less than 0.05 was considered as significantly different.
RESULTS AND DISCUSSION
Figure 1 shows results for micronucleus (MN) formation in fibroblasts derived from the lungs of SD rats at various times after a dose of 12Gy. Two of the groups of rats were given EUK-207 by infusion pump or a genistein diet starting about 1 hr after the irradiation and finishing at 14 weeks post irradiation. Groups of four animals were sacrificed and analysed at various times after irradiation. The results (radiation-only group) demonstrate that the presence of MN is prolonged in the lung fibroblasts after irradiation and that treatment with either EUK-207 or genistein for up to 14 weeks can significantly reduce the number of MN detected in the cells. The EUK-207 infusion appears to be somewhat more effective in this regard since the MN levels decline more rapidly (see 8 week time point) than with genistein diet. However, further studies would be necessary to clarify this effect. The genistein diet caused a similar decline in MN in the lungs of C3H/HeJ mice following fractionated irradiation [20]. Importantly, after the drug treatment is stopped at 14 weeks, the number of MN observed at 28 weeks returns to levels similar to that seen in the radiation-only group. It is possible that death or terminal differentiation of severely damage cells coupled with the influx of myofibrocytes from circulating blood affects the number of MN observed, since the cells are required to undergo one division in vitro for the assay procedure. Such effects, however, could not account for the increase of DNA damage seen at the 28-week time point. These results suggest that generation of DNA damage occurs in the lung fibroblasts post irradiation, presumably due to the production of ROS or RNOS. Such ROS (RNOS) could be produced by the inflammatory cells but possibly also by leakage from damaged mitochondria, activation of NAD oxidases or iNOS in the cells. Considering the reactivity of these radicals, the extent of production of them inside the cell relative to outside (by inflammatory cells) may be critical for DNA damage. Studies of bystander effects in cells in vitro have demonstrated a role of ROS and RNOS in MN formation in bystander cells, although it is unclear how extensive such effects might be in vivo [21].
Figure 1.
Number of micronuclei (MN) per 1000 binucleate (BN) cells in fibroblasts taken from lungs of Sprague-Dawley rats either before irradiation or at the indicated times after a dose of 10 Gy given to the lung. The animals were treated with EUK-207 or genistein starting 1 hr after irradiation and stopped at 14 weeks after irradiation. Modified from [14].
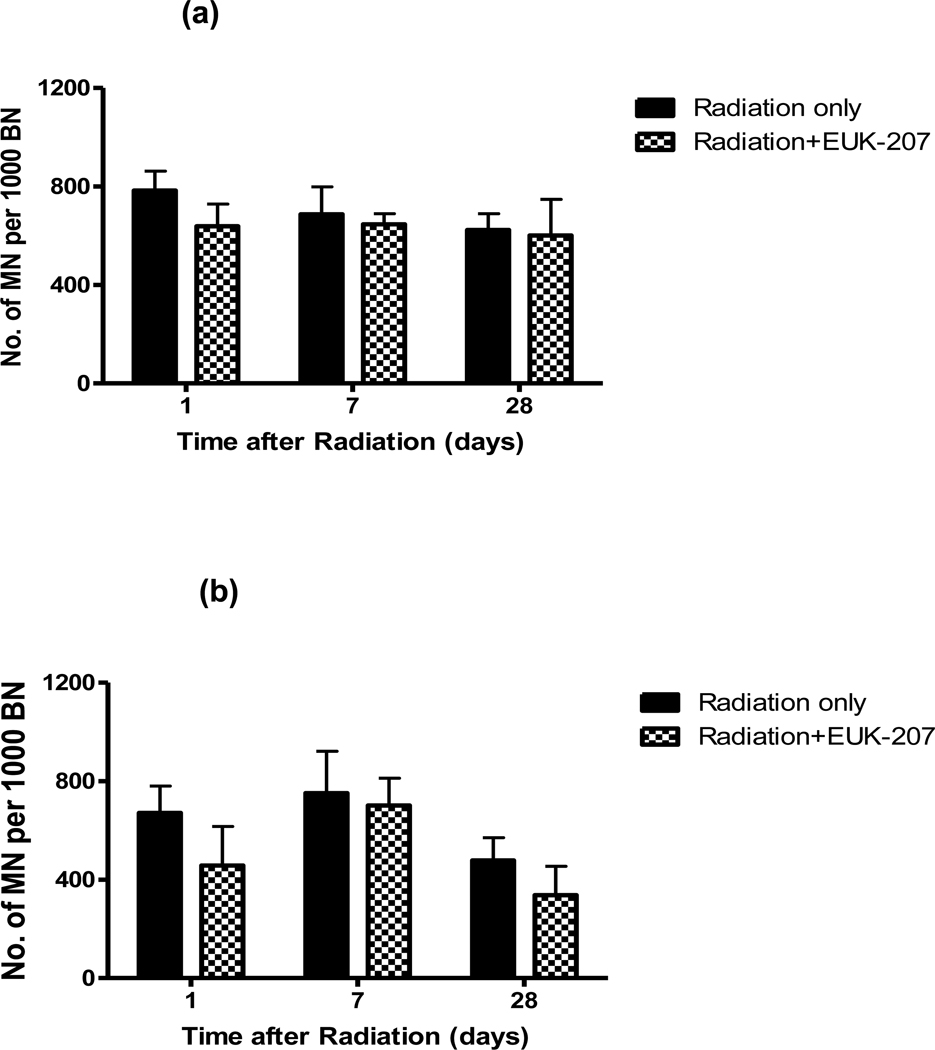
