Abstract
Frailty is associated with a pro-inflammatory state, which has been characterized by elevated levels of systemic inflammatory biomarkers, but has not been related to the number of co-existing chronic diseases associated with inflammation. We sought to determine the extent to which a higher number of inflammatory-related diseases is associated with frailty and to identify the most common disease patterns associated with being frail in older adults. We performed binomial regression analyses to assess whether a higher count of inflammatory-related diseases increases the probability of frailty using data from the Women's Health and Aging Studies I and II, companion cohorts composed of 70–79-year-old community-dwelling older women in Baltimore, Maryland (n=620). An increase of one inflammatory-related disease was associated log-linearly with frailty (Prevalence Ratio (PR)=2.32, 95% Confidence Interval (CI)=1.85–2.92). After adjusting for age, race, education, and smoking status, the probability of frailty remained significant (PR=1.97, 95%CI=1.52–2.55). In the frail population, chronic kidney disease (CKD) and depressive symptoms (Prevalence=22.9%, 95%CI=14.2–34.8%); CVD and depressive symptoms (21.7%, 95%CI=13.2–33.5%); CKD and anemia (18.7%, 95%CI=11.1–29.7%); cardiovascular disease (CVD), CKD, and pulmonary disease (10.7%, 95%CI=5.2–21.0%); CKD, anemia, and depressive symptoms (8.7%, 95%CI=3.9–18.2%); and CVD, anemia, pulmonary disease, and depressive symptoms (5.0%, 95%CI=1.6–14.4%) were among the most frequent disease combinations. Their prevalence percentages were significantly higher in the frail versus non-frail women. A higher inflammatory-related disease count, perhaps reflecting a greater pro-inflammatory burden, increases the likelihood of frailty. Shared mechanisms among specific disease combinations may further contribute to this risk.
Keywords: comorbidity, inflammation, frailty
1. Introduction
Frailty is an important syndrome of advancing age that is recognized as a risk factor for falls, disability, hospitalization, and death in older adults (Fried L.P. et al., 2001, 2004). It is hypothesized to result from a complex interplay of biochemical and multi-systemic changes that may result in decreased physiologic reserves in older adults (Fried et al., 2005). Previous observational studies have found an association between frailty and elevated levels of pro-inflammatory mediators, such as interleukin-6 (IL-6) and C-reactive protein (CRP), (Leng et al., 2007; Walston et al., 2002) implicating a chronic, pro-inflammatory state in the pathogenesis of frailty as well as many age-related chronic diseases that are also independently associated with frailty. These diseases include chronic kidney disease (CKD), cardiovascular disease (CVD), anemia, and diabetes mellitus (DM) (Barzilay et al., 2007; Chaves et al., 2005; Fried et al., 2001; Newman et al., 2001; Shlipak et al., 2004; Wilhelm-Leen et al., 2009).
An association between frailty and a pro-inflammatory state has been described in terms of elevated levels of systemic inflammatory markers, but has not been related to the number of chronic diseases with an inflammatory etiology, which are present in older adults. In this cross-sectional study, we sought to determine whether a higher count of inflammatory-related diseases is associated with a greater likelihood of being frail and to identify the specific disease patterns, based upon the total number of prevalent inflammatory-related diseases, which are most frequently associated with being frail in older adults. Results from this study would provide the basis for investigating shared disease mechanisms which may illuminate potential targets of treatment to prevent or delay frailty.
2. Materials and methods
2. 1. Study population
Data from the Women’s Health and Aging Studies I and II (WHAS), complementary longitudinal cohort studies of community-dwelling older women in Baltimore, MD were merged for this cross-sectional study. WHAS I, which consisted of 1,002 women aged ≥65, represented the one-third most disabled women in Baltimore; WHAS II, composed of 436 women aged 70–79, was recruited from among the two-thirds least disabled in the community.
The two study cohorts were sampled using the Health Care Financing Administration’s Medicare eligibility lists for 12 zip codes that spanned contiguous areas in eastern Baltimore City and Baltimore County. More extensive details about the sampling methods and study eligibility criteria have been previously published (National Institute on Aging, 1995). Seventy-one percent of women in WHAS I and 49.5% of women in WHAS II who fulfilled study eligibility criteria consented to participate. At baseline, they received standardized interviews, physical examinations, and blood testing. Baseline assessment occurred from November 1992 to February 1995 in WHAS I and from August 1994 to February 1996 in WHAS II.
We chose to study the combined baseline data from subjects in WHAS I and WHAS II, who represented the full spectrum of physical function in community-dwelling older women. Only participants aged 70–79 years old with a Mini-Mental State Exam (MMSE) score ≥24 were included. Those with missing data for frailty status (n=6), a Geriatric Depression Scale (GDS) score (n=4), and laboratory data (n=99) were excluded, resulting in a final analytic sample of 620. Written informed consent was obtained from all subjects for their study participation. The Johns Hopkins Medical Institutions Institutional Review Board approved the research protocols.
2.2. Laboratory data collection
Baseline non-fasting blood specimens were collected by certified phlebotomists, either at participants’ homes in WHAS I or at the Johns Hopkins General Clinical Research Center in WHAS II. Laboratory results of blood samples drawn within 90 days of the first round of testing were incorporated into the study analyses. Assessment of IL-6 was performed using the High-Sensitivity Quantikine Kit, R & D Systems, Minneapolis, MN, a commercial enzyme-linked immunosorbent assay. Other laboratory data were analyzed at Quest Diagnostics Laboratories in Teterboro, New Jersey.
2.3. Frailty as the outcome
Study participants were considered frail if they had ≥3 of the following validated measurable criteria: (1) unintentional weight loss of ≥10% since age 60 or a body mass index (BMI) <18.5; (2) slowness in gait, according to height; (3) weakness in dominant-hand grip strength, according to BMI; (4) extreme exhaustion in the past month, as determined by self-reported low energy level, unusual fatigue, or unusual weakness; and (5) self-reported decreased energy expenditure based upon a modified Minnesota Leisure-time Physical Activity Scale (Bandeen-Roche et al., 2006; Fried et al., 2001).
