Abstract
A high prevalence of the sequence variant c.1436C>T in the CPT1A gene has been identified among Alaska Native newborns but the clinical implications of this variant are unknown. We conducted medically supervised fasts in 5 children homozygous for the c.1436C>T variant. Plasma free fatty acids increased normally in these children but their long-chain acylcarnitine and ketone production was significantly blunted. The fast was terminated early in two subjects due to symptoms of hypoglycemia. Homozygosity for the c.1436C>T sequence variant of CPT1A impairs fasting ketogenesis, and can cause hypoketotic hypoglycemia in young children.
Keywords: fatty acid oxidation, carnitine palmitoyltransferase 1A, ketones, acylcarnitines, hypoketotoic, hypoglycemia, fasting
Introduction
Carnitine palmitoyltransferase 1A (CPT1A) catalyzes the synthesis of long chain acylcarnitines and is required for mitochondrial fatty acid oxidation in the liver. CPT1A deficiency is an inherited disorder of hepatic fatty acid oxidation that affects the tolerance to fasting and presents clinically with hypoketotic hypoglycemia. A unique polymorphism in the CPT1A gene (c.1436C>T) is associated with decreased enzyme activity and altered regulation [1], and has been identified among the Canadian and Greenland Inuit, and some Alaska Native populations [2–4]. This sequence variant results in a proline to leucine substitution at amino acid 479 (p.P479L) of the CPT1A protein. The prevalence of this genetic variant is extremely high in some regions, with reports of up to 80% of individuals being homozygous in some Native populations [2, 3]. The observation that the c.1436C>T variant is the most prevalent or “normal” allele within certain populations suggests positive selection for some unknown beneficial effect. The potential benefit of the c.1436C>T variant has not been elucidated, but Greenberg and colleagues [3] have proposed that because the P479L protein has a high residual enzyme activity (20% of normal) and is relatively insensitive to malonyl-CoA inhibition, it may allow some post-prandial hepatic ketogenesis to occur in the context of the traditional high fat, high protein diet consumed by indigenous Arctic people.
There are also potential negative consequences associated with the c.1436C>T variant. CPT1A is required for fasting ketogenesis, and severe deficiency is associated with hypoketotic hypoglycemia, liver dysfunction and sudden infant death [5]. Infant mortality rates are significantly higher in regions where the variant is most prevalent [6, 7], and our preliminary evidence indicates that Alaska Native infants who are homozygous for the variant have an increased risk of infant death [8]. Our hypothesis is that the increased infant mortality rate associated with the c.1436C>T variant is due to its effect on fasting ketogenesis.
During the initial phase of fasting the breakdown of liver glycogen supplies systemic glucose needs. Glycogen depletion leads to a gradual decrease in serum glucose and insulin, with activation of hormone sensitive lipase in adipose tissue and release of free fatty acids into the blood. The liver oxidizes fatty acids to generate ketones, which serve as an alternate energy source, sparing glucose for glucose-requiring tissues. ATP derived from fatty acid oxidation also energizes de novo glucose synthesis from amino acids and other substrates in the liver. CPT1A is required for mitochondrial fatty acid oxidation and is therefore essential in the metabolic response to fasting. In this study we evaluated the fasting response of five children homozygous for the c.1436C>T variant to determine the physiologic significance of the decreased CPT1A activity associated with this polymorphism.
Methods
Subject Recruitment
The Alaska Newborn Screening Program sent a letter to all nine eligible families with children age three and older that had been identified as homozygous for the c.1436C>T variant via newborn screening and lived in the Norton Sound area of Alaska inviting them to participate. Six interested families contacted the principal investigator (MBG), who provided details of the study and handled travel logistics for families. Five families decided to participate and they flew from their home in Alaska to Portland, OR. Informed consent to participate in the study was obtained from the parents of all subjects. The Institutional Review Boards of Oregon Health & Science University (OHSU) and the Alaska Area Indian Health Service (OHSU IRB3556, Alaska Area IRB 2007-9-30, NCT00653666) approved this study. Tribal approval was obtained from the Research Ethics Review Board of the Norton Sound Health Corporation (Nome, Alaska) and the Southcentral Foundation (Anchorage, Alaska).