Since EUK-207 was so effective in lung we also examined its efficacy in skin where we had previous found similar prolonged presence of MN in fibroblasts following irradiation of human, rat and mouse skin [13, 15, 16, 22]. Figure 2 shows results for MN in cells derived from the skin of C3H/HeJ mice analysed at 1 day, 1 week and 4 weeks after dose of 5 Gy. The animals were given one i.p. injection of EUK-207 (30 mg/kg) immediately after irradiation for studying the mitigation effect at 1 day or 5 i.p. injections (daily) of EUK-207 for the analysis at 7 or 28 days. For the protection effect 5 i.p. injections (daily) of EUK-207 were started 4 days before radiation with the last dose of drug delivered 30 mins before irradiation. The results demonstrate that treatment with EUK-207 had only a small effect on MN levels in the skin, even though a larger dose of the drug was used than in the lung studies. Studies in which a whole body dose of 10 Gy, similar to that used in the lung, was given to the mice also showed only a small effect of EUK-207 treatment on MN formation in skin fibroblasts (Jelveh and Hill, 2011, unpublished data). A dose of 5 Gy was used in the current study because our detailed analyses of MN in skin showed that a dose of 5 Gy is in the steeply rising part of the dose response curve, whereas above a dose of 7–8 Gy the MN level is starting to plateau [16].
Figure 2.
Number of micronuclei per 1000 binucleated cells in fibroblasts taken from skin 1, 7 or 28 days after a dose of 5Gy (4mice per group). (a) One i.p. injection of EUK-207 (30mg/kg) was delivered 30 mins after radiation (1 day) or five i.p. injections were given daily starting 30 mins after irradiation (7 and 28 days) (b) Five i.p. injections of EUK-207 (30mg/kg) were delivered daily before irradiation. The last injection was 30 mins before irradiation.
The reason for the difference between lung and skin remains unclear but may reflect different levels or timing of inflammation in lung and skin at the radiation doses used. The results are consistent with our observations that contrary to lung [9, 10] there is no out-of-field effect for MN formation following skin irradiation (Hill, unpublished data). There may also be differences in proliferation and turnover of the fibroblasts in these two tissues following irradiation. Previous studies in skin and oral mucosa of rats and hamster using larger radiation doses have indicated that EUK-207 does provide protection and mitigation against radiation-induced wound healing delay and radiation dermatitis respectively [23]. It is thus unlikely that our results reflect lack of drug distribution to the skin. However, these different results for different endpoints raise the question of the importance of long term DNA damage in fibroblasts to wound healing and radiation dermatitis, although, particularly for wound healing, fibroblasts would be expected to play an important role. A possible explanation may be that there is recruitment of myofibrocytes from the circulation or other sites [24] to assist healing following large radiation doses. Studies in animals bearing labelled bone marrow cells could help to address this issue. Such studies could also examine whether labelled cells recruited from the circulation can pick-up ‘bystander’ DNA damage from ROS produced by radiation-exposed cells in the tissue, either by examining MN formation or by examining γH2AX foci in tissue sections[25].
