Abstract
Naturalistic paradigms such as movie watching or simulated driving that mimic closely real-world complex activities are becoming more widely used in functional magnetic resonance imaging (fMRI) studies both because of their ability to robustly stimulate brain connectivity and the availability of analysis methods which are able to capitalize on connectivity within and among intrinsic brain networks identified both during a task and in resting fMRI data. In this paper we review over a decade of work from our group and others on the use of simulated driving paradigms to study both the healthy brain as well as the effects of acute alcohol administration on functional connectivity during such paradigms. We briefly review our initial work focused on the configuration of the driving simulator and the analysis strategies. We then describe in more detail several recent studies from our group including a hybrid study examining distracted driving and compare resulting data with those from a separate visual oddball task.
The analysis of these data were performed primarily using a combination of group independent component analysis (ICA) and the general linear model (GLM) and in the various studies we highlight novel findings which result from an analysis of either 1) within-network connectivity, 2) inter-network connectivity, also called functional network connectivity, or 3) the degree to which the modulation of the various intrinsic networks were associated with the alcohol administration and the task context.
Despite the fact that the behavioral effects of alcohol intoxication are relatively well known, there is still much to discover on how acute alcohol exposure modulates brain function in a selective manner, associated with behavioral alterations. Through the above studies, we have learned more regarding the impact of acute alcohol intoxication on organization of the brain’s intrinsic connectivity networks during performance of a complex, real-world cognitive operation. Lessons learned from the above studies have broader applicability to designing ecologically valid, complex, functional MRI cognitive paradigms and incorporating pharmacologic challenges into such studies. Overall, the use of hybrid driving studies is a particularly promising area of neuroscience investigation.
Keywords: fMRI, functional, brain, visual perception, alcohol
Introduction
Through most of their history, psychology and neuroscience have been inevitably constrained by laboratory settings. Although essential for controlled, repeated experimental measurements, the laboratory has also been an inescapable stimulus environment, prominent in every subject’s experiences as the context for any experimental task. This is especially true for brain imaging research. Volunteers in fMRI experiments lie supine for up to an hour in a narrow, noisy cylinder, with their heads immobilized in a helmet-like radio frequency coil. Anyone who has ever been a scan subject knows that the scanner itself is a salient background for any experimental task.
The scanner environment and the laboratory context naturally constrain the types of behaviors chosen for study. A wide array of perceptual, cognitive, and motor abilities are presumably invariant across most environments, and cognitive neuroscience has found no shortage of experiments appropriate for the scanner and representative of domains and capacities ubiquitous in cognitive activities. Nonetheless, real behavior and real environments represent combinations and applications more complex than their laboratory versions, and it is not always clear how more basic perceptual and behavioral capacities “add up” in ecologically realistic environments, where many stimuli and many possible responses compete for a subject’s attention and emergent properties of more complex tasks become important to quantify.
A key question we wished to address was our ability to study complex real-world behaviors with imaging techniques developed to examine simpler, more constrained cognitive neuroscience paradigms. Virtual reality (VR) offers methods for dissolving the scanner bore and the laboratory wall. VR can simulate complex, ecologically valid environments that are responsive to multiple complex behaviors. VR can also enable experiments that could not be realized in any other environment, in that it allows simulations of tasks that would be impractical, dangerous, unethical, or even impossible in real contexts. Finally, VR provides a means, consistent with the assumptions of experimental psychology, to provide a “critical mass” of environmental cues for a simulated driving experiment.
In this paper we discuss variations on an experimental paradigm in which we implement VR-based paradigms in the fMRI environment. We highlight a significant methodological problem that emerges as VR experiments achieve increasing perceptual/motor realism. Traditional fMRI interpretive methods rest on a priori hypotheses about the time courses of component brain functions that comprise the experimental task, and frequently reflect experimenters’ assumptions about the functional capacities of particular brain regions and how they act individually versus collectively. For a complex behavior like driving, these assumptions may be questioned; additional complexities exist because multiple brain circuits are not only activated simultaneously, but in a complex manner where a particular region may contribute differentially to several circuits. The multiple responses of skilled driving overlap and interact in ways that make modeling their time course uncertain. Accordingly, we explore the application of a data-driven approach, independent component analysis (ICA), in this complex behavioral context. ICA extracts covarying ensembles of voxel time courses without needing an a priori specification of onsets and offsets. Rather, the onsets and offsets are compared to the time courses estimated using ICA. In our analysis we use group ICA, an approach pioneered by our group, which produces subject-specific maps and timecourses (Calhoun, et al. 2001; Erhardt, et al. In Press). The time courses of the independent components can then be compared to the time courses of multiple behavioral measures to explore how a complex skill like driving emerges from the complex overlapping action of multiple brain areas unconstrained by a priori hypotheses. We also show examples of combining ICA and GLM results, for example in our hybrid study which incorporates a visual oddball paradigm within a simulated driving study.
Several other groups also study simulated driving using neuroimaging. Schier et al. showed in a EEG study that alpha power decreases during driving versus replayed scenarios and also more alpha power was observed in later versus earlier laps (Schier 2000). Walter et al. demonstrated a GLM comparison of driving versus watching (Walter, et al. 2001). Uchiyama evaluated the neural correlates of driving at a safe distance (Uchiyama, et al. 2003). Young et al., Hsieh et al., and Bowyer et al., used a human factors approach and evaluated simulated driving with fMRI and magnetoencephalography (MEG) during a dual driving and distraction task (Bowyer, et al. 2009; Hsieh, et al. 2009; Young, et al. 2004). A similar study of the impact of distraction from an individual speaking to the driver was evaluated by Just et al (Just, et al. 2008). Spier et al. evaluated fMRI activity during a realistic driving simulation using a GLM approach (Spiers and Maguire 2007). Jancke et al. showed more alpha band activity during EEG recordings for fast driving (Jancke, et al. 2008). Mader et al. evaluated the changes associated with familiar versus unfamiliar driving (Mader, et al. 2009) and Callan et al. investigated the neural correlates associated with uncertainty in driver’s decision making (Callan, et al. 2009). Despite the extremely useful information gained in the above fMRI studies, they all used a standard GLM approach, which is limited in that it does not assess functional connectivity networks, nor does it capture variance which is not tightly coupled to the task.
Why study intoxicated driving in an imaging environment?
In the United States alcohol is our most commonly used recreational drug and driving while intoxicated remains prevalent. As a societal and legal problem, more than 2.5 million persons in the U.S. were reported injured and over 40 thousand died in motor vehicle crashes in 2006 (2007). Traffic accidents are the greatest single cause of death in 5–34 year-olds and up to 40% are due to intoxicated drivers (Fillmore, et al. 2008). In 2008, intoxicated driving resulted in ~13,000 alcohol-impaired driving fatalities, representing 1 death every 40 minutes in 2007 (2008). Increased understanding of the dangers and mechanisms of alcohol will ultimately help refine existing social policy. Thus, we need to better understand impairment in behavior and functional neural circuitry caused by alcohol on performance on validated, realistic, simulated driving tasks under various road conditions and at different alcohol blood levels. Important causes of accidents include brief attentional lapses, often due to distraction. Alcohol is believed to exacerbate both of these factors as well as being associated with impaired inhibitory control and overconfidence (Field, et al. 2008; Fillmore, et al. 2009; Kleykamp, et al. 2010; Lee 2008; Lee 2009; Lee, et al. 2009; Lees and Lee 2007) i.e. by interacting with emotional or temperamental factors as well as cognitive ones.
More specifically, acute alcohol administration interferes with performance on neuropsychological tasks assessing a wide variety of cognitive processes, including immediate memory span (Jones 1973; Parker, et al. 1974; Tarter and Jones 1971), short-term memory (Rosen and Lee 1976; Tarter, et al. 1991), conceptual and abstracting processes, and motor speed and coordination (Tarter and Jones 1971), which may relate to prefrontal cortex moderation of complex motor skills (Peterson, et al. 1990). Although some evidence suggests no alcohol-induced differences on attention tests (Tarter and Jones 1971), other findings indicate detrimental effects on attention allocation (Lamb and Robertson 1987). Learning and memory are also negatively affected by alcohol (Mungas, et al. 1994; Ryback 1971).
In addition, psychophysical measures (Ahveninen, et al. 2000; Wegner, et al. 2001) are impaired by greater degrees of intoxication and suggest slower information processing (Colrain, et al. 1993; Krull, et al. 1993). Longer-term neuropsychological and neurological deficits in executive function, visuospatial performance, and functions of gait and balance are detectable as the result of chronic use in alcoholic men even after a month of sobriety (Sullivan, et al. 2002). In general, these acute and chronic studies support deleterious effects of alcohol on cognitive functioning.
Despite these results, there is relatively little evidence examining how exposure to alcohol might transiently modulate brain function in the context of cognitive task performance (Mathew and Wilson 1986; Schwartz, et al. 1993; Tiihonen, et al. 1994; Volkow, et al. 1990; Volkow, et al. 1988). (Haier, et al. 1999). As with all pharmacological challenge studies, working with alcohol adds complexities. Alcohol has vasoactive properties, potentially confounding fMRI studies relying upon hemodynamic changes (Levin, et al. 1998). There may also exist brain activation differences between chronic alcoholics and healthy controls (Pfefferbaum, et al. 2001; Tapert, et al. 2001). Finally, behaviorally there are non-linear behavioral alterations with increasing blood alcohol levels; for example at lower levels subjects are aware of intoxication and tend to overcompensate by making fewer errors; more dangerous driving errors emerge only at higher blood alcohol concentrations.
With regard to experimental design, alcohol effects on cognitive performance and brain activation are most often studied for simpler tasks. We believe that a comprehensive study of alcohol’s effects on driving a complex task should assess “top down”, more complex simulated driving tasks to complement existing literature on simpler “bottom up” cognitive tasks related to specific components of driving in the fMRI scanner. The ability to assess a “whole behavior” such as driving in the scanner is novel and was one of the essential motivations for the work described below.