2.4. Inflammatory-related chronic diseases and conditions
Chronic diseases and conditions were adjudicated and validated by decision-tree algorithms, involving systematic physician review of the study participants’ medical history, as discussed in complete detail elsewhere (National Institute on Aging, 1995). Definite angina pectoris, myocardial infarction, and congestive heart failure were categorized as CVD if ≥1 of these conditions existed (Chaves et al., 2005). Anemia was defined by a hemoglobin concentration of <12 g/dL, according to the World Health Organization (World Health Organization, 1968). CKD was defined by a creatinine clearance of <60 ml/min, which was determined using the Modification of Diet in Renal Disease formula (Levey et al., 1999) and as previously described in WHAS (Matteini et al., 2008). Prevalent depressive symptoms were defined by a GDS score of >9 out of 30 (Yesavage et al., 1982). In the primary care setting, the GDS score has been validated as a reliable instrument for measuring depressive symptomatology in older adult patients (Burke et al., 1992; Norris et al., 1987), demonstrating a high internal consistency (Cronbach's alpha = 0.86), comparing normal to depressed study participants (Penninx et al., 1998).
We identified eight inflammatory-related chronic diseases using previously published methods in WHAS (Chang et al., 2010). CKD, pulmonary disease, CVD, depressive symptoms, anemia, DM, peripheral artery disease (PAD), and rheumatoid arthritis (RA) fulfilled criteria as inflammatory-related diseases based upon whether they were associated with IL-6 and/or CRP, and whether sufficient literature was available to support an inflammatory etiology (Barzilay et al., 2007; Bremmer et al., 2008; Cesari et al., 2003; Choy et al., 2001; Ferrucci et al., 2005; Fried L.F. et al., 2004; Karadag et al., 2008; Kritchevsky et al., 2005; McDermott et al., 2003; Penninx et al., 2003; Tonelli et al., 2005). Several diseases could not be considered for inclusion in our study because of unavailable data in WHAS (e.g., dementia and inflammatory bowel disease) or because they did not meet both criteria of chronic inflammatory conditions (i.e., stroke and cancer). We created a variable based upon the summation of the eight inflammatory-related diseases that were identified and chose to categorize this variable because of its skewed distribution. Based upon a box plot graph of the distribution demonstrating that total counts of ≥4 diseases were outlying values, the variable was classified into four levels, according to a total count of 0, 1, 2, or ≥3 diseases.
2.5. Statistical analyses
Exploratory data analyses, consisting of summary statistics and two-way tabulations, were conducted to organize the pooled WHAS baseline data. Differences between categorical variables were evaluated by Pearson’s chi-square tests. Lowess smoothing plots were also performed to evaluate for linear trends. Multi-collinearity among the chronic diseases, IL-6, and CRP levels were assessed by examining correlation matrices and variance inflation factors. IL-6 and CRP levels were categorized into tertiles due to their skewed distributions.
Binomial regression models were used to estimate the associations between the sum count of inflammatory diseases and other covariates with respect to frailty status (Barros and Hirakata, 2003; Deddens and Petersen, 2008). By estimating prevalence ratios instead of odds ratios, these generalized linear models more closely approximate the probability of an outcome with a known prevalence of >10%, which in this study was frailty (McNutt et al., 2003). Predicted probabilities of frailty were calculated from the prevalence ratios modeled by the binomial regression analyses.
In order to identify the ten most common disease combinations associated with frailty, we performed pattern analyses, which ranked the weighted frequencies of mutually exclusive disease patterns, based upon the total number of inflammatory diseases (1, 2, or ≥3) present in the frail participants. We then determined the observed prevalence and 95% confidence interval of each disease combination by frailty status.
We adjusted for age-stratified sampling and study refusals by incorporating probability weights, referenced to WHAS community-dwelling older women, into the statistical analyses. Potential confounders were tested with bivariate analyses, first examining their associations with each of the chronic diseases and conditions and then subsequently with frailty status. Fully-adjusted models included age, education, race, and smoking. Statistical significance was set at an alpha level of ≤0.05. Stata 9.2 software (Stata Corp, College Station, TX) was used to conduct all of the analyses.
3. Results
3.1. Characteristics of study population
Table 1 presents the major baseline characteristics of the study population; 11.3% (n=67) were frail, whereas 88.7% (n=553) were non-frail. A higher percentage of the frail, older women were black, received less education, had lower cognitive function, and had more inflammatory-related diseases than compared to the non-frail, older women (all p-values <0.05). Of the frail population, 42.8% (n=28) had ≥3 inflammatory-related diseases, compared to 15.3% (n=81) in the non-frail population. Age (p-value=0.06) and smoking status (p-value=0.06) did not differ significantly among the participants according to frailty status. Of the eight inflammatory diseases, depressive symptoms, CVD, anemia, PAD, and RA were remarkably more prevalent in the frail, compared to the non-frail, population (all p-values ≤0.01), except for CKD (54.3% vs. 42.5% in the non-frail, p-value=0.07), pulmonary disease (39.9% vs. 28.7% in the non-frail, p-value=0.07), and DM (17.0% vs. 10.9% in the non-frail, p-value=0.14).
Table 1.
