Abstract
Background
Otitis media is an infectious, inflammatory process involving the middle ear space. Chronic inflammation is associated with fibrosis, scarring and osteogenesis within the middle ear, which may contribute to subsequent hearing loss and increase the difficulty of treatment.
Methods
Heat-killed Streptococcus pneumoniae was injected into the middle ears of 8–12 week old Balb/c mice. Control mice were treated with PBS middle ear injections. Middle ears were harvested at 1, 3, 5 and 7 days following injection (n=8 for each time point). The middle ears were processed using standard RT-PCR techniques. Up- and down-regulation of mRNA expression of various members of the Bone Morphogenetic Protein (BMP), Fibroblast Growth Factor (FGF) and Matrix Metalloproteinase (MMP) families was quantified and compared to PBS treated controls (n = 8 for each time point).
Results
Significant upregulation of MMP2, MMP3 and MMP9 was observed at varying time points (p < 0.05). Significant downregulation of BMP3, BMP4, BMP5 BMP6 and BMP8a was seen at varying time points (p < 0.05). Significant downregulation of FGF3, FGF6, FGF10 and FGFr1 was observed at varying time points (p < 0.05). No significant expression of BMP8b, BMP9, BMP10, FGF5, FGF8, MMP1a, MMP7 and MMP14 was detected within the middle ear.
Conclusions
Inflammation within the middle ear following injection of bacterial products results in changes in the regulation of several tissue remodeling cytokines and proteinases in the mouse model. Further understanding of these molecular processes may allow for the development of treatment modalities aimed at preventing middle ear tissue remodeling.
Keywords: Otitis media, mouse model, tissue remodeling, cytokines
INTRODUCTION
Otitis media is an infectious, inflammatory process involving the middle ear space. Chronic inflammation may lead to mucosal hypertrophy, hypervascularity, osteogenesis and fibrosis within the middle ear, which may in turn contribute to increased symptomatology and hearing loss. Prior studies have demonstrated upregulation of several inflammatory cytokines in mouse models of chronic otitis media (COM) and acute otitis media (AOM).1–5 Evidence of tissue remodeling with osteogenesis, sclerosis and neovascularization has been observed in the COM mouse model.6 Little is known, however, regarding the underlying molecular processes governing these tissue changes in the middle ear.
Members of the Bone Morphogenetic Protein (BMP) and Fibroblast Growth Factor (FGF) families of cytokines are responsible for regulation of tissue growth and remodeling in many normal physiologic processes, such as embryological development, bone growth, wound healing, angiogenesis and regulation of inflammation.7,8 Matrix metalloproteinases (MMPs) act as endopeptidases that regulate remodeling and breakdown of the extracellular matrix. MMP dysregulation plays a role in many pathologic processes, including cancer, arthritis, atherosclerosis, neurodegenerative diseases and cholesteatoma, as well as diseases of mucosal inflammation such as otitis media, chronic rhinosinusitis and asthma.9–18 BMP dysregulation is seen in gastric and colon malignancies, and FGF signaling is known to play a key role in many types of cancer.19–21
The purpose of the current study is to characterize the time-dependent changes that occur in regulation of members of the BMP, FGF and MMP families following induction of the inflammatory process in the AOM mouse model. We have previously described a mouse model of acute otitis media induced by transtympanic injection of heat killed Streptococcus pneumoniae.22 We hypothesize that induction of otitis media will result in several time-dependent changes in regulation of members of the BMP, FGF and MMP families in the hours and days following onset of inflammation.
METHODS
Middle ear injections
BALB/c mice age 8–12 weeks were anesthetized using a subcutaneous injection of ketamine (100 mg/ml; 0.067 mg/gram body weight) and xylazine (20 mg/ml; 0.013 mg/gram body weight). One µl of heat-killed Streptococcus pneumoniae (1 × 1010 cfu/ml) was injected trans-tympanically into both middle ears under direct visualization using a 30 gauge needle. Mice were sacrificed 24 hours, 3 days, 5 days and 7 days following the injection (n = 8 each time point). Visualization of the tympanic membrane was performed at the time of sacrifice in order to detect the presence of middle ear inflammation. The entire middle ear, including the bulla, tympanic membrane and ossicles, was dissected and placed in RNAlater solution (QIAGEN, Inc., Valencia, CA), then stored at −20° C until the time of RNA extraction. Control mice were treated only with trans-tympanic PBS injections and harvested at the same time points (n = 8 each time point).