Initial work on fMRI of simulated driving
Our group has studied fMRI of simulated driving for over a decade. First we describe the simulation environment; we summarize this work in logical sequence below. Next we describe initial work related to the identification of brain networks which are modulated by the driving stimuli. Then we describe a follow up study including many more subjects and move into hybrid paradigms which combine the experience of driving with additional tasks (such as acknowledging a salient stimulus with in the virtual vehicle).
The simulator environment
The incorporation of a virtual reality driving simulator into an fMRI environment provides a highly novel way to study brain activation during simulation. It is one of the most common experimental tools in intoxicated driving research, and many studies to date have employed driving simulators to assess the effects of various abused substances and prescribed medications, as well as the effects of normal aging, sleep deprivation, and other adverse conditions on driving and driving related skills (Arnedt, et al. 2001; Deery and Fildes 1999; Linnoila and Mattila 1973; Rimm, et al. 1982; Verster, et al. 2002). However, there are specific challenges related to the hardware (e.g. the simulator must be non-magnetic, and not generate radio frequency signals which interfere with the fMRI scan), the environment (e.g. typical MRI scanners requires participants to lay on their back while driving), and the analysis (e.g. the brain activation during simulated driving is highly complex due to the multiple cognitive domains being stimulated). In our initial work we demonstrated the validity of a similar, simulated driving environment to evaluate performance measures in sober and alcohol-intoxicated subjects compared directly to real on-road driving (McGinty, et al. 2001).
The driving functional connectome, study of intrinsic networks identified during simulated driving
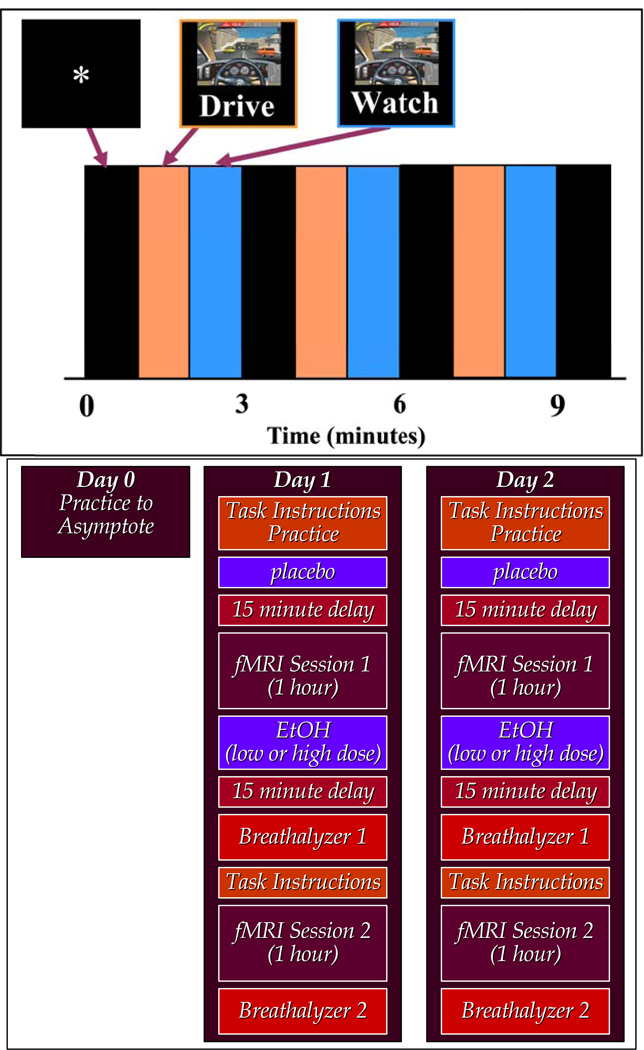
The term “intrinsic network” is used to describe temporally coherent patterns that have been identified both in resting data as well as in task data. During a task the timecourses and spatial patterns are modulated, but on the whole there is considerable similarity in the networks identified at rest and during a task (Calhoun, et al. 2008). Driving is a complex behavior that recruits multiple cognitive elements, probably in complex ways that do not track exactly with the task. Because of this, it makes sense to analyze simulated driving data in a way that evaluates the functional connectivity among regions. Because of this we have from the beginning pursued an analytic approach that enables us to study how the brain is functionally connected and how these so called intrinsic networks are modulated by the driving task, the so called driving functional connectome (Allen, et al. 2011; Biswal, et al. 2010). In our initial work we reported on a study of 12 individuals performing a driving paradigm within the fMRI environment (Calhoun, et al. 2002). In all studies subjects received brain scanning after training to asymptote performance on the driving task. The experimental paradigm is presented in Figure 1. Simulated driving involved a ten-minute task consisting of 1-minute epochs of (a) an asterisk fixation task, (b) active simulated driving, and (c) watching a simulated driving scene (while randomly moving fingers over the controller). Subjects were instructed to stay in the right lane except to pass other vehicles, to avoid collisions, to stay within a speed range of 100–140 (the units were not specified to the participant) and to drive normally. While in the scanner, participants held a Nintendo-like controller, shielded in copper foil, with all ferromagnetic components removed. Aggregate driving performance was rated on eight different measures: vehicle collisions, near vehicle collisions, number of lane deviations, duration of lane deviations, number of instances over maximum speed limit, total time over maximum speed limit, number of instances below minimum speed limit, and total time below minimum speed limit.
Figure 1.
Timeline of the Simulated Driving Paradigm and Outline of 2-day Study Design for EtOH Experiments: The driving paradigm (top) consisted of ten, one-minute epochs of (a) a fixation target, (b) driving the simulator, and (c) watching a simulation while randomly moving fingers over the controller. Two-day study design (bottom) for EtOH experiments consists of two scan sessions on each day.
In analyzing the data, we were interested in moving beyond a block-design GLM approach, which, though useful in certain contexts, can be quite limited in the context of such a complex task (Walter, et al. 2001). When performed naturalistically, driving is particularly unsuitable to GLM analytic paradigms, since the task generally involves adaptation to a changing environment by altering the integration of multiple perceptual, attentional and motor behaviors. This would involve activation of multiple circuits simultaneously with particular brain regions likely playing different simultaneous roles in multiple functions. We decomposed the data using group independent component analysis (ICA) also known as “blind source separation,” an iterative algorithm that separates a mixed signal into source components while maximizing statistical independence from one another (Calhoun, et al. 2001; Erhardt, et al. In Press). Applied to fMRI, each independent component is an ensemble of voxels, a “brain network,” that varies in activity over time. The total fMRI signal, then, is the sum of contributions from the varying components. The advantage of ICA is that, contrary to the GLM, a given set of component regions have a similar time course (hence providing a measure of functional connectivity), in addition ICA enables us to detect components whose time courses may be related to the paradigm, but in a more complex manner.
Given the wide use of ICA, we now provide a brief comment on the use of ICA on fMRI data. The selection of ICA components has matured since ICA of fMRI was introduced over twelve years ago. One key parameter that needs to be selected is the number of components. Many studies have used around twenty-five components, which we call a low model order analysis. There are information theoretic approaches, implemented in the most widely used software packaged which can determine the number of components (e.g. (Li, et al. 2007)). More recently there has been interest in doing higher order ICA, with some evidence that around seventy-five components is optimal (Abou-Elseoud, et al. 2010; Kiviniemi, et al. 2009). This latter approach is quite useful as it splits up somewhat distinct networks, and through evaluation of inter-component correlation (i.e. functional network connectivity) one can still see natural groupings of the networks that would be revealed in low model order ICA (e.g parts of the default mode network tend to be more correlated with one another than with other components) (Allen, et al. 2011). There are also several large studies which can be used as a referene for selecting components. For example, we have recently released component images from twenty-eight intrinsic networks derived from over six-hundred healthy individuals as well as all seventy-five components, see http://mialab.mrn.org/data (Allen, et al. 2011).
Results showed we could successful decompose the activation into interpretable pieces using a novel, generally applicable approach, based upon independent component analysis. Results were quite interesting and we showed that some regions turn on or off, others exhibit a gradual decay, and yet others turn on transiently when starting or stopping driving. Signal in the anterior cingulate cortex, an area often associated with error monitoring and inhibition, decreased exponentially with a rate proportional to driving speed, while decreases in frontoparietal regions, implicated in vigilance and later coined the default mode network (Raichle, et al. 2001), correlated with speed. Increases in cerebellar and occipital areas, presumably related to complex visuomotor integration, were activated during driving but not associated with driving speed. This initial study was quite encouraging and demonstrated the wealth of information that could be captured during simulated driving within an imaging environment. We moved ahead from this point to begin studying the impact of alcohol on these intrinsic networks associated with simulated driving.
First study of EtOH and simulated driving
In our next study we evaluated nine participants who were scanned in two sessions on two different days (Calhoun, et al. 2004). Sessions were run at the same time of day (mid-morning). An outline of the study design is show in Figure 1 (bottom). For the first scan session, participants received a placebo after which they were removed from the scanner and administered a dose of beverage alcohol individualized to participant body weight, age, and sex, calculated using a published algorithm (Kapur 1989), and designed to produce a blood alcohol content (BAC) of 0.04% or 0.08%. BAC’s were determined immediately before drinking and before and after all scan sessions, using a hand-held breath meter (Intoximeters, Inc.); subjects were blind to their BAC’s.
