Abstract
It has been proposed that constitutive expression of endothelial NO synthase (eNOS) protects against myocardial ischemia/reperfusion injury in the naive (unstressed) state and that eNOS plays a critical role in the early phase of ischemic preconditioning (PC). We addressed these issues using both a genetic approach (i.e., eNOS null [eNOS−/−]) mice and a pharmacologic approach (with the NOS inhibitor N(omega)-nitro-l-arginine [L-NA]). We found that in the nonpreconditioned state, both of the available strains of eNOS−/− mice (C57BL6 and B6129) exhibited infarct sizes similar to the corresponding wild-type (WT) mice (63.3±2.2% [group I, n = 15] vs. 59.7±1.4% [group VI, n = 10] of the risk region and 60.9±3.6% [group IX, n = 14] vs. 68.2±2.5% [group X, n = 9], respectively). When WT mice were preconditioned with either one or six cycles of 4-min coronary occlusion (O)/reperfusion (R) 10 min prior to the 30-min O, infarct size was markedly reduced (28.5±3.3% [group II, one O/R cycle, n = 10] and 19.7±2.6% [group III, six O/R cycles, n = 7] of the risk region, respectively), indicating the development of a robust early PC effect. In eNOS−/− mice preconditioned with the same protocol, the reduction in infarct size was similar (24.9±2.9% and 15.3±2.4% of the risk region, after one [group VII, n = 9] or six O/R cycles [group VIII, n = 10], respectively), indicating that the PC effect was intact. When WT mice were pretreated with L-NA 30 min before sham PC (group IV, n = 7) or PC (group V, six O/R cycles, n = 7), infarct size was not different from untreated control and PC groups. We conclude that, in the mouse, basal eNOS activity does not modulate infarct size in the nonpreconditioned state and is not necessary for the cardioprotective effects of early PC. Early PC is not eNOS-dependent, at least in this species.
Keywords: Ischemia, Reperfusion, Myocardial infarction, Nitric oxide synthase, eNOS−/−
1. Introduction
Although nitric oxide (NO) is known to be a powerful cardioprotectant [1,2], the role of endothelial NO synthase (eNOS) (the major constitutive source of NO in the heart) remains poorly understood. Specifically, two fundamental issues pertaining to the effect of eNOS in myocardial ischemia/reperfusion injury are unresolved. The first issue relates to the role of eNOS in protecting the heart against infarction under basal (unstressed) conditions. One study [3] found no change in infarct size in eNOS−/− mice with deletion of the calmodulin-binding domain [4], suggesting that constitutive expression of this protein does not modulate ischemia/reperfusion injury. Similarly, we have recently reported that in this strain of eNOS−/− mice infarct size is similar to wild-type (WT) controls [5]. In contrast, two independent studies have reported that the heart of this strain of eNOS−/− mice is protected, both in vivo [6] and in vitro [7], and have ascribed this to upregulation of inducible NOS (iNOS). To further compound the controversy, another strain of eNOS−/− mice (with deletion of the NADPH ribose and adenine binding sites [8]) has been found to exhibit no upregulation of iNOS and an increase (rather than a decrease) in infarct size [6]. Thus, there is considerable uncertainty as to whether deletion of eNOS exacerbates ischemia/reperfusion injury in the naive state.
The second issue is the role of eNOS in ischemic preconditioning (PC). It is well established that the protective effects of PC occur in two distinct phases: an early phase that develops rapidly after the stimulus but dissipates within 2–3 h, and a late phase that becomes apparent 12–24 h later and persists for approximately 72 h [9–20]. The role of eNOS in these two phases needs to be examined separately. While previous studies have found that the late phase of ischemic PC is triggered by eNOS-derived NO [5,16,21], the contribution of eNOS to the early phase of PC has not been resolved. A recent study has suggested that activation of eNOS (secondary to activation of the PI3 kinase/Akt pathway [22]) is necessary for the initiation of early PC [23]. This hypothesis, however, is based upon experiments with pharmacologic inhibitors of PI3 kinase, Akt, and eNOS [23]. Furthermore, previous pharmacologic studies had arrived at the opposite conclusion, i.e., that pharmacologic blockade of NOS does not prevent the early phase of ischemic PC [24–26]. The only investigation that has used genetic deletion of eNOS to study the early phase of PC [27] has concluded that eNOS is not required for early PC when the stimulus is robust (multiple brief ischemic episodes) but is necessary when the stimulus is weak, just above threshold (i.e., two or three brief ischemic episodes). This study [27], however, was conducted in an isolated mouse heart model with a short (30 min) reperfusion time, a preparation in which measurements of infarct size have limitations. Therefore, the role of eNOS in the early phase of ischemic PC remains uncertain.
The objective of the present study was to determine the role of eNOS in the early phase of ischemic PC and in cardioprotection in general using a genetic approach. Two specific questions were addressed in a well-established murine model of myocardial infarction [28–30]: (i) Does constitutive expression of eNOS modulate infarct size? and (ii) Is eNOS necessary for the early phase of ischemic PC? Although our recent work [13,20,5] indicates that eNOS does not modulate infarct size in the nonpreconditioned state in eNOS−/− mice with deletion of the calmodulin-binding domain [4], in that study we did not examine the other available strain of eNOS−/− mice (mice with deletion of the NADPH ribose and adenine binding sites [8]), in which eNOS deletion has been found to exacerbate ischemia/reperfusion injury [6,7]. Furthermore, in that study we did not use pharmacologic inhibitors of NOS. Accordingly, to comprehensively interrogate the role of eNOS in myocardial ischemia/reperfusion injury under basal conditions, in the present investigation we examined both strains of eNOS−/− mice as well as pharmacologic inhibition of NOS in the same mouse model.