Quantitative RT-PCR
RNA for RT-PCR was extracted from dissected middle ear tissue using the RNeasy Mini Kit according to the manufacturer’s instructions (QIAGEN, Inc.). 200 µg of RNA was used to synthesize the cDNA probe using the C-03 RT2 first strand kit (SABiosciences, Frederick, MD). Standardized custom PCR kits optimized for each cytokine, including the primer and probe, were utilized (SABiosciences). Custom arrays were obtained for the following cytokines: BMP1, BMP3, BMP4, BMP6, BMP7, BMP8a, BMP8b, BMP9, BMP10, FGFr1, FGF1, FGF3, FGF5, FGF6, FGF8, FGF10, MMP1a, MMP2, MMP3, MMP7, MMP8, MMP9, MMP12 and MMP14. The method of quantitative real-time RTPCR was utilized, and reverse transcription was performed using an ABI Step One Plus system (Applied Biosystems, Inc., Foster City, CA). Using this system, the parameter CT (threshold cycle) is defined as the fractional cycle number at which the reporter fluorescence generated by cleavage of the probe passes a fixed threshold above baseline. A plot of the log of initial target copy number for a set of standards versus CT is a straight line. Quantitation of the amount of target in samples is done by measuring CT and using the standard curve to determine starting copy number. Calculation of CTs, preparation of a standard curve, determination of the starting copy number and calculation of statistical significance was performed using the SABiosciences program. A two-tailed t-test assuming equal variance was performed using the 2^-delta Ct values as input. 18S RNA was used as the comparator.
All animal procedures were approved by the OHSU Institutional Animal Care and Use Committee.
RESULTS
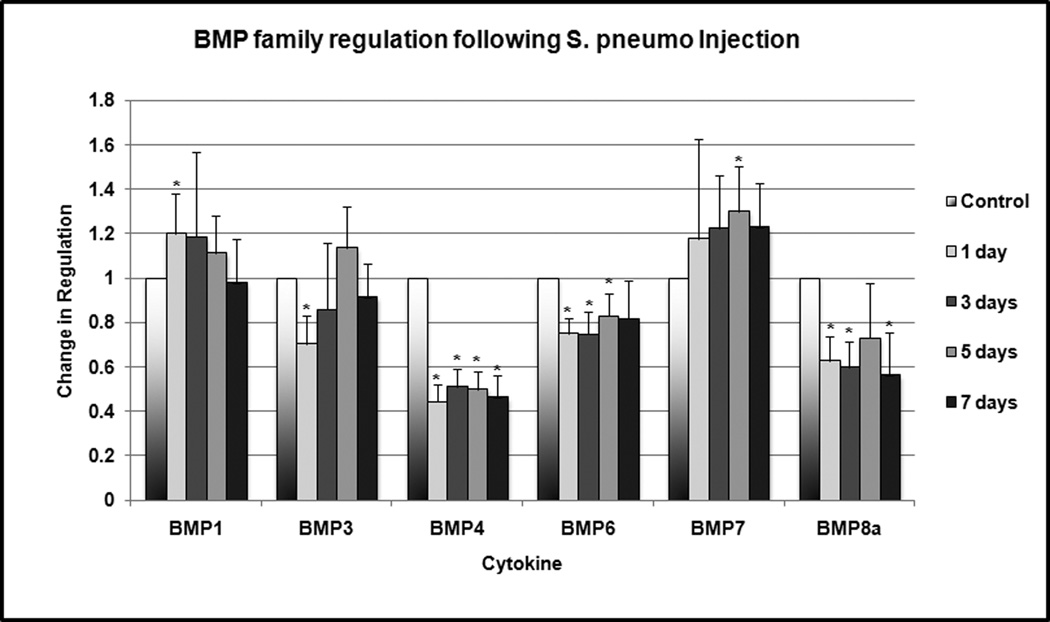
Statistically significant downregulation of several members of the BMP family was observed at multiple time points (Figure 1). BMP 3 was downregulated to 70% of normal expression (p = 0.004) at 24 hours. BMP4 was downregulated to 44–50% of normal expression at 24 hours, 3 days, 5 days and 7 days (p < 0.001). BMP6 was downregulated to 74–82% of normal expression at 24 hours, 3 days and 5 days (p < 0.001). BMP8a was downregulated to 60% of normal expression at 24 hours, 3 days and 5 days (p = 0 .005). No significant expression of BMP8b, 9 and 10 was detected in the middle ear tissue. BMP1 and 7 were slightly, but significantly, upregulated at 1 and 5 days, respectively.