Compared to sober baseline, at the lower BAC, behavioral performance slightly improved and participants reduced average speed. At the higher BAC, subjects drove at a higher average speed (p<0.008, corrected). Additional details on scanning techniques and fMRI preprocessing may be found in previous reports (Calhoun, et al. 2001; Calhoun, et al. 2002; Calhoun, et al. 2004). At the lower BAC (mean 0.041 ± 0.016) on the 5-point analog scale (where 5 indicated maximal intoxication), participants indicated subjective intoxication of mean 1.0 ± 0.7 and at the higher BAC (mean 0.096 ± 0.040), participants self-rated intoxication of mean 3.1 ± 0.8. The difference on the subjective intoxication scores was significant (p<0.000001).
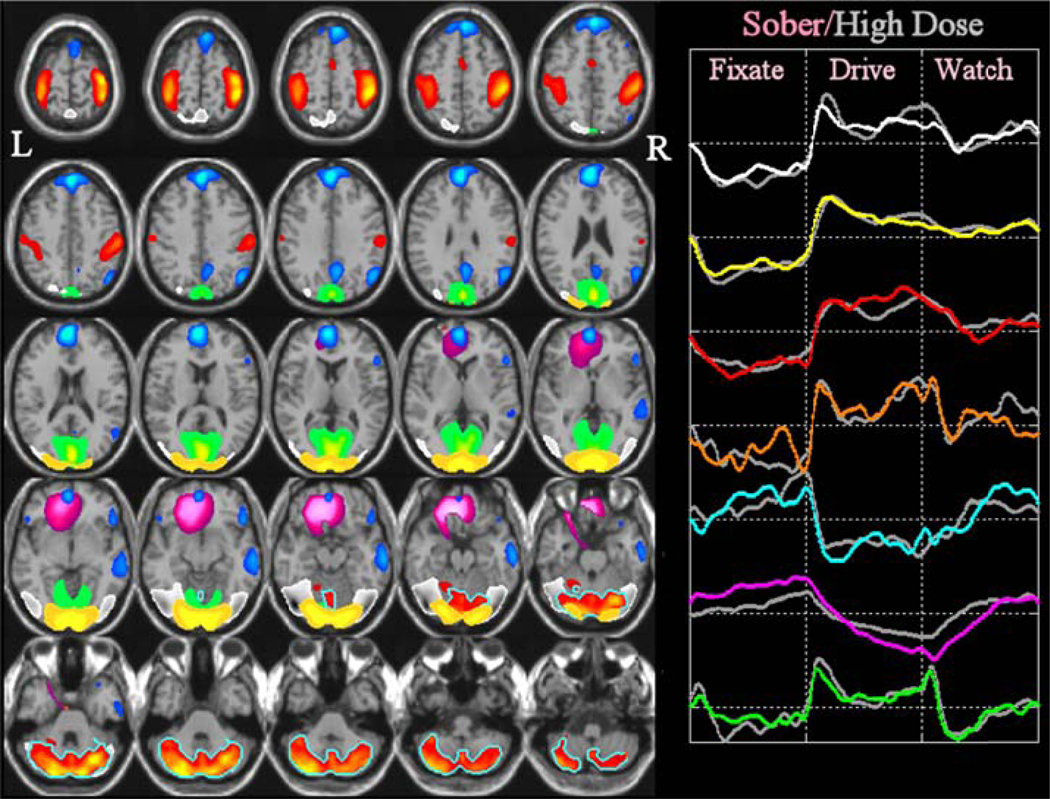
ICA imaging results from the analysis are summarized in Figure 2, with different colors coding for each component. The average independent component time courses for each experimental condition are presented in the right panel of Figure 2. For visualization, the three epoch cycles are averaged together and are presented as ‘fixation’, ‘drive’, and ‘watch’. The left panel of Figure 2 displays the average components-of-interest determined from the sober-condition data. Cerebellar networks are depicted in the same color as the motor and supplementary motor regions and are differentiated by a turquoise border surrounding the region. The right panel displays the average time courses from the sober-condition and high-dose-condition, color-coded as in the spatial component maps, with the alcohol condition indicated in gray.
Figure 2.
Results from the alcohol intoxication study: Group fMRI maps are thresholded at p<0.005 (corrected for multiple comparisons). A total of seven components are presented. A “green” component extends on both sides of the parieto-occipital sulcus including portions of cuneus, precuneus, and the lingual gyrus. A “yellow” component contains mostly occipital areas. A “white” component contains bilateral visual association and parietal areas; and a component consisting of motor areas is depicted in red. Cerebellar areas are also depicted in red (but with a turquoise border). Orbitofrontal and anterior cingulate areas identified are depicted in pink. Finally, a component including medial frontal, parietal, and posterior cingulate regions is depicted in blue. Group averaged time courses (right) for the fixate-drive-watch order are depicted with similar colors for the sober versus high-dose conditions (the drug condition is shown in gray). The three repeated epochs are averaged and presented as fixation, drive, and watch.
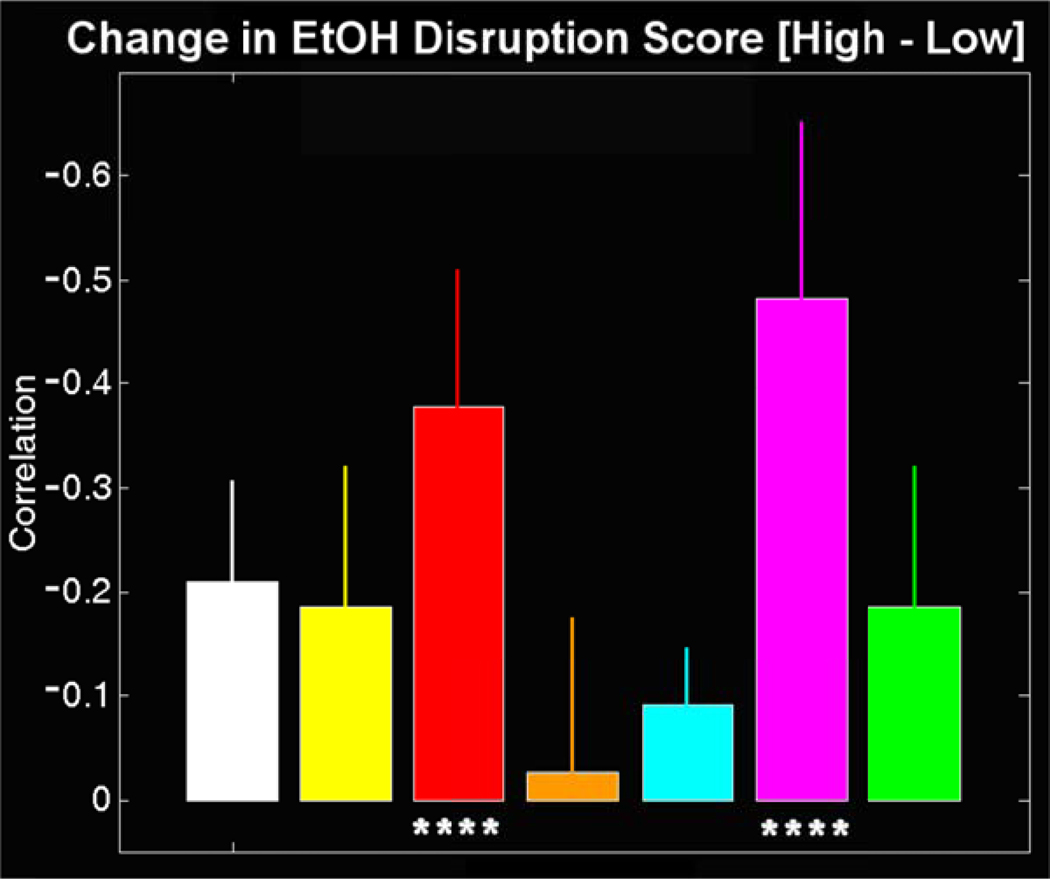
We examined the extent of alteration in the average component time courses as an effect of alcohol dose, to calculate a “disruption score” for each condition. This is a score indicating the degree to which alcohol intoxication changed the fMRI activity compared to the same-day sober scan. The time courses from the same-day sober scan were correlated to the corresponding intoxication scan. The disruption score is the difference in this correlation from high-dose to low-dose. The change in the component disruption score with respect to dose is shown in Figure 3 (color-coded as in Figure 2). All components were found to be more disrupted for the high-dose experiment than for the low-dose experiment (the changes are all negative). Significant differences were found for the regions indicated in the first analysis, namely, the orbitofrontal component and the primary motor/supplemental motor area (SMA) component (p<0.001). This is suggestive of a global change in the time course shape induced by intoxication, however there was no significant change in the amplitude of the BOLD signal, as reported in experiments involving sensory stimuli (Calhoun, et al. 2004; Levin, et al. 1998).
Figure 3.
Differences in disruption scores for the ICA time courses: Within-day correlations were computed between the sober condition and the drug condition on the same day as a measure of the amount of disruption induced by the EtOH. The differences in these correlations are presented for each component with color corresponding to Figure 2. The high dose condition was in all cases less correlated with its sober counterpart than was the low dose condition (all values are negative). Significant (p<0.001) differences were observed for the pink (orbitofrontal/anterior cingulate) and red (motor) components only.
A direct comparison of significant aggregate behavioral measures (vehicular collisions and speed over 140), alcohol dose, and fMRI signal, was performed by computing the dose-related correlation of the behavior results with the component time courses. All p values were greater than 0.2 except for two results: First, the cerebellar component exhibited a significant dose-related effect with driving greater than 140 (p<0.001, corrected). This may reflect less efficient processing of the complex motor coordination required at higher speeds. The dose-related cerebellar involvement is also consistent with previous studies implicating a detrimental effect of alcohol upon complex motor control (Peterson, et al. 1990) and alcohol associated decreases in cerebellar blood flow (Volkow, et al. 1988). Second, because their visual and attentional fields narrow, inebriated drivers are less responsive to peripheral events and often exhibit depressed perceptual and motor functioning (Mascord, et al. 1995).