Baseline Characteristics of the Women's Health and Aging Studies (WHAS) I & II Study Participants Aged 70–79
| Frail | Non-frail | Total | p-valuea | |
|---|---|---|---|---|
| (n=67) | (n=553) | (n=620)b | ||
| Age (years), % (n) | 0.06 | |||
| 70–74 | 42.9 (31) | 55.1 (328) | 53.7 (359) | |
| 75–79 | 57.1 (36) | 44.9 (225) | 46.3 (261) | |
| Race, % (n) | 0.04 | |||
| Black | 29.8 (20) | 18.9 (109) | 20.1 (129) | |
| White, not Hispanic | 70.3 (47) | 81.1 (444) | 79.8 (491) | |
| Education, % (n) | <0.001 | |||
| 0–8th grade | 40.8 (27) | 20.3 (107) | 22.6 (134) | |
| 9–11th grade | 28.1 (19) | 18.2 (99) | 19.3 (118) | |
| 12th grade or GEDc equivalent | 16.2 (11) | 27.4 (155) | 26.1 (166) | |
| Higher education beyond 12th grade or GED | 15.0 (10) | 34.1 (192) | 32.0 (202) | |
| Smoking, % (n) | 0.06 | |||
| Current | 18.4 (13) | 10.7 (59) | 11.6 (72) | |
| Former | 43.5 (29) | 37.2 (210) | 37.9 (239) | |
| Never | 38.1 (25) | 52.1(284) | 50.5 (309) | |
| MMSEd score (24–30), mean ± SDe | 27.71 ± 1.9 | 28.50 ± 1.8 | 28.40 ± 1.8 | <0.01 |
| Single inflammatory-related diseases, % (n) | ||||
| Chronic kidney disease | 54.3 (36) | 42.5 (228) | 43.8 (264) | 0.07 |
| Pulmonary disease | 39.9 (26) | 28.7 (155) | 30.0 (181) | 0.07 |
| Cardiovascular disease | 42.5 (28) | 22.4 (119) | 24.7 (147) | <0.001 |
| Depressive symptoms | 46.3 (31) | 13.3 (74) | 17.0 (105) | <0.001 |
| Anemia | 28.5 (19) | 11.3 (60) | 13.3 (79) | <0.001 |
| Diabetes mellitus | 17.0 (12) | 10.9 (61) | 11.6 (73) | 0.14 |
| Peripheral artery disease | 19.8 (13) | 9.5 (48) | 10.7 (61) | 0.01 |
| Rheumatoid arthritis | 6.6 (4) | 1.2 (7) | 1.8 (11) | <0.01 |
| Total inflammatory-related disease count, % (n) | <0.001 | |||
| 0 | 2.8 (2) | 24.1 (141) | 21.7 (143) | |
| 1 | 13.8 (10) | 35.2 (192) | 32.7 (202) | |
| 2 | 40.7 (27) | 25.5 (139) | 27.2 (166) | |
| ≥3 | 42.8 (2) | 15.3 (81) | 18.4 (109 |
Based upon chi-squared test for categorical variables
The sample size (n) reflects the unweighted number of participants.
General Educational Development
Mini Mental State Examination
Standard deviation
3.2. Association between total inflammatory-related disease count and frailty
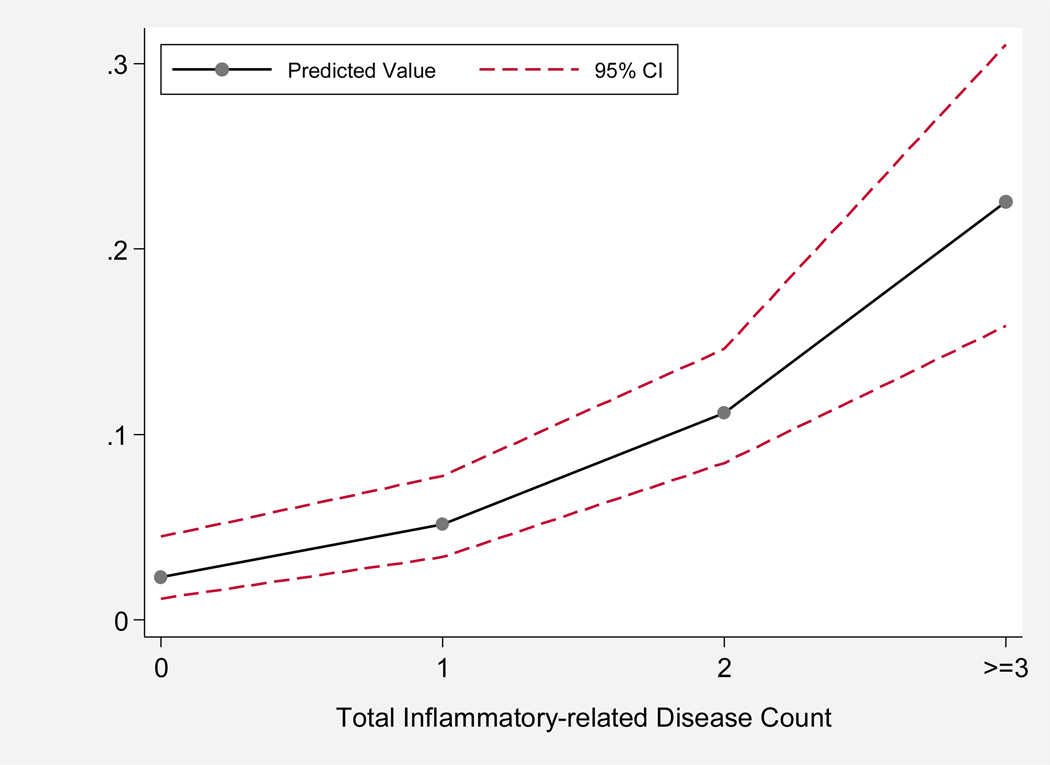
An increase of one inflammatory-related disease was independently associated with a two-fold increased probability of being frail (PR=2.28, 95%CI=1.81–2.87). After adjusting for age, race, education, and smoking, the probability of being frail remained significant (PR= 1.97, 95%CI=1.52–2.55). Figure 1 graphically shows the log-linear relationship between the predicted probabilities of being frail by the total inflammatory-related disease count, adjusted for race, age, education, and smoking. The predicted values were derived from the adjusted prevalence ratios of being frail, according to the sum count of inflammatory-related diseases.
