Abstract
Purpose
Although chemoradiation using 5-fluorouracil (5-FU) and Mitomycin-C (MMC) is the standard of care in the treatment of anal cancer, many patients are unable to tolerate MMC. This phase II clinical trial was performed to determine if cisplatin could replace MMC in the treatment of anal cancer.
Methods and Materials
33 patients with localized anal cancer were enrolled. One patient registered but never received any assigned therapy and is excluded from all analyses. Between 2/1/93 to 7/21/93, 19 patients were accrued to cohort 1. Radiation consisted of 45 Gy to the primary tumor and pelvic nodes, followed by a boost to the primary and involved nodes to 59.4 Gy. A planned two week treatment break was utilized after 36 Gy. Concurrent chemotherapy consisted of 5-FU, 1000 mg/m2 /day on days 1-4 and cisplatin, 75 mg/m2 on day 1. A second course of 5-FU and cisplatin was given after 36 Gy, when the patient resumed radiation therapy. Between 4/4/96 and 9/23/96 an additional 13 patients (cohort 2) were accrued on study with the same treatment except without the planned treatment break.
Results
Complete response (CR) was seen in 78% (90% CI: 63% to 89%) of patients and was higher in patients who did not get a planned treatment break (92% vs. 68%. The overall grade 4 toxicity rate was 31%. One treatment related death (grade 5) occurred in a patient who developed sepsis. The 5-year overall survival was 69%.
Conclusions
Radiation therapy, cisplatin, and 5-FU resulted in an overall objective response (CR + PR) of 97%. Although the 5-year progression-free survival was only 55%, the overall 5-year survival was 69%. Given the excellent salvage provided by surgery, this study affirms that cisplatin-based regimens may be an alternative for patients who cannot tolerate the severe hematological toxicities associated with mitomycin-based chemoradiation regimens.
Keywords: cisplatinum, CDDP, sphincter preservation, induction chemotherapy, maintenance chemotherapy
Introduction
Anal cancer is a rare malignancy of the anogenital tract that requires multi-disciplinary management by a radiation oncologist, medical oncologist, and surgical oncologist to ensure the best patient outcome. In 2009, the American Cancer Society (ACS) estimated that there were 5,290 new cases of cancer of the anus, anal canal, and anorectum as well as 710 deaths from this disease. (1) According to SEER data collected between 1973 and 2000, the incidence of this malignancy has increased from 10 to 20 patients per million.(2) Risk factors for anal cancer are well known and include human papillomavirus (HPV) infection, multiple sexual partners, immunosuppression, and smoking.
The treatment paradigm for anal cancer changed dramatically in the 1980s when surgical resection consisting of an abdominoperineal resection (APR) with the creation of a permanent colostomy was replaced with concurrent chemoradiation therapy using 5-FU, MMC, and radiation. The use of primary chemoradiation, with surgery reserved for salvage, allowed almost two-thirds of patients to be cured of their disease as well as maintain sphincter preservation. Randomized Phase III trials conducted by the United Kingdom Coordinating Committee on Cancer (UKCCCR) and the European Organization for the Research and Treatment of Cancer (EORTC) showed the superiority of concurrent chemoradiotherapy over radiotherapy alone in terms of local control and colostomy-free survival.(3) (4, 5)
While clinical trials have established concurrent 5-FU, MMC, and RT as the standard of care for anal cancer,(4) it remains an imperfect regimen. The 5-year disease free survival from this therapy is only 63% and MMC has severe, life-threatening, or fatal hematologic and lung toxicities as well as an increased incidence of hemolytic-uremic syndrome (HUS). Unfortunately, previous efforts to remove MMC have proven unsuccessful. In a phase III clinical trial there was a lower colostomy-free survival (59% vs. 71%) and lower disease-free survival (51% vs. 73%) in patients treated with 5-FU and RT as compared to those treated with MMC, 5-FU, RT at four years.(4)
If removing MMC from the treatment regimen yielded inferior clinical results, then the next logical question was whether a safer, more efficacious agent could replace it? Cisplatin has proven efficacy in the treatment of squamous cell carcinoma (SCC) of the head and neck, esophagus and cervix. At the inception of this study there were pilot studies showing promising local control rates of 85% at two years.(6, 7) Moreover, there was also evidence that higher doses of radiation resulted in improved local control. (8) In 1993, the Eastern Cooperative Oncology Group (ECOG) conducted a prospective, Phase II study (ECOG E4292) of higher dose radiation (59.4 Gy), 5-FU, and cisplatin in patients with localized anal cancer. The objective of this study was to assess this regimen with regard to response rates and toxicity before consideration of this regimen as a potential arm for a randomized clinical trial. The initial results of E4292 were reported by Martenson et al. in 1996 and showed a promising overall response rate of 95%.(9) Subsequently, a parallel RTOG study which evaluated the use of high dose, split course radiation with 5-FU and MMC showed higher rates of local failure and therefore 13 additional patients were accrued (cohort 2) without the use of the planned treatment break.