Based on the anatomic regions which contribute most to each components, we can interpret them in terms of well-known neurophysiological networks as discussed in (Calhoun, et al. 2002). The seven components can be divided into four patterns with alcohol and speed-related effects (Calhoun, et al. 2002). Hypothesized functions (and short anatomic description) of these networks are 1) vigilance (fronto parietal); one of the first presentations of what was later coined the default model network, 2) error monitoring and inhibition (anterior cingulate and medial frontal), 3) motor (primary motor cortex), 4) higher order motor (cerebellar), 5) visual (lingual, cuneus), 6) higher order visual (fusiform, middle occipital), and 7) visual monitoring (cuneus, lingual, posterior cingulate). We delineated the networks affected by driving speed in the previous study, as well as the networks affected by alcohol dose in the current study. We have discussed the involvement of these circuits in the context of simulated driving in detail previously, and the reader is referred to this work for further discussion (Calhoun, et al. 2002; Groeger 2000).
Improved in-scanner simulation environment
Since this initial work was performed, we have developed a custom driving simulator which records continuous behavior during the driving task described above (Carvalho, et al. 2006). The driving interface and environment has also been modified. The hand-held controller has been replaced with a steering wheel and gas and brake pedals similar to those found in modern automobiles. The pedals are arranged in a position near the feet comparable to automobile pedals, and the steering wheel is placed just outside the scanner on a plastic table mounted above the participant. Both steering wheel and pedals are connected to computers outside the MRI room via a shielded cable. This allows continuous information on steering and pedal activity to be recorded. As before, the display is visible through a mirror mounted above the participant which lying prone in the MRI scanner. Using this new software and hardware, we can compare the dynamic fMRI time courses to the continuous behavior of each participant. This, in contrast to previous results comparing aggregate behavioral scores averaged across the entire session, enables us to compare behavior to brain activity at a much higher temporal resolution.
Follow-up study of EtOH and simulated driving
We recently replicated and significantly extended our earlier fMRI results on the impact of EtOH on brain activity during simulated driving. Our initial study involved 9 participants scanned on a 1.5T scanner using our initial simulator setup (Calhoun, et al. 2004). The more recent study included 40 participants (20 males) scanned on a 3T scanner using our custom simulator environment (Meda, et al. 2009). We used two complementary image analysis techniques to investigate alcohol-related changes in temporal dynamics of the driving circuitry at two dosage levels compared to placebo. We report five crucial networks including, orbito-frontal/anterior cingulate, fronto-temporal, primary/secondary motor, cerebellar and the resting state networks as being modulated by alcohol in a dose-related manner. As before, each subject received 3 separate single blind doses of beverage alcohol individualized to weight, and sex (Kapur 1989) designed to produce BACs of 0.05% (moderate) or 0.10% (high) and one placebo dose. 3T functional magnetic resonance imaging (fMRI) scanning along with continuous behavioral measurement was performed on subjects during simulated driving. Brain function was assessed and compared using both ICA and a conventional GLM analysis. ICA results replicated and significantly extended our previous 1.5T study (Calhoun, et al. 2004). GLM analysis revealed significant functional differences in several areas between the three doses, complementing the ICA results. The GLM analysis revealed a significant dose-dependent response in areas including the amygdala and parahippocampus. Further, we found consistent behavioral changes while driving intoxicated supporting our imaging results. Further, driving behaviors such as opposite white line crossings and mean speed independently demonstrated a significant dose-dependent change. Behavior-based factors also predicted the frontal-basal-temporal circuit to have a functional impairment with alcohol dosage across baseline scaled, good and poorly performing drivers. In this study we were able to reveal neural correlates of driving behavior and found dose related spatio-temporal disruptions in critical driving-associated regions including the superior, middle and orbito frontal gyri, ant erior cingulate, primary/supplementary motor areas, basal ganglia, and cerebellum.
A synthesis of the two major papers from our group and their correspondence with event-related driving data from Spiers et al 2007 is shown below in Table 1 (Spiers and Maguire 2007). These combined data allow us to make specific hypotheses regarding intoxicated driving. For example, alcohol disrupts the fidelity of visuospatial performance, especially for more complex images, likely by interfering with higher order visual and parietal circuits and interpretation of complex images may be more affected by alcohol intoxication (Leone and McCourt 2010; Miller and Fillmore 2010). In addition, a frontal-parietal network was identified by (Van Horn, et al. 2006) as most affected by alcohol consumption, and modulated by visual feedback. Thus we predict that alcohol’s impact on the "white” circuit from (Calhoun, et al. 2004) will be associated with a failure to appreciate and to deal with road obstacles and other vehicles in a timely manner, leading to accidents. Such an association would only be apparent in an event-related design and was therefore not detected in our work to date. If validated, such a hypothesis would be useful, as it links a putative global effect of alcohol on the neural basis of perception to "downstream" effects on other neural circuits, validated by behavioral measurements. Additionally we would predict that goal directed visuo-motor performance, for example a task involving steering accurately around cones, would be particularly disrupted by alcohol and involve significant alterations in FNC between the higher-order visual and error monitoring/inhibition/attention span and executive/attention/motor planning circuitry delineated in Table 1.
Table 1.
Major driving-related regions and the associated behavioral effects of high-dose alcohol in our prior research (Calhoun, et al. 2004; Meda, et al. 2009). Because each component/network comprises multiple regions, these are referred to in “shorthand” in the table by the color code from Meda 2009 shown in the left-most column above. Using functional network connectivity we showed alcohol-related connectivity disruption between the pink and green circuits, the correlated with unstable steering (Rzepecki, et al. 2010).
| Component | Major Regions | Role In Driving | Alcohol Disrupts |
Alcohol Behavioral Consequences (our work) |
Role in Spiers |
|
|---|---|---|---|---|---|---|
| (a) Meda (Meda, et al. 2009) | (b) Calhoun (Calhoun, et al. 2004) | |||||
| Blue “Motor” | Red | Pre + post-central gyri, Frontal- sup. & middle SPL/IPL cingulate | Gross/Fine Motor Control | (a) (b) | Crashes, Median Crossings | Avoiding Collisions |
| Pink “Cerebellar” | Orange/ Turquois | Cerebellum | Find Motor Control | (b) | FNC uncorrelated, unstable steering | Action Planning |
| Orange “Default Mode” | Blue | PCC, Precuneus, ACC | Baseline Resting State | (b) | Avoiding Collisions, Swerving | |
| Green “Frontal- Basal Ganglia” | Not Identified | Medial occipital, IPL, cingulate, striatum, ACC, STG, Insula, MFG/MEdFG/SFG, thalamus | Executive/Attention, Motor Planning | (b) | Higher Speed, Steering Weave, Passenger Line Cross | Monitoring Traffic, Action Planning |
| Red “Orbito Frontal” | Pink | Frontal- Sup/Inf/Med (inc. BA 9, 46), ACC, Orbito-Frontal | Error Monitoring, Inhibition, Attention Span | (a) | Avoiding Collisions | |
| Identified but un- affected by alcohol | (White) | Visual-Association, Parietal | Higher Order Visual | Action Planning, Monitoring Traffic | ||
| As Above | (Yellow) | Occipital | Primary Visual | Monitoring Traffic | ||
An important question is how best to integrate our collective observations to date into modern cognitive neuroscience paradigms. AR Laird (personal communication) using the BrainMap analysis approach (Laird, et al. 2009) recently examined multiple literature-derived circuits in BrainMap, each of which is commonly co-activated under similar task conditions. The corresponding circuit to our Blue "motor" network (Meda, et al. 2009) in Laird’s unpublished work for example includes primary sensorimotor cortices for the upper extremities and was associated with action and somesthesis corresponding to hand movements (such as finger tapping, grasping, pointing and tactile stimulation) and included such tasks as grasping and pointing that comprised ventral precentral gyri, central sulci, postcentral gyri, superior and inferior cerebellum and primary sensorimotor cortices representing the upper extremities that corresponds to one or more circuits we have identified using ICA.
Overall, results suggest that alcohol causes functional impairments localized to brain regions related to motor planning and control, goal directedness, error monitoring and memory. Overall, our findings might imply a significant impairment in attention, cognitive, goal direction, motor planning and emotional/working memory related functional capabilities while driving under the influence of alcohol.
Hybrid driving and visual oddball task
Prior studies report that accidents involving intoxicated drivers are more likely to occur during performance of secondary tasks. We studied this phenomenon, using a dual-task paradigm, involving performance of a visual oddball (VO) task while driving in an alcohol challenge paradigm (Allen, et al. 2009). Previous functional MRI (fMRI) studies of the VO task have shown activation in the anterior cingulate, hippocampus, and prefrontal cortex. Thus, we predicted dose-dependent decreases in activation of these areas during VO performance. Forty healthy social drinkers were administered three different doses of alcohol, individually tailored to their gender and weight. Participants performed a VO task while operating a virtual reality driving simulator in a 3T fMRI scanner. Analysis showed a dose-dependent linear decrease in blood oxygenation-level dependent activation during task performance, primarily in hippocampus, anterior cingulate, and dorsolateral prefrontal areas, with the least activation occurring during the high dose. Behavioral analysis showed a dose-dependent linear increase in reaction time, with no effects associated with either correct hits or false alarms. In all dose conditions, driving speed decreased significantly after a VO stimulus. However, at the high dose this decrease was significantly less. Passenger-side line crossings significantly increased at the high dose. These results suggest that driving impairment during secondary task performance may be associated with alcohol-related effects on the above brain regions, which are involved with attentional processing/decision-making. Drivers with high blood alcohol concentrations may be less able to orient or detect novel or sudden stimuli such as road obstacles during driving. The hippocampus is known to be associated with visuospatial memory (Burgess 2002) in the case of our observations, this represents likely underpinning of the ability to remember the vehicle's spatial location on the road prior to target-related distraction. The decreased alcohol-associated hippocampal activation we measured is likely linked to our observation that more driving errors occurred following target stimuli.
