Abstract
Rationale
Numerous neuroimaging studies have demonstrated lower neural tissue density in chronic cocaine users, which may be linked to cognitive dysfunction.
Objectives
The goal of this study was to determine whether neural tissue density was also impaired in individuals abstinent from cocaine and whether any observed changes were associated with cognitive performance.
Methods
A total of 73 participants were included: 24 active cocaine users, 24 abstainers (abstinent for at least 1 month), and 25 nondrug-abusing controls rigorously matched for age, gender, and IQ. All participants performed a cognitive assessment battery and received an MRI which was analyzed using voxel-based morphometry.
Results
The abstainers had significantly higher gray matter density than the current cocaine users in neocortical areas including the frontal and temporal cortex. In contrast to the users, there was no difference in white matter density in the abstainers relative to the controls. The abstainers performed better than current users on several behavioral tasks. Within users and abstainers, cortical density was correlated with performance on memory and reaction time tasks. Subcortical gray matter density was lower in both the users and abstainers relative to the controls. Within abstainers, subcortical tissue density was correlated with the ability to set-shift.
Conclusions
These data suggest that individuals able to remain abstinent from cocaine for at least 1 month have elevated neocortical tissue density and perform better on multiple cognitive tests, relative to current cocaine users. Larger, longitudinal studies are needed to address this interaction between abstinence, cognition, and cortical tissue density directly.
Keywords: Addiction, Neuroimaging, Cocaine, Cognition, Substance abuse, Myelin, Abstinence
Introduction
Individuals with a history of chronic cocaine use often have significant deficits in multiple cognitive domains including poor response inhibition, impaired decision-making skills, deficits in abstract reasoning, nonverbal problem solving, and both spatial and auditory processing, as well as other impairments of executive function (Hoff et al. 1996; Manschreck et al. 1990; Rogers et al. 1999). These cognitive deficits have been linked to functional abnormalities in multiple cortical and subcortical regions of human addicts (Bolla et al. 2004, Goldstein et al. 2004, Hester and Garavan 2004).
In addition to functional abnormalities, cocaine use and abuse has also been associated with structural abnormalities. Franklin et al. (2002) were the first to report neuroanatomical evidence of lower gray matter density in cocaine users using voxel-based morphometry (Franklin et al. 2002). Lower gray matter density was observed in the insular cortex, medial orbitofrontal cortex, superior temporal cortex, and the right anterior cingulate. Sim et al. (2007) recently reported lower white matter density in the right cerebellum as well as lower gray matter density in the premotor cortex, the temporal cortex, the left thalamus, the cerebellum, and the frontal cortex in current cocaine users (Sim et al. 2007). These results are consistent with prior studies that demonstrated reduced gray matter volume in the frontal cortex of cocaine users (Bartzokis et al. 1999; Fein et al. 2002; Lim et al. 2002). Few attempts have been made, however, to relate these abnormalities to specific cognitive functions.
Cocaine-dependent individuals that have been abstinent for several weeks also have significantly lower brain tissue density than nondrug-using controls. Matochik et al. (2003), for example, investigated gray and white matter densities in individuals abstinent from cocaine for 20 days (Matochik et al. 2003). These relatively short-term abstainers had lower gray matter density in the cingulate gyrus, lateral prefrontal cortex, and medial and lateral aspects of the orbitofrontal cortex than controls. A study of polydrug abusers in a residential treatment facility documented lower gray matter volume in the left and right orbitofrontal cortex of abstainers relative to controls (Tanabe et al. 2009). Furthermore, Volkow et al. (1992) demonstrated that lower glucose metabolism in the frontal cortex of cocaine users may persist for up to 3 months of cocaine abstinence (Volkow et al. 1992).
While the neurostructural integrity of cocaine abstainers has not been directly compared to active cocaine users and nondrug-using controls, independent reports demonstrate that both active cocaine users and short-term cocaine abstainers have significantly lower tissue density in several overlapping brain regions, namely, the cingulate, orbitofrontal, and insular cortices. These regions are implicated in multiple cognitive and limbic functions such as assigning emotional valence (Nielen et al. 2009; Prohovnik et al. 2004), decision making (Bechara et al. 2001), and behavioral inhibition (Knutson et al. 2000; Koechlin et al. 1999), processes which are impaired in current cocaine users. Deficits in executive functioning in nontreatment-seeking users relative to matched controls appear to be most evident when users have a negative rather than positive urine drug screen on the day of testing (Woicik et al. 2009). This suggests that recent cocaine use may actually mask additional cognitive deficits in these users. Few studies, however, have addressed the trajectory of cognitive function through extended abstinence. After 3 months of abstinence, for example, former cocainedependent users had lower academic achievement, verbal memory, and abstraction measures than nondrug-using controls (Block et al. 2002). DiSclafani et al. (2002) demonstrated that, independent of depression scores and alcohol dependence status, individuals abstinent from cocaine for 6 weeks had significantly lower scores on attention, executive function, spatial processing, and memory and reaction time tasks than nondrug-using controls. After 6 months, 52% of the abstaining participants had relapsed. The individuals that maintained sobriety, however, had significantly higher cognitive performance at the end of the treatment than the beginning, with the largest improvement being in the memory domain (DiSclafani et al. 2002).
While these aforementioned studies of cocaine abstinence have documented abnormalities in both brain structure and cognitive performance compared to nondrug-using healthy controls, the extent to which these abnormalities in neurostructural integrity compare to current cocaine users has not been addressed. The purpose of this study, therefore, was first to determine whether structural abnormalities documented in current cocaine users relative to well-matched nondrug-using controls were also present in individuals that have been abstinent from cocaine for over 1 month. A second purpose was to determine whether there was a relationship between cognitive performance and neurostructural integrity in these cocaine users and abstinent individuals.