Figure 1.
Predicted probabilities and 95% confidence intervals (95% CIs) of frailty by the total inflammatory disease count (0, 1, 2, and ≥3). The predicted values were derived from prevalence ratios of frailty, adjusted for age, race, education, and smoking.
The predicted probability of being frail was at its highest of 0.23 (95%CI=0.16–0.31) when ≥3 diseases were present. Participants with only one of the inflammatory-related diseases present had a smaller predicted probability of being frail (0.05, 95%CI=0.03–0.08) as did those in the study population who did not have any of the eight inflammatory-related diseases (0.02, 95%CI=0.01–0.04). The maximum number of chronic inflammatory-related diseases was six, which was present in only 0.86% (n=2) of study participants.
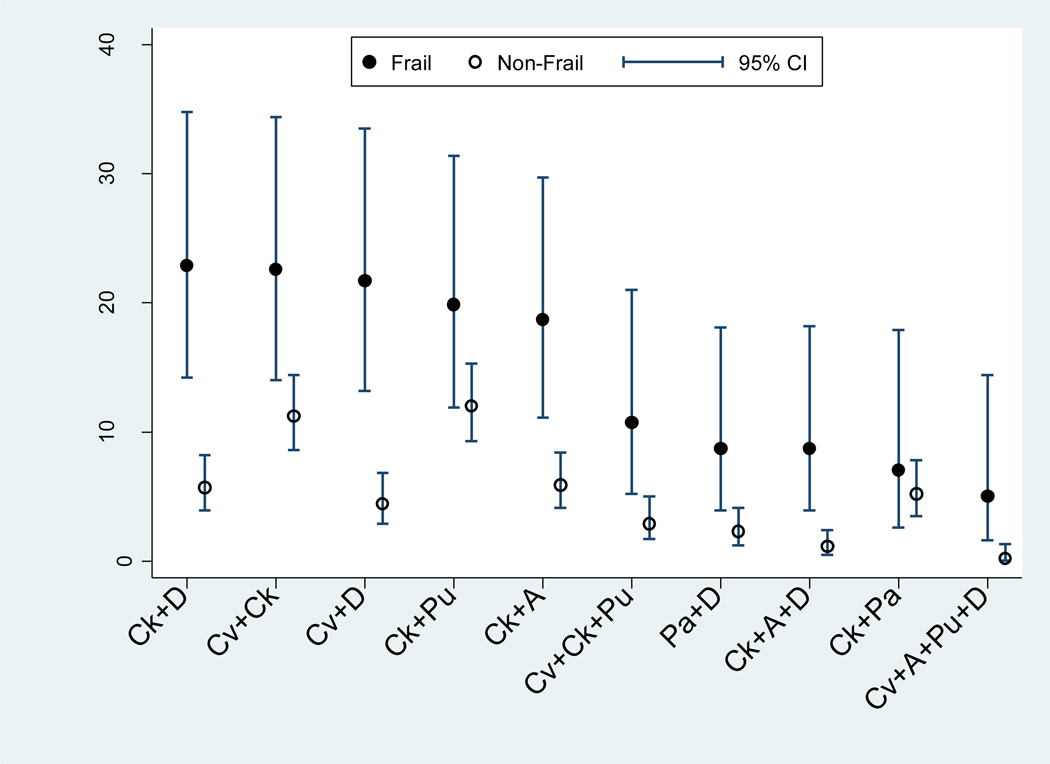
3.3. Ten most common disease patterns in frailty
Of all the disease combinations associated with frailty, the ten most frequent were (1) CKD and depressive symptoms, (2) CVD and CKD, (3) CVD and depressive symptoms, (4) CKD and pulmonary disease, (5) CKD and anemia (6) CVD, CKD, and pulmonary disease, (7) PAD and depressive symptoms, (8) CKD, anemia, and depressive symptoms, (9) CKD and PAD, and (10) CVD, anemia, pulmonary disease, and depressive symptoms, respectively. The prevalence percentages of these disease combinations in the frail vs. non-frail populations are displayed in Figure 2. Compared to the non-frail participants, those who were frail demonstrated significantly higher prevalence percentages of the following six disease combinations: CKD and depressive symptoms (Prevalence in frail=22.9%, 95%CI=14.2–34.8%); CVD and depressive symptoms (21.7%, 95%CI=13.2–33.5%); CKD and anemia (18.7%, 95%CI=11.1–29.7%); CVD, CKD, and pulmonary disease (10.7%, 95%CI=5.2–21.0%); CKD, anemia, and depressive symptoms (8.7%, 95%CI=3.9–18.2%); and CVD, anemia, pulmonary disease, and depressive symptoms (5.0%, 95%CI=1.6–14.4%). The prevalence percentages of the other four disease combinations did not differ significantly between the frail and the non-frail groups; their 95% confidence intervals overlapped in each of the disease combinations. These included CVD and CKD (14–34.4% frail vs. 8.6–14.4% non-frail), CKD and pulmonary disease (11.9–31.4% frail vs. 9.3–15.3% non-frail), PAD and depressive symptoms (3.9–18.1% frail vs. 1.2–4.1% non-frail), and CKD and PAD (2.6–17.9% frail vs. 3.5–7.8% non-frail).
Figure 2.
Prevalence percentages of the ten most common inflammatory disease combinations associated with frailty from the Women's Health and Aging Studies I and II, according to frailty status. The disease combinations are listed in order from left to right based upon their highest to lowest frequencies in the frail population. Inflammatory disease combinations with prevalence percentages less than 5.0% among frail women are not displayed.
Abbreviations: 95% CI=95% confidence interval. Ck=chronic kidney disease; D=depressive symptoms; Cv=cardiovascular disease; Pu=pulmonary disease; A=anemia; Pa=peripheral artery disease.