We also evaluated brain activity during a go no-go task collected separately from the simulated driving task (Anderson, et al. 2011). Fifty-one healthy volunteers were studied. Alcohol increased time needed to respond to stimuli and frequency of inappropriate error responses, i.e. false alarm errors in a dose-dependent manner. FMRI showed alcohol decreased activity in both the anterior cingulate and inferior frontal gyrus during correct rejections and false alarm responses and this also occurred in a dose-dependent manner. No statistically significant reaction time impairments were visible at the moderate alcohol dose, consistent with the adoption of a breath alcohol concentration of 0.08% as the legal driving limit in many states in the USA. Overall, these findings suggest that acute alcohol use impairs cognitive control through a dose-dependent decrease in cortical activation and that regions implicated in error monitoring are affected by alcohol and associated with impaired behavioral performance. Both anterior cingulate and inferior frontal gyrus were also identified in our previous simulated driving studies, as well as being associated with aspects of error processing.
Inter-network analysis
In the Meda study (Meda, et al. 2009), our group identified five, independent critical driving-associated brain circuits whose inter-regional connectivity was disrupted by alcohol intoxication. Moving beyond within-network connectivity, we can also evaluated the connectivity among networks, called functional network connectivity (FNC) (Jafri, et al. 2008). We had previously reported (Anderson, et al. 2011) that alcohol compromised cerebellar activation but with no differences between moderate and high alcohol doses in the context of a cognitive control task. This region is implicated in numerous limbic, sensory and motor functions (Schmahmann, et al. 2007) its decreased activation under acute alcohol conditions is previously documented (Gundersen, et al. 2008; Van Horn, et al. 2006) and may underlie alcohol-provoked impairment seen in mood, behavior, cognition and motor activity (Volkow, et al. 2008). In particular, decreased cerebral activity may be directly linked with the poor motor coordination often associated with alcohol intoxication.
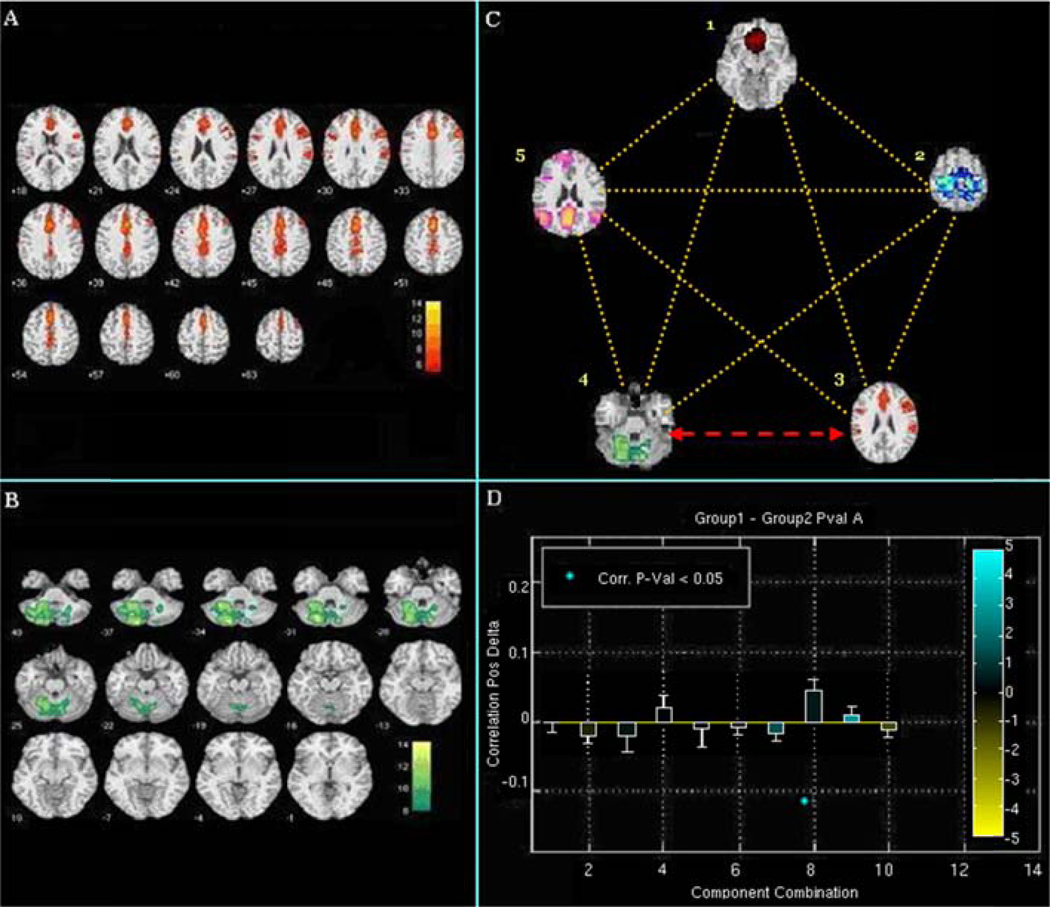
In recent work, we were able to explore the above ideas in more depth. We evaluated the FNC between driving-related circuits in order to determine how these networks communicate with each other during sober and alcohol-intoxicated states (Rzepecki, et al. 2010). We explored such differences in connections between the above brain circuits and driving behavior, in forty individuals under the influence of alcohol versus placebo. During the active dose, we found specific disruptions of functional network connectivity between the frontal cortex-basal ganglia and the cerebellar circuits (see Figure 7). The temporal connectivity between these two circuits was found to be less correlated (p <0.05) when driving under the influence of alcohol. This disconnection was also associated with an abnormal driving behavior (unstable motor vehicle steering). Connections between frontal-basal ganglia and cerebellum have recently been explored; these may be responsible in part for maintaining normal motor behavior by integrating their overlapping motor control functions. These connections appear to be disrupted by alcohol intoxication, in turn associated with an explicit type of impaired driving behavior.
Figure 7.
Correlation differences of circuit combinations for sober versus alcohol condition: (A) Axial slices of basal ganglia component. (B) Axial slices of cerebellum component. (C) Image of all five independent critical driving-associated brain circuits (1) anterior cingulate, middle and orbito frontal gyri, (2) primary/secondary motor cortex, (3) fronto-basal ganglia, (4) cerebellum, and (5) resting state. Yellow dotted lines show network connections which do not differ significantly between baseline sober and alcohol intoxication conditions; the red arrow shows the network connection between the fronto-basal ganglia and cerebellar circuits which differs significantly between baseline sober and alcohol intoxication conditions (D) Graphical representation of correlations between different network combinations.
Combining imaging and behavior
In our newer simulation environment we are able to record continuous behavior. We thus have information about multiple behavioral measures in addition to the multiple intrinsic networks that can be extracted from the brain imaging data. Such data is highly suitable for analysis with multivariate approaches, hence enabling a fusion of the imaging and behavioral data. We have successfully used the group ICA approach to evaluate the relationship of continuous driving behavior with the driving related intrinsic networks (Carvalho, et al. 2006).
In other work, we apply a novel statistical method, multi-set canonical correlation analysis (M-CCA), to study the fMRI datasets acquired during the simulated driving task (Li, et al. In Press). The M-CCA method jointly decomposes fMRI datasets from different subjects/sessions into brain activation maps and their associated time courses, such that the correlation in each group of estimated activation maps across datasets is maximized. Therefore, the functional activations across all datasets are extracted in the order of consistency across different dataset. On the other hand, M-CCA preserves the uniqueness of the functional maps estimated from each dataset by avoiding concatenation of different datasets in the analysis. Hence, the cross-dataset variation of the functional activations can be used to test the hypothesis of functional-behavioral association. We studied 120 simulated driving fMRI datasets and identify parietal-occipital regions and frontal lobe as the most consistently engaged areas across all the subjects and sessions during simulated driving. The functional behavioral association study indicates that all the estimated brain activations are significantly correlated with the steering operation during the driving task. M-CCA thus provides a new approach to investigate the complex relationship between the brain functions and multiple behavioral variables, especially in naturalistic tasks as demonstrated by the simulated driving study.
The future
fMRI driving studies still require improvement in design and strategy. For example, our prior research did not employ maximally realistic driving software, multiple driving scenarios or an event-related design. With regard to the latter, from a cognitive neuroscience viewpoint, the multiple task-relevant cognitive networks involved in driving are required to change rapidly to keep pace with the varying, (sometimes rapidly) environmental demands of this complex task, implying the possibility of moment-to-moment reallocation of brain regions among varied task components. Parsing these changes and inter-relationships optimally requires event-related designs; however few investigators have used such paradigms in this specific context. Another facet of this question is that motor vehicle accidents are rare events that are essential to study because of their importance, yet cannot be captured in block design fMRI, because when occurring uncommonly they are averaged into signals produced by non-emergency everyday driving scenarios. On the other hand when presented frequently in “blocks” to allow conventional analysis, they become predictable by research subjects and are no longer naturalistic These observations emphasize the need for event-related driving scenarios, whose importance lies in part in the recognition that serious accidents are robustly linked to the occasional errors and momentary lapses seen when real-life driving behavior is closely examined in non-virtual paradigms (Boyle, et al. 2008). These lapses are believed to be the proximate cause of failure to react effectively to road hazards or similar changes in driving conditions. To date, the single published fMRI driving study to use an event-related design (Spiers and Maguire 2007) was a significant step forward, but neither examined intoxicated driving, nor did it use an ICA-based design to fully assess data; it was also based on a driving videogame not designed to examine accident conditions in detail.