We next examined the role of TNF alpha, one of the major proinflammatory cytokines, in the radiation-induced inflammatory process. The effects of the genistein diet on the mRNA expression of this cytokine at various times after irradiation (10 Gy) of the lower lung of SD rats are shown in Figure 3. They confirm previous work indicating a cyclic pattern of increased expression following irradiation [26–28] and show an early peak at about 7 hours followed by a second rise at 5–12 days and a third rise at 4–20 weeks. Notably this pattern of expression of TNF alpha is the same in the upper (shielded) region of the lung as in the lower irradiated region indicating that the inflammatory response is organ-wide. This result is consistent with our previous findings that irradiation of the lower lung results in the production of MN in fibroblasts from the shielded upper part of the lung and that this damage can be reduced by ROS (RNOS) scavengers [9, 10]. When the animals were put on a genistein diet, this largely prevented the increase in TNF alpha over the whole time period. These results are consistent with our observations of a reduction in MN described above.
Figure 3.
Expression of TNF-alpha mRNA relative to GAPDH in the upper or lower region of the lungs of groups (4 rats per group) of Sprague-Dawley rats given a dose of 10 Gy to the lower lung. Some of the rats were put on a genistein diet immediately after irradiation and maintained on the diet throughout the experiment.
rad upper/lower = upper or lower lung of rats which received radiation only. gen upper/lower = upper or lower lung of rats which received radiation and the genistein diet.
We further analysed the role of TNF alpha by examining the effects of lung irradiation (10 Gy) on breathing rate in C57Bl6 mice knocked out for TNF-alpha or its two receptors TNFR1 and TNFR2. The results (Figure 4) show that knocking out either receptor has little effect on the overall increase in breathing rate which occurred after the irradiation (peaking at about 12 weeks), although the results show that the increase in breathing rate occurred significantly earlier for the TNFR1 knock-out mice. These findings are inconsistent with a recent report of early protection of lung function in mice at 4–8 weeks by knocking out TNFR1[29]. Most notably in our studies the animals knocked out for TNF alpha showed a significantly damped increase in breathing rate following irradiation over the whole time course of the study. These results are consistent with our previous observations in SD rats using genistein [12] and suggests that TNF alpha plays a significant role in the induction of radiation-induced pneumonitis. Previous studies have reported that TNF blockade reduces production of VEGF by macrophages following irradiation, which could reduce protection of vasculature [30]. Analysis of MN formation in lungs of mice knocked out for TNF-alpha is needed to determine if the cytokine also plays a role in the prolongation of DNA damage.
Figure 4.
Mean breathing rate (+/− SD) for groups of mice as a function of time after being given either 0 or10 Gy to the whole lung at time zero. The solid lines and black symbols indicate the non-irradiated mice. The grey dashed lines and symbols indicate the irradiated animals.
All mice have a C57Bl6 background WT = wild type, TNFR1−/− = TNFR1 K/O, TNFR2−/− = TNFR2 K/O, TNF alpha −/− + TNF alpha K/O.
