Abstract
Photodynamic therapy (PDT) is an FDA-approved modality for the treatment of early-stage disease and palliation of late-stage disease. Pre-clinical studies using mouse models and clinical studies in patients have demonstrated that PDT is capable of influencing the immune system. The effect of PDT on the generation of anti-tumor immunity is regimen-dependent and is tightly linked to the degree and nature of inflammation induced by PDT. However, the precise mechanism underlying PDT regulated adaptive anti-tumor immunity remains unclear. This review will focus on the current knowledge of immune regulation by PDT.
PDT Efficacy is Dependent upon Anti-Tumor Immunity
Photodynamic therapy (PDT) is an anti-tumor modality that rapidly destroys tumors by a multi-faceted mechanism. The efficacy of PDT has been shown to be dependent upon the induction of anti-tumor immunity. Initial studies in mice models demonstrated that long-term tumor control is diminished in immuno-compromised mice1,2. PDT treatment of EMT6 tumors at a curative dose in BALB/c mice exhibited no long-term protection of tumors in either scid, which lack T and B cells, or nude mice, which lack T cells. The curative effect of PDT on EMT6 tumors was restored when scid mice were reconstituted with splenic T cells or bone marrow cells from BALB/c mice.
The dependence of PDT efficacy on the induction of anti-tumor immunity has also been demonstrated in clinical studies. Topical PDT of immuno-suppressed actinic keratoses and Bowen’s disease transplant patients had comparable initial response with those in immuno-competent patients 3. However, immuno-suppressed patients had an increased propensity to develop new lesions soon after treatment. Patient vulval intraepithelial neoplasia (VIN) biopsies expressing major histocompatibility class I molecules (MHC I) were more likely to respond to ALA-PDT than tumors that had down-regulated MHC I molecules 4. Recognition of MHC I is critical for CD8+ T cell activation and one mechanism used by tumors to evade immune recognition is down-regulation of MHC I molecules 5. The infiltration of CD8+ T cells was significantly increased in VIN responders compared with nonresponders following PDT.
PDT Augments Anti-Tumor Immunity
The induction of anti-tumor immunity by PDT was first established by Canti et al.6 who demonstrated that transfer of TDLN cells from PDT treated tumors to naïve hosts conferred resistance upon subsequent tumor challenge. In this study, PDT-treated mice that remained tumor free for 100 days were able to effectively control subsequent tumor challenge suggesting the presence of immune memory. Korbelik and Dougherty 7 later demonstrated that PDT treatment of murine tumors resulted in the generation of immune memory. Reconstitution of scid mice with PDT-cured splenocytes protected the mice against tumor rechallenge. Recent reports have demonstrated that clinical PDT also augments anti-tumor immunity. PDT treatment of basal cell carcinoma (BCC) increased systemic anti-tumor immunity to a BCC-associated tumor antigen 8. A recent study demonstrated that treatment of multifocal angiosarcoma of the head and neck with PDT resulted in increased immune cell reactivity against distant untreated tumors that correlated with tumor regression 9.
Although PDT can activate both humoral and cell-mediated adaptive anti-tumor immunity 10, the importance of the humoral arm of anti-tumor immunity remains unclear. The efficacy of PDT in mice and humans is dependent upon CD8+ T cells 2,4,11. Therefore most mechanistic studies have focused on PDT modulation of cell-mediated adaptive immunity.