2. Methods
2.1. Animals
We used eNOS−/− mice with deletion of either the calmodulin-binding domain [4] (University of North Carolina [UNC] eNOS−/− mice) or deletion of the NADPH ribose and adenine binding sites [8] (Harvard eNOS−/− mice). Wild-type (WT) control mice were C57BL/6 (B6) and B6129SF2 (B6129), respectively (The Jackson Laboratory). All mice were maintained in sterile micro-isolator cages under pathogen-free conditions. The genotype of the eNOS−/− strain was verified by PCR, as previously described, using DNA prepared from tail samples taken at the end of the experiments [28].
2.2. Experimental preparation
The murine model of ischemic PC has been previously described in detail [13,20,28–30]. Briefly, a nontraumatic balloon occluder was implanted around the mid-left anterior descending coronary artery in pentobarbital-anesthetized mice. To prevent hypotension, blood from a donor mouse was given during surgery. Rectal temperature was maintained close to 37 °C throughout the experiment.
2.3. Experimental protocol
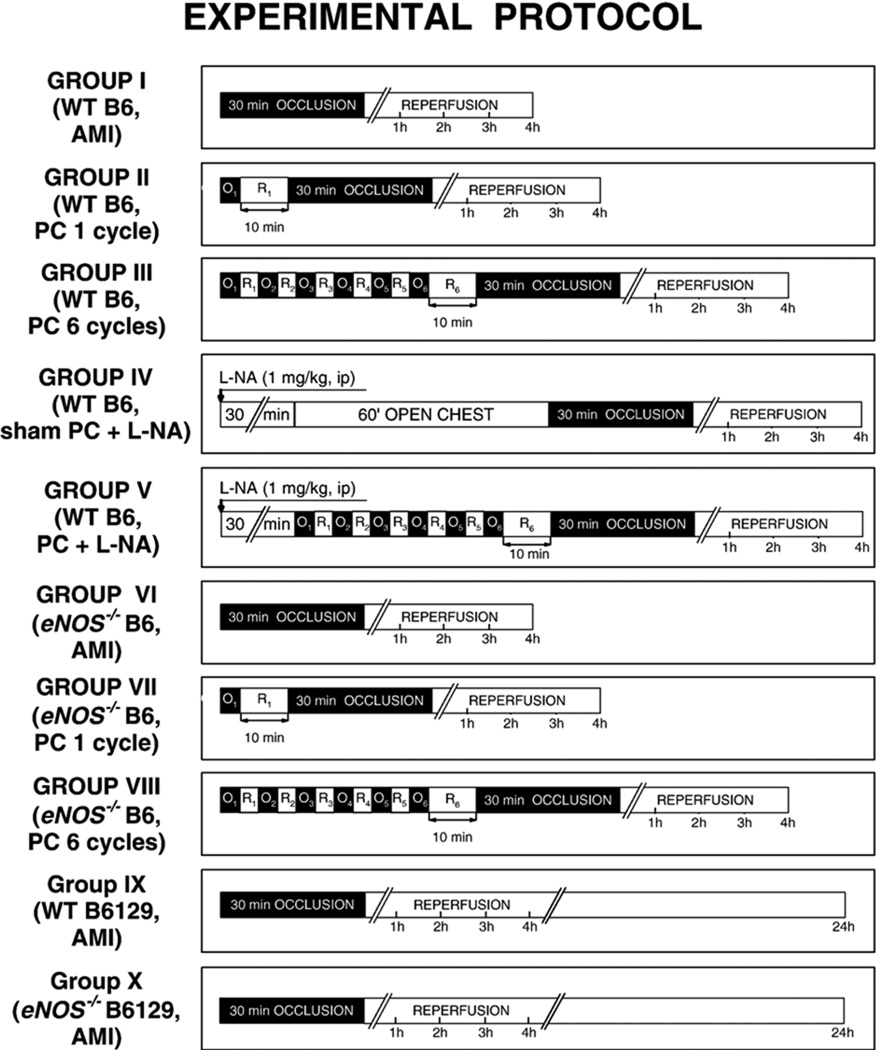
To thoroughly examine the role of eNOS in early PC elicited either by a mild or by a robust ischemic stress, we used two different protocols; thus, ischemic PC was elicited either by a single 4-min coronary occlusion/4-min reperfusion (O/R) cycle or by a sequence of six 4-min O/R cycles (Fig. 1). Ten minutes after the last reperfusion, myocardial infarction (MI) was induced with a 30-min coronary occlusion followed by 4 or 24 h of reperfusion. Control mice underwent the acute MI (AMI) protocol, a 30-min coronary occlusion followed by 4 or 24 h of reperfusion. The nonselective NOS inhibitor N(omega)-nitro-l-arginine (L-NA) was given 30 min before sham PC or PC (1 mg/kg ip) (Fig. 1). At the conclusion of the study, the occluded/reperfused vascular bed and the infarct were identified by postmortem perfusion of the heart with triphenyltetrazolium chloride and phthalo blue, respectively (Fig. 1). Infarct size was calculated as a percentage of the region at risk [13,20,30].