Fasting Protocol
Fasting studies were done on the inpatient unit of the OHSU Pediatric Clinical and Translational Research Center in Doernbecher Children’s Hospital. Subjects were fasted for 18 hours following an evening meal, with continuous heart rate and pulse oximetry monitoring. Subjects received maintenance IV fluids with ½ normal saline and were allowed to drink calorie-free beverages. Blood samples were obtained after 1, 6, 12 and 18 hours of fasting for electrolytes, glucose, insulin, free fatty acids, acylcarnitines and ketones. Beginning at 6 hours of fasting, serum glucose was measured hourly on the inpatient unit with a glucose oxidase analyzer (Yellow Springs Instruments, Yellow Springs, OH). The fast was terminated after 18 hours and subjects were allowed to eat and drink ad-lib. If the blood glucose dropped below 60 mg/dl monitoring was increased to every 30 minutes. If the blood glucose dropped below 40 mg/dl or the subject developed clinical signs of hypoglycemia, 2 ml/kg body weight of 25% dextrose was given via IV push and the fast was terminated. Subjects and their families were allowed to return home the following day.
Biochemical Analysis
Whole blood was centrifuged within 20 minutes of collection and aliquots of serum samples were stored at −80°C. Serum ketones (3-hydroxybutyrate and acetoacetate) were measured by stable isotope dilution gas chromatography-mass spectrometry (GC-MS)[9]. Serum carnitine levels and acylcarnitine profiles were quantified by tandem mass spectrometry (MS/MS) and expressed as µmol/L[10]. Plasma samples were isolated via centrifugation of whole blood collected in EDTA tubes containing a lipase inhibitor (tetrahydrolipostatin) and stored at −80°C. Plasma free fatty acids (FFA) were measured using the NEFA-HR(2) kit (Waco Chemicals, Richmond, VA).
Data Analysis
Group data is given as the mean ± the standard deviation of the mean for all subjects (n=5) unless otherwise stated. Because of the availability of published normal data and the ethical concerns of fasting control children, data from our subjects were compared to published data of children who were free of a fatty acid oxidation (FAO) disorder [11, 12].
Results
Subject Characteristics
Each of the five children were homozygous for the c.1436C>T variant. Height and weight of all subjects were within normal percentiles for age, and the mean body mass index (BMI) was 19.6 kg/m2 (92%ile) (Table 1). All subjects were developmentally normal and had a normal physical exam at the time of the study. Blood chemistries (AST, ALT, bilirubin, albumin, BUN/Cr) were normal in all subjects. To our knowledge, the subjects were unrelated. Parents of all subjects reported that they consumed a regular pediatric diet at home prior to entering the study. Alaska Native families in the Norton Sound region typically follow a diet that includes large amounts fish, land and sea mammals, but is modified from the traditional diet by the inclusion of increased amounts of carbohydrate. During their stay in the pediatric CTRC, subjects were allowed to choose foods from the regular pediatric hospital menu.
Table 1.
Demographic Data of Subjects
| Subjects | Plasma Acylcarntitine Profiles (μmol/L) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Post-prandially | Termination of fast | ||||||||||||
| Age (years) |
Gender | Height (cm) |
Weight (kg) |
BMI | Prior Symptoms | Free Carnitine |
C16:0 | C18:0 | C0/ (C16+C18) |
C16:0 | C18:0 | C0/ (C16+C18) |
|
| 1 | 4 | M | 109.0 | 20.3 | 24.0 | none | 44.8 | 0.026 | 0.021 | 307 | 0.059 | 0.025 | 156 |
| 2 | 3 | M | 92.0 | 16.6 | 19.6 | hypoglycemia & seizures with illness | 45.7 | 0.067 | 0.042 | 127 | 0.059 | 0.025 | 151 |
| 3 | 4 | M | 111.5 | 23.9 | 19.2 | none | 55.4 | 0.052 | 0.041 | 195 | 0.059 | 0.029 | 165 |
| 4 | 3 | M | 98.0 | 16.4 | 17.1 | none | 33.6 | 0.031 | 0.027 | 202 | 0.061 | 0.041 | 108 |
| 5 | 4 | F | 110.5 | 21.9 | 17.9 | lethargy with illness | 51.7 | 0.041 | 0.050 | 163 | 0.083 | 0.040 | 103 |
Individual subject data are presented. cm = centimeter; kg=kilograms; BMI=body mass index calculated as weight (kg)/ height (m2); prior symptoms = parental reports of symptoms prior to study enrollment; plasma acylcarnitine concentrations of free carnitine, C16:0 and C18:0 acylcarnitine and the C0/C16+C18 ratio one hour after a meal, and C16:0, C18:0, acylcarnitine and the C0/C16+C18 ratio at the time the fast was terminated. The fast was terminated at 18 hours of fasting for subjects 1, 3 and 4, at 16 hours of fasting for subject 2 and at 17 hours of fasting for subject 5.