Mechanism of PDT Enhanced Anti-Tumor Immunity
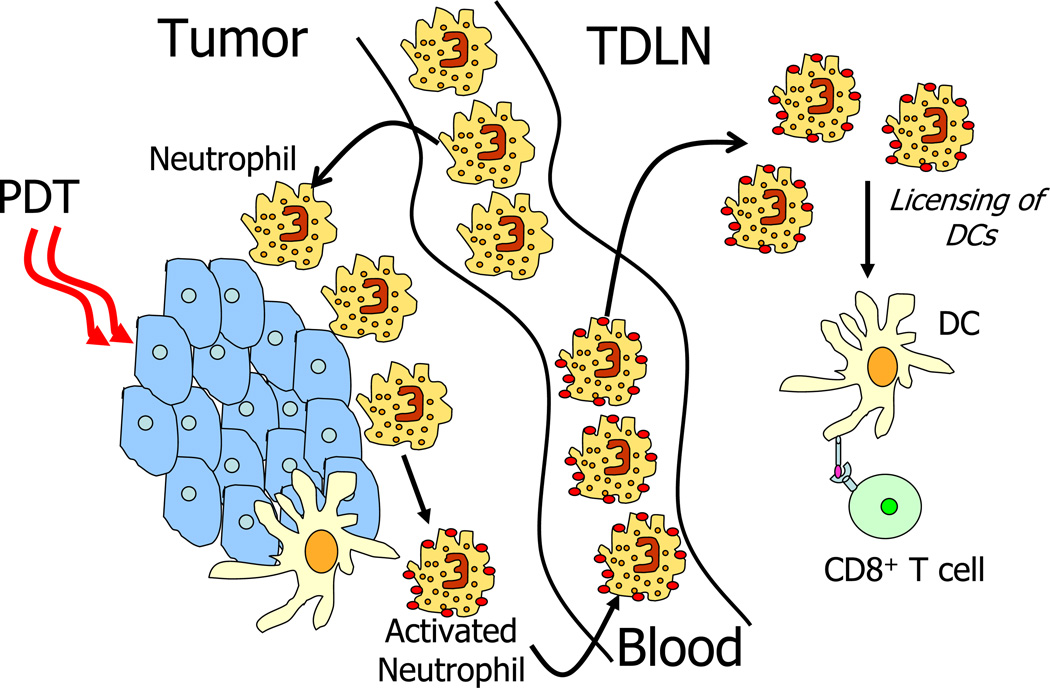
A hallmark of PDT is the induction of acute inflammation that contributes considerably to the overall long-term control of tumor growth 12. High-inflammatory PDT regimens induce significant acute inflammation characterized by increased expression of several pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6 13–16, adhesion molecules E-selectin and ICAM-1 16, and rapid accumulation of leukocytes into the treated tumor bed 1,7,17 and TDLNs 18. The accumulation of leukocytes to the tumor is dominated by Gr-1 expressing leukocytes that morphologically resemble neutrophils. Depletion of Gr-1-expressing leukocytes resulted in diminished long-term tumor growth by PDT 2,12,17,19.
Studies in pre-clinical and clinical settings have demonstrated that the degree of inflammation influences the anti-tumor adaptive immune response. PDT was initially considered a local treatment that generated a local inflammatory response. Subsequent studies have identified PDT as a systemic response characterized by increased systemic neutrophilia 20, systemic release of pro-inflammatory cytokines 16,21–25, induction of acute phase proteins 16,20, and increased complement protein expression 26–28. These acute inflammatory mediators augment the presence and activation of innate and adaptive immunity and the generation of anti-tumor immunity.
PDT enhancement of anti-tumor immunity appears to involve the stimulation of DCs by dying tumor cells 10,29. Induction of acute inflammation by PDT results in the maturation and activation of dendritic cells 30 and migration to the TDLNs where they are believed to stimulate T cell activation 30,31. Studies have shown that incubation of PDT-treated tumor cells with immature DCs leads to enhanced DC maturation, activation, and ability to stimulate T cell activation 29,32. A recent report demonstrated that PDT-generated tumor cell lysate induces IL-1α, IL-1β, and IL-6 secretion from DCs, suggesting PDT enhanced anti-tumor immunity is due in part to increased DC activation 33.
It is thought that DC activation by PDT is the result of sensing endogenous danger signals released by dying tumor cells 30,34–37. The danger model was initially proposed by Matzinger 38 and suggests that cells express endogenous danger signals in response to damage or physical/chemical insult/stress. These danger signals are referred to as damage-associated molecular patterns (DAMPs) that are immunostimulatory by interacting with pattern-recognition receptors (PRRs) expressed on innate immune cells. These specialized receptors are non-clonal, germline-encoded molecules classified as RIG-I-like receptors (RLRs), NOD-like receptors (NLRs), and Toll-like receptors (TLRs) 39. It appears that PDT treatment of tumor cells induces expression of multiple danger signals capable of activating antigen presenting cells and generating anti-tumor immunity. Heat shock protein 70 (HSP70) is a danger signal that interacts with TLRs (Toll-like receptors) 2 and 4 40. PDT induces expression of HSP70 41 and the level of expression correlates with DC maturation 42 and the extent of inflammation 36. Other HSPs and danger signals released by PDT treated tumor cells have been reported to stimulate DC activation 29,43. Additionally, complement opsonization of PDT-treated tumor cells augments immune recognition of PDT-generated vaccines 44.