Figure 1.
Change in BMP family regulation following S. pneumo injection as compared to PBS-only treated controls. Asterisks indicate statistically significant values as compared to control.
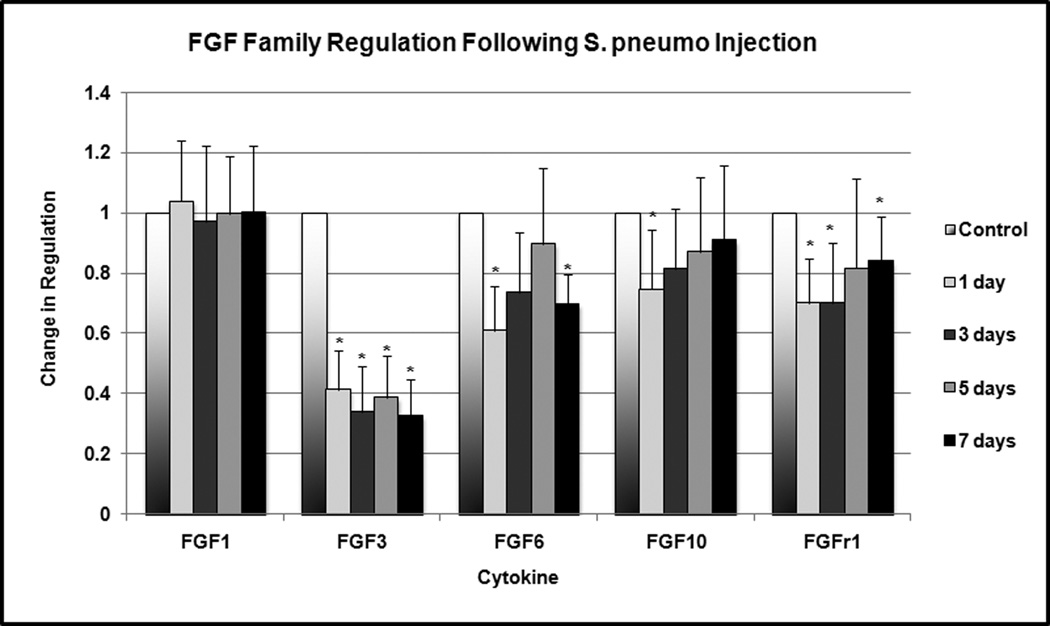
Several members of the FGF family exhibited statistically significant downregulation at various time points (Figure 2). FGF3 was downregulated to 32–41% of normal expression at 24 hours, 3 days, 5 days and 7 days (p < 0.001). FGF6 was downregulated to 60–70% of normal expression at 24 hours and 7 days (p = 0.002). FGF10 was downregulated to 75% of normal expression at 24 hours (p = 0.04). FGFr1 was downregulated to 69–82% of normal expression at 24 hours, 3 days and 5 days (p < 0.02). No significant expression of FGF5 and FGF8 was detected within the middle ear tissue.
Figure 2.
Change in FGF family regulation following S. pneumo injection as compared to PBS-only treated controls. Asterisks indicate statistically significant values as compared to control.