Fig. 1.
Experimental protocol. Ten groups of mice were studied. Mice in groups I (WT B6, AMI; n = 15) and VI (eNOS−/− B6, AMI; n = 10) underwent a 30-min coronary occlusion followed by 4 h of reperfusion. Mice in groups II (WT B6, PC 1 cycle; n = 10) and VII (eNOS−/− B6, PC 1 cycle; n = 9) were preconditioned with a 4-min coronary occlusion (O) and, 10 min later, underwent a 30-min coronary occlusion followed by 4 h of reperfusion. In groups III (WT B6, PC 6 cycles; n = 7) and VIII (eNOS−/− B6, PC 6 cycles; n = 10), mice were preconditioned with a sequence of six 4-min coronary occlusion (O)/4-min reperfusion (R) cycles; 10 min later, they underwent a 30-min coronary occlusion followed by 4 h of reperfusion. Mice in group IV (WT B6, sham PCNA; n = 7) were given L-NA (1 mg/kg ip) 30 min before 1 h of open-chest state (sham PC), and then underwent a 30-min coronary occlusion followed by 4 h of reperfusion. Mice in group V (WT B6, PCNA; n = 7) received L-NA (1 mg/kg ip) 30 min before 6 cycles of 4-min O/R; 10 min later, animals were subjected a 30-min coronary occlusion followed by 4 h of reperfusion. Mice in groups IX (WT B6129, AMI; n = 14) and X (eNOS−/− B6129, AMI; n = 9) underwent a 30-min coronary occlusion followed by 24 h of reperfusion. WT, wild-type mice; B6, C57BL6/J; B6129, B6129SF2; AMI, acute myocardial infarction; PC, ischemic preconditioning; O, occlusion; R, reperfusion.
For Western immunoblotting analysis of iNOS, cytosolic, membranous, and nuclear fractions were prepared as previously described [28,29]. Western immunoblotting analysis was performed using standard techniques [28,29]. Equal loading was confirmed by staining with Ponceau-S [28,29].
2.4. Statistical methods
Data are reported as means±SEM. Measurements were analyzed by unpaired Student’s t-tests with the Bonferroni correction. In all Western analyses, the content of the specific protein of interest was expressed as a percentage of the corresponding protein in the anterior LV wall of control (WT) mice [28,29].
3. Results
A total of 206 mice were used: 98 mice were used for studies of infarct size, 98 mice were used as blood donors, and 10 mice were used for Western immunoblotting analyses.
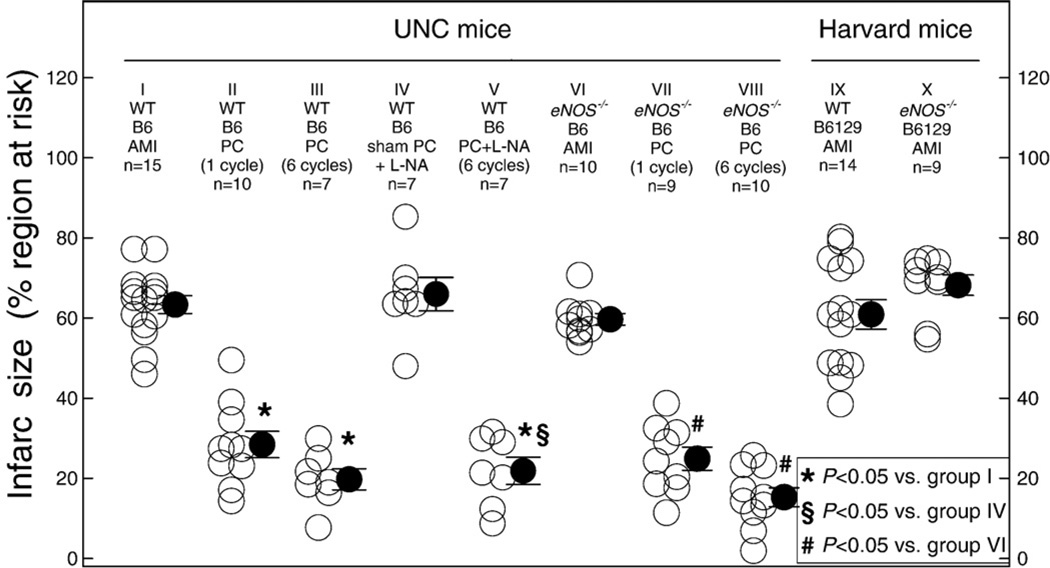
There were no significant differences among the ten groups of mice subjected to infarction with respect to heart weight, size of the region at risk, rectal temperature, or heart rate (Tables 1 and 2). The two groups of WT control mice (WT B6 AMI [group I, n = 15] and WT B6129 AMI [group IX, n = 14]) exhibited similar infarct size (63.3±2.2% and 60.9±3.6% of the risk region, respectively [Fig. 2]). In mice undergoing sham PC that were pretreated with L-NA (B6 sham PCNA, group IV, n = 7 [Fig. 2]), infarct size did not differ from the WT acute MI mice (group I) not given L-NA (66.0±4.2% of the risk region), indicating that NOS inhibition did not affect the extent of ischemia/reperfusion injury. In mice undergoing PC that were pretreated with L-NA (B6, PCNA, group V, n = 7 [Fig. 2]), infarct size was 21.9±3.4% of the risk region, which also was not different compared with WT preconditioned mice (groups II and III), indicating that non-isoform-selective NOS inhibition did not block the early PC effect.