Of the ten most frequent disease combinations associated with frailty, seven were disease pairs, three were composed of three diseases, and one included four diseases. CKD was the most common inflammatory-related disease that was found in seven disease combinations, of which four were significant. The next most common inflammatory-related condition that was present in five disease combinations, of which also four were significant, was depressive symptoms, followed by CVD in four disease combinations, of which three were significant. Anemia was also included in three significant disease combinations; whereas, peripheral artery disease was only part of two significant disease combinations. Of the eight inflammatory-related diseases, neither DM nor RA was present in any of the ten most frequent disease patterns associated with frailty.
4. Discussion
This population-based study of older women highlights the new finding that the probability of frailty increased in a dose-response fashion as the number of chronic diseases that are associated with inflammation increased, accounting for age, race, education, and smoking. Many of the most frequent inflammatory-related disease combinations in frail older adults were also considerably less prevalent in non-frail older adults. Altogether, these results suggest not only that a higher inflammatory-related disease count is a risk factor for frailty, but also that specific disease combinations, possibly through their shared mechanistic pathways, further contribute to the onset of frailty. It is known from prior large cohort studies that multi-morbidity commonly co-exists with frailty (Barzilay et al., 2007; Fried L.P. et al., 2004; Woods et al., 2005). However, these observations have been based primarily upon prevalence percentages and do not examine the extent that multi-morbidity is related to frailty. This work provides evidence that there is a dose-response relationship between the sum count of inflammatory-related diseases and the likelihood of frailty in older women.
A key clinical implication of these findings is that a higher sum count of inflammatory-related diseases in older adults should alert clinicians to the possibility that their patients, if not already frail, have an increased probability of becoming frail. Similarly, the likelihood of frailty should be considered in older adults with multi-morbid inflammatory-related diseases that include specific disease combinations, which are most frequently associated with frailty. Identification of these vulnerable older adults is an essential first step for maximizing strategies that would prevent or reduce sarcopenia, improve energy, and benefit physical function. Factors known to precipitate or accelerate frailty, such as decreased physical activity and function, poor nutritional intake, and polypharmacy, may be addressed with resistance exercises, nutritional supplementation, and a routine review of medications to ensure that the benefits outweigh their side effects (Crentsil et al., 2010; Fried et al., 2005). One recent randomized clinical trial on frail, older women indicated that regular low-impact exercise supplemented by daily oral dehydroepiandrosterone, an anabolic steroid hormone which decreases with aging, may modestly improve lower extremity strength and physical performance over a 6-month interval (Kenny et al., 2010). While the long-term clinical impact of such an intervention in frail older adults has not yet been determined, these findings are still promising.
In this current study, we identified 1) CKD and depressive symptoms, 2) CVD and depressive symptoms, and 3) CKD and anemia as the three most prevalent, significant disease combinations in frail older adults, respectively. Characterization of the specific chronic conditions most frequently associated with frailty adds to the growing literature on multimorbidity in frail older adults. Previous research has linked depression in CKD to a 68% prevalence of frailty among incident dialysis patients. (Johansen et al., 2007). Major depressive episodes in patients with CKD have been found to increase the risks of dialysis initiation and frailty-related outcomes, such as hospitalizations and death, even after adjusting for CKD severity and co-existing medical conditions (Hedayati et al., 2010). Depression also commonly co-occurs across the clinical spectrum of CVD and has been similarly associated with major frailty-related outcomes, including disability, as well as heightened rates of CVD events in patients with pre-existing disease (Celano et al., 2011; Hance et al., 1996). Decreased physical activity associated with depressive symptoms and the net deficit in skeletal muscle protein synthesis associated with a heightened catabolic state and underlying inflammatory changes in CKD and CVD can facilitate muscle mass atrophy, which ultimately may lead to a frail state (Adams et al., 2006; Persinger et al., 2003; Rantanen et al., 2000; Schapp et al., 2006). Furthermore, advanced stages of CKD have been known to cause anemia from impaired renal synthesis of erythropoietin with poorer kidney function corresponding with lower hemoglobin levels (Astor et al., 2002). Because lower hemoglobin levels diminish oxygen delivery to major organ systems, anemia can negatively impact the ability of CKD patients to engage in physical activity. As a consequence, patients with co-existing CKD and anemia may experience accelerated loss of muscle mass, subsequently resulting in sarcopenia and the development of frailty. While it is not surprising that having more than one chronic condition is worse than having just one, we have identified the importance of these specific disease patterns based on both their prevalence rates and associations with frailty.
Another striking result in our study was that depressive symptoms, CVD, and anemia were among the most common of the eight single inflammatory-related diseases that were found in significant disease patterns of frail older adults. This provides further evidence suggesting that frailty often co-exists with disease combinations that include depressive symptoms, CVD, or anemia. However, whether frailty is a cause or consequence of specific disease combinations remains uncertain. To determine the role of disease-related mechanisms in the development of frailty, the temporal relationship between frailty and significant disease combinations, such as CKD or CVD and depressive symptoms, needs further elucidation as does the extent to which frailty affects important health outcomes in older adults with these specific co-existing conditions.
Potential approaches to delay or prevent the development of frailty may include the following: 1) periodic screening for depressive symptoms and improved treatment of depression in older adults who have CKD or CVD, 2) multi-pronged management of vascular risk factors that contribute to the manifestation of renal insufficiency and cardiovascular disease, and 3) more rigorous evaluation for potentially treatable causes of newfound anemia in older adults, particularly in anemic patients with progressing CKD who may clinically benefit from erythropoietin-stimulating agents. Subsequent studies which compare the risk reduction of frailty using different strategies or treatments for co-managing specific inflammatory-related chronic diseases in older adults would serve as the basis for developing improved interventions for frailty.