Since the initial report of this study, a Phase III randomized trial (RTOG 98-11) showed that neoadjuvant, cisplatin-based chemoradiotherapy regimen resulted in an increased colostomy rate as compared to a MMC-based regimen (19% vs. 10%, p = .02). There was, however, no significant difference in the 5- year overall survival between these two groups (75%, MMC-based group vs. 70%, cisplatin-based group, p = .10).Severe hematologic toxicity was worse in the mitomycin-based treatment (p < .001) (10) There remains a population of patients for whom mitomycin-C remains too toxic and alternative treatment regimens are needed. We report the final results of a Phase II trial that replaced MMC with cisplatin as well as used radiation dose intensification to a total dose of 59.4 Gy to improve rates of local control and survival in patients with non metastatic anal cancer.
Materials and Methods
Study Population
Between 2/1/93 to 7/21/93, nineteen patients (cohort 1) with biopsy-proven, measurable anal cancer without evidence of distant metastasis were accrued to this study. Tumors had to be squamous, cloacogenic or basoloid in histology. Patients had to have an Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2. Patients had adequate hematologic (absolute neutrophil count ≥ 1.8 × 109/liter; platelet count ≥ 100 × 109/liter) and renal function (serum creatinine within normal limits or creatinine clearance of ≥ 60 ml/min). Patients who were human immunodeficiency virus (HIV)-positive or who had a prior history of chemotherapy, pelvic RT, or invasive cancer were ineligible for this study.
Radiation Therapy
The protocol required that the initial fields for RT include the pelvis (with a superior border at the L5-Sl interspace), the inguinal lymph nodes, and the anus. This initial field received 30.6 Gy at 1.8 Gy per day. Lateral inguinal nodes were treated with the anterior photon field, and supplementary irradiation fields were used to boost the daily dose to lateral inguinal nodes. After 30.6 Gy the superior border of the photon fields was dropped to the level of the inferior sacroiliac joints. An additional 5.4 Gy was given (cumulative dose of 36 Gy), followed by a 2-week treatment break in cohort 1. When treatment was resumed, treatment of lateral inguinal nodes was discontinued unless they were grossly involved. The reduced pelvic field was continued to a total dose of 45 Gy. This was then followed by a boost to the primary tumor and any involved inguinal nodes to an additional dose of 14.4 Gy. A 2.5-cm margin of normal tissue between tumor and the field edge was required for boost fields. The total cumulative dose to primary site and metastatic lymph nodes was 59.4 Gy in 33 fractions and the duration of treatment was 60 days. In the last 13 patients enrolled on this study (cohort 2), the two week treatment break was eliminated.
Chemotherapy
Chemotherapy was started on day 1 of RT and consisted of 5-FU, 1000 mg/m2 /day by continuous intravenous infusion on days 1- 4. Cisplatin, 75 mg/m2 intravenously was given on day 1 of RT. A second course of 5-FU (1000 mg/m2 /day by continuous infusion for 4 days and cisplatin 75 mg/m2 was given when radiation was resumed following the planned 2-week break. Therapy was completed on day 60. In the 13 patients without the planned treatment break (cohort 2), chemotherapy was given weeks 1 and 5.
Tumor Response
Patients were reevaluated 8 weeks after completion of treatment for assessment of response to treatment. Complete disappearance of all clinically detectable disease constituted a complete response. Partial response was defined as a decrease of 50% or more in the product of the longest perpendicular diameter for tumors that were measurable in two dimensions. For lesions that were measurable in one dimension, a decrease of 30% or more in linear tumor measurement was considered a partial response. An additional biopsy after completion of therapy was not required nor routinely performed. The National Cancer Institute Common Toxicity Criteria (2) were used in the assessment of adverse side effects of treatment. (11)
Statistical Analysis
Demographic and clinical characteristics of the patients were summarized with frequencies and percentages or means and standard deviations. Statistical analysis of the time to event data was done using Kaplan-Meier plots of overall and progression-free survival.
Results
Patient Demographics and Clinical Characteristics
From 2/1/93 to 9/23/96 thirty-three patients with non metastatic anal cancer participated in this study between. One patient is excluded from this analysis as he never received any protocol therapy. The remaining 32 patients are analyzed for response, toxicity, and survival. The median progression-free survival for the group was 48 months.