Table 1.
Size of left ventricle, risk region, and infarct
| Group | Body Wt, g |
Heart Wt, g |
LV Wt, mg |
Heart Wt/ body Wt |
Risk region Wt, mg |
Infarct Wt, mg |
Risk region, % of LV |
Infarct,% of risk region |
Infarct, % of LV |
|---|---|---|---|---|---|---|---|---|---|
| Group I (WT B6, AMI) | 30.7±0.8 | 156.5±6.4 | 116.8±5.4 | 0.51±0.02 | 46.6±3.8 | 29.1±2.3 | 39.8±2.8 | 63.3±2.2 | 25.0±1.7 |
| Group II (WT B6, PC 1 cycle) | 26.5±0.7 | 132.6±4.9 | 100.9±4.3 | 0.46±0.02 | 35.8±2.3 | 10.2±1.3a | 35.7±2.2 | 28.5±3.3a | 10.3±1.3a |
| Group III (WT B6, PC 6 cycles) | 31.4±0.7 | 161.0±7.8 | 125.4±5.5 | 0.51±0.02 | 49.1±2.1 | 9.9±1.7a | 39.5±2.1 | 19.8±2.6a | 7.8±1.2a |
| Group IV (WT B6, sham PCNA) | 26.8±0.7 | 134.0±6.2 | 99.3±4.5 | 0.50±0.02 | 36.7±1.8 | 24.6±2.7 | 37.0±1.1 | 66.0±4.2 | 24.4±1.6 |
| Group V (WT B6, PC 6 cyclesNA) | 26.9±0.6 | 127.3±5.1 | 95.4±4.8 | 0.48±0.03 | 30.2±2.6 | 6.3±1.0a,b | 31.8±2.5 | 21.9±3.4a,b | 6.5±0.8a,b |
| Group VI (eNOS−/− B6, AMI) | 28.4±1.1 | 155.7±6.9 | 119.1±5.3 | 0.55±0.03 | 46.3±3.3 | 27.8±2.4 | 38.8±2.2 | 59.7±1.4 | 23.3±1.6 |
| Group VII (eNOS−/− B6, PC 1 cycle) | 28.8±0.8 | 132.2±5.1 | 104.8±3.8 | 0.50±0.01 | 34.5±3.0 | 8.6±1.3c | 33.0±3.0 | 24.9±2.9c | 8.1±1.1c |
| Group VIII (eNOS−/− B6, PC 6 cycles) | 30.3±0.8 | 165.8±10.4 | 132.8±10.5 | 0.55±0.03 | 43.1±5.6 | 6.9±1.5c | 32.3±3.3 | 15.3±2.4c | 5.1±0.9c |
| Group IX (WT B6129, AMI) | 29.0±0.8 | 165.2±7.3 | 118.6±5.8 | 0.57±0.03 | 40.7±2.4 | 24.6±2.1 | 34.4±1.3 | 60.9±3.6 | 20.9±1.4 |
| Group X (eNOS−/− B6129, AMI) | 29.7±1.3 | 163.7±8.9 | 119.3±7.2 | 0.56±0.03 | 30.6±1.5 | 25.3±2.6 | 30.6±1.5 | 68.2±2.5 | 21.0±1.5 |
Data are means±SEM. Heart Wt, total heart weight (ventricles and atria); LV, left ventricle. WT, wild-type mice; B6, C57BL6/J; B6129, B6129SF2; AMI, acute myocardial infarction; PC, early ischemic preconditioning.
P<0.05 vs. group I,
P<0.05 vs. group IV,
P<0.05 vs. group VI.
Table 2.