PDT regimens that lead to acute inflammation induce a rapid influx of Gr-1hiCD11b+F4/80−CD11c− neutrophils into treated tumors leading to enhanced anti-tumor immunity 12,18 and strong primary and memory T cell responses 18. Furthermore, the efficacy of PDT is critically dependent on Gr-1 expressing leukocytes 12,19. Mice defective in neutrophil homing (Cxcr2−/−) to secondary lymphoid tissue lack the ability to mount strong CD8+ T cell responses following PDT 18. We therefore postulate that neutrophils are instrumental in modulating anti-tumor immunity following PDT. The mechanism by which neutrophils instruct adaptive anti-tumor immunity is an area of extensive investigation. These leukocytes have been shown to regulate anti-pathogen immunity through (1) secretion of chemokines and granule proteins that recruit monocytes and dendritic cells 45 (2) activation of DCs via cell-to-cell contact and secretion of TNF-α 46,47 (3) secretion of IFN-γ that stimulates monocytes and T cell differentiation 45,48. We have previously demonstrated that neutrophils express cell-surface TNF-α following PDT 18. Neutrophils rapidly accumulate within the treated tumor bed and peak between 4 and 8 hours post treatment. These leukocytes acquire cell-surface expression of TNF-α and accumulate in the TDLN by 4h post treatment. We predict that these TNF-α expressing leukocytes directly interact with DCs in the TDLN, licensing them and promoting T cell activation and increased adaptive anti-tumor immunity. Generation of CD8+ effector and memory T cell formation is generally dependent on CD4+ T cells 49–51. However, PDT-induced anti-tumor immunity may be CD4+ T cell independent and augmented by NK cells 52. Furthermore, Korbelik et al. 1 demonstrated that transfer of CD8+ T cells alone could significantly restore PDT efficacy in SCID mice.
In addition to directly augmenting anti-tumor immunity by enhanced DC and T cell activation, PDT can also affect tumor-derived immuno-suppression. Immunosuppressive Tregulatory cells (Tregs) dampen the generation of anti-tumor immunity 53,54. A recent study demonstrated that PDT in combination with low-dose cyclophosphamide depletes Tregs, potentiating the immune response leading to increased cures and the generation of immune memory 55. Myeloid-derived suppressor cells (MDSCs) are immature myeloid cells recruited by the tumor and suppress adaptive anti-tumor immunity 56. Although the role of MDSCs in PDT has not been reported, it is tempting to speculate that PDT causes the destruction of MDSCs through a similar mechanism of tumor destruction initiated by PDT. Myeloid cells are extremely phagocytic, capable of phagocytosing photosensitizer and upon tumor illumination become apoptotic and necrotic. One could predict that PDT-initiated MDSC destruction may potentiate anti-tumor immunity.
The effects of PDT on the immune system appear not only to augment immune cell reactivity against tumors but also to suppress immune cell activation. Although the precise mechanism of immuno-suppression is unclear, the effect of PDT on the immune system appears dependent on the treatment regimen, the photosensitizer type, and the treatment area 18,57. PDT induced immune suppression of cutaneous and transdermal PDT involves a large surface area 57–59. Immune suppression generated by PDT can be adoptively transferred to naïve recipients and is mediated primarily by T cells 60.
Conclusion
PDT is an effective therapy for a growing number of malignancies in the US and Europe. Pre-clinical and clinical studies have demonstrated that PDT eliminates tumors by direct tumor cell death and indirectly by augmenting anti-tumor immunity. The mechanism underlying PDT enhancement of anti-tumor immunity remains unclear and is under current investigation. Defining the mechanism by which PDT augments anti-tumor immunity will allow the development of protocols to eliminate primary tumor growth while augmenting systemic anti-tumor immunity to control disseminating disease. We hope to develop protocols to use PDT as an adjuvant in combination with other treatment modalities. PDT was shown to be an effective adjuvant in combination with surgery in patients with non-small-cell lung caner with plural spread 61. A recent report using a pre-clinical mouse model of reticulum cell sarcoma demonstrated that PDT can be effectively combined with low-dose cyclophosphamide to generate durable anti-tumor immunity while augmenting long-term cures 55. PDT is a promising anti-tumor modality that is receiving considerable attention as a therapeutic tool alone and in combination with other treatment regimens.