Patient demographics and clinical characteristics are summarized in Table 1. Cohort 1 consisted of 19 patients of whom 7 were male and 12 were female. The mean age for this group was 58 years with a range of 28 to 74 years. Stage distribution of these 19 patients according to American Joint Committee on Cancer (AJCC) staging, 4th edition, was as follows: T1-2 NO, 11 patients; T3-4 N0, 6 patients; T1-2 N1-3, 1 patient; T3-4 N1-3, 1 patient. Cohort 2 consisted of patients who did not have the planned two week treatment break and consisted of 13 patients of whom two were male and 11 were female. The mean age for this group was 53 with a range of 35 to 73. Stage distribution of cohort 2 was as follows: T1-2 NO, 4 patients; T3-4 N0, 5 patients; T1-2 N1-3, 3 patients; T3-4 N1-3, 1 patient.
Table 1. Patient demographic and clinical characteristics.
| Patient Cohort | Total | |||||
|---|---|---|---|---|---|---|
| Cohort I | Cohort II | |||||
| N | Col % | N | Col % | N | Col % | |
| Number of patients | 19 | 100 | 13 | 100.0 | 32 | 100.0 |
| Gender | ||||||
| Male | 7 | 36.8 | 2 | 15.4 | 9 | 28.1 |
| Female | 12 | 63.2 | 11 | 84.6 | 23 | 71.9 |
| Total | 19 | 100 | 13 | 100.0 | 32 | 100.0 |
| Age | ||||||
| Under 55 | 9 | 47.4 | 6 | 46.2 | 15 | 46.9 |
| 55-69 | 6 | 31.6 | 6 | 46.2 | 12 | 37.5 |
| 70+ | 4 | 21.1 | 1 | 7.7 | 5 | 15.6 |
| Total | 19 | 100.0 | 13 | 100.0 | 32 | 100.0 |
| Race | ||||||
| White | 16 | 84.2 | 11 | 84.6 | 27 | 84.4 |
| Black | 3 | 15.8 | 1 | 7.7 | 4 | 12.5 |
| Other | 0 | 0 | 1 | 7.7 | 1 | 3.1 |
| Total | 19 | 100.0 | 13 | 100.0 | 32 | 100.0 |
| PS | ||||||
| 0 | 13 | 68.4 | 8 | 61.5 | 21 | 65.6 |
| 1 | 6 | 31.6 | 5 | 38.5 | 11 | 34.4 |
| Total | 19 | 100.0 | 13 | 100.0 | 32 | 100.0 |
| Stage | ||||||
| T1-2 N0 | 11 | 57.9 | 4 | 30.8 | 15 | 46.9 |
| T3-4 N0 | 6 | 31.6 | 5 | 38.5 | 11 | 34.4 |
| T1-2 N1-3 | 1 | 5.3 | 3 | 23.1 | 4 | 12.5 |
| T3-4 N1-3 | 1 | 5.3 | 1 | 7.7 | 2 | 6.3 |
| Total | 19 | 100.0 | 13 | 100.0 | 32 | 100.0 |
| Prior Surgery | ||||||
| No | 12 | 63.2 | 11 | 84.6 | 23 | 71.9 |
| Yes | 7 | 36.8 | 2 | 15.4 | 9 | 28.1 |
| Total | 19 | 100.0 | 13 | 100.0 | 32 | 100.0 |
| Histology | ||||||
| Squamous | 11 | 57.9 | 10 | 76.9 | 21 | 65.6 |
| Basaloid | 6 | 31.6 | 2 | 15.4 | 8 | 25 |
| Other | 2 | 10.5 | 1 | 7.7 | 3 | 9.4 |
| Total | 19 | 100.0 | 13 | 100.0 | 32 | 100.0 |
| Differentiation | ||||||
| Well | 3 | 33.3 | 0 | 0 | 3 | 15 |
| Moderate | 3 | 33.3 | 5 | 45.5 | 8 | 40 |
| Poor | 3 | 33.3 | 6 | 54.5 | 9 | 45 |
| Total | 9 | 100.0 | 11 | 100.0 | 20 | 100.0 |
| Location of Primary | ||||||
| Anal Canal (AC) Only | 8 | 42.1 | 4 | 30.8 | 12 | 37.5 |
| AC Extending to Perianal Skin | 3 | 15.8 | 2 | 15.4 | 5 | 15.6 |
| AC Extending to Rectum | 4 | 21.1 | 7 | 53.8 | 11 | 34.4 |
| AC Extending to Perianal Skin & Rectum | 2 | 10.5 | 0 | 0 | 2 | 6.3 |
| Perianal Skin Only | 2 | 10.5 | 0 | 0 | 2 | 6.3 |
| Total | 19 | 100.0 | 13 | 100.0 | 32 | 100.0 |
| Vaginal Wall involvement | ||||||
| No | 18 | 94.7 | 11 | 84.6 | 29 | 90.6 |
| Yes | 1 | 5.3 | 2 | 15.4 | 3 | 9.4 |