Rectal temperature and heart rate on the day of the 30-min coronary occlusion
| Pre-occlusion | Occlusion | Reperfusion | |||||
|---|---|---|---|---|---|---|---|
| 5 min | 15 min | 30 min | 5 min | 10 min | 15 min | ||
| Temperature (°C) | |||||||
| Group I (WT B6, AMI) | 37.0±0.1 | 37.2±0.1 | 37.0±0.0 | 37.1±0.0 | 37.0±0.1 | 37.1±0.1 | 37.0±0.1 |
| Group II (WT B6, PC 1 cycle) | 37.0±0.1 | 37.0±0.1 | 37.1±0.1 | 36.9±0.0 | 37.0±0.1 | 37.0±0.1 | 37.0±0.1 |
| Group III (WT B6, PC 6 cycles) | 37.0±0.1 | 37.0±0.1 | 36.9±0.1 | 36.9±0.1 | 37.0±0.1 | 37.0±0.1 | 36.9±0.1 |
| Group IV (WT B6, sham PCNA) | 37.0±0.2 | 37.1±0.2 | 37.0±0.2 | 37.2±0.2 | 37.0±0.2 | 36.9±0.2 | 36.9±0.1 |
| Group V (WT B6, PC 6 cyclesNA) | 37.0±0.2 | 37.0±0.2 | 36.9±0.1 | 37.1±0.1 | 37.0±0.1 | 37.1±0.3 | 36.9±0.1 |
| Group VI (eNOS−/− B6, AMI) | 36.9±0.1 | 37.0±0.1 | 37.0±0.1 | 37.0±0.1 | 37.0±0.1 | 37.0±0.1 | 37.0±0.1 |
| Group VII (eNOS−/− B6, PC 1 cycle) | 36.9±0.1 | 37.1±0.0 | 37.0±0.1 | 37.0±0.1 | 36.9±0.1 | 36.9±0.1 | 37.0±0.1 |
| Group VIII (eNOS−/− B6, PC 6 cycles) | 37.0±0.1 | 37.0±0.1 | 37.0±0.0 | 37.0±0.1 | 36.9±0.1 | 37.0±0.1 | 37.1±0.1 |
| Group IX (WT B6129, AMI) | 36.9±0.1 | 37.0±0.1 | 37.0±0.1 | 37.1±0.1 | 37.0±0.1 | 37.2±0.1 | 37.1±0.1 |
| Group X (eNOS−/− B6129, AMI) | 37.2±0.1 | 37.2±0.1 | 37.1±0.0 | 37.1±0.0 | 37.1±0.1 | 37.1±0.1 | 37.1±0.1 |
| Heart rate (beats/min) | |||||||
| Group I (WT B6, AMI) | 527±17 | 556±16 | 549±19 | 570±17 | 575±18 | 570±17 | 591±19 |
| Group II (WT B6, PC 1 cycle) | 477±9 | 487±10 | 494±11 | 493±11 | 513±17 | 507±14 | 522±12 |
| Group III (WT B6, PC 6 cycles) | 533±9 | 542±13 | 534±10 | 535±8 | 543±13 | 544±14 | 556±21 |
| Group IV (WT B6, sham PCNA) | 491±42 | 527±39 | 487±31 | 496±45 | 514±49 | 521±59 | 513±65 |
| Group V (WT B6, PC 6 cyclesNA) | 461±18 | 486±46 | 474±48 | 489±38 | 516±51 | 507±53 | 485±35 |
| Group VI (eNOS−/− B6, AMI) | 520±11 | 533±14 | 520±14 | 508±11 | 522±18 | 523±20 | 535±15 |
| Group VII (eNOS−/− B6, PC 1 cycle) | 480±9 | 493±9 | 501±9 | 504±11 | 519±20 | 517±11 | 543±19 |
| Group VIII (eNOS−/− B6, PC 6 cycles) | 522±15 | 529±14 | 538±14 | 538±14 | 553±20 | 547±19 | 572±18 |
| Group IX (WT B6129, AMI) | 515±16 | 544±13 | 543±12 | 557±14 | 559±15 | 551±17 | 546±13 |
| Group X (eNOS−/− B6129, AMI) | 573±22 | 616±16 | 597±15 | 602±17 | 593±16 | 619±15 | 597±15 |
Data are means±SEM. Measurements of rectal temperature and heart rate were taken before the 30-min coronary occlusion (pre-occlusion), at 5, 15 and 30 min into the 30-min occlusion, and at 5, 15 and 30 min after reperfusion. Rectal temperature was continuously monitored and carefully controlled throughout the experiment, as detailed in the text. WT, wild-type mice; B6, C57BL6/J; B6129, B6129SF2; AMI, acute myocardial infarction; PC, early ischemic preconditioning. The experimental protocols for the ten groups of mice are specified in Fig. 1.
Fig. 2.
Disruption of the eNOS gene or administration of L-NA does not alter infarct size in the nonpreconditioned state and does not interfere with the protective effects of early PC against myocardial infarction. WT, wild-type mice; B6, C57BL6/J; B6129, B6129SF2; AMI, acute myocardial infarction; PC, early ischemic preconditioning. Data are means±SEM.