Methods and materials
Participants
Data from of 73 participants were compiled across two existing databases previously acquired for functional neuroimaging investigations of cocaine users and cocaine abstainers. Participants were recruited from the Winston-Salem, North Carolina area via television advertisements. Participants were divided into three groups: current cocaine users meeting the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) criteria for cocaine dependence (n=24), current cocaine abstainers (n=24), and demographically matched, nondrug-abusing controls (n=25). Pertinent demographic variables of all three groups are presented in Table 1. At the time of screening, all participants were given a psychiatric examination (Structured Clinical Interview for DSM-IV Axis I Disorders [SCID]), completed a basic medical history inventory, provided urine for a urine drug screen, and completed several surveys including a drug use inventory and an IQ test (Wechsler Abbreviated Scale of Intelligence). Participants were excluded for past or current psychological disorders (including alcohol dependence) by DSM-IV criteria, a past or current neurological disorder (e.g., head trauma, seizure, extended loss of consciousness), gross anatomic brain abnormalities, presence of metal objects in the body, and pregnancy. In addition to demographic data and cocaine use histories, high-resolution structural MRIs of each subject’s brain were obtained (details below). Including image acquisition, the evaluation lasted no longer than 4 h. The study was approved by the Wake Forest University Institutional Review Board and all participants provided written consent for participation. The current cocaine users reported that cocaine was their primary drug of choice using crack or both crack and powdered cocaine. Participants had all been using cocaine for at least 12 months, administering the drug at least once per week. Cocaine use up to 72 h prior to our evaluation was verified using a urine toxicology screen that tested positive for cocaine metabolites on the day of the MRI scan. Participants were instructed not to use the drug after midnight the night before our evaluation. The current cocaine abstainers group also reported that cocaine had been their primary drug of choice using crack or both crack and powdered cocaine. Participants had a history of cocaine dependence and weekly cocaine use from 1 to 25 years. These individuals were currently enrolled in one of two local stepped rehabilitation programs. They had been abstinent for a period of time ranging from 30 to 114 days. Abstinence was confirmed by semiweekly urine toxicology screens at the rehabilitation center. Abstinence was further confirmed at the time of this study via both urine drug screen and self-report. The control group was rigorously selected to match the cocaine user and abstainer groups on the basis of age, sex, and IQ. Participants were excluded from the control group if they had ever used any illicit drugs other than marijuana.
Table 1.
Demographics of the study participants
| Cocaine users (24) | Cocaine abstainers (24) | Healthy controls (25) | |
|---|---|---|---|
| Age | 38.9±0.9 | 37.0±1.5 | 36.2±1.0 |
| Gender (male/female) | 17/7 | 15/9 | 12/13 |
| IQ (verbal) | 82.3±3.6 | 89.7±4.8 | 94.2±2.1 |
| IQ (spatial) | 85.1±5.8 | 96.7±6.7 | 97.1±2.0 |
| Age at first use (years) | 23.8±1.4 | 20.5±1.0 | – |
| Days per week using | 3.5±0.4 | 4.6±0.5 | – |
| Years of use | 11.1±1.2 | 10.2±1.5 | – |
| Days since last use* | 1.9±0.4 | 243.8±56.5 | – |
| Alcohol risk (AUDIT) | 12.3±2.6 | 9.2±3.4 | 3.3±1.5 |
| Cigarette usea (%) | 92 | 68 | 40 |
| Marijuana useb (%) | 35 | 9 | 0 |
| Depression (BDI)* | 20.3±3.0 | 10.4±4.8 | 3.2±0.4 |
| Anxiety (Speilberger State) | 36.0±2.0 | 35.3±1.8 | 34.4±2.7 |
Values are expressed as group means±standard error. Sample size is indicated after group name
p<0.05, significant difference among groups
Percentage that smoke at least one pack per week
Percentage that smoke at least one joint per week
Cognitive assessment
Neuropsychological assessment was carried out with the selected tests from the Cambridge Neuropsychological Test Automated Battery (CANTAB). The CANTAB battery included: reaction time, intradimensional/extradi-mensional (IDED) set-shift, delayed match to sample, pattern recognition memory, and the Stockings of Cambridge planning task. These tests evaluated psychomotor performance, recognition memory, working memory, attentional set-shifting, and planning, respectively (for more detail, see http://www.cantab.com/cantab). In addition to cognitive assessment, a measure of depressive symptoms and both state and trait anxiety were gathered for all participants. Depressive symptoms were measured with the Beck Depression Inventory (BDI; Beck et al. 1996). While the classification of depression based on BDI cutoff scores is an area of debate, the following guidelines were used to interpret our results: <10=none or minimal depression; 10–18=mild to moderate depression; 19–29=moderate to severe depression; 30–63=severe depression. Anxiety was measured with the Spielberger State-Trait Anxiety Inventory at the time of the MRI scan (Spielberger 1983).
Statistical analysis
To determine whether there were any differences in cognitive performance among groups, analyses of variance were performed for each of the five CANTAB tests. CANTAB generates multiple dependent measures for each of the five tests. One measure was chosen from each test as the best indicator of overall performance for that task (motor: reaction time, pattern recognition memory: percent correct, delayed match to sample: percent correct on the longest delay (12 s), set-shifting: total errors adjusted for stages completed, planning: stages completed). Bonferroni tests were used for post hoc analysis to account for multiple comparisons (p<0.05).
Brain tissue density assessment
MRI acquisition
Structural T1-weighted images were acquired with a 3-D SPGR on a 1.5-T GE magnetic resonance imaging scanner (Milwaukee, WI) at Wake Forest University Baptist Medical Center. The acquisition parameters were TE/TR=9.56/2.98 ms, flip angle=20°, matrix=256×256, with 124 slices of 1.5 mm thickness (with no interslice gap) through the entire brain.
Voxel-based morphometric analysis
Data were analyzed on a Dell Precision 690 Workstation (Dell Inc., Round Rock, TX) using SPM 05 software (Wellcome Department of Cognitive Neurology, London, UK) running on MATLAB 7.0.4 (The Mathworks, Natick, MA) according to the method described by Ashburner and Friston (2005). Voxel-based morphometry requires an ordered sequence of preprocessing steps including segmentation, spatial normalization, and smoothing. Following preprocessing, statistical parametric comparisons were made. These steps are explained further below.
Segmentation
Structural T1-weighted images were received in DICOM format, converted to Analyze format, interpolated to 0.94 mm3 voxels, and stored as axial slices. These original MRI images were segmented into gray matter, white matter, and CSF images using a clustering algorithm that recognizes particular tissue classes by voxel intensity. All nonbrain voxels of scalp, muscle, bone, and venous sinuses were extracted from the segmented images. Segmentation is bias corrected for magnetic field inhomogeneity.