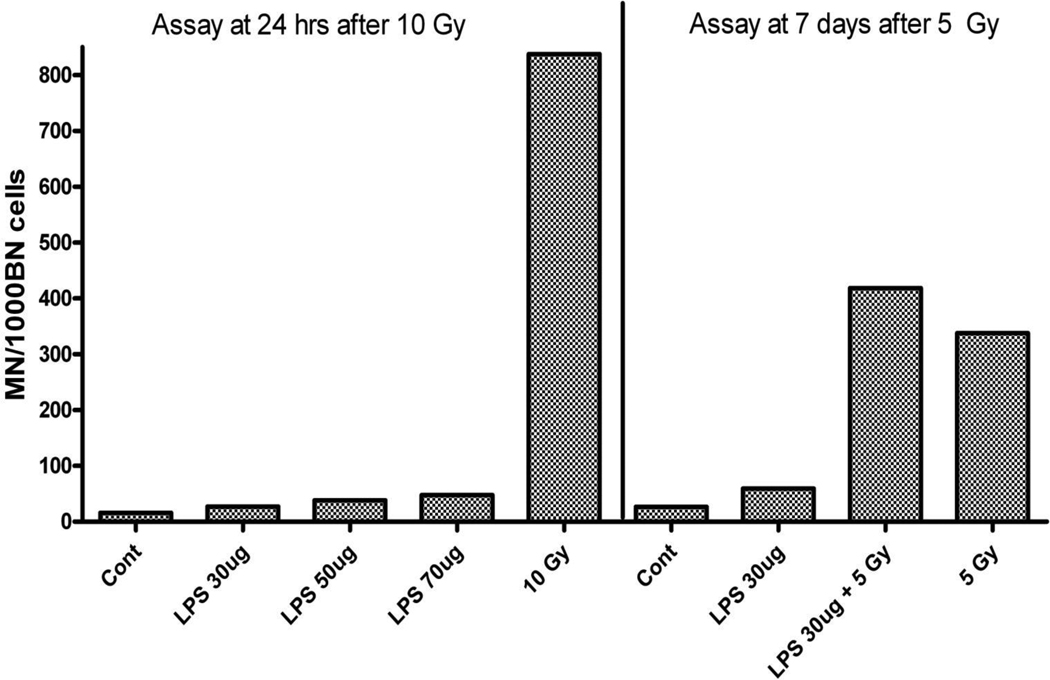
Finally we examined whether further stimulation of the inflammatory response through the TNF pathway by exposure of the animals to LPS 1 hr post irradiation would further enhance the effects of irradiation. We first studied whether LPS given intratracheally would induce MN formation in the lung fibroblasts. Results of this experiment, shown in figure 5, indicate a very small effect in comparison to that observed following irradiation, but the data do suggest that at 1 week the LPS treatment may induce a small additive increase to the level of MN observed with irradiation alone. However, only a small number of animals were used in this study and while the data clearly indicate that LPS treatment is much less effective than irradiation in MN formation, it is not sufficient to determine whether there is a true additive increase. Despite the small effect on MN formation, analysis of cytokine levels in the lung did indicated a significant inflammatory effect of the doses of LPS used in this treatment (data not shown).
Figure 5.
Micronuclei (MN) per 1000 binucleate (BN) cells in fibroblasts from lungs of C57WT mice at 1day or 7 days after exposure to radiation and or lipopolysaccharide (LPS) at different doses as indicated. Each column represent mean data from 2 mice.
We next determined whether additive LPS treatment would affect breathing rate changes associated with lung irradiation of C57WT mice. The results (Figure 6) indicate that the LPS exposure actually mitigated the increased breathing rate, contrary to what might have been expected based on the previous data. These results raise the possibility that treatment with LPS may modify the expression of inflammatory cytokines following irradiation. It has been reported that it is the secondary waves of chronic inflammation rather than the early induction of cytokines that are more important in the induction of radiation–induced pneumonitis. [31]. Analysis of cytokine levels at different times after lung irradiation and exposure to LPS are needed to determine whether combination treatment modifies these secondary waves of chronic inflammation following irradiation. A similar effect of exposure to LPS following irradiation has been reported for early damage to the intestine in mice [32], however, prior irradiation has been reported to sensitize lung to increased inflammation following late (6–15 months post irradiation) exposure LPS [7, 33].
Figure 6.
Mean breathing rate (+/− SD) for groups of C57WT mice as a function of time after being given either 0 or10 Gy to the whole lung at time zero. The solid black lines and square symbols indicate the non-irradiated mice. The dark grey triangles and dashed lines indicate the irradiated animals. The light grey dotted lines and inverted triangles indicate animals given LPS (30 ug) intratracheally 1 hr after irradiation.
CONCLUSION
The MN damage observed in fibroblasts from lung both in and out of the radiation field and the finding that MN levels increase again when treatment with a ROS(RNOS) scavenger is halted suggests that this DNA damage is due to ROS(RNOS) arising from radiation-induced inflammatory effects in lung. The inability of ROS(RNOS) scavengers to affect MN formation in skin and the lack of an out-of-field effect in skin suggests that the lung mounts a much more extensive inflammatory response than skin following irradiation. ROS(RNOS) can be produced by inflammatory cells but also by leakage from damaged mitochondria, activation of NAD oxidases or iNOS inside the cells. Considering the reactivity of such radicals the extent of production of them inside the cell relative to outside (by inflammatory cells) may be critical for DNA damage observed. The balance of these effects may be different in lung and skin although the absolute levels of MN formed in-field are similar in the two tissues following the same radiation dose.