| Total | 19 | 100.0 | 13 | 100.0 | 32 | 100.0 |
| Prostate involvement | ||||||
| No | 19 | 100.0 | 12 | 92.3 | 31 | 96.9 |
| Yes | 0 | 0 | 1 | 7.7 | 1 | 3.1 |
| Total | 19 | 100.0 | 13 | 100.0 | 32 | 100.0 |
| Inguinal LN involvement | ||||||
| No | 16 | 88.9 | 8 | 72.7 | 24 | 82.8 |
| Yes | 2 | 11.1 | 3 | 27.3 | 5 | 17.2 |
| Total | 18 | 100.0 | 11 | 100.0 | 29 | 100.0 |
Radiation Treatment
Table 2 summarizes the details of radiation treatment. In cohort 1 (planned treatment break), 89% of patients completed the planned radiation, as compared to 92% of patients in cohort 2 (no planned break). In both groups, the reason RT was not completed as planned was due to toxicity. Additionally, more patients in cohort 2 (no planned break) experienced a moist exudative skin reaction as compared to cohort I (69% vs. 41%).
Table 2. Details of radiation treatment.
| Patient Cohort | Total | |||||
|---|---|---|---|---|---|---|
| Cohort I | Cohort II | |||||
| N | Col % | N | Col % | N | Col % | |
| Split Course Used | ||||||
|
|
||||||
| No | 4 | 25.0 | 10 | 83.3 | 14 | 50.0 |
|
| ||||||
| Yes | 12 | 75.0 | 2 | 16.7 | 14 | 50.0 |
|
| ||||||
| Total | 16 | 100.0 | 12 | 100.0 | 28 | 100.0 |
|
| ||||||
| Bone Marrow Depression | ||||||
|
| ||||||
| No | 6 | 37.5 | 6 | 54.5 | 12 | 44.4 |
|
| ||||||
| Yes | 10 | 62.5 | 5 | 45.5 | 15 | 55.6 |
|
| ||||||
| Total | 16 | 100.0 | 11 | 100.0 | 27 | 100.0 |
|
| ||||||
| Unplanned RT Interruptions | ||||||
|
| ||||||
| No | 9 | 52.9 | 4 | 30.8 | 13 | 43.3 |
|
| ||||||
| Yes | 8 | 47.1 | 9 | 69.2 | 17 | 56.7 |
|
| ||||||
| Total | 17 | 100.0 | 13 | 100.0 | 30 | 100.0 |
|
| ||||||
| Planned RT dose/fraction adhered to | ||||||
|
| ||||||
| No | 2 | 11.1 | 1 | 7.7 | 3 | 9.7 |
|
| ||||||
| Yes | 16 | 88.9 | 12 | 92.3 | 28 | 90.3 |
|
| ||||||
| Total | 18 | 100.0 | 13 | 100.0 | 31 | 100.0 |
|
| ||||||
| Total planned RT dose delivered | ||||||
|
| ||||||
| No | 2 | 11.1 | 1 | 7.7 | 3 | 9.7 |
|
| ||||||
| Yes | 16 | 88.9 | 12 | 92.3 | 28 | 90.3 |
|
| ||||||
| Total | 18 | 100.0 | 13 | 100.0 | 31 | 100.0 |
|
| ||||||
| Reason RT not completed as planned | ||||||
|
| ||||||
| Excessive reactions | 1 | 100.0 | 1 | 100.0 | 2 | 100.0 |
| Total | 1 | 100.0 | 1 | 100.0 | 2 | 100.0 |
| RT Acute Skin Reaction | ||||||
| None | 2 | 11.8 | 0 | 0 | 2 | 6.7 |
| Erythema & Desquamation | 2 | 11.8 | 3 | 23.1 | 5 | 16.7 |
| Dry Desquamation Reaction | 3 | 17.6 | 1 | 7.7 | 4 | 13.3 |
| Desquamation & Blistering | 3 | 17.6 | 0 | 0 | 3 | 10.0 |
| Moist Exudative Reaction | 7 | 41.2 | 9 | 69.2 | 16 | 53.3 |
| Total | 17 | 100.0 | 13 | 100.0 | 30 | 100.0 |
Response Rates
The overall objective response rates (complete response + partial response) were 97% (n=32), 95% (cohort 1, n=19) and 100% (cohort 2, n=13). Complete response (CR) to therapy was seen in 78% (90% CI: 63% to 89%) of all patients. The complete response rates were higher in patients who did not get a planned treatment break (cohort 2) versus those who did get a break (92% vs. 68%, respectively). (Table 3)
Table 3. Objective response.