Spatial normalization
Segmented gray and white matter images were spatially normalized by linear and nonlinear transformations to the same stereotaxic coordinate space developed at the Montreal Neurological Institute (MNI). This is done in order to account for global brain differences in shape and size. In order to preserve volume changes that may be created during the normalization process, segmented images are modulated by the Jacobian determinants created during the normalization process. This step accounts for the absolute volumes as they compare to the relative density during the standard voxel-based morphometry process.
Smoothing
Each segmented, normalized, modulated gray/white matter image was then smoothed using an isotropic full width at half maximum 8 mm3 Gaussian kernel. This is done in order to reduce the effects of image noise and to conform the images to the assumptions of random field theory for subsequent statistical analysis.
Statistical analyses
The effects of aging on the structure of the brain have been well documented (Davis et al. 2009; Madden et al. 2009; Milton et al. 1991), and in order to account for these effects, age was included in all comparisons as a nuisance variable, removing the effects due to age on brain structure. Voxel-based statistics were used to compare differences in signal intensity between groups (users, abstainers, and controls) using the voxel-based morphometry toolbox in SPM5. Contrast maps were passed through an initial threshold of p<0.001. Clusters of adjacent voxels that passed an initial uncorrected threshold of p<0.001 were considered statistically significant at a threshold of p<0.05 with correction for multiple comparisons. Final images were then created with a minimum cluster size of 200 voxels, the smallest significant cluster, in order to show only those regions of statistical significance.
The relationship between cognitive performance and brain tissue density was investigated independently for gray matter and white matter. Performance on all five CANTAB tests were incorporated into the design and all participants in the study were included in the regressions. Alcohol use (AUDIT score) was modeled as a covariate of no interest. As the cognitive data revealed a significant interaction between group and task performance, the results of the regressions with tissue density are reported for each group independently. Furthermore, linear correlations were performed to determine whether duration of use and/or duration of abstinence were associated with differences in gray and white matter densities in users and abstainers. For all correlation analyses, clusters of brain regions that passed a voxel threshold of p<0.001 and reached a cluster threshold of p<0.05 corrected for multiple comparisons were considered significant.
Post hoc analysis
Based on the observation that the average BDI scores were within the range of mild to moderate depressive symptoms (BDI=11–19) for both the cocaine users and the abstainers, post hoc analyses were used to determine whether BDI score was related to neurostructural integrity and cognitive performance among these individuals. For the neurostructural analysis, BDI was incorporated as a covariate into the design matrices for both gray matter and white matter maps. Data for the cocaine users and abstainers were included in the analysis. An analysis of variance was performed to assess the effect of BDI score on cognitive performance in these individuals. The distribution of BDI scores for the controls violated the assumption of normality and was, therefore, not included in the regressions.
Results
Brain tissue density
Cocaine users relative to controls
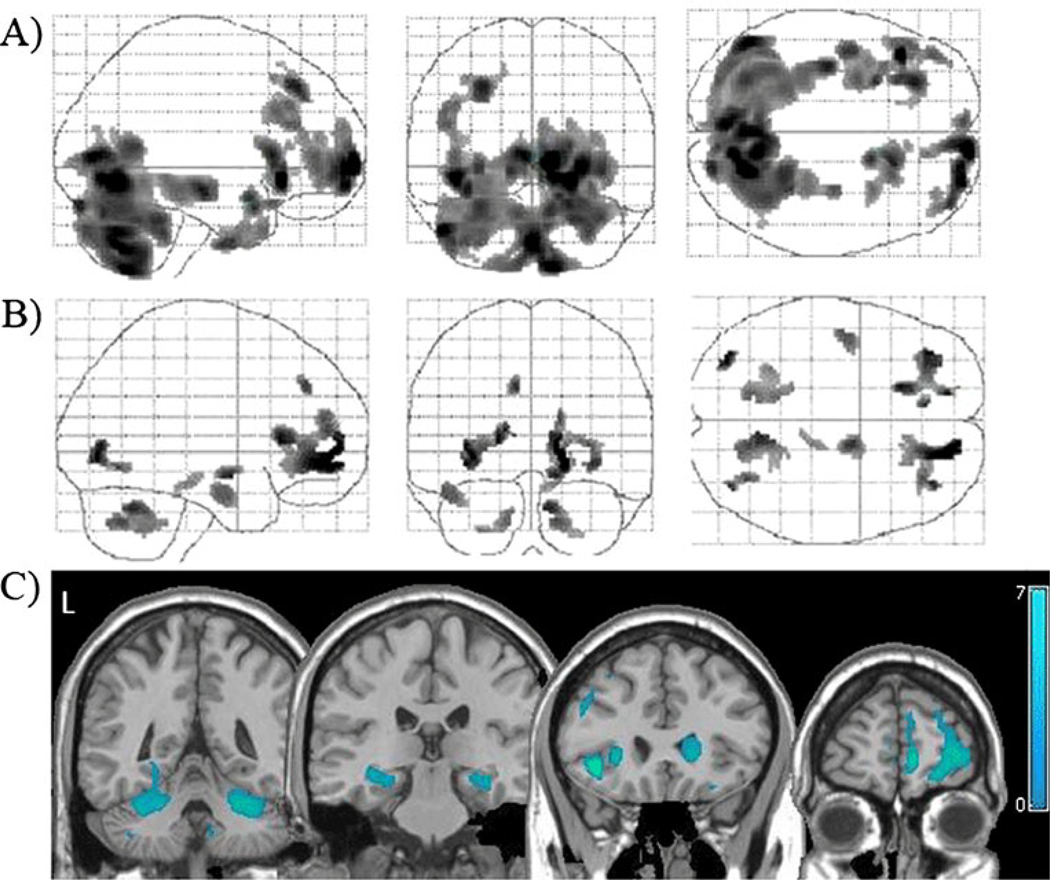
The current cocaine users had significantly lower tissue density than controls in several areas of gray matter (Table 2, Fig. 1a) and white matter (Table 3, Fig. 1b). These differences were observed in both cortical and subcortical areas (Fig. 1c). Current users had lower cortical gray matter density in the right medial, left middle, and bilateral inferior frontal gyri relative to controls. The users had lower subcortical gray matter density in the right caudate and the bilateral cerebellum. There were no regions in which the cocaine users had higher gray or white matter density than controls.
Table 2.