In terms of analytic approaches, the study of naturalistic tasks, simulated driving in particular, would benefit from more functional connectivity studies. In our own work we have found that this provides an extremely rich source of information which has led to the generation of new hypotheses as well as to a more complete study of the brain dynamics involved in a complex task that is afforded by a standard GLM analysis (Calhoun, et al. 2002). To date, very few other studies of simulated driving have use connectivity or network-based approaches, it is critical for such studies to bring additional analytic approaches to bear in order to fully capitalize on the complexity of the functional dynamics involved. This is even more important when studying modifications to these dynamics (such as in the case of alcohol intoxication that we have discussed in this article).
As we have reviewed, studying intoxicated driving allows one to determine how a widely socially used intoxicant (that is straightforward and safe to administer and measure in laboratory conditions) affects the above interactions, using functional network connectivity measures in a socially relevant context. Acute administration of any psychoactive substance however inevitably involves complex interactions between drug, internal set and experimental setting, extending beyond, in this case, blood alcohol concentrations and the nature of the driving paradigm, including temperamental characteristics of the driver, mood and triggers by other drivers’ behavior. All of these are potentially amenable to future study, (e.g. in the latter case by manipulating avatars in VR) and in terms of affective components, by examination of mood-related circuitry that is extractable from fMRI signal even in non-affectively-based tasks using ICA strategies.
Figure 4.
Screen Shot from Driving Simulator and Snapshot of Hardware setup: The picture on the left shows a typical screen shot taken from the driving simulation program. Pedestrians are a relatively common sight, as are cars, especially at intersections. The picture on the right shows the inside of the scanner room where the participant is scanned. The steering wheel is located just outside the scanner in a position comfortable for the participant. Pedals are located where the feet naturally fall in a position comparable to vehicle pedals.
Figure 5.
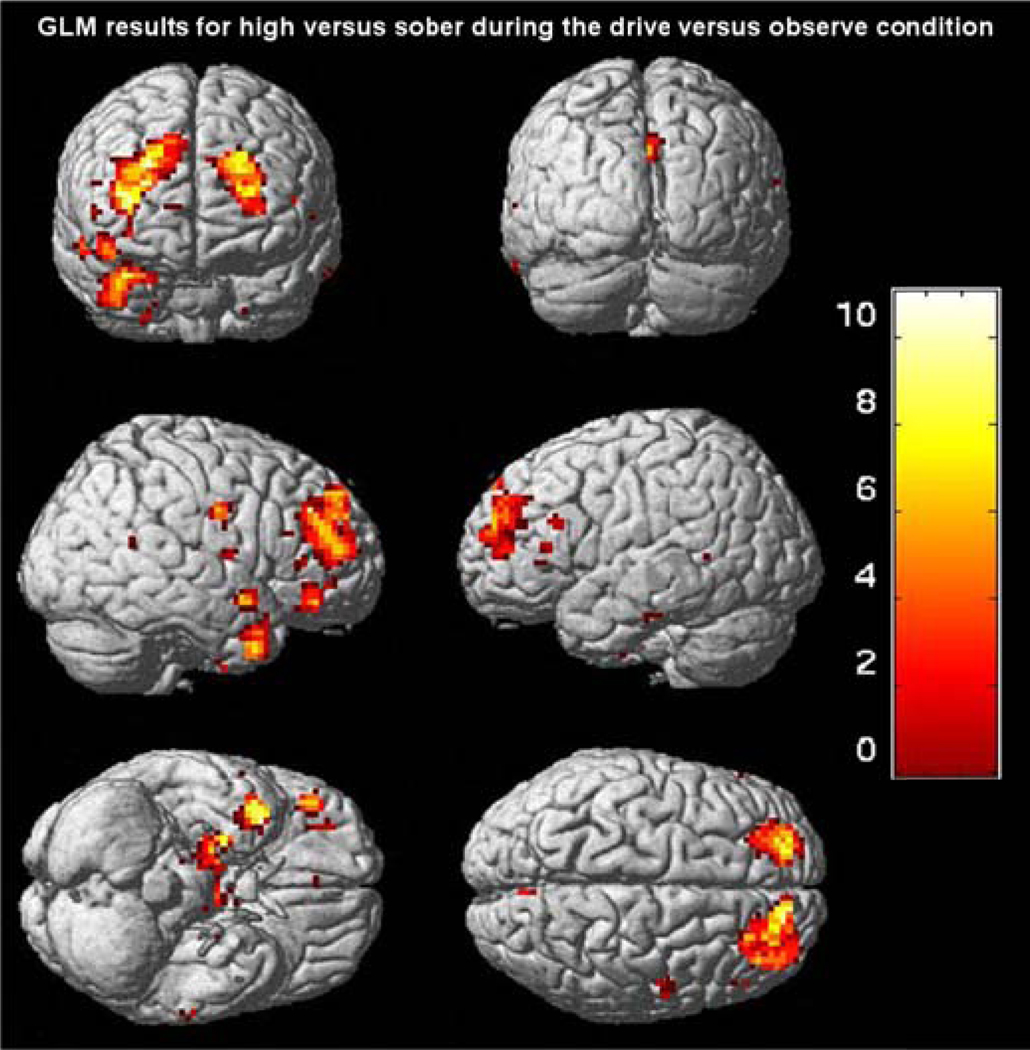
GLM results for high versus sober during the drive versus observe condition: Regions that demonstrated a significantly lower functional activation (thresholded at p = 0.05 FDR corrected) during the high dose relative to placebo condition. Maps were derived from a random effects (RFX) repeated measures ANOVA comparing the three dosages conducted through a standard GLM analysis in SPM2
Figure 6.
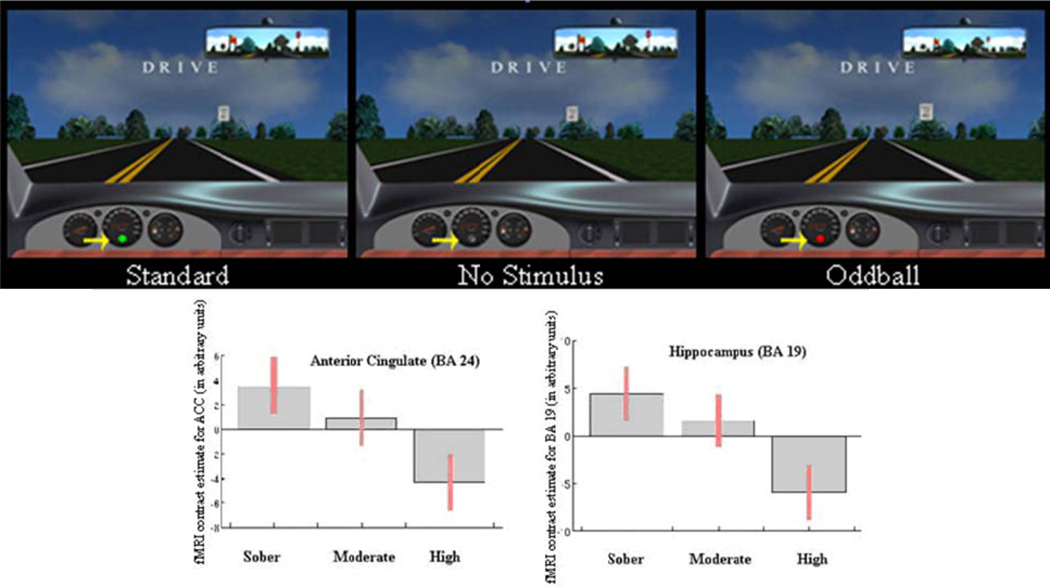
Response to oddball task at different EtOH doses: (top) Screen shots of the driving software, with arrow pointing to: 1. standard presentation; 2. no stimulus presentation; 3. oddball presentation. (bottom) Contrast plots showing the dose-dependent linear trend of the noted brain activations (BA 19, BA 24) in the targets vs. standard comparison.
Figure 8.
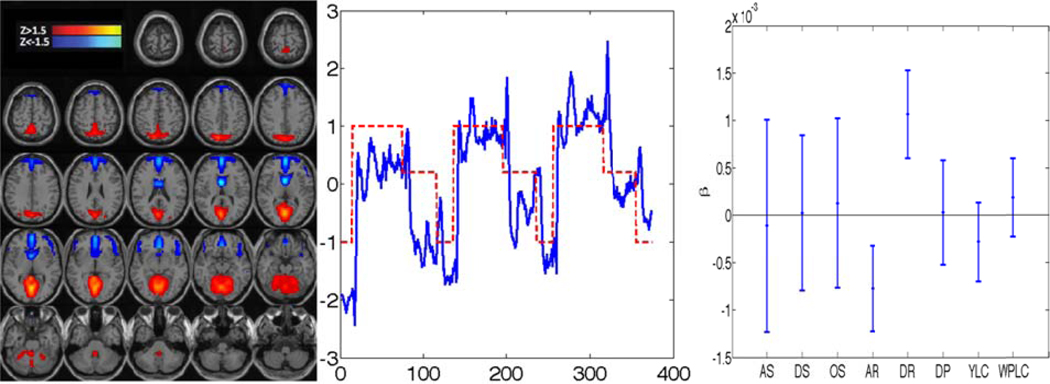
Example of CCA results integrating behavioral and imaging data: The group activation map is shown on the left, mean time course overlaid with the paradigm for three repetitions of [F]ixation-[D]riving-[W]atching in the middle, and confidence interval (CI) of behavioral correlation on the right. This map shows correlation in parieto-occipital regions and anticorrelation in medial frontal regions. The time course has high regression coefficients associated with the driving paradigm (0.48 ± 0.20)—indicating that the frontal and parieto-occipital brain regions are highly consistent across all the subjects when performing the driving task. Among the eight behavioral factors defined in Section 3.5, this component has significant association with the average and differential of steering weave.
Acknowledgement
The research was support by the National Institutes of Health under grants R01 EB 000840 (to VDC) and by RO1 AA015615 and R01AA016599 (to GDP).