Metabolic Response to Fasting
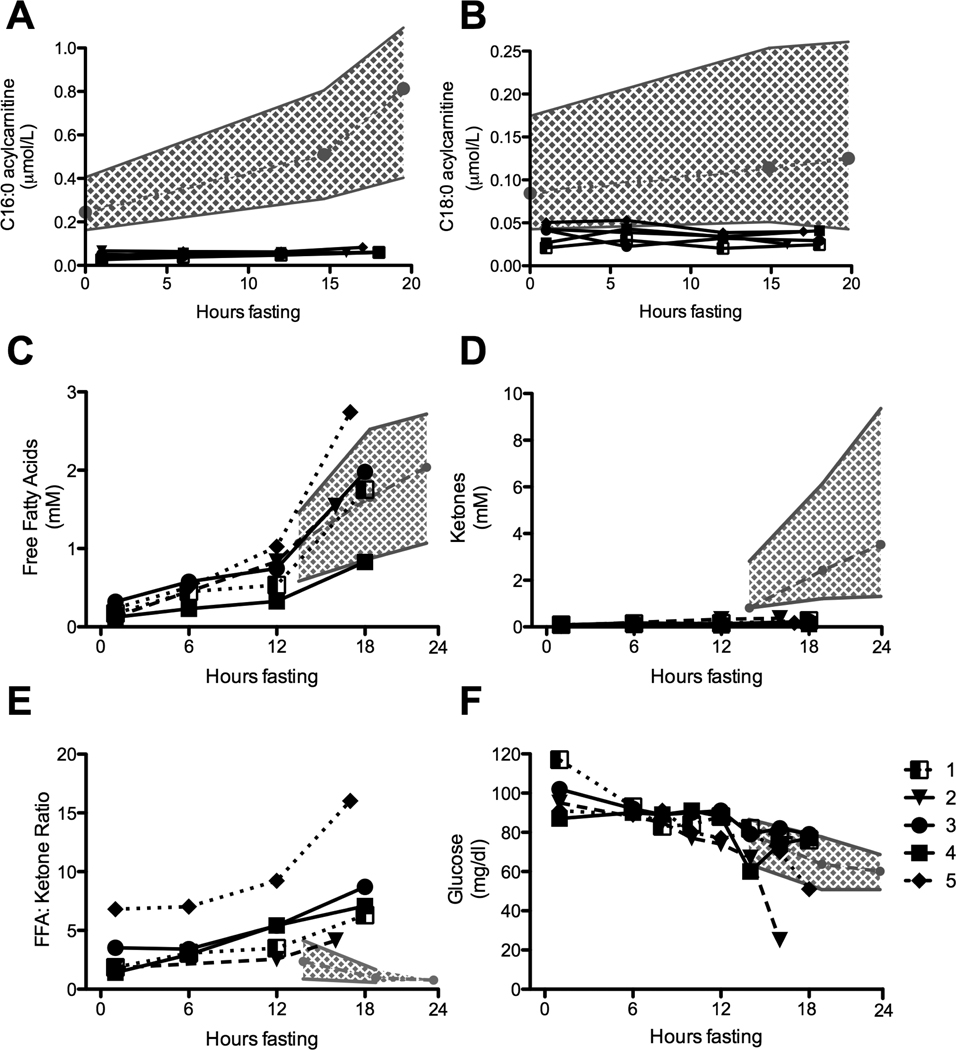
Baseline levels of total (54 ± 10 µmol/L) and free (46 ±8 µmol/L) carnitine were normal in all subjects. In contrast, the ratio of free carnitine (C0) to the sum of the C16 and C18 acylcarnitines (C0/C16+C18) was significantly elevated (mean 199 ± 68, normal < 130 in infants), which is characteristic of CPT1A deficiency, reflecting the decreased ability to generate long chain acylcarnitines[13]. This ratio is used diagnostically to identify infants with CPT1A deficiency by newborn screening, including the five subjects in this study. Over the course of the fast, the expected rise in levels of long chain acylcarnitines and concomitant decrease in free carnitine was reduced (Figure 1A and 1B; Table 1) [12, 14].