Locations of clusters with significant differences in gray matter density
| Significant clusters* | Brodmann | MNI coordinates | Maximuma | ||||
|---|---|---|---|---|---|---|---|
| Users vs controls | |||||||
| Lower signal intensity | R | Medial frontal gyrus | 10/32 | 10 | 66 | 2 | 6.18 |
| L | Middle frontal gyrus | 8/9 | −28 | 34 | 44 | 4.79 | |
| R | Inferior frontal gyrus | 44/45 | 16 | 8 | −16 | 4.64 | |
| L | Inferior frontal gyrus | 44/45 | −42 | 26 | −6 | 5.99 | |
| L | Superior temporal gyrus | 38 | −28 | 10 | −22 | 4.6 | |
| R | Caudate | 16 | 20 | 6 | 4.82 | ||
| R, L | Cerebellum | 0 | −62 | −44 | 5.39 | ||
| Higher signal intensity | – | No significant clusters | – | – | – | – | |
| Abstainers vs controls | |||||||
| Lower signal intensity | R | Parahippocampal gyrus | 30 | 22 | −52 | 8 | 5.09 |
| R | Caudate | 18 | −34 | 24 | 4.37 | ||
| R, L | Cerebellum | −6 | −62 | −58 | 4.61 | ||
| Higher signal intensity | – | No significant clusters | – | – | – | – | |
| Users vs abstainers | |||||||
| Lower signal intensity | R | Orbitofrontal cortex | 10/11 | 32 | 34 | −12 | 5.98 |
| R | Medial frontal gyrus | 10/32 | 2 | 38 | 52 | 4.87 | |
| L | Inferior temporal gyrus | 21 | −42 | 4 | −44 | 4.78 | |
| R | Superior frontal gyrus | 9 | 24 | 14 | 60 | 4.43 | |
| R | Middle frontal gyrus | 8/9 | −28 | 32 | 44 | 4.41 | |
| Higher signal intensity | – | No significant clusters | – | – | – | – | |
p<0.01 corrected cluster level, 200 voxels minimum
t value at the voxel level
Fig. 1.
Tissue density in active cocaine users relative to controls. Locations with significantly lower gray matter (a) and white matter (b) densities in cocaine users relative to controls (p<0.001, corrected). These statistical gray and white matter maps have been superimposed to demonstrate the distribution of lower tissue density in the users (c). Color bar represents t values from the statistical maps. The left side of the brain appears on the left side of the image (L)
Table 3.
Locations of clusters with significant differences in white matter density
| Significant clusters* | MNI coordinates | Maximuma | ||||
|---|---|---|---|---|---|---|
| Users vs controls | ||||||
| Lower signal intensity | R | Superior longitudinal fasciculus | 16 | 54 | 0 | 5.17 |
| R | Middle frontal gyrus (BA 6, 8) | 34 | 42 | −6 | 4.11 | |
| R | Medial frontal gyrus | 16 | 48 | 18 | 3.91 | |
| R | Uncinate fasciculus | 14 | −4 | −12 | 4.14 | |
| Higher signal intensity | – | No significant clusters | – | – | – | – |
| Abstainers vs controls | ||||||
| Lower signal intensity | – | No significant clusters | – | – | – | – |
| Higher signal intensity | – | No significant clusters | – | – | – | – |
| Users vs abstainers | ||||||
| Lower signal intensity | L | Insula | −38 | 12 | 12 | 6.39 |
| L | Middle frontal gyrus | −34 | 44 | −4 | 5.60 | |
| R | Middle frontal gyrus | 36 | 42 | −6 | 5.51 | |
| L | Medial frontal gyrus | −16 | 52 | 14 | 5.23 | |
| R | Medial frontal gyrus | 10 | 42 | 36 | 5.15 | |
| Higher signal intensity | – | No significant clusters | – | – | – | – |
If a cluster is within the corona radiata, the nearest gray matter is listed as the cluster label
p<0.01 corrected cluster level, 200 voxels minimum
t value at the voxel level
Cocaine abstainers relative to controls
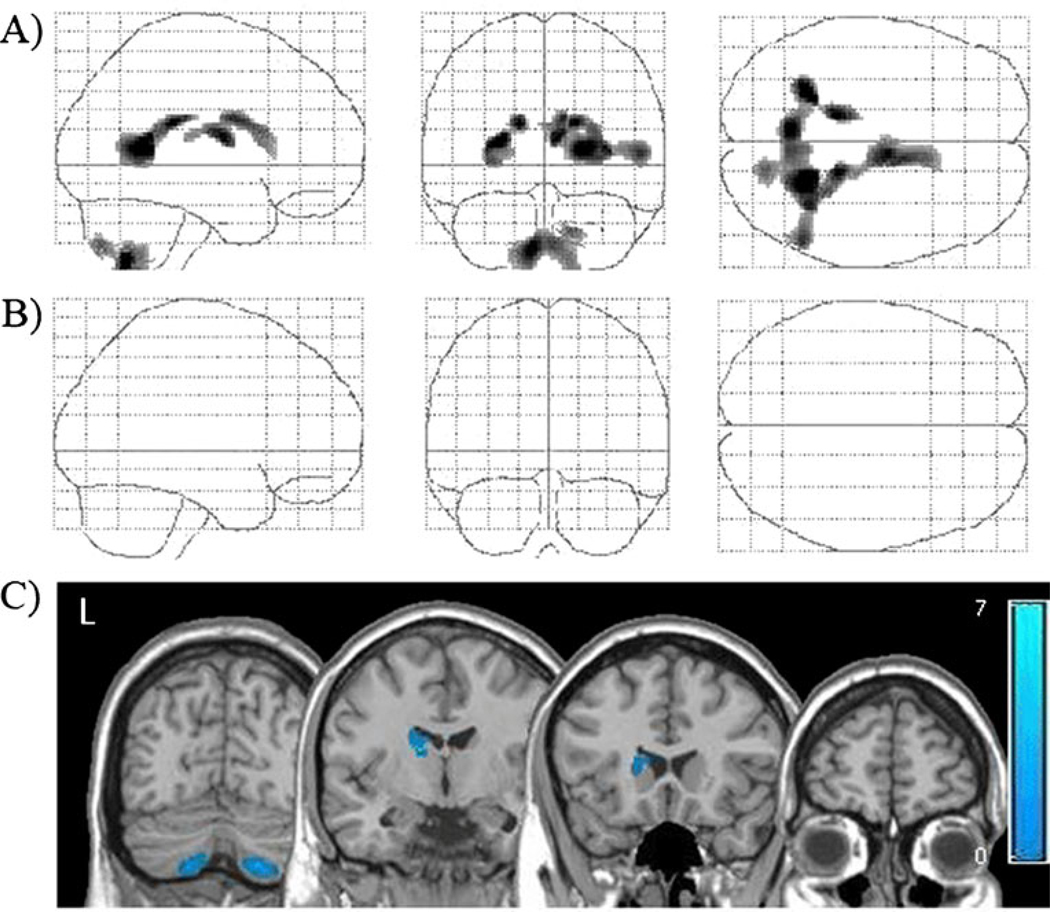
The cocaine abstainers had significantly lower tissue density than controls in several areas of subcortical gray matter (Table 2, Fig. 2a), but no significant differences were observed in white matter (Table 3, Fig. 2b). Similar to the cocaine users, there were significant differences in subcortical gray matter density; specifically, the cocaine abstainers had lower gray matter density in the right caudate/putamen and the bilateral cerebellum than controls (Fig. 2c). There were no areas in which the cocaine abstainers had higher gray matter density than controls. There were also no areas of higher white matter density in the abstainers.