Extensive data in the literature suggest that radiation-induced lung damage arises as a result of the interaction of a cycle of chronic inflammation and oxidative damage and may be modified by ROS scavengers [3, 7, 24, 26–28, 34–41]. Our results with the TNF-alpha knock-out mice are consistent with this concept, although the results with LPS treatment seem contrary to this idea. This might reflect the fact that LPS-induced inflammation is normally short-lived [42]. and that the early induction of inflammation following irradiation in mice is reported to play little role in the later development of pneumonitis [31]. However, a study in patients with FDG, which is taken up in areas of inflammation, has suggested that increased uptake at 1–2 weeks during lung radiotherapy may reflect the later development of pneumonitis [43]. Possibly, as noted above, treatment with LPS may modify the expression of inflammatory cytokines following irradiation. ROS(RNOS) can produce oxidation of lipids and proteins as well as DNA damage and these effects in lung cells may play a more important role in functional endpoints, such as pneumonitis, than DNA damage in fibroblasts. However, the DNA damage we observed in lung fibroblasts, particularly in shielded regions, presumably reflects the inflammatory environment in the irradiated lung. The production of ROS(RNOS) intracellularly vs extracellularly (by inflammatory cells) may be critical for the balance between the development of functional effects and DNA damage in surviving lung cells.
Acknowledgements
This work was supported by funds from an NIAID/NIH U19 program (U19 AI-067734) and by funds from the Canadian Institutes of Health Research (#144089). Partial support was also provided by the Ontario Ministry of Health and Long Term Care. The views expressed do not necessarily reflect those of OMHLTC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts
The authors declare no conflicts of interest.
REFERENCES
- 1.Rodemann HP. Molecular radiation biology: perspectives for radiation oncology. Radiother Oncol. 2009;92(3):293–298. doi: 10.1016/j.radonc.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 2.Stewart FA, Dorr W. Milestones in normal tissue radiation biology over the past 50 years: from clonogenic cell survival to cytokine networks and back to stem cell recovery. Int J Radiat Biol. 2009;85(7):574–586. doi: 10.1080/09553000902985136. [DOI] [PubMed] [Google Scholar]
- 3.Fleckenstein K, et al. Using biological markers to predict risk of radiation injury. Semin Radiat Oncol. 2007;17(2):89–98. doi: 10.1016/j.semradonc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Robbins ME, Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: a review. Int J Radiat Biol. 2004;80(4):251–259. doi: 10.1080/09553000410001692726. [DOI] [PubMed] [Google Scholar]
- 5.Zhao W, Robbins ME. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr Med Chem. 2009;16(2):130–143. doi: 10.2174/092986709787002790. [DOI] [PubMed] [Google Scholar]
- 6.Ghafoori P, et al. Radiation-induced lung injury. Assessment, management, and prevention. Oncology (Williston Park) 2008;22(1):37–47. discussion 52–3. [PubMed] [Google Scholar]
- 7.Johnston CJ, et al. Inflammatory cell recruitment following thoracic irradiation. Exp Lung Res. 2004;30(5):369–382. doi: 10.1080/01902140490438915. [DOI] [PubMed] [Google Scholar]
- 8.Schaue D, McBride WH. Links between innate immunity and normal tissue radiobiology. Radiat Res. 2010;173(4):406–417. doi: 10.1667/RR1931.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan MA, Hill RP, Van Dyk J. Partial volume rat lung irradiation: an evaluation of early DNA damage. Int J Radiat Oncol Biol Phys. 1998;40(2):467–476. doi: 10.1016/s0360-3016(97)00736-0. [DOI] [PubMed] [Google Scholar]
- 10.Khan MA, et al. Partial volume rat lung irradiation; assessment of early DNA damage in different lung regions and effect of radical scavengers. Radiother Oncol. 2003;66(1):95–102. doi: 10.1016/s0167-8140(02)00325-0. [DOI] [PubMed] [Google Scholar]