Figure 1. Metabolic Response to Fasting in children with CPT1A deficiency.
(A) Change in C16:0 acylcarnitine (B) C18:0 acylcarnitine in 5 subjects homozygous for the c.1436C>T variant of CPT1A are compared to published data for children free of a FAO disorder. Normal control data is presented as the mean (•---•---•) and the range (grey shading) at 0, 15, and 20 hours of fasting [12]. (C) Change in free fatty acids (FFA) (D) ketones (E) the FFA:ketone ratio and (F) glucose in each of 5 subjects who are homozygous for the c.1436C>T variant of CPT1A are compared to published normal data. Normal control data is presented as the mean (•---•---•) and the 10th and 90th percentiles (grey shading) at 15, 18 and 24 hours of fasting [11].
The blunted rise in acylcarnitines was not due to a lack of substrate. Plasma FFA levels rose to a mean of 1.5 ± 0.6 mmol/L after 18 hours of fasting, which is similar to levels reported in normal children (Figure 1C)[11]. In contrast, at the termination of the fast the mean level of serum ketones was decreased (0.21 ± 0.08 mmol/L vs. 2.4 mmol/L), and the FFA:ketone ratio was markedly increased (8.45 ± 4.5, range 4.1 – 16.0, vs. < 2.5) in all study subjects when compared to controls at 20 hours of fasting (Figure 1D and 1E).
Two of the five subjects in our study developed symptoms of hypoglycemia (lethargy and tachycardia) prior to completing the 18-hour fast. Blood glucose dropped to 25 mg/dl after 16 hours in subject 2, and to 50 mg/dl at 17 hours in subject 5 (Figure 1F). Symptoms resolved rapidly in both subjects following IV glucose administration and resumption of oral calorie intake. The remaining three subjects remained asymptomatic and maintained a normal serum glucose level (>50 mg/dl) throughout the 18-hour fast.
Discussion
The c.1436C>T variant in CPT1A was identified in Alaska Native infants following the implementation of expanded newborn using MS/MS [2]. The subsequent discovery that up to 80% of infants born in some regions are homozygous for this variant has raised significant questions about its health consequences, and whether identification via newborn screening is necessary. The goal of this study was to determine if young children who are homozygous for the c.1436C>T variant in CPT1A identified by newborn screening have an abnormal fasting response. All five of the children in our study had an abnormal metabolic response to fasting, as demonstrated by markedly elevated FFA:ketone ratios. The FFA:ketone ratio is a strong indicator of the capacity to oxidize fatty acids and generate ketones, and the elevated ratios seen in our subjects are characteristic of patients with fatty acid oxidation disorders [11, 12, 15]. In addition to hypoketosis, patients with disorders of fatty acid oxidation often develop hypoglycemia during fasting, which occurred in two of our study subjects. The impairment of both ketogenesis and glucose homeostasis observed in our subjects indicates that the c.1436C>T variant can have a physiologically relevant impact on CPT1A activity.
It is estimated that more than 700 Alaska Native infants homozygous for the c.1436C>T variant are born each year [2], and yet reports of hypoketotic hypoglycemia are relatively rare and most often associated with fasting related to illness (personal observation – MH and DMK). This suggests that under normal circumstances the impaired fasting ability associated with homozygosity for the variant is rarely symptomatic. This may not be true of prolonged fasting associated with viral illness and other stressors. However, we do not know whether all homozygous children are at risk. Within our small sample only two subjects developed symptoms of hypoglycemia, and there were no obvious differences in baseline lab or anthropomorphic data between them and the other three, suggesting it may not be possible to identify children most at risk. Parents of these two subjects did report prior symptoms in their children at the time of enrollment (Table 1). There are a number of inherited metabolic diseases where only a portion of affected individuals ever present with disease-specific symptoms, and then only when associated with environmental stress. These include other fatty acid oxidation disorders such as medium chain acyl-CoA dehydrogenase deficiency (MCADD) and acute porphyrias.