Fig. 2.
Tissue density in cocaine abstainers relative to controls. Locations with significantly lower gray matter (a) and white matter (b) densities in cocaine abstainers relative to matched controls (p<0.001, corrected). These statistical gray and white matter maps have been superimposed to demonstrate the distribution of lower tissue density in the users (c). Color bar represents t values from the statistical maps
Current cocaine users relative to cocaine abstainers
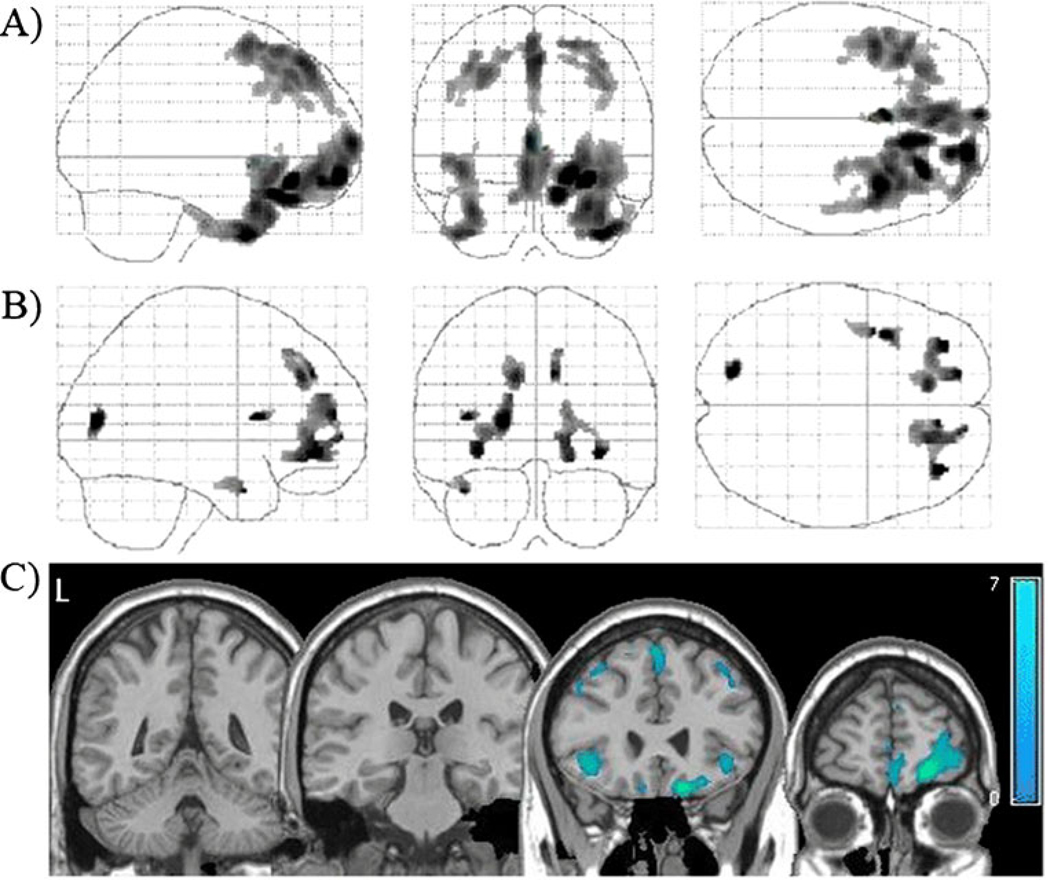
Cocaine users had significantly lower tissue density than abstainers and controls in multiple frontal and temporal cortical areas (Table 2, Fig. 3a) as well as lower white matter density in the vicinity of the insula and the middle and medial frontal gyri (Table 3, Fig. 3b). Areas with the largest differences between current users and abstainers were all cortical: the right orbitofrontal cortex, right medial frontal gyrus, left inferior temporal gyrus, right superior frontal gyrus, and right middle frontal gyrus (Fig. 3c). There were no areas in which the cocaine users had higher gray or white matter density than the abstainers.
Fig. 3.
Tissue density in active cocaine users relative to cocaine abstainers. Locations with significantly lower gray matter (a) and white matter (b) densities in cocaine users relative to abstainers (p<0.001, corrected). These statistical gray and white matter maps have been superimposed to demonstrate the distribution of lower tissue density in the users (c). Color bar represents t values from the statistical maps
Duration of cocaine use and abstinence
There were no clusters of gray or white matter that significantly correlated with length of cocaine use in the current cocaine users or the cocaine abstainers (p>0.05, corrected). In addition, duration of abstinence was not correlated with measures of density in the cocaine abstaining group.