- 11.Calveley VL, et al. Genistein can mitigate the effect of radiation on rat lung tissue. Radiat Res. 2010;173(5):602–611. doi: 10.1667/RR1896.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calveley VL, et al. Partial volume rat lung irradiation: temporal fluctuations of in-field and out-of-field DNA damage and inflammatory cytokines following irradiation. Int J Radiat Biol. 2005;81(12):887–899. doi: 10.1080/09553000600568002. [DOI] [PubMed] [Google Scholar]
- 13.Hill RP, et al. Studies of the in vivo radiosensitivity of human skin fibroblasts. Radiother Oncol. 2007;84(1):75–83. doi: 10.1016/j.radonc.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahmood J, et al. Mitigation of radiation-induced lung injury by Genistein and EUK-207. International Journal of Radiation Biology. 2011;87(8) doi: 10.3109/09553002.2011.583315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaspler P, Pintilie M, Hill RP. Dynamics of micronuclei in rat skin fibroblasts after X irradiation. Radiat Res. 2009;172(1):106–113. doi: 10.1667/RR1649.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaspler P, et al. Biodosimetry using Radiation-Induced Micronuclei in Skin Fibroblasts. International Journal of Radiation Biology. 2011;87(8) doi: 10.3109/09553002.2011.582927. p.?? [DOI] [PubMed] [Google Scholar]
- 17.Langan AR, et al. Partial volume rat lung irradiation: the protective/mitigating effects of Eukarion-189, a superoxide dismutase-catalase mimetic. Radiother Oncol. 2006;79(2):231–238. doi: 10.1016/j.radonc.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Rosenthal RA, et al. Orally available Mn porphyrins with superoxide dismutase and catalase activities. J Biol Inorg Chem. 2009;14(6):979–991. doi: 10.1007/s00775-009-0550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clarkson R, et al. Characterization of image quality and image-guidance performance of a preclinical microirradiator. Med Phys. 2010;38(2):845–856. doi: 10.1118/1.3533947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Para AE, et al. Effects of genistein following fractionated lung irradiation in mice. Radiother Oncol. 2009;92(3):500–510. doi: 10.1016/j.radonc.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Shao C, et al. Bystander signaling between glioma cells and fibroblasts targeted with counted particles. Int J Cancer. 2005;116(1):45–51. doi: 10.1002/ijc.21003. [DOI] [PubMed] [Google Scholar]
- 22.Kaspler P, Hyrien O, Hill RP. Dynamics of micronuclei in mouse skin fibroblasts after gamma irradiation. Health Phys. 2010;98(2):228–233. doi: 10.1097/HP.0b013e3181b02f90. [DOI] [PubMed] [Google Scholar]
- 23.Rosenthal RA, et al. Salen Mn Complexes Mitigate Radiation Injury in Normal Tissues. Anticancer Agents Med Chem. 2011 doi: 10.2174/187152011795677490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yarnold J, Brotons MC. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol. 2010;97(1):149–161. doi: 10.1016/j.radonc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 25.Bhogal N, et al. Late residual gamma-H2AX foci in murine skin are dose responsive and predict radiosensitivity in vivo. Radiat Res. 2010;173(1):1–9. doi: 10.1667/RR1851.1. [DOI] [PubMed] [Google Scholar]
- 26.Johnston CJ, et al. Early and persistent alterations in the expression of interleukin-1 alpha, interleukin-1 beta and tumor necrosis factor alpha mRNA levels in fibrosis-resistant and sensitive mice after thoracic irradiation. Radiat Res. 1996;145(6):762–767. [PubMed] [Google Scholar]
- 27.Hong JH, et al. Rapid induction of cytokine gene expression in the lung after single and fractionated doses of radiation. Int J Radiat Biol. 1999;75(11):1421–1427. doi: 10.1080/095530099139287. [DOI] [PubMed] [Google Scholar]