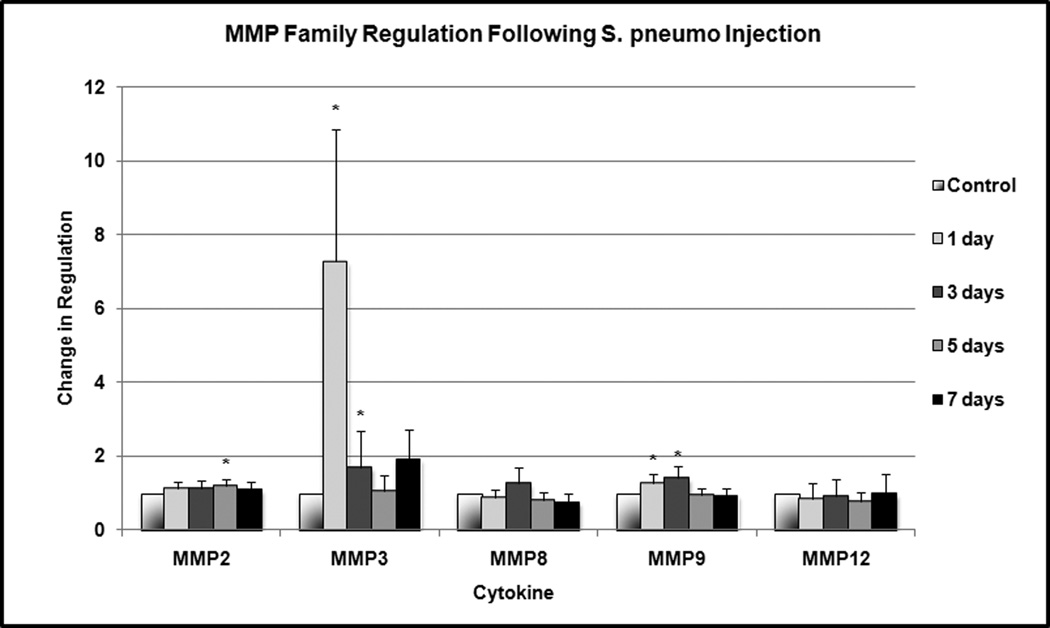
Several members of the MMP family were upregulated in a statistically significant manner at several time points (Figure 3). MMP2 was upregulated by a factor of 1.2 at 5 days (p = 0.004). MMP3 was upregulated by a factor of 7.3 at 24 hours (p < 0.001) and 1.7 at 3 days (p = 0.04). MMP9 was upregulated by 1.3–1.4 at 24 hours and 3 days (p = 0.01). No significant expression of MMP1a, MMP7 and MMP14 was detected within the middle ear tissue.
Figure 3.
Change in MMP family regulation following S. pneumo injection as compared to PBS-only treated controls. Asterisks indicate statistically significant values as compared to control.
All mice injected with heat-killed bacteria exhibited bulging and erythema of the TM upon otomicroscopic examination, consistent with the presence of middle ear inflammation. Examination of the PBS injected middle ears revealed subtle findings of TM erythema, mild in contrast to the experimental ears.
DISCUSSION
In this study we observed changes in regulation of various members of the BMP, FGF and MMP families in a time-dependent manner following onset of inflammation in the AOM mouse model. Immunohistochemistry results indicate that these cytokines and MMPs are produced by cells within the middle ear mucosa. In many cases, these changes in regulation persisted over all observed time points within the 7 day study period. In some cases, such as MMP3, these changes were seen at only 1 or 2 time points. This suggests that changes in regulation of these cytokines occurs in a rapid, complex and dynamic fashion.
We observed downregulation of several members of the FGF and BMP families at various time points in the AOM mouse model. Little is known regarding the role of these cytokines in otitis media. To our knowledge, the FGF and BMP families of cytokines have not been studied in relation to otitis media. While it may seem counterintuitive that many of these cytokines are downregulated rather than upregulated, we have observed that these changes are dynamic over time, and additional changes may be observed at further time points. Additionally, members of these cytokine families interact in a complex manner with some members acting as negative regulators of other factors. For example, some authors have suggested that BMP3 acts as a negative regulator of other BMP cytokines.23 Also, the transient suppression of many tissue remodeling cytokines may be required for the mucosal hyperplasia and neovascularization that occur during the acute inflammatory response. Further study of these changes in a chronic OM mouse model is ongoing.