| Patient Cohort | Total | |||||
|---|---|---|---|---|---|---|
| Cohort I | Cohort II | |||||
| N | Col % | N | Col % | N | Col % | |
| Best Overall Objective Response | ||||||
|
|
||||||
| CR | 13 | 68.4 | 12 | 92.3 | 25 | 78.1 |
|
| ||||||
| PR | 5 | 26.3 | 1 | 7.7 | 6 | 18.8 |
|
| ||||||
| NC | 1 | 5.3 | 0 | 0 | 1 | 3.1 |
|
| ||||||
| Total | 19 | 100.0 | 13 | 100.0 | 32 | 100.0 |
Toxicities
Toxicity data is summarized in Table 4. The overall grade 4 toxicity rate was 31% (10 of 32 patients). Grade 4 toxicity consisted of granulocytopenia, thrombocytopenia, diarrhea, and skin reaction. There was one treatment related death which occurred in a patient who developed sepsis. Only 4 of 13 patients in the second cohort suffered grade 4 toxicities consisting of infection, diarrhea, hypotension and skin reaction. There were no grade 5 toxicities in cohort 2. The protocol specified that if 4 or fewer grade 4-5 toxicities were seen in 10 patients in cohort 2, it would be concluded that removal of the two week treatment break did not result in an increase in toxicity. Only 4 of 13 patients in cohort 2 experienced grade 4 toxicity, thereby meeting the pre-specified protocol criterion of no added toxicity with elimination of the planned treatment break. There were no late grade 3 or 4 toxicities.
Table 4. Toxicity.
| Treatment Arm | Treatment Arm | Treatment Arm | ||||||
|---|---|---|---|---|---|---|---|---|
| A (n=32) | A (n=19) | A (n=13) | ||||||
| Grade | Grade | Grade | ||||||
| 3 | 4 | 5 | 3 | 4 | 5 | 3 | 4 | |
| Toxicity Type | (n) | (n) | (n) | (n) | (n) | (n) | (n) | (n) |
| Leukopenia | 17 | - | - | 9 | - | - | 8 | - |
| Granulocytopenia | 13 | 4 | - | 6 | 4 | - | 7 | - |
| Thrombocytopenia | - | 1 | - | - | 1 | - | - | - |
| Anemia | 1 | - | - | 1 | - | - | - | - |
| Infection | 2 | 1 | 1 | 1 | - | 1 | 1 | 1 |
| GU | 1 | - | - | 1 | - | - | - | - |
| Nausea/vomiting | 4 | - | - | 2 | - | - | 2 | - |
| Vomiting | 2 | - | - | 1 | - | - | 1 | - |
| Diarrhea | 7 | 3 | - | 3 | 1 | - | 4 | 2 |
| Stomatitis | 2 | - | - | 1 | - | - | 1 | - |
| Liver | 1 | - | - | 1 | - | - | - | - |
| Hypotension | - | 1 | - | - | - | - | - | 1 |
| Skin | 13 | 2 | - | 3 | 1 | - | 10 | 1 |
| Neuro-clinical | 1 | - | - | 1 | - | - | - | - |
| Anorexia | 1 | - | - | - | - | - | 1 | - |
| Dehydration | - | 1 | - | - | - | - | - | 1 |
| WORST DEGREE | 16 | 10 | 1 | 8 | 6 | 1 | 8 | 4 |
Overall Survival and Progression-Free Survival
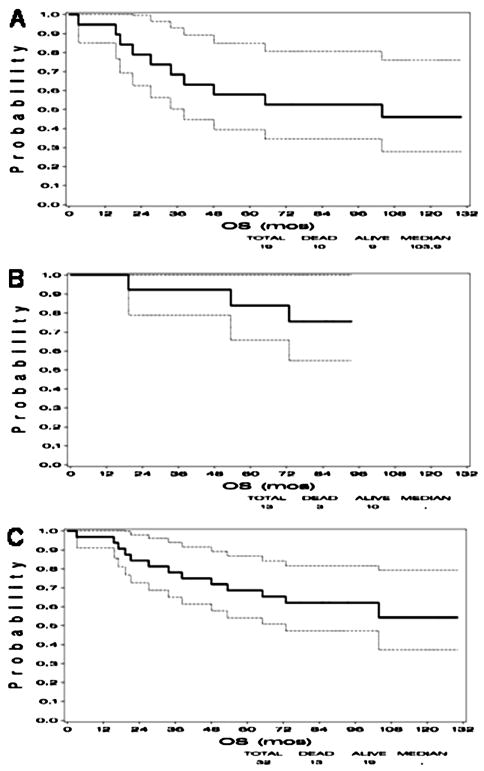