Newborn screening for MCADD has been shown to be highly sensitive and to decrease infant morbidity and mortality due to this disorder when treatment is initiated in screened populations [16–19]. Treatment for MCADD only requires avoiding prolonged fasting and close monitoring during illness. In our clinical experience, the same approach is also effective for avoiding symptoms of CPT1A deficiency. We predict that only a small fraction of the children identified via newborn screening as homozygous for the c.1436C>T variant will ever have an episode of hypoketotic hypoglycemia. However, in contrast to MCADD, newborn screening by MS/MS identifies only a small fraction (~10%) of the infants homozygous for the c.1436C>T variant due to low sensitivity and specificity [2]. Determination of the need for full ascertainment, which would require DNA testing of all infants born in Alaska, will require epidemiologic data to determine the extent and severity of clinical events, including death, associated with the variant allele.
Regardless of future policies regarding the merits of identifying infants with CPT1A deficiency due to the c.1436C>T variant by newborn screening, our results support continuation of the combined educational efforts of the State of Alaska Newborn Screening Program and healthcare providers from the Alaska Native Health System regarding proper care of affected infants and children. These efforts could significantly decrease infant mortality in the Alaska Native population, in which case identification of the c.1436C>T variant by newborn screening would be yet another example of the public health benefits of newborn screening.
Acknowledgements
Supported by the National Institutes of Health grant K01 NIDDK DK071869 (MBG) and the Oregon Clinical and Translational Research Institute (OCTRI) grant ULI RR024140 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research.
Abbreviations
- CPT1A
carnitine palmitoyltransferase 1A
- BMI
body mass index
- FAO
fatty acid oxidation
- MCADD
medium chain acyl-CoA dehydrogenase deficiency
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brown NF, Mullur RS, Subramanian I, Esser V, Bennett MJ, Saudubray JM, Feigenbaum AS, Kobari JA, Macleod PM, McGarry JD, Cohen JC. Molecular characterization of L-CPT I deficiency in six patients: insights into function of the native enzyme. J Lipid Res. 2001;42:1134–1142. [PubMed] [Google Scholar]
- 2.Gessner BD, Gillingham MB, Johnson MA, Richards CS, Lambert WE, Sesser D, Rien LC, Hermerath CA, Skeels MR, Birch S, Harding CO, Wood T, Koeller DM. Prevalence and distribution of the c.1436C-->T sequence variant of carnitine palmitoyltransferase 1A among Alaska Native infants. J Pediatr. 2011;158:124–129. doi: 10.1016/j.jpeds.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg CR, Dilling LA, Thompson GR, Seargeant LE, Haworth JC, Phillips S, Chan A, Vallance HD, Waters PJ, Sinclair G, Lillquist Y, Wanders RJ, Olpin SE. The paradox of the carnitine palmitoyltransferase type Ia P479L variant in Canadian Aboriginal populations. Mol Genet Metab. 2009;96:201–207. doi: 10.1016/j.ymgme.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Rajakumar C, Ban MR, Cao H, Young TK, Bjerregaard P, Hegele RA. Carnitine palmitoyltransferase IA polymorphism P479L is common in Greenland Inuit and is associated with elevated plasma apolipoprotein A-I. J Lipid Res. 2009;50:1223–1228. doi: 10.1194/jlr.P900001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett MJ, Narayan S. Carnitine Palmitoyltransferase 1A Deficiency, Genereviews. 2005 [Google Scholar]