We observed upregulation of several members of the MMP family in the AOM mouse model. MMPs are endopeptidases produced by stromal cells, macrophages and neutrophils that are responsible for breakdown of the extracellular matrix. Prior studies have implicated MMP2, MMP3 and MMP9 in otitis media, and they have been observed in higher concentrations in mucoid middle ear effusions than in serous effusions. Others have shown that increased MMP activity is present in infections less than 1 week duration. Increased MMP2 expression has also been shown to be associated with increased chronicity of otitis media.10,11,25
MMP3 is also known as stromelysin 1. MMP3 acts upon fibronectin, laminin, elastin and various collagens in promoting their breakdown within the extracellular matrix.17 MMP3 has been studied in relation to cholesteatoma, and increased expression of MMP3 has been shown to be associated with greater invasive behavior in cholesteatoma.15 MMP3 expression has also been associated with joint destruction in rheumatoid arthritis.13
From a clinical perspective, these early changes in regulation of tissue remodeling factors may contribute to mucosal hyperplasia, scarring, fibrosis and osteoneogenesis in OM. Further evaluation of these changes at later time points on a molecular and histologic level in a chronic OM model may help to better define the role of these cytokines and proteinases in the pathogenesis of OM. With further understanding, novel therapies may be designed to specifically block these molecular changes leading to tissue remodeling in OM.
CONCLUSION
Changes in expression of various members of the BMP, FGF and MMP families occur in a complex and dynamic fashion following onset of inflammation in the AOM mouse model. Further study is necessary in order to better determine the association between these cytokines and tissue remodeling within the middle ear.
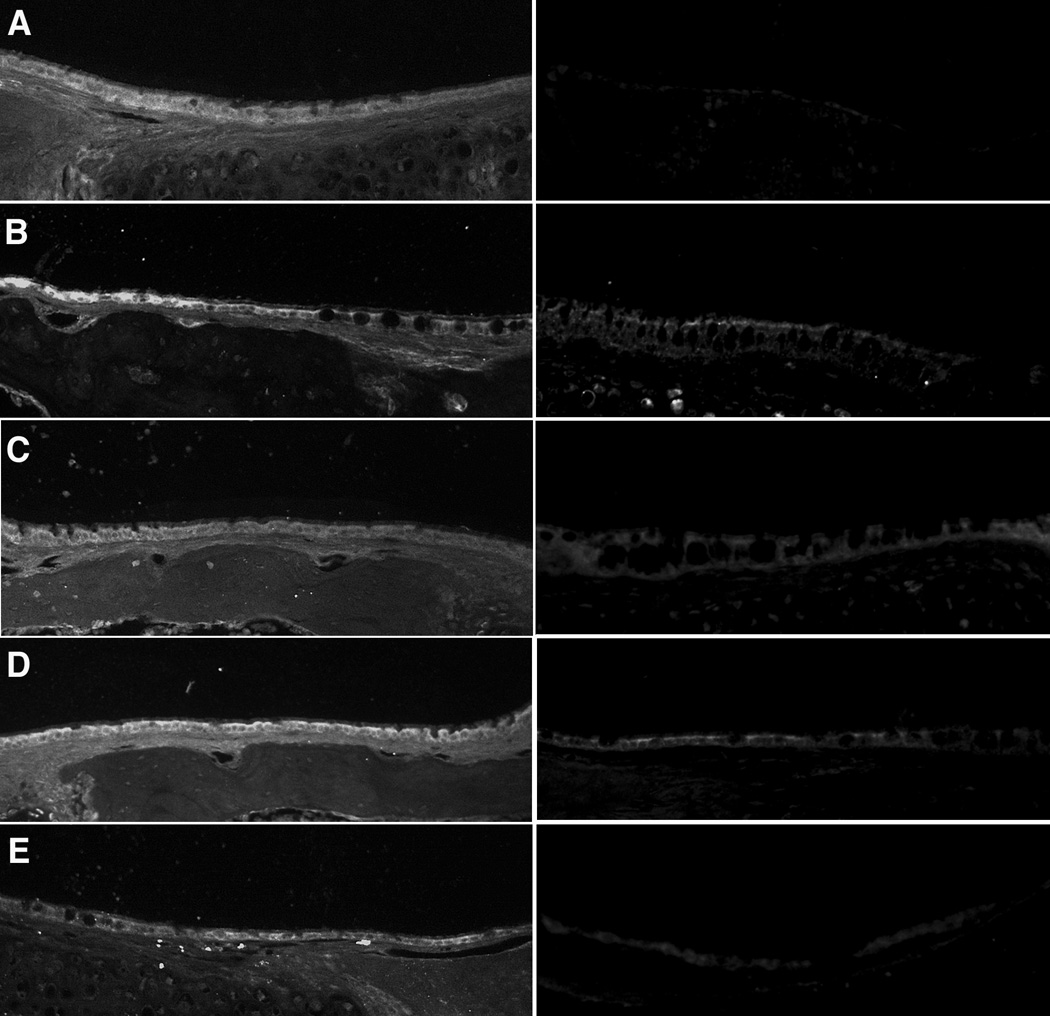
Figure 4.