Cognitive performance
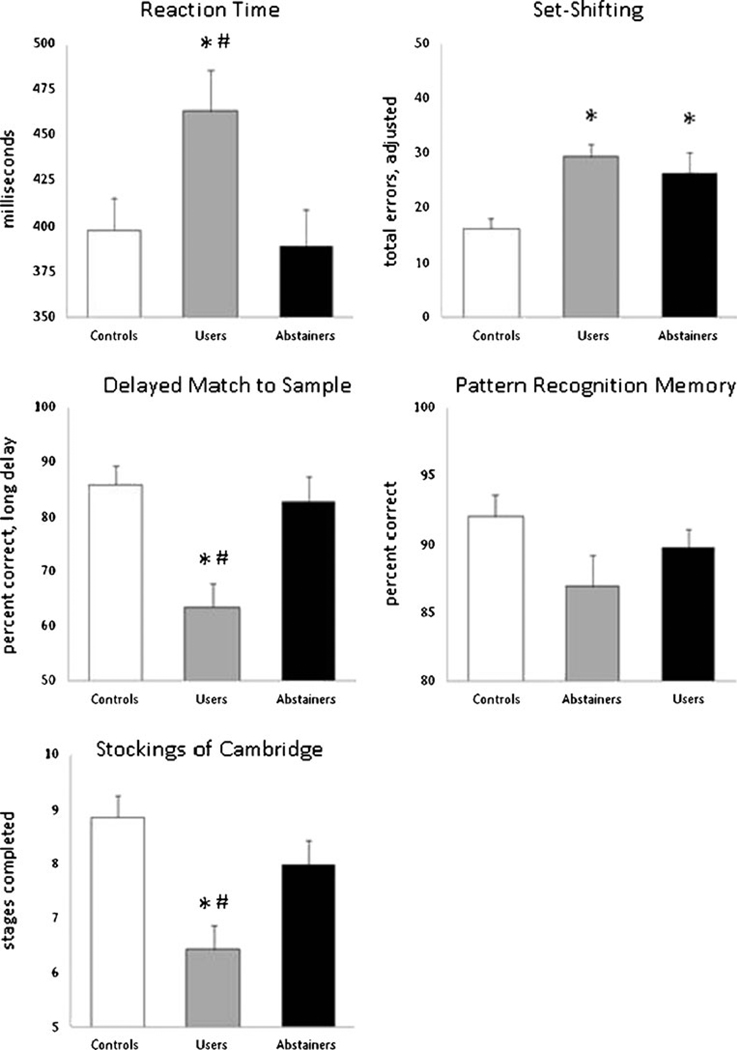
There was a significant difference in cognitive performance across the groups on four of the five the CANTAB subtests: reaction time (F(2,70) =4.078, p=0.022), delayed match to sample performance on the long delay (F(2,70)=7.434, p<0.001), IDED set-shifting errors (F(2,70)=8, p=0.034), and Stockings of Cambridge problems completed (F(2,70)=7.281, p<0.001), In addition, there was a clear trend in pattern recognition memory accuracy (F(2,70)=2.949, p=0.06). Relative to both controls and abstainers, current cocaine users had slower reaction times and lower accuracy on pattern recognition memory, delayed match to sample, and planning (Fig. 4).
Fig. 4.
Cognitive performance in current cocaine users, abstainers, and matched controls. Average scores (+SE) for the cocaine abstainers (black), cocaine users (gray), and age- and IQ-matched controls (white) are displayed for five subtests of the CANTAB. *p<0.05 corrected, relative to controls; #p<0.05 corrected, relative to abstainers
In contrast, on the IDED set-shifting task, both current cocaine users and abstainers made significantly more errors than controls. There was no difference in performance between the current users and cocaine abstainers. Therefore, the IDED shift was the only test on which cocaine abstainers performed significantly more poorly than matched controls. Further analyses of the performance on the IDED task revealed that the cocaine abstainers made significantly less extradimensional shifting errors than the users (F(1,46) =3.609, p=0.033), but overall error rate was significantly higher than that of the matched controls (F(1,47) =3.30, p=0.028).
Relationship between brain tissue density and cognitive performance
To determine whether differences in tissue density among the participants were related differences in cognitive performance, a multiple regression was performed for all groups. Within the healthy controls, there was no significant relationship between cognitive performance and gray matter density. Within the current cocaine users and the abstainers, there was a negative correlation between performance on the delayed match to sample task (errors at the long delay) and gray matter density in the medial prefrontal cortex (cluster, p=0.036, coordinates of the maximum, x,y,z=7, 42, −12) and middle temporal gyrus (p=0.042, x,y,z=−35, 10, −44). There was also a negative correlation between reaction time and gray matter density in the superior prefrontal cortex (p=0.035, x,y,z=15, 14, 60), extending medially through the supplementary motor area. Stockings of Cambridge performance was not significantly correlated with any specific clusters of gray matter density in the study. There were no significant correlations between white matter density and performance in the cognitive tests for any of the groups.
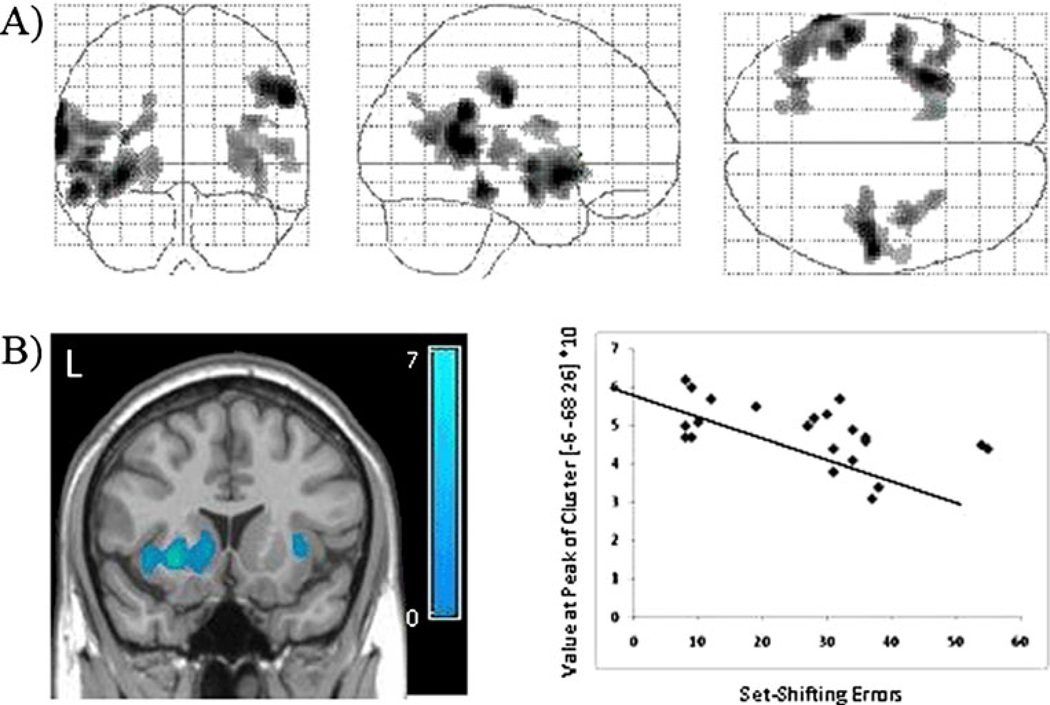
Although the cocaine users and abstainers both made significantly more errors on the set-shifting task, within the cocaine abstainers alone, there was a significant relationship between performance on the set-shifting task in several cortical gray matter areas including the left anterior insula and left and right temporal cortices (Fig. 5a). In addition to these cortical areas, the lower error rates on the IDED task were associated with significantly higher tissue density in the left caudate and putamen (Fig. 5b).