Figure 1.
References
- 1.Korbelik M, Krosl G, Krosl J, Dougherty GJ. The role of host lymphoid populations in the response of mouse EMT6 tumor to photodynamic therapy. Cancer Res. 1996;56:5647–5652. [PubMed] [Google Scholar]
- 2.Korbelik M, Cecic I. Contribution of myeloid and lymphoid host cells to the curative outcome of mouse sarcoma treatment by photodynamic therapy. Cancer Lett. 1999;137:91–98. doi: 10.1016/s0304-3835(98)00349-8. [DOI] [PubMed] [Google Scholar]
- 3.Dragieva G, Hafner J, Dummer R, Schmid-Grendelmeier P, Roos M, Prinz BM, Burg G, Binswanger U, Kempf W. Topical photodynamic therapy in the treatment of actinic keratoses and Bowen's disease in transplant recipients. Transplantation. 2004;77:115–121. doi: 10.1097/01.TP.0000107284.04969.5C. [DOI] [PubMed] [Google Scholar]
- 4.Abdel-Hady ES, Martin-Hirsch P, Duggan-Keen M, Stern PL, Moore JV, Corbitt G, Kitchener HC, Hampson IN. Immunological and viral factors associated with the response of vulval intraepithelial neoplasia to photodynamic therapy. Cancer Res. 2001;61:192–196. [PubMed] [Google Scholar]
- 5.Maeurer MJ, Gollin SM, Storkus WJ, Swaney W, Karbach J, Martin D, Castelli C, Salter R, Knuth A, Lotze MT. Tumor escape from immune recognition: loss of HLA-A2 melanoma cell surface expression is associated with a complex rearrangement of the short arm of chromosome 6. Clin. Cancer Res. 1996;2:641–652. [PubMed] [Google Scholar]
- 6.Canti G, Lattuada D, Nicolin A, Taroni P, Valentini G, Cubeddu R. Immunopharmacology studies on photosensitizers used in photodynamic therapy (PDT) Proc. SPIE Photodyn. Ther. Cancer. 1994;2078:268–275. [Google Scholar]
- 7.Korbelik M, Dougherty GJ. Photodynamic therapy-mediated immune response against subcutaneous mouse tumors. Cancer Res. 1999;59:1941–1946. [PubMed] [Google Scholar]
- 8.Kabingu E, Oseroff AR, Wilding GE, Gollnick SO. Enhanced systemic immune reactivity to a basal cell carcinoma associated antigen following photodynamic therapy. Clin. Cancer Res. 2009;15:4460–4466. doi: 10.1158/1078-0432.CCR-09-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thong PS, Ong KW, Goh NS, Kho KW, Manivasager V, Bhuvaneswari R, Olivo M, Soo KC. Photodynamic-therapy-activated immune response against distant untreated tumours in recurrent angiosarcoma. Lancet Oncol. 2007;8:950–952. doi: 10.1016/S1470-2045(07)70318-2. [DOI] [PubMed] [Google Scholar]
- 10.Preise D, Oren R, Glinert I, Kalchenko V, Jung S, Scherz A, Salomon Y. Systemic antitumor protection by vascular-targeted photodynamic therapy involves cellular and humoral immunity. Cancer Immunol. Immunother. 2009;58:71–84. doi: 10.1007/s00262-008-0527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krosl G, Korbelik M, Krosl J, Dougherty GJ. Potentiation of photodynamic therapy-elicited antitumor response by localized treatment with granulocyte-macrophage colony-stimulating factor. Cancer Res. 1996;56:3281–3286. [PubMed] [Google Scholar]
- 12.Henderson BW, Gollnick SO, Snyder JW, Busch TM, Kousis PC, Chene RT, Morgan J. Choice of oxygen-conserving treatment regimen determines the inflammatory response and outcome of photodynamic therapy of tumors. Cancer Res. 2004;64:2120–2126. doi: 10.1158/0008-5472.can-03-3513. [DOI] [PubMed] [Google Scholar]
- 13.Evans S, Matthews W, Perry R, Fraker D, Norton J, Pass HI. Effect of photodynamic therapy on tumor necrosis factor production by murine macrophages. J. Natl. Cancer Inst. 1990;82:34–39. doi: 10.1093/jnci/82.1.34. [DOI] [PubMed] [Google Scholar]