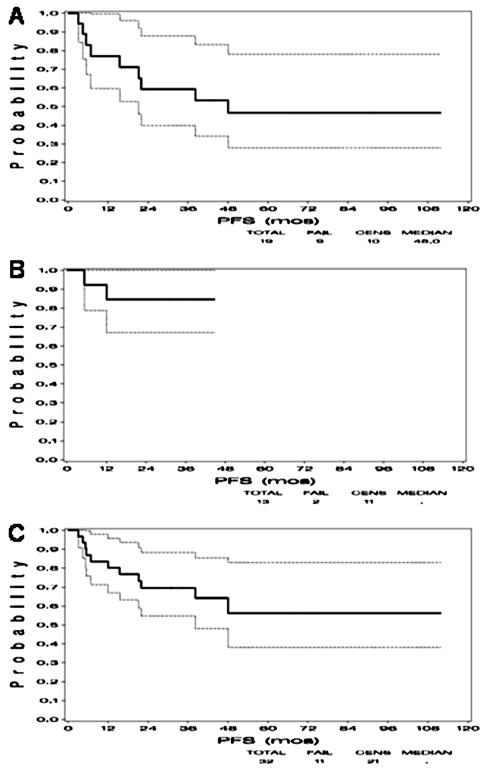
Kaplan-Meier plots of overall survival by cohorts and combined are shown in Figure 1. The 5-year overall survival for all 32 patients was 69% (cohort 1: 58% and cohort 2: 84%). Progression-free survival for both cohorts is shown in Figure 2. The progression free survival for the cohort 1 was 53% and 85% in cohort 2.
Figure 1.
Kaplan-Meier curves demonstrating overall survival in A) Cohort 1 consisting of 19 patients who received a 2-week planned treatment break, B) Cohort 2 consisting of 13 patients without a planned break, and C) both cohorts combined.
Figure 2.
Kaplan-Meier curves demonstrating progression-free survival (PFS) in A) Cohort 1 consisting of 19 patients who received a 2-week planned treatment break, B) Cohort 2 consisting of 13 patients who did not receive a planned treatment break, and C) both cohorts combined.
Discussion
Although it has been clearly demonstrated that abdominoperineal resection leading to permanent colostomy is no longer required for the curative treatment of anal cancer, the best agents as well as radiation doses remains unclear. The optimal chemotherapy to utilize with radiation to maximize rates of local control and sphincter function remains an area of active investigation. Since the initial publication of E4292, the Radiation Therapy Oncology Group completed a randomized Phase III study (RTOG 98-11) that showed MMC, 5-FU, and RT results in better disease -free survival and colostomy-free survival in patients with anal cancer than a regimen of induction cisplatin, 5-FU, and RT. There was no significant difference in 5-year overall survival between the mitomycin-based group and the cisplatin-based group (75% vs. 70%, p =.10). The primary end point was 5-year disease free survival with secondary endpoints being overall survival and time to relapse. Although the authors of the study concluded that cisplatin should not replace MMC in combined modality treatment of anal cancer, another interpretation of these findings is that cisplatin-based induction chemotherapy followed by concurrent chemoradiation is not as effective as primary chemoradiation. In the RTOG study, the rate of acute non-hematologic grade 3 or 4 toxicity was the same (74%) in both the mitomycin- and cisplatin-based groups. A post hoc test, however, was statistically significant for differences in severe acute hematologic toxicity (grade 3 or 4, 61% in the mitomycin-based group and 42% in the cisplatin-based group; P < .001).