10x micrographs of middle ear mucosa following immunohistochemical staining for BMP3 (Fig 4a), BMP7 (Fig 4b), FGF3 (Fig 4c), FGF7 (Fig 4d) and MMP3 (Fig 4e). Experimental middle ears 24 hours following S. pneumo injection are represented in the left column, and untreated controls are seen on the right.
Acknowledgments
Grant Support: NIH-NIDCD R01 DC009455 and DC009455-S1 (ARRA) (DRT)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest disclosure: None
WORKS CITED
- 1.Ghaheri BA, Kempton JB, Pillers DA, Trune DR. Cochlear cytokine gene expression in murine chronic otitis media. Otolaryngol Head Neck Surg. 2007;137(2):332–337. doi: 10.1016/j.otohns.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Ghaheri BA, Kempton JB, Pillers DA, Trune DR. Cochlear cytokine gene expression in murine acute otitis media. Laryngoscope. 2007;117(1):22–29. doi: 10.1097/01.mlg.0000240170.48584.73. [DOI] [PubMed] [Google Scholar]
- 3.MacArthur CJ, Pillers DM, Pang J, Kempton JB, Trune DR. Altered expression of middle and inner ear cytokines in mouse otitis media. Laryngoscope. doi: 10.1002/lary.21349. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trune DR, Larrain BE, Hausman F, Kempton JB, MacArthur CJ. Simultaneous measurement of multiple mouse ear proteins with multiplex ELISA assays. Hearing Research. doi: 10.1016/j.heares.2010.11.009. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacArthur CJ, Hausman F, Kempton JB, Trune DR. Murine middle ear inflammation and ion homeostasis gene expression. Otology & Neurotology. doi: 10.1097/MAO.0b013e31820e6de4. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacArthur CJ, Hefeneider SH, Kempton B, Trune DR. C3H/HeJ mouse model for spontaneous chronic otitis media. Laryngoscope. 2006;116:1071–1079. doi: 10.1097/01.mlg.0000224527.41288.c4. [DOI] [PubMed] [Google Scholar]
- 7.Xiao YT, Xiang XL, Shao JZ. Bone morphogenetic protein. Biochem Biophys Res Comm. 2007;362:550–553. doi: 10.1016/j.bbrc.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 8.Turner N, Grose R. Fibroblast growth factor signaling: from development to cancer. Nature Reviews: Cancer. 2010;10(2):116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 9.Wilmoth JG, Schultz GS, Antonelli PJ. Tympanic membrane metalloproteinase inflammatory response. Otolaryngol Head Neck Surg. 2003;129:647–654. doi: 10.1016/S0194-59980301388-3. [DOI] [PubMed] [Google Scholar]
- 10.Jennings CR, Guo L, Collins HM, Birchall JP. Matrix metalloproteinase's 2 and 9 in otitis media with effusion. Clin Otolaryngol. 2001;26:491–494. doi: 10.1046/j.1365-2273.2001.00510.x. [DOI] [PubMed] [Google Scholar]
- 11.Jang CH, Shin SH, Cho HH, Moon SJ, Cho YB. Expression of matrix metalloproteinase 9 and 2 in pediatric chronic otitis media with effusion. Int J Ped Otorhinolaryngol. 2006;70:1155–1158. doi: 10.1016/j.ijporl.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Moon SK, Linthicum FH, Yang HD, et al. Activities of matrix metalloproteinases and tissue inhibitor of metalloproteinase-2 in idiopathic hemotympanum and otitis media with effusion. Acta Otolaryngologica. 2008;128:144–150. doi: 10.1080/00016480701477610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gosset M, Pigenet A, Salvat C, Berenbaum F, Jacques C. Inhibition of matrix metalloproteinase 3 and 13 synthesis induced by IL-1 in chondrocytes from mice lacking microsomal prostaglandin E synthase-1. J Immunol. 2010;185:6244–6252. doi: 10.4049/jimmunol.0903315. [DOI] [PubMed] [Google Scholar]