- 6.Adams MM. The descriptive epidemiology of sudden infant deaths among natives and whites in Alaska. Am J Epidemiol. 1985;122:637–643. doi: 10.1093/oxfordjournals.aje.a114143. [DOI] [PubMed] [Google Scholar]
- 7.Fleshman JK, Peterson DR. The sudden infant death syndrome among Alaskan natives. Am J Epidemiol. 1977;105:555–558. doi: 10.1093/oxfordjournals.aje.a112419. [DOI] [PubMed] [Google Scholar]
- 8.Gessner BD, Gillingham MB, Birch S, Wood T, Koeller DM. Evidence for an association between infant mortality and a carnitine palmitoyltransferase 1A genetic variant. Pediatrics. 2010;126:945–951. doi: 10.1542/peds.2010-0687. [DOI] [PubMed] [Google Scholar]
- 9.Shoemaker JD, Elliott WH. Automated screening of urine samples for carbohydrates, organic and amino acids after treatment with urease. J Chromatogr. 1991;562:125–138. doi: 10.1016/0378-4347(91)80571-s. [DOI] [PubMed] [Google Scholar]
- 10.Smith EH, Matern D. Acylcarnitine analysis by tandem mass spectrometry Chapter 17. Curr Protoc Hum Genet. 2010;(Unit 17 18):11–20. doi: 10.1002/0471142905.hg1708s64. [DOI] [PubMed] [Google Scholar]
- 11.Bonnefont JP, Specola NB, Vassault A, Lombes A, Ogier H, de Klerk JB, Munnich A, Coude M, Paturneau-Jouas M, Saudubray JM. The fasting test in paediatrics: application to the diagnosis of pathological hypo- and hyperketotic states. Eur J Pediatr. 1990;150:80–85. doi: 10.1007/BF02072043. [DOI] [PubMed] [Google Scholar]
- 12.Costa CC, de Almeida IT, Jakobs C, Poll-The BT, Duran M. Dynamic changes of plasma acylcarnitine levels induced by fasting and sunflower oil challenge test in children. Pediatr Res. 1999;46:440–444. doi: 10.1203/00006450-199910000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Fingerhut R, Roschinger W, Muntau AC, Dame T, Kreischer J, Arnecke R, Superti-Furga A, Troxler H, Liebl B, Olgemoller B, Roscher AA. Hepatic carnitine palmitoyltransferase I deficiency: acylcarnitine profiles in blood spots are highly specific. Clin Chem. 2001;47:1763–1768. [PubMed] [Google Scholar]
- 14.Frohlich J, Seccombe DW, Hahn P, Dodek P, Hynie I. Effect of fasting on free and esterified carnitine levels in human serum and urine: correlation with serum levels of free fatty acids and beta-hydroxybutyrate. Metabolism. 1978;27:555–561. doi: 10.1016/0026-0495(78)90022-7. [DOI] [PubMed] [Google Scholar]
- 15.van Maldegem BT, Duran M, Wanders RJ, Waterham HR, de Koning TJ, Rubio E, Wijburg FA. Fasting and fat-loading tests provide pathophysiological insight into short-chain acyl-coenzyme a dehydrogenase deficiency. J Pediatr. 156:121–127. doi: 10.1016/j.jpeds.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Wilcken B, Hammond J, Silink M. Morbidity and mortality in medium chain acyl coenzyme A dehydrogenase deficiency. Arch Dis Child. 1994;70:410–412. doi: 10.1136/adc.70.5.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horvath GA, Davidson AG, Stockler-Ipsiroglu SG, Lillquist YP, Waters PJ, Olpin S, Andresen BS, Palaty J, Nelson J, Vallance H. Newborn screening for MCAD deficiency: experience of the first three years in British Columbia, Canada. Can J Public Health. 2008;99:276–280. doi: 10.1007/BF03403754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold GL, Saavedra-Matiz CA, Galvin-Parton PA, Erbe R, Devincentis E, Kronn D, Mofidi S, Wasserstein M, Pellegrino JE, Levy PA, Adams DJ, Nichols M, Caggana M. Lack of genotype-phenotype correlations and outcome in MCAD deficiency diagnosed by newborn screening in New York State. Mol Genet Metab. 99:263–268. doi: 10.1016/j.ymgme.2009.10.188. [DOI] [PubMed] [Google Scholar]
- 19.Marsden D. Expanded newborn screening by tandem mass spectrometry: the Massachusetts and New England experience Southeast Asian. J Trop Med Public Health. 2003;34 Suppl 3:111–114. [PubMed] [Google Scholar]