In ECOG E4292, cisplatin-based chemoradiotherapy resulted in a relatively low rate of severe hematologic toxicity (16%, 5 of 32). Furthermore, radiation therapy, cisplatin, and 5-FU resulted in an overall objective response (complete response + partial response) of 97%. Although the 5-year progression free survival was only 55%, the overall 5-year survival remains 69%, which compares favorably to standard MMC-based chemoradiation therapy. The recently completed Cancer Research United Kingdom Anal Cancer Trial (ACT II) is a phase III trial that randomized patients using a 2 × 2 factorial design. It attempts to answer the question of whether cisplatin can replace MMC in the primary treatment of anal cancer. The second randomization asks whether maintenance cisplatin/5FU will improve on these results. Patients are first randomized to either MMC, 5-FU, and RT or cisplatin, 5-FU, and RT. Regardless of the type of chemotherapy the patients received with radiation, both groups were further randomized to receive either two cycles of cisplatin/5-FU maintenance therapy or no additional therapy. Complete response rate at six months was 95% in the cisplatin arm as well as the MMC arm. Recurrence-free survival during the maintenance phase was an identical 75% at three years in patients who received maintenance and those who did not receive maintenance chemotherapy. In contrast to the results of RTOG 98-11, the colostomy rate was actually lower in the cisplatin arm (11% with cisplatin, 14% with MMC). In the ACT II trial, as in RTOG 98-11, grade 3-4 acute hematological toxicity occurred significantly more often in the mitomycin arm (25% versus 13%, P< 0.001), whereas the frequency of non-hematologic toxicity did not differ between the two arms (60% versus 65%). Interestingly, the authors of the ACT II trial conclude that MMC-based therapy should remain the standard-of-care, despite the fact that both regimens had similar recurrence-free survival and overall survival.(12)
In both studies (RTOG 98-11 and ACT II) the authors have concluded that MMC-based combination therapy remains the standard of care. We believe that this conclusion may be misleading. In RTOG 98-11, cisplatin/FU was given as induction therapy followed by concurrent cisplatin/FU/RT. Therefore, an alternative conclusion could be that neoadjuvant cisplatin based chemotherapy is not indicated in anal cancer. It does not address the question of the role of primary concurrent chemoradiation using cisplatin based chemotherapy.
The recently completed Cancer Research United Kingdom Anal Cancer Trial (ACT II) is one of the largest trials of anal cancer. It used a 2 × 2 factorial design to compare concurrent chemoradiation using 5-FU/cisplatin with 5-FU/MMC with a second randomization to maintenance 5-FU/cisplatin. Preliminary analysis shows no difference in complete response rates between 5-FU/MMC and 5-FU/cisplatin (94% vs. 95%; p = 0.53).There was also no difference in recurrence-free survival (hazard ratio 0.89, 95% confidence interval 0.68-1.18; p = 0.42) and overall survival (hazard ratio 0.79, 95% confidence interval 0.56-1.12; p = 0.19) with or without maintenance therapy. There was significantly higher hematologic toxicities with the use of MMC (25% vs. 13%; p < 0.001). (12) Unfortunately, the second randomization of two additional courses of cisplatin/5FU maintenance chemotherapy after completion of primary chemoradiation makes answering the question of primary chemoradiation using cisplatin/FU vs. MMC/FU in the primary treatment of anal cancer more difficult. Although we would agree with the authors conclusion that 5-FU/MMC without maintenance remains the standard of care for the management of anal cancer, given the more favorable side effect profile, cisplatin based chemoradiation regimens should still be considered as an alternative chemotherapy backbone on which to add biologics in clinical trials that seek to improve the local control results of patients with anal cancer.
Squamous cell carcinomas from other sites express the epidermal growth factor receptor (EGFR) and its overexpression is a negative prognostic factor. EGFR is overexpressed in the majority of anal cancers(13) and K-ras mutations are rare. Thus, treatment with EGFR inhibitors such as cetuximab should be potentially effective. Early results in patients with metastatic anal cancer have shown promising response rates with cetuximab monotherapy. (14) A phase I study of cetuximab in combination with cisplatin and radiation for locally advanced anal cancer has shown that this combination is tolerable. Seven of nine (78%) patients achieved a complete response. (15) An ongoing phase II ECOG study is evaluating the efficacy of cetuximab plus cisplatin, 5-fluorouracil and radiation therapy (E3205).(16) Given the results of large randomized studies that have shown no benefit to the addition of either induction cisplatin-based chemotherapy (10) or maintenance cisplatin-based chemotherapy (12), E3205 employs cetuximab, 5-FU and radiation without the use of either induction or maintenance chemotherapy. A similar study is accruing HIV positive patients to concurrent cetuximab with cisplatin and 5-fluorouracil through the AIDS Associated Malignancies Clinical Trials Consortium (NCT00324415). (17)Cetuximab has been shown to enhance the effectiveness of cisplatin and radiation therapy and our hope is that this combination of therapies will have a synergistic effect in the treatment of anal cancer to result in improved local control with less toxicity. In addition to testing novel biologic agents, ongoing clinical trials in anal cancer are also incorporating newer radiation techniques such as intensity modulated radiation (IMRT) to help decrease acute toxicity while improving local control.