References
- Motor vehicle traffic crash fatality counts and estimates of people injured for 2006. Administration NHTS. 2007 [Google Scholar]
- Traffic Safety Facts-2007. Administration NHTS. 2008 [Google Scholar]
- Abou-Elseoud A, Starck T, Remes J, Nikkinen J, Tervonen O, Kiviniemi V. The effect of model order selection in group PICA. Hum Brain Mapp. 2010;31(8):1207–1216. doi: 10.1002/hbm.20929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahveninen J, Jaaskelainen IP, Pekkonen E, Hallberg A, Hietanen M, Naatanen R, Sillanaukee P. Global field power of auditory N1 correlates with impaired verbal-memory performance in human alcoholics. Neurosci.Lett. 2000;285(2):131–134. doi: 10.1016/s0304-3940(00)01041-7. [DOI] [PubMed] [Google Scholar]
- Allen AJ, Meda S, Skudlarski P, Calhoun VD, Astur R, Ruopp K, Pearlson GD. Effects of alcohol on performance on a distraction task during simulated driving. Alcoholism: Clinical & Experimental Research. 2009;33(1):1–9. doi: 10.1111/j.1530-0277.2008.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E, Erhardt E, Damaraju E, Gruner W, Segall J, Silva R, Havlicek M, Rachakonda S, Fries J, Kalyanam R, Michael A, Turner J, Eichele T, Adelsheim S, Bryan A, Bustillo JR, Clark VP, Feldstein S, Filbey FM, Ford C, Hutchison K, Jung R, Kiehl KA, Kodituwakku P, Komesu Y, Mayer AR, Pearlson GD, Phillips J, Sadek J, Stevens M, Teuscher U, Thoma RJ, Calhoun VD. A baseline for the multivariate comparison of resting state networks. Frontiers in Human Neuroscience. 2011;1:12. doi: 10.3389/fnsys.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B, Stevens MC, Meda S, Jordan M, Calhoun VD, Pearlson GD. Functional Imaging of Cognitive Control During Acute Alcohol Intoxication. Alcoholism: Clinical and Experimental Research. 2011;35(1):156–165. doi: 10.1111/j.1530-0277.2010.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnedt JT, Wilde GJ, Munt PW, MacLean AW. How do prolonged wakefulness and alcohol compare in the decrements they produce on a simulated driving task? Accid.Anal.Prev. 2001;33(3):337–344. doi: 10.1016/s0001-4575(00)00047-6. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SA, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang L, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107(10):4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer SM, Hsieh L, Moran JE, Young RA, Manoharan A, Liao CC, Malladi K, Yu YJ, Chiang YR, Tepley N. Conversation effects on neural mechanisms underlying reaction time to visual events while viewing a driving scene using MEG. Brain Res. 2009;1251:151–161. doi: 10.1016/j.brainres.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle LN, Tippin J, Paul A, Rizzo M. Driver Performance in the Moments Surrounding a Microsleep. Transp Res Part F Traffic Psychol Behav. 2008;11(2):126–136. doi: 10.1016/j.trf.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N. The hippocampus, space, and viewpoints in episodic memory. Q J Exp Psychol A. 2002;55(4):1057–1080. doi: 10.1080/02724980244000224. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A Method for Making Group Inferences from Functional MRI Data Using Independent Component Analysis. Hum.Brain Map. 2001;14(3):140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD. Modulation of Temporally Coherent Brain Networks Estimated using ICA at Rest and During Cognitive Tasks. Hum Brain Mapp. 2008;29(7):828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Pekar JJ, McGinty VB, Adali T, Watson TD, Pearlson GD. Different activation dynamics in multiple neural systems during simulated driving. Hum.Brain Map. 2002;16(3):158–167. doi: 10.1002/hbm.10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Pekar JJ, Pearlson GD. Alcohol Intoxication Effects on Simulated Driving: Exploring Alcohol-Dose Effects on Brain Activation Using Functional MRI. Neuropsychopharmacology. 2004;29:2097–2107. doi: 10.1038/sj.npp.1300543. [DOI] [PubMed] [Google Scholar]
- Callan AM, Osu R, Yamagishi Y, Callan DE, Inoue N. Neural correlates of resolving uncertainty in driver's decision making. Hum Brain Mapp. 2009;30(9):2804–2812. doi: 10.1002/hbm.20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho K, Pearlson GD, Astur RS, Calhoun VD. Simulated Driving and Brain Imaging: Combining Behavior, Brain Activity, and Virtual Reality. CNS Spectrum. 2006;11(1):52–62. doi: 10.1017/s1092852900024214. [DOI] [PubMed] [Google Scholar]
- Colrain IM, Taylor J, McLean S, Buttery R, Wise G, Montgomery I. Dose dependent effects of alcohol on visual evoked potentials. Psychopharmacology (Berl) 1993;112(2–3):383–388. doi: 10.1007/BF02244937. [DOI] [PubMed] [Google Scholar]
- Deery HA, Fildes BN. Young novice driver subtypes: relationship to high-risk behavior, traffic accident record, and simulator driving performance. Hum.Factors. 1999;41(4):628–643. doi: 10.1518/001872099779656671. [DOI] [PubMed] [Google Scholar]
- Erhardt E, Rachakonda S, Bedrick E, Adali T, Calhoun VD. Comparison of multi-subject ICA methods for analysis of fMRI data. Hum Brain Mapp. doi: 10.1002/hbm.21170. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Schoenmakers T, Wiers RW. Cognitive processes in alcohol binges: a review and research agenda. Curr Drug Abuse Rev. 2008;1(3):263–279. doi: 10.2174/1874473710801030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Blackburn JS, Harrison EL. Acute disinhibiting effects of alcohol as a factor in risky driving behavior. Drug Alcohol Depend. 2008;95(1–2):97–106. doi: 10.1016/j.drugalcdep.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillmore MT, Ostling EW, Martin CA, Kelly TH. Acute effects of alcohol on inhibitory control and information processing in high and low sensation-seekers. Drug Alcohol Depend. 2009;100(1–2):91–99. doi: 10.1016/j.drugalcdep.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeger J. Understanding Driving: Applying cognitive psychology to a complex everyday task. New York: Psychology Press; 2000. [Google Scholar]
- Gundersen H, Gruner R, Specht K, Hugdahl K. The effects of alcohol intoxication on neuronal activation at different levels of cognitive load. Open Neuroimag J. 2008;2:65–72. doi: 10.2174/1874440000802010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haier R, Schandler S, MacLachlan A, Soderling E, Buchsbaum M, Cohen M. Alcohol induced changes in regional cerebral glucose metabolic rate during divided attention. Personality & Individual Differences. 1999;26(3):425–439. [Google Scholar]
- Hsieh L, Young RA, Bowyer SM, Moran JE, Genik RJ, 2nd, Green CC, Chiang YR, Yu YJ, Liao CC, Seaman S. Conversation effects on neural mechanisms underlying reaction time to visual events while viewing a driving scene: fMRI analysis and asynchrony model. Brain Res. 2009;1251:162–175. doi: 10.1016/j.brainres.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Jafri M, Pearlson GD, Stevens M, Calhoun VD. A Method for Functional Network Connectivity Among Spatially Independent Resting-State Components in Schizophrenia. NeuroImage. 2008;39:1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancke L, Brunner B, Esslen M. Brain activation during fast driving in a driving simulator: the role of the lateral prefrontal cortex. Neuroreport. 2008;19(11):1127–1130. doi: 10.1097/WNR.0b013e3283056521. [DOI] [PubMed] [Google Scholar]
- Jones BM. Memory impairment on the ascending and descending limbs of the blood alcohol curve. J.Abnorm.Psychol. 1973;82(1):24–32. doi: 10.1037/h0034872. [DOI] [PubMed] [Google Scholar]
- Just MA, Keller TA, Cynkar J. A decrease in brain activation associated with driving when listening to someone speak. Brain Res. 2008;1205:70–80. doi: 10.1016/j.brainres.2007.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur BM. Computer Blood Alcohol Calculator v1.20 ARF Software. Toronto, Canada: Addiction Research Foundation; 1989. [Google Scholar]
- Kiviniemi V, Starck T, Remes J, Long X, Nikkinen J, Haapea M, Veijola J, Moilanen I, Isohanni M, Zang YF. Functional segmentation of the brain cortex using high model order group PICA. Hum Brain Mapp. 2009;30:3865–3886. doi: 10.1002/hbm.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleykamp BA, Griffiths RR, Mintzer MZ. Dose effects of triazolam and alcohol on cognitive performance in healthy volunteers. Exp Clin Psychopharmacol. 2010;18(1):1–16. doi: 10.1037/a0018407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krull KR, Smith LT, Sinha R, Parsons OA. Simple reaction time event-related potentials: effects of alcohol and sleep deprivation. Alcohol Clin.Exp.Res. 1993;17(4):771–777. doi: 10.1111/j.1530-0277.1993.tb00839.x. [DOI] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, Robinson JL, Lancaster JL, Fox PT. ALE Meta-Analysis Workflows Via the Brainmap Database: Progress Towards A Probabilistic Functional Brain Atlas. Front Neuroinformatics. 2009;3:23. doi: 10.3389/neuro.11.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb MR, Robertson LC. Effect of acute alcohol on attention and the processing of hierarchical patterns. Alcohol Clin.Exp.Res. 1987;11(3):243–248. doi: 10.1111/j.1530-0277.1987.tb01298.x. [DOI] [PubMed] [Google Scholar]