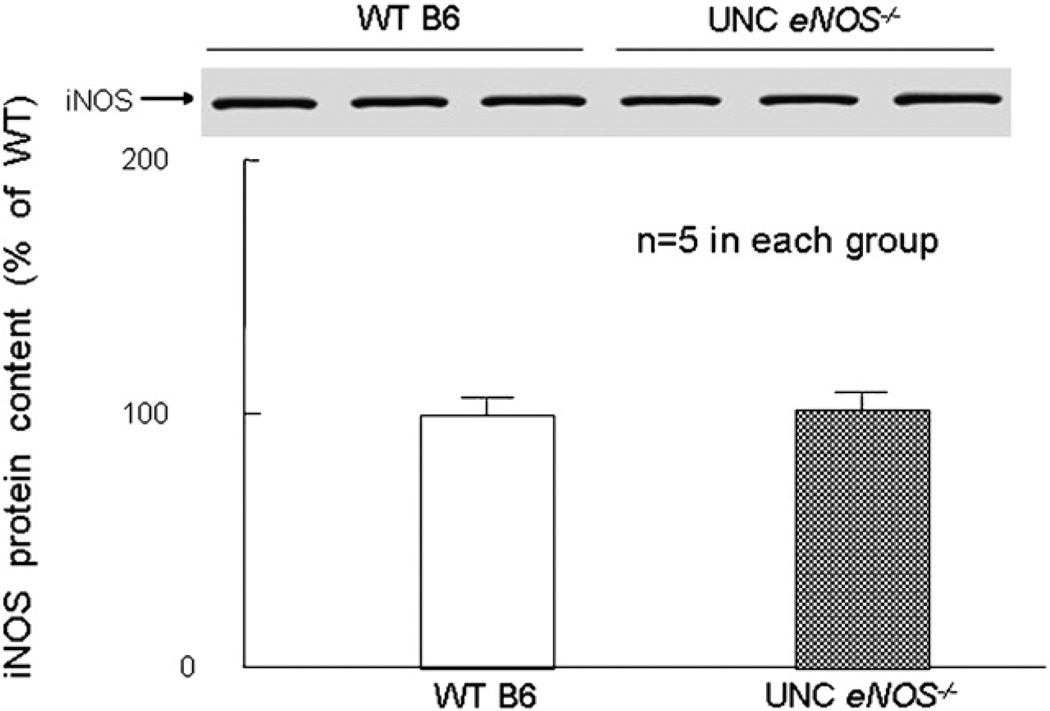
In both the nonpreconditioned UNC eNOS−/− mice (eNOS−/− B6 AMI, group VI, n = 10 [Fig. 2]) and the nonpreconditioned Harvard eNOS−/− mice (eNOS−/− B6129 AMI, group X, n = 9 [Fig. 2]), infarct size did not differ from the corresponding WT mice (59.7±1.4% and 68.2±2.5% of the risk region, respectively), indicating that neither eNOS mutation affected the extent of ischemia/reperfusion injury. Western blot analysis demonstrated that iNOS was not upregulated in the UNC eNOS−/− mice (Fig. 3).
Fig. 3.
iNOS protein content in myocardium. There was no change in the basal levels of iNOS protein expression in the myocardium either in WT B6 (C57BL/6) or in UNC eNOS−/− mice (n = 5/group). Data are means±SEM.
When UNC WT (B6) mice were preconditioned with either one or six cycles of 4-min coronary O/R 10 min prior to the 30-min coronary occlusion, infarct size was markedly reduced to 28.5±3.3% (PC one cycle, group II, n = 10) and 19.8±2.6% (PC six cycles, group III, n = 7) of the risk region, respectively (Fig. 2), indicating the development of a robust early PC effect. Moreover, when UNC eNOS−/− mice were preconditioned with one O/R cycle (group VII, n = 9) or six O/R cycles (group VIII, n = 10), a similar reduction in infarct size was observed (Fig. 2), indicating that the PC effect was intact.
4. Discussion
The present study was conducted in a well-established in vivo murine model of myocardial infarction in which measurements of infarct size are highly reproducible and major physiological variables that affect ischemia/reperfusion injury (arterial blood gases, arterial blood gases, arterial hematocrit, and body temperature) are carefully monitored and controlled [13,20,30]. The results demonstrate that (i) under basal conditions, genetic ablation of eNOS by two different methods (deletion of the calmodulin-binding domain [UNC eNOS−/− mice] or the NADPH ribose and adenine binding sites [Harvard eNOS−/− mice]) as well as pharmacologic inhibition of NOS does not affect infarct size, i.e., it neither exacerbates nor alleviates injury, (ii) contrary to previous reports, genetic ablation of eNOS in the UNC eNOS−/− mice does not result in upregulation of iNOS, and (iii) contrary to previous reports, eNOS is not necessary for the development of the cardioprotective effects of the early phase of PC. Administration of the non-isoform-selective NOS inhibitor L-NA yielded results that were similar to those obtained in eNOS null mice, indicating that strain differences or unanticipated effects of genetic manipulations cannot explain our results in eNOS null mice. To our knowledge, this is the first in vivo demonstration that eNOS is not necessary for early PC, either when a mild (one 4-min O/R cycle) or a robust (six 4-min O/R cycles) ischemic PC stimulus is used, refuting (at least in the mouse) the theory [23,27] that eNOS is an obligatory component of the mechanism that triggers the early phase of ischemic PC.
Two previous studies, one in vivo [6] and one in vitro [7], have concluded that the eNOS−/− mice developed at UNC by Shesely et al. [4] exhibit a cardioprotected phenotype, which was ascribed to upregulation of iNOS [6,7]. However, a third study [3] (performed in vivo) found no change in infarct size in UNC eNOS−/− mice. In contrast, the eNOS−/− mice developed at Harvard University by Huang et al. [8] have been reported to exhibit no upregulation of iNOS and an actual increase in infarct size [6]. Contrary to these previous reports, we found that neither the UNC eNOS−/− mice nor the Harvard eNOS−/− mice exhibited infarct sizes different from those found in the corresponding WT mice (Fig. 2) and that there was no significant difference in iNOS expression between the UNC eNOS−/− mice and their WT controls (Fig. 3). The results obtained in Harvard eNOS−/− mice with 24 h of reperfusion were similar to those obtained in UNC eNOS−/− mice with 4 h of reperfusion (Fig. 2). The data in UNC eNOS−/− mice are consistent with those recently reported by our group in a separate communication [5]. Collectively, these findings demonstrate that, at least in the mouse, basal eNOS activity does not modulate myocardial ischemia/reperfusion injury.