Fig. 5.
Correlation between set-shifting performance and gray matter density in cocaine abstainers. Statistical maps of the distribution of voxels that are negatively correlated with errors on the IDED set-shifting task in abstainers are displayed (a) (p<0.05, corrected). The correlation between total errors in the set-shifting task relative to the gray matter intensity value at the peak of the most significant cluster (striatum) is displayed (b). The color bar represents t values from the voxel-based correlation
Post hoc analysis
BDI score was not significantly correlated with either gray or white matter density among the cocaine users or abstainers, as revealed by the general linear model (p>0.05, corrected). There was also no main effect or interaction between BDI score and CANTAB performance in these individuals. The control group was not included in these analyses due to low levels and limited distribution of BDI scores.
Discussion
The results of this preliminary investigation demonstrate that there are significant differences in gray and white matter densities of individuals that have abstained from cocaine for at least 1 month, relative to current cocaine users. Consistent with prior studies, current cocaine users had significantly lower tissue density in both cortical and subcortical structures relative to controls. While the abstainers also had significantly lower tissue density in subcortical areas (caudate and cerebellum), tissue density in the cortex did not significantly differ from controls. Furthermore, cortical gray matter density was correlated with performance on multiple cognitive tests in which abstainers performed better than cocaine users. Together, these data suggest that individuals that are able to remain abstinent for more than a month may have higher cortical gray matter and better cognitive performance than individuals that currently use cocaine.
One interpretation of these data is that lower gray matter density observed in current cocaine users may return to control levels with protracted abstinence. This reversal is well documented in individuals abstaining from alcohol. Lower gray and white matter volumes in alcohol-dependent individuals recover after several weeks of abstinence from alcohol (Gazdzinski et al. 2005; Pfefferbaum et al. 1995; Pfefferbaum et al. 1998). Gray matter density also increases with abstinence from methamphetamine use in the striatum, accumbens, and parietal cortex (Jernigan et al. 2005), and positron emission tomography studies have revealed elevated glucose metabolism throughout the cortex of methamphetamine abstainers, with the greatest increase (≥20%) in the parietal cortex (Berman et al. 2008; London et al. 2004). Additionally, dopamine transporter availability in users reverses with prolonged abstinence (Volkow et al. 2001). These reports in alcohol and methamphetamine abusers are consistent with the possibility that structural abnormalities associated with cocaine use may be reversed following extended abstinence.
An alternate interpretation of the preserved neocortical tissue density of abstinent cocaine users is that this group may represent a “survivor effect.” That is, among cocaine users, there is a subpopulation with more intact cortical tissue density that may play a role in improving their ability to achieve and maintain abstinence. Such individuals would be over-represented in the abstinence group as our current data suggests. Should individuals in this cohort seek treatment, higher cortical integrity at the initiation of abstinence could lead to better long-term outcomes than the high relapse rates typical of most cocaine users. In the present study, tissue deficits observed in current users were largely within the corticolimbic system, deficits of which are associated with compulsive drug-seeking behaviors (Volkow and Fowler 2000), poor behavioral inhibition (Devinsky et al. 1995), and cocaine dependence (Bolla et al. 2004; Matochik et al. 2003). Cocaine users with higher tissue density, especially within this system, are more likely to have better behavioral inhibition and less likely to express and/or act on compulsive drug behaviors. As a result, this cohort may have the improved capacity to maintain abstinence over prolonged periods of time. Finally, an additional factor is the better cognitive performance of the abstainers, compared to the current cocaine users, which may reflect the high cortical tissue density of the abstainers. This may also contribute to their ability to maintain abstinence.
In order to evaluate this hypothesis further, gray and white matter tissue densities were regressed with length of use for all individuals in the cocaine user and abstainer groups. Consistent with prior studies, there was no correlation between length of use and gray matter density in the frontal cortex (Matochik et al. 2003), suggesting that cocaine users who successfully abstain represent a unique population of cocaine-dependent individuals. A later study found that bilateral cerebellar gray matter volumes were negatively correlated with length of cocaine use (Sim et al. 2007). A possible explanation for this difference is that volumetric measurements and tissue density measurements are not equivalent, since a change in volume within a region could occur without a change in tissue density (Franklin et al. 2002). While it would be valuable to assess the effect of length of abstinence with cognitive performance and brain structure directly, the limited sample size and cross-sectional design of this study did not have enough power to make that comparison.
Another important observation is that the abstainers had significantly fewer depressive symptoms than the current users in this investigation. While none of the cocaine users met the criteria for current or past major depressive disorder, current users had significantly higher scores on BDI than the abstainers and the controls. The relationship between depression and cocaine addiction has been well documented, as has the reduction in symptoms in the first weeks of treatment (Weddington et al. 1990). However, because the present study was cross-sectional in nature, it is not possible to conclude that longer durations of abstinence produced an improvement in depressive symptoms. Several reports suggest that cocaine abusers with comorbid depression may have greater difficulty in remaining abstinent and suffer from more intense cocaine cravings (Elman et al. 2002). An alternative explanation for the lower depression scores observed in these abstainers is that individuals with higher depressive symptoms are less likely to remain in treatment programs. Recent data suggests, however, that there is no significant relationship between depressive symptoms and treatment retention (Aharonovich et al. 2006). Furthermore, post hoc analyses did not reveal a significant relationship between BDI score and either CANTAB performance or gray or white matter density in these data. There may, however, be a synergistic relationship among decreased symptoms of depression, elevated cognitive function, cortical density, and continued abstinence.