- 14.Kick G, Messer G, Goetz A, Plewig G, Kind P. Photodynamic therapy induces expression of interleukin 6 by activation of AP-1 but not NF-kB DNA binding. Cancer Res. 1995;55:2373–2379. [PubMed] [Google Scholar]
- 15.Gollnick SO, Liu X, Owczarczak B, Musser DA, Henderson BW. Altered expression of interleukin 6 and interleukin 10 as a result of photodynamic therapy in vivo. Cancer Res. 1997;57:3904–3909. [PubMed] [Google Scholar]
- 16.Gollnick SO, Evans SE, Baumann H, Owczarczak B, Maier P, Vaughan L, Wang WC, Unger E, Henderson BW. Role of cytokines in photodynamic therapy-induced local and systemic inflammation. Br. J. Cancer. 2003;88:1772–1779. doi: 10.1038/sj.bjc.6600864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korbelik M. Induction of tumor immunity by photodynamic therapy. J. Clin. Laser Med. & Surg. 1996;14:329–334. doi: 10.1089/clm.1996.14.329. [DOI] [PubMed] [Google Scholar]
- 18.Kousis PC, Henderson BW, Maier PG, Gollnick SO. Photodynamic therapy (PDT) enhancement of anti-tumor immunity is regulated by neutrophils. Can. Res. 2007;67:10501–10510. doi: 10.1158/0008-5472.CAN-07-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Vree WJ, Essers MC, De Bruijn HS, Star WM, Koster JF, Sluiter W. Evidence for an important role of neutrophils in the efficacy of photodynamic therapy in vivo. Cancer Res. 1996;56:2908–2911. [PubMed] [Google Scholar]
- 20.Cecic I, Parkins CS, Korbelik M. Induction of systemic neutrophil response in mice by photodynamic therapy of solid tumors. Photochem. Photobiol. 2001;74:712–720. doi: 10.1562/0031-8655(2001)074<0712:iosnri>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 21.Nseyo UO, Whalen RK, Duncan MR, Berman B, Lundahl SL. Urinary cytokines following photodynamic therapy for bladder cancer. Urology. 1990;36:167–171. doi: 10.1016/0090-4295(90)80220-h. [DOI] [PubMed] [Google Scholar]
- 22.Ziolkowski P, Symonowicz K, Milach J, Szkudlarek T. In vivo tumor necrosis factor-alpha induction following chlorin e6-photodynamic therapy in Buffalo rats. Neoplasma. 1996;44:192–196. [PubMed] [Google Scholar]
- 23.de Vree WJ, Essers MC, Koster JF, Sluiter W. Role of interleukin 1 and granulocyte colony-stimulating factor in photofrin-based phtodynamic therapy of rat rhabdomyosarcoma tumors. Cancer Res. 1997;57:2555–2558. [PubMed] [Google Scholar]
- 24.Cecic I, Korbelik M. Mediators of peripheral blood neutrophilia induced by photodynamic therapy of solid tumors. Cancer Lett. 2002;183:43–51. doi: 10.1016/s0304-3835(02)00092-7. [DOI] [PubMed] [Google Scholar]
- 25.Yom SS, Busch TM, Friedberg JS, Wileyto EP, Smith D, Glatstein E, Hahn SM. Elevated serum cytokine levels in mesothelioma patients who have undergone pleurectomy or extrapleural pneumonectomy and adjuvant intraoperative photodynamic therapy. Photochem. Photobiol. 2003;78:75–81. doi: 10.1562/0031-8655(2003)078<0075:esclim>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 26.Cecic I, Serrano K, Gyongyossy-Issa M, Korbelik M. Characteristics of complement activation in mice bearing Lewis lung carcinomas treated by photodynamic therapy. Cancer Lett. 2005;225:215–223. doi: 10.1016/j.canlet.2004.11.059. [DOI] [PubMed] [Google Scholar]
- 27.Stott B, Korbelik M. Activation of complement C3, C5, and C9 genes in tumors treated by photodynamic therapy. Cancer Immunol. Immunother. 2006 doi: 10.1007/s00262-006-0221-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cecic I, Sun J, Korbelik M. Role of complement anaphylatoxin C3a in photodynamic therapy-elicited engagement of host neutrophils and other immune cells. Photochem. Photobiol. 2006;82:558–562. doi: 10.1562/2005-09-09-RA-681. [DOI] [PubMed] [Google Scholar]