Previous pharmacologic investigations have arrived at conflicting conclusions regarding the role of eNOS in early PC [23–26]. Using rats, Costa et al. [23] concluded that eNOS is required for the mediation of early PC, whereas three other studies found NOS not to be necessary in rabbits [24,25] or pigs [26]. Only one study has used genetic deletion of eNOS, and the results have been equivocal [27]. This study in Harvard eNOS−/− mice [27] concluded that eNOS is necessary for early PC induced by two or three 5-min ischemic episodes but not by four 5-min episodes. These observations [27], however, were made in isolated hearts reperfused for only 30 min; it is unknown whether measurements of infarct size after 30 min of reperfusion in this in vitro setting reliably reflect the size of an infarct in vivo. Our data, obtained with both a genetic and a pharmacologic approach in an in vivo model, show that deletion of eNOS failed to block the early phase of PC induced by either one cycle or six cycles of 4-min coronary O/R (Fig. 2 and Table 1). The use of only pharmacologic vs. genetic and pharmacologic inhibition of eNOS is a major difference between previous studies [23–26] and the present investigation. It is also possible that the difference between the present data and those of previous studies [23,27] may be due to differences in the species (rats [23] and rabbits [24,25] vs. mice) and/or protocols used for inducing ischemic PC (two–four 5-min episodes of global ischemia [27] vs. one or six 4-min episodes of regional ischemia). Regardless of the exact reason(s) for the differences, the present results demonstrate that early PC can be fully manifested in the absence of eNOS, indicating that eNOS does not play a necessary role, at least in the mouse. Although our results cannot be extrapolated to other species, genetic deletion of eNOS has thus far been performed only in mice, and so it would be very difficult to conclusively establish the role of this enzyme in other species.
In conclusion, we have demonstrated that, in contrast to previous reports, basal eNOS activity does not modulate infarct size in the nonpreconditioned (naive) state and is not necessary for the cardioprotective effects of the early phase of ischemic PC. This latter result impels a reassessment of recent paradigms [23,27] implicating eNOS as an essential component of the signaling pathway that leads to early PC.
Acknowledgments
Supported in part by NIH grants R01 HL-65660, HL-55757, HL-68088, HL-70897, HL-76794, and P01 HL-78825.
References
- 1.Bolli R. Cardioprotective function of inducible nitric oxide synthase and role of nitric oxide in myocardial ischemia and preconditioning: an overview of a decade of research. J Mol Cell Cardiol. 2001;33:1897–1918. doi: 10.1006/jmcc.2001.1462. [DOI] [PubMed] [Google Scholar]
- 2.Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Yang XP, Liu YH, Shesely EG, Bulagannawar M, Liu F, Carretero OA. Endothelial nitric oxide gene knockout mice: cardiac phenotypes and the effect of angiotensin-converting enzyme inhibitor on myocardial ischemia/reperfusion injury. Hypertension. 1999;34:24–30. doi: 10.1161/01.hyp.34.1.24. [DOI] [PubMed] [Google Scholar]
- 4.Shesely EG, Maeda N, Kim HS, Desai KM, Krege JH, Laubach VE, Sherman PA, Sessa WC, Smithies O. Elevated blood pressures in mice lacking endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1996;93:13176–13181. doi: 10.1073/pnas.93.23.13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G, Bolli R. Endothelial nitric oxide synthase plays an obligatory role in the late phase of ischemic preconditioning by activating the protein kinase C{epsilon}-p44/42 mitogen-activated protein kinase-pSer-signal transducers and activators of transcription 1/3 pathway. Circulation. 2007;116:535–544. doi: 10.1161/CIRCULATIONAHA.107.689471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharp BR, Jones SP, Rimmer DM, Lefer DJ. Differential response to myocardial reperfusion injury in eNOS-deficient mice. Am J Physiol Heart Circ Physiol. 2002;282:H2422–H2426. doi: 10.1152/ajpheart.00855.2001. [DOI] [PubMed] [Google Scholar]
- 7.Kanno S, Lee PC, Zhang Y, Ho C, Griffith BP, Shears LL, 2nd, Billiar TR. Attenuation of myocardial ischemia/reperfusion injury by superinduction of inducible nitric oxide synthase. Circulation. 2000;101:2742–2748. doi: 10.1161/01.cir.101.23.2742. [DOI] [PubMed] [Google Scholar]
- 8.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 9.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 10.Marber MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation Sep. 1993;88(3):1264–1272. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- 11.Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–983. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- 12.Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 13.Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, et al. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci U S A. 1999;96:11507–11512. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ping P, Zhang J, Qiu Y, Tang XL, Manchikalapudi S, Cao X, et al. Ischemic preconditioning induces selective translocation of protein kinase C isoforms epsilon and eta in the heart of conscious rabbits without subcellular redistribution of total protein kinase C activity. Circ Res. 1997;81:404–414. doi: 10.1161/01.res.81.3.404. [DOI] [PubMed] [Google Scholar]