- Lee JD. Fifty years of driving safety research. Hum Factors. 2008;50(3):521–528. doi: 10.1518/001872008X288376. [DOI] [PubMed] [Google Scholar]
- Lee JD. Engineering. Can technology get your eyes back on the road? Science. 2009;324(5925):344–346. doi: 10.1126/science.1168085. [DOI] [PubMed] [Google Scholar]
- Lee YC, Lee JD, Boyle LN. The interaction of cognitive load and attention-directing cues in driving. Hum Factors. 2009;51(3):271–280. doi: 10.1177/0018720809337814. [DOI] [PubMed] [Google Scholar]
- Lees MN, Lee JD. The influence of distraction and driving context on driver response to imperfect collision warning systems. Ergonomics. 2007;1250(8):1264–1286. doi: 10.1080/00140130701318749. [DOI] [PubMed] [Google Scholar]
- Leone L, McCourt ME. The effect of acute ethanol challenge on global visuospatial attention: exaggeration of leftward bias in line bisection. Laterality. 2010;15(3):327–342. doi: 10.1080/13576500902781745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin JM, Ross MH, Mendelson JH, Kaufman MJ, Lange N, Maas LC, Mello NK, Cohen BM, Renshaw PF. Reduction in BOLD fMRI response to primary visual stimulation following alcohol ingestion. Psychiatry Res. 1998;82(3):135–146. doi: 10.1016/s0925-4927(98)00022-5. [DOI] [PubMed] [Google Scholar]
- Li Y, Adali T, Calhoun VD. Estimating the number of independent components for fMRI data. Hum.Brain Map. 2007;28(11):1251–1266. doi: 10.1002/hbm.20359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Adali T, Eichele T, Calhoun VD. Group study of simulated driving fMRI data by multiset canonical correlation analysis. IEEE Journal of Signal Proc Sys. doi: 10.1007/s11265-010-0572-8. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnoila M, Mattila MJ. Interaction of alcohol and drugs on psychomotor skills as demonstrated by a driving simulator. Br.J.Pharmacol. 1973;47(3):671P–672P. [PMC free article] [PubMed] [Google Scholar]
- Mader M, Bresges A, Topal R, Busse A, Forsting M, Gizewski ER. Simulated car driving in fMRI--Cerebral activation patterns driving an unfamiliar and a familiar route. Neurosci Lett. 2009;464(3):222–227. doi: 10.1016/j.neulet.2009.08.056. [DOI] [PubMed] [Google Scholar]
- Mascord D, Walls J, Starmes G, Hartley L. Fatigue and alcohol: interactive effects on human performance in driving-related tasks. Fatigues and Driving: Driver impairment, driver fatigue, and driving simulation. London: Taylor and Francis; 1995. pp. 189–205. [Google Scholar]
- Mathew RJ, Wilson WH. Regional cerebral blood flow changes associated with ethanol intoxication. Stroke. 1986;17(6):1156–1159. doi: 10.1161/01.str.17.6.1156. [DOI] [PubMed] [Google Scholar]
- McGinty VB, Shih RA, Garrett ES, Calhoun VD, Pearlson GD. Assessment of Intoxicated Driving with a Simulator: A Validation Study with on Road Driving. Proc.Human Centered Trans.Sim.Conf. 2001:11–19. [Google Scholar]
- Meda S, Calhoun VD, Astur R, Turner B, Ruopp K, Pearlson GD. Alcohol dose effects on brain circuits during simulated driving: An fMRI study. Hum Brain Mapp. 2009;30(4):1257–1270. doi: 10.1002/hbm.20591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Fillmore MT. The effect of image complexity on attentional bias towards alcohol-related images in adult drinkers. Addiction. 2010;105(5):883–890. doi: 10.1111/j.1360-0443.2009.02860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungas D, Ehlers CL, Wall TL. Effects of acute alcohol administration on verbal and spatial learning. Alcohol Alcohol. 1994;29(2):163–169. [PubMed] [Google Scholar]
- Parker ES, Alkana RL, Birnbaum IM, Hartley JT, Noble EP. Alcohol and the disruption of cognitive processes. Arch.Gen.Psychiatry. 1974;31(6):824–828. doi: 10.1001/archpsyc.1974.01760180064008. [DOI] [PubMed] [Google Scholar]
- Peterson JB, Rothfleisch J, Zelazo PD, Pihl RO. Acute alcohol intoxication and cognitive functioning. Journal of Studies on Alcohol. 1990;51(2):114–122. doi: 10.15288/jsa.1990.51.114. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. Neuroimage. 2001;14(1 Pt 1):7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc.Natl.Acad.Sci.U.S.A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm DC, Sininger RA, Faherty JD, Whitley MD, Perl MB. A balanced placebo investigation of the effects of alcohol vs. alcohol expectancy on simulated driving behavior. Addict.Behav. 1982;7(1):27–32. doi: 10.1016/0306-4603(82)90021-1. [DOI] [PubMed] [Google Scholar]
- Rosen LJ, Lee CL. Acute and chronic effects of alcohol use on organizational processes in memory. J.Abnorm.Psychol. 1976;85(3):309–317. doi: 10.1037//0021-843x.85.3.309. [DOI] [PubMed] [Google Scholar]
- Ryback RS. The continuum and specificity of the effects of alcohol on memory. A review. Q.J.Stud.Alcohol. 1971;32(4):995–1016. [PubMed] [Google Scholar]
- Rzepecki CI, Meda SA, Calhoun VD, Jafri MJ, Astur RS, Pearlson GD. Disruptions in Functional Network Connectivity during Alcohol Intoxicated Driving. Alcoholism: Clinical and Experimental Research. 2010;34(3):479–487. doi: 10.1111/j.1530-0277.2009.01112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier MA. Changes in EEG alpha power during simulated driving: a demonstration. Int.J.Psychophysiol. 2000;37(2):155–162. doi: 10.1016/s0167-8760(00)00079-9. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of the cerebellum -insights from the clinic. Cerebellum. 2007;6(3):254–267. doi: 10.1080/14734220701490995. [DOI] [PubMed] [Google Scholar]
- Schwartz JA, Speed NM, Gross MD, Lucey MR, Bazakis AM, Hariharan M, Beresford TP. Acute effects of alcohol administration on regional cerebral blood flow: the role of acetate. Alcohol Clin.Exp.Res. 1993;17(6):1119–1123. doi: 10.1111/j.1530-0277.1993.tb05217.x. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA. Neural substrates of driving behaviour. Neuroimage. 2007;36(1):245–255. doi: 10.1016/j.neuroimage.2007.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Desmond JE, Lim KO, Pfefferbaum A. Speed and efficiency but not accuracy or timing deficits of limb movements in alcoholic men and women. Alcohol Clin.Exp.Res. 2002;26(5):705–713. [PubMed] [Google Scholar]
- Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcohol Clin.Exp.Res. 2001;25(2):236–245. [PubMed] [Google Scholar]
- Tarter RE, Arria AM, Van Thiel DH. Hepatic encephalopathy coexistent with alcoholism. Recent Dev.Alcohol. 1991;9:205–224. [PubMed] [Google Scholar]
- Tarter RE, Jones BM. Absence of intellectual deterioration in chronic alcoholics. J.Clin.Psychol. 1971;27(4):453–455. [PubMed] [Google Scholar]
- Tiihonen J, Kuikka J, Hakola P, Paanila J, Airaksinen J, Eronen M, Hallikainen T. Acute ethanol-induced changes in cerebral blood flow. Am.J.Psychiatry. 1994;151(10):1505–1508. doi: 10.1176/ajp.151.10.1505. [DOI] [PubMed] [Google Scholar]
- Uchiyama Y, Ebe K, Kozato A, Okada T, Sadato N. The neural substrates of driving at a safe distance: a functional MRI study. Neurosci Lett. 2003;352(3):199–202. doi: 10.1016/j.neulet.2003.08.072. [DOI] [PubMed] [Google Scholar]
- Van Horn JD, Yanos M, Schmitt PJ, Grafton ST. Alcohol-induced suppression of BOLD activity during goal-directed visuomotor performance. Neuroimage. 2006;31(3):1209–1221. doi: 10.1016/j.neuroimage.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Verster JC, Volkerts ER, Verbaten MN. Effects of alprazolam on driving ability, memory functioning and psychomotor performance: a randomized, placebo-controlled study. Neuropsychopharmacology. 2002;27(2):260–269. doi: 10.1016/S0893-133X(02)00310-X. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wolf AP, Logan J, Fowler JS, Christman D, Dewey SL, Schlyer D, Burr G, Vitkun S. Acute effects of ethanol on regional brain glucose metabolism and transport. Psychiatry Res. 1990;35(1):39–48. doi: 10.1016/0925-4927(90)90007-s. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Ma Y, Zhu W, Fowler JS, Li J, Rao M, Mueller K, Pradhan K, Wong C, Wang GJ. Moderate doses of alcohol disrupt the functional organization of the human brain. Psychiatry Res. 2008;162(3):205–213. doi: 10.1016/j.pscychresns.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Mullani N, Gould L, Adler SS, Guynn RW, Overall JE, Dewey S. Effects of acute alcohol intoxication on cerebral blood flow measured with PET. Psychiatry Res. 1988;24(2):201–209. doi: 10.1016/0165-1781(88)90063-7. [DOI] [PubMed] [Google Scholar]
- Walter H, Vetter SC, Grothe J, Wunderlich AP, Hahn S, Spitzer M. The neural correlates of driving. Neuroreport. 2001;12(8):1763–1767. doi: 10.1097/00001756-200106130-00049. [DOI] [PubMed] [Google Scholar]
- Wegner AJ, Gunthner A, Fahle M. Visual performance and recovery in recently detoxified alcoholics. Alcohol Alcohol. 2001;36(2):171–179. doi: 10.1093/alcalc/36.2.171. [DOI] [PubMed] [Google Scholar]
- Young RA, Hsieh L, Graydon FX, Genik R, Benton M, Green CC, Bowyer S, Moran JE, Tepley N. Mind-on-the-Drive: Real-time Functional Neuroimaging of Cognitive Brain Mechanisms Underlying Driver Performance and Distraction. SAE 05B-263. 2004 [Google Scholar]