A plausible mechanism for the association between multi-morbidity and the likelihood of frailty is the induction of multiple overlapping pro-inflammatory pathways associated with many disease-related systemic changes. This activated pro-inflammatory cascade is theorized to dysregulate a critical mass of metabolic and physiologic systems, which undermine homeostatic adaptive capacity, precipitating frailty (Fried et al., 2009; Gruenwald et al., 2009). Elevations in systemic inflammatory biomarkers have been associated with the frailty measures and its potential risk factors (Hubbard et al., 2009; Payette et al., 2003), which include not only inflammatory-related chronic conditions, but also socio-demographic factors and health-associated behaviors, such as advancing age and smoking (Bruunsgaard et al., 2001; Levitzky et al., 2008). To this effect, a heightened pro-inflammatory state may play a key role in the multi-systemic dysregulation associated with frailty. Although our study population did not demonstrate any statistically significant differences for age group (70–74 vs. 75–79 years old) and smoking status (current, former, and never) between the frail and the non-frail women (both p-values= 0.06), general trends for these characteristics indicated that higher frequencies of frail, compared to non-frail, participants were older and current or former smokers. These trends in prevalence rates appear to support prior studies showing that advancing age and active smoking are associated with frailty (Hubbard et al., 2009; Song et al., 2010).
Thus far, assessment of the total pro-inflammatory burden in older adults and its relationship with the progression of frailty has remained a methodologic challenge (Bandeen-Roche et al., 2009). Perhaps, the sum count of inflammatory-related chronic diseases in older adults may reflect the magnitude of an induced pro-inflammatory state. As a next step, it will be important to assess whether a sum count of inflammatory-related diseases may have prognostic utility in the development of a prediction index for incident frailty and its associated clinical outcomes, including disability and death. Results from these prospective cohort studies would demonstrate whether a greater inflammatory-related disease burden is indeed causally-related to the risk of frailty and its associated health outcomes. These studies may also reveal the extent to which current treatment strategies that effectively co-manage multi-morbid inflammatory-related diseases affect the risk of frailty.
Several caveats limit the conclusions that can be drawn from this study. First, we cannot establish a casual relationship between a higher count of inflammatory-related chronic diseases and the risk of frailty because of the study’s cross-sectional design. Our study population was also exclusively composed of community-dwelling older women. As a result, further research is needed in the minority of older adults who are men, since gender influences body composition and size, which may lead to differences in prevalence rates of inflammatory-related chronic diseases and in the progression rate of frailty (Cawthon et al., 2007). The internal validity of this study, however, remains preserved. Next, the data were lacking in chronic diseases and conditions which were non-inflammatory in origin. Therefore, we could not feasibly perform parallel analyses investigating whether the sum count of non-inflammatory diseases is associated with frailty. An insignificant association would have further strengthened the specificity of our findings. We also could not capture the inflammatory contribution of undiagnosed, subclinical diseases. This may render the inflammatory-related disease count conservative and thereby result in underestimation of frailty in older adults with underlying inflammatory-related chronic conditions. Perhaps, this is one reason why participants who did not have any of the eight inflammatory-related diseases were found to have a statistically significant predicted probability of frailty (0.02, 95%CI=0.01–0.04), albeit extremely low. Finally, we could not evaluate the relationship of cognitive impairment or dementia to frailty. Underlying inflammatory mechanisms also may contribute to the development of cognitive impairment, which has been shown to independently increase the risk of frailty (Raji et al., 2010). Because participants who were initially enrolled into WHAS were cognitively intact with a MMSE score ≥24, data were not available to study older women who had baseline cognitive impairment or dementia.
4.1. Conclusions
We demonstrated that the likelihood of frailty increases in a dose-response fashion as the chronic inflammatory-related disease count increases. Shared mechanisms among specific disease combinations may further contribute to this risk and facilitate the progression of frailty. Longitudinal ascertainment of chronic inflammatory-related diseases with respect to incident frailty in older adults will help establish a temporal relationship between a higher total disease count and the risk of frailty. Additionally, future research using larger cohorts to determine the extent that specific disease combinations effect frailty would lay the groundwork for evaluating likely disease mechanisms for therapeutic interventions. This, in turn, could lead to the development of improved treatment strategies to manage co-morbid diseases more effectively and potentially prevent or delay the progression of frailty in older adults.
Acknowledgements
This work was supported by the National Institute on Aging (grant numbers N01 AG12112, R01 AG11703, R37 AG19905, and T32 AG00247 to SSC); the National Institutes of Health-National Center for Research Resources (grant number UL1 RR 025005); the John A. Hartford Foundation Center of Excellence in Geriatric Medicine at Yale University (grant number 2007-0009 to SSC); the Robert Wood Johnson Foundation Amos Medical Faculty Development Program to COW; and the Johns Hopkins Older Americans Independence Center (grant number P30-AG02133 to QLX). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official view of the National Institute on Aging and National Institutes of Health-National Center for Research Resources, John A. Hartford Foundation, or the Robert Wood Johnson Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None.
Contributor Information
Carlos O. Weiss, Email: cweiss9@jhmi.edu.
Qian-Li Xue, Email: qxue@jhsph.edu.
Linda P. Fried, Email: lpfried@columbia.edu.