- 29.Jalili A, Makowski M, Switaj T, Nowis D, Wilczynski GM, Wilczek E, Chorazy-Massalska M, Radzikowska A, Maslinski W, Bialy L, Sienko J, Sieron A, Adamek M, Basak G, Mroz P, Krasnodebski IW, Jakobisiak M, Golab J. Effective photoimmunotherapy of murine colon carcinoma induced by the combination of photodynamic therapy and dendritic cells. Clin. Cancer Res. 2004;10:4498–4508. doi: 10.1158/1078-0432.CCR-04-0367. [DOI] [PubMed] [Google Scholar]
- 30.Gollnick SO, Owczarczak B, Maier P. Photodynamic therapy and anti-tumor immunity. Lasers Surg. Med. 2006;38:509–515. doi: 10.1002/lsm.20362. [DOI] [PubMed] [Google Scholar]
- 31.Sur BW, Nguyen P, Sun CH, Tromberg BJ, Nelson EL. Immunophototherapy using PDT combined with rapid intratumoral dendritic cell injection. Photochem. Photobiol. 2008;84:1257–1264. doi: 10.1111/j.1751-1097.2008.00356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gollnick SO, Vaughan LA, Henderson BW. Generation of effective anti-tumor vaccines using photodynamic therapy. Cancer Res. 2002;62:1604–1608. [PubMed] [Google Scholar]
- 33.Kushibiki T, Tajiri T, Tomioka Y, Awazu K. Photodynamic therapy induces interleukin secretion from dendritic cells. Int. J. Clin. Exp. Med. 2010;3:110–114. [PMC free article] [PubMed] [Google Scholar]
- 34.Castano AP, Mroz P, Hamblin MR. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer. 2006;6:535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Canti G, De Simone A, Korbelik M. Photodynamic therapy and the immune system in experimental oncology. Photochem. Photobiol. Sci. 2002;1:79–80. doi: 10.1039/b109007k. [DOI] [PubMed] [Google Scholar]
- 36.Korbelik M, Sun J, Cecic I. Photodynamic therapy-induced cell surface expression and release of heat shock proteins: relevance for tumor response. Cancer Res. 2005;65:1018–1026. [PubMed] [Google Scholar]
- 37.Korbelik M, Stott B, Sun J. Photodynamic therapy-generated vaccines: relevance of tumour cell death expression. Br. J. Cancer. 2007;97:1381–1387. doi: 10.1038/sj.bjc.6604059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 39.Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28:429–436. doi: 10.1016/j.it.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Vabulas RM, Wagner H, Schild H. Heat shock proteins as ligands of toll-like receptors. Curr. Top. Microbiol. Immunol. 2002;270:169–184. doi: 10.1007/978-3-642-59430-4_11. [DOI] [PubMed] [Google Scholar]
- 41.Gomer CJ, Ryter SW, Ferrairo A, Ryffel N, Woodard A, Fisher AMR. Photodynamic therapy-mediated oxidative stress can induce expression of heat shock proteins. Cancer Res. 1996;56:2355–2360. [PubMed] [Google Scholar]
- 42.Gollnick SO, Kabingu E, Kousis PC, Henderson BW. Stimulation of the host immune response by photodynamic therapy (PDT) 2004:60–70. Ref ID: 8330. [Google Scholar]
- 43.Korbelik M. Complement upregulation in photodynamic therapy-treated tumors: Role of Toll-like receptor pathway and NFkappaB. Cancer Lett. 2009;281:232–238. doi: 10.1016/j.canlet.2009.02.049. [DOI] [PubMed] [Google Scholar]
- 44.Korbelik M, Sun J. Photodynamic therapy-generated vaccine for cancer therapy. Cancer Immunol. Immunother. 2006;55:900–909. doi: 10.1007/s00262-005-0088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 46.van Gisbergen KPJM, Sanchez-Hernandez M, Geijtenbeek TBH, van Kooyk Y. Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J. Exp. Med. 2005;201:1281–1292. doi: 10.1084/jem.20041276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bennouna S, Denkers EY. Microbial antigen triggers rapid mobilization of TNF-alpha to the surface of mouse neutrophils transforming them into inducers of high-level dendritic cell TNF-alpha production. J Immunol. 2005;174:4845–4851. doi: 10.4049/jimmunol.174.8.4845. [DOI] [PubMed] [Google Scholar]