Acknowledgments
This study was conducted by The Eastern Cooperative Oncology Group and supported in part by Public Health Service grants CA13650, CA23318, CA15488, CA14548, CA17145 and CA21115 from the National Cancer Institute, National Institutes of Health, and the Department of Health and Human Services. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Conflict of Interest Notification: The authors deny any actual or potential conflict of interest pertaining to this research.
References
- 1.ACS. American Cancer Society: Cancer Facts and Figures 2009. 2009;2009 [Google Scholar]
- 2.Johnson LG, Madeleine MM, Newcomer LM, et al. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973-2000. Cancer. 2004;101:281–288. doi: 10.1002/cncr.20364. [DOI] [PubMed] [Google Scholar]
- 3.Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet. 1996;348:1049–1054. [PubMed] [Google Scholar]
- 4.Flam M, John M, Pajak TF, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol. 1996;14:2527–2539. doi: 10.1200/JCO.1996.14.9.2527. [DOI] [PubMed] [Google Scholar]
- 5.Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997;15:2040–2049. doi: 10.1200/JCO.1997.15.5.2040. [DOI] [PubMed] [Google Scholar]
- 6.Rich TA, Ajani JA, Morrison WH, et al. Chemoradiation therapy for anal cancer: radiation plus continuous infusion of 5-fluorouracil with or without cisplatin. Radiother Oncol. 1993;27:209–215. doi: 10.1016/0167-8140(93)90076-k. [DOI] [PubMed] [Google Scholar]
- 7.Gerard JP, Ayzac L, Hun D, et al. Treatment of anal canal carcinoma with high dose radiation therapy and concomitant fluorouracil-cisplatinum. Long-term results in 95 patients. Radiother Oncol. 1998;46:249–256. doi: 10.1016/s0167-8140(97)00192-8. [DOI] [PubMed] [Google Scholar]
- 8.Hughes LL, Rich TA, Delclos L, et al. Radiotherapy for anal cancer: experience from 1979-1987. Int J Radiat Oncol Biol Phys. 1989;17:1153–1160. doi: 10.1016/0360-3016(89)90520-8. [DOI] [PubMed] [Google Scholar]
- 9.Martenson JA, Lipsitz SR, Wagner H, Jr, et al. Initial results of a phase II trial of high dose radiation therapy, 5-fluorouracil, and cisplatin for patients with anal cancer (E4292): an Eastern Cooperative Oncology Group study. Int J Radiat Oncol Biol Phys. 1996;35:745–749. doi: 10.1016/0360-3016(96)00146-0. [DOI] [PubMed] [Google Scholar]
- 10.Ajani JA, Winter KA, Gunderson LL, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA. 2008;299:1914–1921. doi: 10.1001/jama.299.16.1914. [DOI] [PubMed] [Google Scholar]
- 11.NCI/CTC Common Toxicity Criteria
- 12.James R, Wan S, Glynne-Jones R, et al. A randomized trial of chemoradiation using mitomycin or cisplatin, with or without maintenance cisplatin/5FU in squamous cell carcinoma of the anus (ACT II) ASCO Meeting Abstracts. 2009;27:LBA 4009. [Google Scholar]
- 13.Le LH, Chetty R, Moore MJ. Epidermal growth factor receptor expression in anal canal carcinoma. American Journal of Clinical Pathology. 2005;124:20–23. doi: 10.1309/X4UADHVN317V2XMW. [DOI] [PubMed] [Google Scholar]
- 14.Lukan N, Strobel P, Willer A, et al. Cetuximab-Based Treatment of Metastatic Anal Cancer: Correlation of Response with KRAS Mutational Status. Oncology. 2009;77:293–299. doi: 10.1159/000259615. [DOI] [PubMed] [Google Scholar]
- 15.Olivatto LO, Meton F, Bezerra A, et al. Phase I study of cetuximab (CET) in combination with 5-fluorouracil (5-FU), cisplatin (CP), and radiotherapy (RT) in patients with locally advanced squamous cell anal carcinoma (LAAC) J Clin Oncol. 2008;26 [Google Scholar]
- 16.E3205 E. Phase II Trial of Cetuximab Plus Cisplatin, 5-Fluorouracil and Radiation in Immunocompetent Patients with Anal Carcinoma. 2010. [Google Scholar]
- 17.AMC45. Phase II Study of Cisplatin, Fluorouracil, Cetuximab, and Radiotherapy in Patients With HIV-Associated Stage I-IIIB Anal Carcinoma. 2010. [Google Scholar]