References
- Adams GR, Vaziri ND. Skeletal muscle dysfunction in chronic renal failure: effects of exercise. Am J Physiol Renal Physiol. 2006;290:F753–F761. doi: 10.1152/ajprenal.00296.2005. [DOI] [PubMed] [Google Scholar]
- Astor BC, Muntner P, Levin A, Eustace JA, Coresh J. Association of kidney function with anemia. Arch Intern Med. 2002;162:1401–1408. doi: 10.1001/archinte.162.12.1401. [DOI] [PubMed] [Google Scholar]
- Bandeen-Roche K, Xue QL, Ferrucci L, Walston J, Guralnik JM, Zeger SL, Fried LP. Phenotype of frailty: characterization in the Women's Health and Aging Studies. J Gerontol A Biol Sci Med Sci. 2006;61:262–266. doi: 10.1093/gerona/61.3.262. [DOI] [PubMed] [Google Scholar]
- Bandeen-Roche K, Walston JD, Huang Y, Semba RD, Ferrucci L. Measuring systemic inflammatory regulation in older adults: evidence and utility. Rejuvenation Res. 2009;12:403–410. doi: 10.1089/rej.2009.0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilay JI, Blaum C, Moore T, Xue QL, Hirsch CH, Walston JD, Fried LP. Insulin resistance and inflammation as precursors of frailty: The Cardiovascular Health Study. Arch Intern Med. 2007;167:635–641. doi: 10.1001/archinte.167.7.635. [DOI] [PubMed] [Google Scholar]
- Bremmer MA, Beekman ATF, Deeg DJH, Penninx BW, Dik MG, Hack CE, Hoogendijk WJ. Inflammatory markers in late-life depression: Results from a population-based study. J Affect Disord. 2008;106:249–255. doi: 10.1016/j.jad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Pedersen M, Pedersen BK. Aging and pro-inflammatory cytokines. Curr Opin Hematol. 2001;8:131–136. doi: 10.1097/00062752-200105000-00001. [DOI] [PubMed] [Google Scholar]
- Burke WJ, Nitcher RL, Roccaforte WH, Wengel SP. A prospective evaluation of the Geriatric Depression Scale in an outpatient geriatric assessment center. J Am Geriatr Soc. 1992;40:1227–1230. doi: 10.1111/j.1532-5415.1992.tb03647.x. [DOI] [PubMed] [Google Scholar]
- Cawthon PM, Marshall LM, Michael Y, Dam TT, Ensrud KE, Barrett-Connor E, Orwoll ES. Frailty in older men: Prevalence, progression, and relationship with mortality. J Am Geriatr Soc. 2007;55:1216–1223. doi: 10.1111/j.1532-5415.2007.01259.x. [DOI] [PubMed] [Google Scholar]
- Celano CM, Huffman JC. Depression and cardiac disease: A review. Cardiol Rev. 2011;19:130–142. doi: 10.1097/CRD.0b013e31820e8106. [DOI] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB, Pahor M. Inflammatory markers and onset of cardiovascular events: Results from the Health ABC study. Circulation. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- Chang SS, Weiss CO, Xue QL, Fried LP. Patterns of comorbid inflammatory diseases in frail older women: the Women's Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2010;65:407–413. doi: 10.1093/gerona/glp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves PH, Semba RD, Leng SX, Woodman RC, Ferrucci L, Guralnik JM, Fried LP. Impact of anemia and cardiovascular disease on frailty status of community-dwelling older women: the Women's Health and Aging Studies I and II. J Gerontol A Biol Sci Med Sci. 2005;60:729–735. doi: 10.1093/gerona/60.6.729. [DOI] [PubMed] [Google Scholar]
- Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- Crentsil V, Ricks MO, Xue QL, Fried LP. A pharmacoepidemiologic study of community-dwelling, disabled older women: Factors associated with medication use. Am J Geriatr Pharmacother. 2010;8:215–224. doi: 10.1016/j.amjopharm.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Deddens JA, Petersen MR. Approaches for estimating prevalence ratios. Occup Environ Med. 2008;65:501–506. doi: 10.1136/oem.2007.034777. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Guralnik JM, Woodman RC, Bandinelli S, Lauretani F, Corsi AM, Chaves PH, Ershler WB, Longo DL. Proinflammatory state and circulating erythropoietin in persons with and without anemia. Am J Med. 2005;118:1288.e11–1288.e19. doi: 10.1016/j.amjmed.2005.06.039. [DOI] [PubMed] [Google Scholar]
- Fried LF, Solomon C, Shlipak M, Seliger S, Stehman-Breen C, Bleyer AJ, Chaves P, Furberg C, Kuller L, Newman A. Inflammatory and prothrombotic markers and the progression of renal disease in elderly individuals. J Am Soc Nephrol. 2004;15:3184–3191. doi: 10.1097/01.ASN.0000146422.45434.35. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- Fried LP, Hadley EC, Walston JD, Newman AB, Guralnik JM, Studenski S, Harris TB, Ershler WB, Ferrucci L. From bedside to bench: Research agenda for frailty. Sci Aging Knowl Environ. 2005;2005:pe24. doi: 10.1126/sageke.2005.31.pe24. [DOI] [PubMed] [Google Scholar]
- Fried LP, Xue QL, Cappola AR, Ferrucci L, Chaves P, Varadhan R, Guralnik JM, Leng SX, Semba RD, Walston JD, Blaum CS, Bandeen-Roche K. Nonlinear multisystem physiological dysregulation associated with frailty in older women: Implications for etiology and treatment. J Gerontol A Biol Sci Med Sci. 2009;64:1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenewald TL, Seeman TE, Karlamangla AS, Sarkisian CA. Allostatic load and frailty in older adults. J Am Geriatr Soc. 2009;57:1525–1531. doi: 10.1111/j.1532-5415.2009.02389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hance M, Carney RM, Freedland KE, Skala J. Depression in patients with coronary heart disease. A 12-month follow-up. Gen Hosp Psychiatry. 1996;18:61–65. doi: 10.1016/0163-8343(95)00100-x. [DOI] [PubMed] [Google Scholar]
- Hedayati SS, Minhajuddin AT, Afshar M, Toto RD, Trivedi MH, Rush AJ. Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA. 2010;303:1946–1953. doi: 10.1001/jama.2010.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard RE, O'Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13:3103–3109. doi: 10.1111/j.1582-4934.2009.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard RE, Searle SD, Mitnitski A, Rockwood R. Effect of smoking on the accumulation of deficits, frailty and survival in older adults: A secondary analysis from the Canadian Study of Health and Aging. J Nutr Health Aging. 2009;13:468–472. doi: 10.1007/s12603-009-0085-y. [DOI] [PubMed] [Google Scholar]
- Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960–2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- Karadag F, Karul AB, Cildag O, Yilmaz M, Ozcan H. Biomarkers of systemic inflammation in stable and exacerbation phases of COPD. Lung. 2008;186:403–409. doi: 10.1007/s00408-008-9106-6. [DOI] [PubMed] [Google Scholar]
- Kenny AM, Boxer RS, Kleppinger A, Brindisi J, Feinn R, Burleson JA. Dehydroepiandrosterone combined with exercise improves muscle strength and physical function in frail older women. J Am Geriatr Soc. 2010;58:1707–1714. doi: 10.1111/j.1532-5415.2010.03019.x. [DOI] [PubMed] [Google Scholar]
- Kritchevsky SB, Cesari M, Pahor M. Inflammatory markers and cardiovascular health in older adults. Cardiovascular Research. 2005;66:265–275. doi: 10.1016/j.cardiores.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Leng SX, Xue Q, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55:864–871. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- Levitzky YS, Guo CY, Rong J, Larson MG, Walter RE, Keaney JF, Sutherland PA, Vasan D, Lipinska I, Evans JC, Benjamin EJ. Relation of smoking status to a panel of inflammatory markers: The Framingham Offspring. 2008;201:217–224. doi: 10.1016/j.atherosclerosis.2007.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteini AM, Walston JD, Fallin MD, Bandeen-Roche K, Kao WH, Semba RD, Allen RH, Guralnik J, Fried LP, Stabler SP. Markers of B-vitamin deficiency and frailty in older women. J Nutr Health Aging. 2008;12:303–308. doi: 10.1007/BF02982659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott MM, Green D, Greenland P, et al. Relation of levels of hemostatic factors and inflammatory markers to the ankle brachial index. Am J Cardiol. 2003;92:194–199. doi: 10.1016/s0002-9149(03)00537-x. [DOI] [PubMed] [Google Scholar]
- McNutt L, Wu C, Xue X, Hafner JP, Liu K, Criqui MH, Chan C, Guralnik JM, Pearce WH, Ridker PM, Taylor L, Rifai N, Schneider JR. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157:940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- Mitch WE, Price SR. Mechanisms activated by kidney disease and the loss of muscle mass. Am J Kidney Dis. 2001;38:1337–1342. doi: 10.1053/ajkd.2001.29249. [DOI] [PubMed] [Google Scholar]
- National Institute on Aging. Bethesda, MD: National Institutes of Health (NIH) Publication No. 95-4009; 1995. The Women's Health and Aging Study: Health and Social Characteristics of Older Women with Disability. [Google Scholar]
- Newman AB, Gottdiener JS, Mcburnie MA, Hirsch CH, Kop WJ, Tracy R, Walston JD, Fried LP Cardiovascular Health Study Research Group. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- Norris JT, Gallagher D, Wilson A, Winograd CH. Assessment of depression in geriatric medical outpatients: the validity of two screening measures. J Am Geriatr Soc. 1987;35:989–995. doi: 10.1111/j.1532-5415.1987.tb04001.x. [DOI] [PubMed] [Google Scholar]
- Payette H, Roubenoff R, Jacques PF, Dinarello CA, Wilson PW, Abad LW, Harris T. Insulin-like growth factor-1 and interleukin 6 predict sarcopenia in very old community-living men and women: the Framingham Heart Study. J Am Geriatr Soc. 2003;51:1237–1243. doi: 10.1046/j.1532-5415.2003.51407.x. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Guralnik JM, Simonsick EM, Kasper JD, Ferrucci L, Fried LP. Emotional vitality among disabled older women: the Women's Health and Aging Study. J Am Geriatr Soc. 1998;46:807–815. doi: 10.1111/j.1532-5415.1998.tb02712.x. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S, Ferrucci L, Harris T, Pahor M. Inflammatory markers and depressed mood in older persons: Results from the Health, Aging, and Body Composition Study. Biol Psychiatry. 2003;54:566–572. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]
- Persinger R, Janssen-Heininger Y, Wing SS, Matthews DE, LeWinter MM, Toth MJ. Effect of heart failure on the regulation of skeletal muscle protein synthesis, breakdown, and apoptosis. Am J Physiol Endocrinol Metab. 2003;284:E1001–E1008. doi: 10.1152/ajpendo.00517.2002. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Penninx BW, Masaki K, Lintunen T, Foley D, Guralnik JM. Depressed mood and body mass index as predictors of muscle strength decline in old men. J Am Geriatr Soc. 2000;48:613–617. doi: 10.1111/j.1532-5415.2000.tb04717.x. [DOI] [PubMed] [Google Scholar]
- Raji MA, Al Snih S, Ostir GV, Markides KS, Ottenbacher KJ. Cognitive status and future risk of frailty in older Mexican Americans. J Gerontol A Biol Sci Med Sci. 2010;65:1228–1234. doi: 10.1093/gerona/glq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapp LA, Pluijm SMF, Deeg DJH, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119:526.e9–526.e17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- Shlipak MG, Stehman-Breen C, Fried LF, Song X, Siscovick D, Fried LP, Psaty BM, Newman AB. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004;43:861–867. doi: 10.1053/j.ajkd.2003.12.049. [DOI] [PubMed] [Google Scholar]
- Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58:681–687. doi: 10.1111/j.1532-5415.2010.02764.x. [DOI] [PubMed] [Google Scholar]
- Tonelli M, Sacks F, Pfeffer M, Jhangri GS, Curhan G. Cholesterol and Recurrent Events (CARE) Trial Investigators. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int. 2005;68:237–245. doi: 10.1111/j.1523-1755.2005.00398.x. [DOI] [PubMed] [Google Scholar]
- Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Gottdiener J, Fried LP. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Geneva, Switzerland: World Health Organization; 1968. Nutritional Anaemias: Report of a WHO Scientific Group. [PubMed] [Google Scholar]
- Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, Masaki K, Murray A, Newman AB. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]