- 15.Bolli R, Manchikalapudi S, Tang XL, Takano H, Qiu Y, Guo Y, et al. The protective effect of late preconditioning against myocardial stunning in conscious rabbits is mediated by nitric oxide synthase. Evidence that nitric oxide acts both as a trigger and as a mediator of the late phase of ischemic preconditioning. Circ Res. 1997;81:1094–1107. doi: 10.1161/01.res.81.6.1094. [DOI] [PubMed] [Google Scholar]
- 16.Bolli R, Bhatti ZA, Tang XL, Qiu Y, Zhang Q, Guo Y, et al. Evidence that late preconditioning against myocardial stunning in conscious rabbits is triggered by the generation of nitric oxide. Circ Res. 1997;81:42–52. doi: 10.1161/01.res.81.1.42. [DOI] [PubMed] [Google Scholar]
- 17.Qiu Y, Ping P, Tang XL, Manchikalapudi S, Rizvi A, Zhang J, et al. Direct evidence that protein kinase C plays an essential role in the development of late preconditioning against myocardial stunning in conscious rabbits and that epsilon is the isoform involved. J Clin Invest. 1998;101:2182–2198. doi: 10.1172/JCI1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xuan YT, Tang XL, Banerjee S, Takano H, Li RC, Han H, et al. Nuclear factor-kappaB plays an essential role in the late phase of ischemic preconditioning in conscious rabbits. Circ Res. 1999;84:1095–1109. doi: 10.1161/01.res.84.9.1095. [DOI] [PubMed] [Google Scholar]
- 19.Shinmura K, Tang XL, Wang Y, Xuan YT, Liu SQ, Takano H, et al. Cyclooxygenase-2 mediates the cardioprotective effects of the late phase of ischemic preconditioning in conscious rabbits. Proc Natl Acad Sci U S A. 2000;97:10197–10202. doi: 10.1073/pnas.97.18.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo Y, Bao W, Wu WJ, Shinmura K, Tang XL, Bolli R. Evidence for an essential role of cyclooxygenase-2 as a mediator of the late phase of ischemic preconditioning in mice. Basic Res Cardiol. 2000;95:479–484. doi: 10.1007/s003950070024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xuan YT, Tang XL, Qiu Y, Banerjee S, Takano H, Han H, Bolli R. Biphasic response of cardiac NO synthase isoforms to ischemic preconditioning in conscious rabbits. Am J Physiol Heart Circ Physiol. 2000;279:H2360–H2371. doi: 10.1152/ajpheart.2000.279.5.H2360. [DOI] [PubMed] [Google Scholar]
- 22.Kis A, Yellon DM, Baxter GF. Second window of protection following myocardial preconditioning: an essential role for PI3 kinase and p70S6 kinase. J Mol Cell Cardiol. 2003;35:1063–1071. doi: 10.1016/s0022-2828(03)00208-6. [DOI] [PubMed] [Google Scholar]
- 23.Costa AD, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV, et al. Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res. 2005;97:329–336. doi: 10.1161/01.RES.0000178451.08719.5b. [DOI] [PubMed] [Google Scholar]
- 24.Qin Q, Yang XM, Cui L, Critz SD, Cohen MV, Browner NC, et al. Exogenous NO triggers preconditioning via a cGMP- and mitoKATP-dependent mechanism. Am J Physiol Heart Circ Physiol. 2004;287:H712–H718. doi: 10.1152/ajpheart.00954.2003. [DOI] [PubMed] [Google Scholar]
- 25.Nakano A, Liu GS, Heusch G, Downey JM, Cohen MV. Exogenous nitric oxide can trigger a preconditioned state through a free radical mechanism, but endogenous nitric oxide is not a trigger of classical ischemic preconditioning. J Mol Cell Cardiol. 2000;32:1159–1167. doi: 10.1006/jmcc.2000.1152. [DOI] [PubMed] [Google Scholar]
- 26.Post H, Schulz R, Behrends M, Gres P, Umschlag C, Heusch G. No involvement of endogenous nitric oxide in classical ischemic preconditioning in swine. J Mol Cell Cardiol. 2000;32:725–733. doi: 10.1006/jmcc.2000.1117. [DOI] [PubMed] [Google Scholar]
- 27.Bell RM, Yellon DM. The contribution of endothelial nitric oxide synthase to early ischaemic preconditioning: the lowering of the preconditioning threshold. An investigation in eNOS knockout mice. Cardiovasc Res. 2001;52:274–280. doi: 10.1016/s0008-6363(01)00394-7. [DOI] [PubMed] [Google Scholar]
- 28.Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G, Messing RO, et al. Role of the protein kinase C-epsilon-Raf-1-MEK-1/2-p44/42 MAPK signaling cascade in the activation of signal transducers and activators of transcription 1 and 3 and induction of cyclooxygenase-2 after ischemic preconditioning. Circulation. 2005;112:1971–1978. doi: 10.1161/CIRCULATIONAHA.105.561522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xuan YT, Guo Y, Han H, Zhu Y, Bolli R. An essential role of the JAKSTAT pathway in ischemic preconditioning. Proc Natl Acad Sci U S A. 2001;98:9050–9055. doi: 10.1073/pnas.161283798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo Y, Wu WJ, Qiu Y, Tang XL, Yang Z, Bolli R. Demonstration of an early and a late phase of ischemic preconditioning in mice. Am J Physiol. 1998;275:H1375–H1387. doi: 10.1152/ajpheart.1998.275.4.H1375. [DOI] [PMC free article] [PubMed] [Google Scholar]