- 14.Juhasz A, Sziklai I, Rakosy Z, et al. Elevated levels of tenascin and matrix metalloproteinase 9 correlates with the bone destruction capacity of cholesteatomas. Otol Neurotol. 2009;30:559–565. doi: 10.1097/MAO.0b013e31819fe6ed. [DOI] [PubMed] [Google Scholar]
- 15.Choufani G, Ghanooni R, Decaestecker C, et al. Detection of macrophage migration inhibitory factor (MIF) in human cholesteatomas and functional implications of correlations to recurrence status and to expression of matrix metalloproteinases-3/9, retinoic acid receptor-beta, and anti-apoptotic galectin-3. Laryngoscope. 2001;111(9):1656–1662. doi: 10.1097/00005537-200109000-00031. [DOI] [PubMed] [Google Scholar]
- 16.Kostamo K, Toskala E, Tervahartiala T, Sorsa T. Role of matrix metalloproteinases in chronic rhinosinusitis. Curr Opin All Clin Immunol. 2008;8:21–27. doi: 10.1097/ACI.0b013e3282f3f461. [DOI] [PubMed] [Google Scholar]
- 17.De S, Fenton JE, Jones AS. Matrix metalloproteinases and their inhibitors in non-neoplastic otorhinolaryngological disease. J Laryngol Otol. 2005;119:436–442. doi: 10.1258/0022215054273269. [DOI] [PubMed] [Google Scholar]
- 18.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nature Reviews. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 19.Bleuming SA, He XC, Kodach LL, et al. Bone morphogenetic protein signaling suppresses tumorigenesis at gastric epithelial transition zones in mice. Cancer Research. 2007;67(17):8149–8155. doi: 10.1158/0008-5472.CAN-06-4659. [DOI] [PubMed] [Google Scholar]
- 20.Kodach LL, Wiercinska E, de Miranda NF, et al. The bone morphogenetic protein pathway is inactivated in the majority of sporadic colorectal cancers. Gastroenterology. 2008;134(5):1332–1341. doi: 10.1053/j.gastro.2008.02.059. [DOI] [PubMed] [Google Scholar]
- 21.Turner N, Grose R. Fibroblast growth factor signaling: from development to cancer. Nature Reviews. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]
- 22.MacArthur CJ, Hefeneider SH, Kempton JB, Parrish SK, McCoy SL, Trune DR. Evaluation of the mouse model for acute otitis media. Hearing Research. 2006;219(1–2):12–23. doi: 10.1016/j.heares.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Daluiski A, Engstrand T, Bahamonde ME, et al. Bone morphogenetic protein-3 is a negative regulator of bone density. Nature Genetics. 2001;27:84–88. doi: 10.1038/83810. [DOI] [PubMed] [Google Scholar]
- 24.Avidano MA, Cotter CS, Stringer SP, Schultz GS. Analysis of protease activity in human otitis media. Otolaryngol Head Neck Surg. 1998;119(4):346–351. doi: 10.1016/S0194-5998(98)70076-2. [DOI] [PubMed] [Google Scholar]
- 25.Lauhio A, Rezes S, Tervahartiala T, Sziklai I, Pitkäranta A, Sorsa T. Matrix metalloproteinase-8/collagenase-2 in childhood otitis media with effusion. Ann Med. 2010 doi: 10.3109/07853890.2010.530684. (e-pub ahead of print). [DOI] [PubMed] [Google Scholar]