- 48.Ethuin F, Gerard B, Benna JE, Boutten A, Gougereot-Pocidalo MA, Jacob L, Chollet-Martin S. Human neutrophils produce interferon gamma upon stimulation by interleukin-12. Lab Invest. 2004;84:1363–1371. doi: 10.1038/labinvest.3700148. [DOI] [PubMed] [Google Scholar]
- 49.Marzo AL, Vezys V, Klonowski KD, Lee S-J, Muralimohan G, Moore M, Tough DF, Lefrançois L. Fully functional memory CD8 T cells in the absence of CD4 T cells. J. Immunol. 2004;173:969–975. doi: 10.4049/jimmunol.173.2.969. [DOI] [PubMed] [Google Scholar]
- 50.Wang J, Santosuosso M, Ngai P, Zganiacz A, Xing Z. Activation of CD8 T cells by mycobacterial vaccination protects against pulmonary tuberculosis in the absence of CD4 T cells. J. Immunol. 2004;173:4590–4597. doi: 10.4049/jimmunol.173.7.4590. [DOI] [PubMed] [Google Scholar]
- 51.Castellino F, Germain RN. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu. Rev. Immunol. 2006;24:519–540. doi: 10.1146/annurev.immunol.23.021704.115825. [DOI] [PubMed] [Google Scholar]
- 52.Kabingu E, Vaughan L, Owczarczak B, Ramsey KD, Gollnick SO. CD8+ T cell-mediated control of distant tumours following local photodynamic therapy is independent of CD4+ T cells and dependent on natural killer cells. Br. J. Cancer. 2007;96:1839–1848. doi: 10.1038/sj.bjc.6603792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamaguchi T, Sakaguchi S. Regulatory T cells in immune surveillance and treatment of cancer. Semin. Cancer Biol. 2006;16:115–123. doi: 10.1016/j.semcancer.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 54.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804–811. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 55.Castano AP, Mroz P, Wu MX, Hamblin MR. Photodynamic therapy plus low-dose cyclophosphamide generates antitumor immunity in a mouse model. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5495–5500. doi: 10.1073/pnas.0709256105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hunt DW, Levy JG. Immunomodulatory aspects of photodynamic therapy. Expert. Opin. Investig. Drugs. 1998;7:57–64. doi: 10.1517/13543784.7.1.57. [DOI] [PubMed] [Google Scholar]
- 58.Henderson BW, Gollnick SO. Mechanistic principles of photodynamic therapy. In: Vo-Dinh T, editor. Biomedical Photonics Handbook. Boca Raton, FL: CRC Press; 2003. pp. 36-36-27. [Google Scholar]
- 59.Matthews YJ, Damian DL. Topical photodynamic therapy is immunosuppressive in humans. Br. J. Dermatol. 2010;162:637–641. doi: 10.1111/j.1365-2133.2009.09562.x. [DOI] [PubMed] [Google Scholar]
- 60.Yusuf N, Katiyar SK, Elmets CA. The immunosuppressive effects of phthalocyanine photodynamic therapy in mice are mediated by CD4+ and CD8+ T cells and can be adoptively transferred to naive recipients. Photochem. Photobiol. 2008;84:366–370. doi: 10.1111/j.1751-1097.2007.00270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Friedberg JS, Mick R, Stevenson JP, Zhu T, Busch TM, Shin D, Smith D, Culligan M, Dimofte A, Glatstein E, Hahn SM. Phase II trial of pleural photodynamic therapy and surgery for patients with non-small-cell lung cancer with pleural spread. J. Clin. Oncol. 2004;22:2192–2201. doi: 10.1200/JCO.2004.07.097. [DOI] [PubMed] [Google Scholar]