- 28.Rube CE, et al. The bronchiolar epithelium as a prominent source of pro-inflammatory cytokines after lung irradiation. Int J Radiat Oncol Biol Phys. 2005;61(5):1482–1492. doi: 10.1016/j.ijrobp.2004.12.072. [DOI] [PubMed] [Google Scholar]
- 29.Zhang M, et al. Inhibition of the tumor necrosis factor-alpha pathway is radioprotective for the lung. Clin Cancer Res. 2008;14(6):1868–1876. doi: 10.1158/1078-0432.CCR-07-1894. [DOI] [PubMed] [Google Scholar]
- 30.Meng Y, et al. Blockade of tumor necrosis factor alpha signaling in tumor-associated macrophages as a radiosensitizing strategy. Cancer Res. 2010;70(4):1534–1543. doi: 10.1158/0008-5472.CAN-09-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hong JH, et al. Can short-term administration of dexamethasone abrogate radiation-induced acute cytokine gene response in lung and modify subsequent molecular responses? Int J Radiat Oncol Biol Phys. 2001;51(2):296–303. doi: 10.1016/s0360-3016(01)01702-3. [DOI] [PubMed] [Google Scholar]
- 32.Riehl TE, et al. TNFR1 mediates the radioprotective effects of lipopolysaccharide in the mouse intestine. Am J Physiol Gastrointest Liver Physiol. 2004;286(1):G166–G173. doi: 10.1152/ajpgi.00537.2002. [DOI] [PubMed] [Google Scholar]
- 33.Johnston CJ, et al. Effect of Total Body Irradiation on Late Lung Effects: Hidden Dangers. International Journal of Radiation Biology. 2011;87(8) doi: 10.3109/09553002.2011.573439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finkelstein JN, et al. Early alterations in extracellular matrix and transforming growth factor beta gene expression in mouse lung indicative of late radiation fibrosis. Int J Radiat Oncol Biol Phys. 1994;28(3):621–631. doi: 10.1016/0360-3016(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 35.Fleckenstein K, et al. Temporal onset of hypoxia and oxidative stress after pulmonary irradiation. Int J Radiat Oncol Biol Phys. 2007;68(1):196–204. doi: 10.1016/j.ijrobp.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gauter-Fleckenstein B, et al. Comparison of two Mn porphyrin-based mimics of superoxide dismutase in pulmonary radioprotection. Free Radic Biol Med. 2008;44(6):982–989. doi: 10.1016/j.freeradbiomed.2007.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong JH, et al. Bronchoalveolar lavage and interstitial cells have different roles in radiation-induced lung injury. Int J Radiat Biol. 2003;79(3):159–167. doi: 10.1080/0955300031000076894. [DOI] [PubMed] [Google Scholar]
- 38.Ostrau C, et al. Lovastatin attenuates ionizing radiation-induced normal tissue damage in vivo. Radiother Oncol. 2009;92(3):492–499. doi: 10.1016/j.radonc.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 39.Rabbani ZN, et al. Long-term administration of a small molecular weight catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation-induced injury. Int J Radiat Oncol Biol Phys. 2007;67(2):573–580. doi: 10.1016/j.ijrobp.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rube CE, et al. Irradiation induces a biphasic expression of pro-inflammatory cytokines in the lung. Strahlenther Onkol. 2004;180(7):442–448. doi: 10.1007/s00066-004-1265-7. [DOI] [PubMed] [Google Scholar]
- 41.Rube CE, et al. Modulation of radiation-induced tumour necrosis factor alpha (TNF-alpha) expression in the lung tissue by pentoxifylline. Radiother Oncol. 2002;64(2):177–187. doi: 10.1016/s0167-8140(02)00077-4. [DOI] [PubMed] [Google Scholar]
- 42.Rojas M, et al. Endotoxin-induced lung injury in mice: structural, functional, and biochemical responses. Am J Physiol Lung Cell Mol Physiol. 2005;288(2):L333–L341. doi: 10.1152/ajplung.00334.2004. [DOI] [PubMed] [Google Scholar]
- 43.De Ruysscher D, et al. Increased (18)F-deoxyglucose uptake in the lung during the first weeks of radiotherapy is correlated with subsequent Radiation-Induced Lung Toxicity (RILT): a prospective pilot study. Radiother Oncol. 2009;91(3):415–420. doi: 10.1016/j.radonc.2009.01.004. [DOI] [PubMed] [Google Scholar]