It is important to note that the preliminary nature of this investigation results in several limitations. While the use of a control group rigorously matched for age and IQ is an important contribution to the literature on cognitive and neurostructural characteristics of cocaine abstainers, a larger cohort of abstainers that could be followed up in a prospective manner would expand the implications of these data. Additionally, the abstainers were part of drug abuse programs in the community that also required them to abstain from alcohol. As a result, increased neocortical density in abstainers could be the result of concomitant abstinence from the combination of alcohol and cocaine. Given that alcohol abstinence has large effects on the cerebellum (Gazdzinski et al. 2005), however, which remains lower than controls in the cocaine abstainers, alcohol abstinence cannot alone account for the spatial distribution of the data. To address this concern, AUDIT scores were used as a covariate in all group analyses and correlations between behavior and brain structure and were not found to be an important factor. The acute effects of cocaine could also influence these data, as the cocaine users all tested positive for cocaine at the time of image acquisition. While this may have affected their cognitive performance, there is no evidence to suggest that the acute physiological effects of cocaine could account for a selective spatial distribution of changes in voxel-based morphometric measures. Furthermore, although gender differences in neural structure and function are well established among both controls and substance-abusing populations, the relatively low percentage of female users that chose to participate in this study precludes us from conducting meaningful analyses about the effects of gender.
In conclusion, this study reveals that, in a cohort of individuals abstinent from cocaine for at least 1 month, there are few differences in cognitive performance or cortical gray matter relative to age- and IQ-matched control participants. However, relative to cocaine users, these abstainers performed significantly better on tests of memory and sensorimotor control and had higher cortical gray matter density despite similar low gray matter density in the striatum and the cerebellum. This is the first study to directly compare cognitive performance and neurostructural integrity in cocaine abstainers to both current users and nondrug-using controls matched for age and IQ. Considered together, these data suggest that individuals able to abstain from cocaine for more prolonged periods of time may have higher neocortical tissue density than current users despite similar subcortical deficits. Furthermore, the absence of cortical deficits may be an important factor in the ability to remain abstinent, as well as their improved cognitive performance. Longitudinal and behavior studies are needed to address this interpretation directly.
Acknowledgements
The authors thank Marla Torrence and Mack Miller for their assistance in patient recruitment and data processing.
This work was supported by the National Institute on Drug Abuse grants DA027756 (CAH), DA020074 (LJP), and DA06634 (LJP). The funding source had no further role in the study design, collection, analysis, interpretation, or in the decision to submit the paper for publication.
Footnotes
The authors have no conflicts of interest to declare.
Contributor Information
Colleen A. Hanlon, Department of Physiology and Pharmacology, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157-1083 USA Department of Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC 29425, USA.
Darin L. Dufault, Department of Physiology and Pharmacology, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157-1083 USA
Michael J. Wesley, Department of Physiology and Pharmacology, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157-1083 USA
Linda J. Porrino, Department of Physiology and Pharmacology, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157-1083 USA Email: lporrino@wfubmc.edu.
References
- Aharonovich E, Garawi F, Bisaga A, Brooks D, Raby WN, Rubin E, Nunes EV, Levin FR. Concurrent cannabis use during treatment for comorbid ADHD and cocaine dependence: effects on outcome. Am J Drug Alcohol Abuse. 2006;32:629–635. doi: 10.1080/00952990600919005. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Wirshing DA, Lu PH, Foster JA, Mintz J. Choreoathetoid movements in cocaine dependence. Biol Psychiatry. 1999;45:1630–1635. doi: 10.1016/s0006-3223(98)00238-8. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Beck A, Steer R, Brown G. Psychological Corporation. 2nd edn. San Antonio: Harcourt Brace & Company; 1996. Beck Depression Inventory. [Google Scholar]
- Berman SM, Voytek B, Mandelkern MA, Hassid BD, Isaacson A, Monterosso J, Miotto K, Ling W, London ED. Changes in cerebral glucose metabolism during early abstinence from chronic methamphetamine abuse. Mol Psychiatry. 2008;13:897–908. doi: 10.1038/sj.mp.4002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block RI, Erwin WJ, Ghoneim MM. Chronic drug use and cognitive impairments. Pharmacol Biochem Behav. 2002;73:491–504. doi: 10.1016/s0091-3057(02)00816-x. [DOI] [PubMed] [Google Scholar]
- Bolla K, Ernst M, Kiehl K, Mouratidis M, Eldreth D, Contoreggi C, Matochik J, Kurian V, Cadet J, Kimes A, Funderburk F, London E. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16:456–464. doi: 10.1176/appi.neuropsych.16.4.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46:530–541. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. Review. [DOI] [PubMed] [Google Scholar]
- DiSclafani V, Tolou-Shamas M, Price LJ, Fein G. Neuropsy-chological performance of individuals dependent on crack-cocaine, or crack-cocaine and alcohol, at 6 weeks and 6 months of abstinence. Drug and Alcohol Dependence. 2002;66:161–171. doi: 10.1016/s0376-8716(01)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Karlsgodt KH, Gastfriend DR, Chabris CF, Breiter HC. Cocaine-primed craving and its relationship to depressive symptomatology in individuals with cocaine dependence. J Psychopharmacol. 2002;16:163–167. doi: 10.1177/026988110201600207. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Meyerhoff DJ. Prefrontal cortical volume reduction associated with frontal cortex function deficit in 6-week abstinent crack-cocaine dependent men. Drug Alcohol Depend. 2002;68:87–93. doi: 10.1016/s0376-8716(02)00110-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Studholme C, Song E, Banys P, Meyerhoff DJ. Quantitative brain MRI in alcohol dependence: preliminary evidence for effects of concurrent chronic cigarette smoking on regional brain volumes. Alcohol Clin Exp Res. 2005;29:1484–1495. doi: 10.1097/01.alc.0000175018.72488.61. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, Wang GJ, Fowler JS, Volkow ND. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsycho-logia. 2004;42(11):1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff AL, Riordan H, Morris L, Cestaro V, Wieneke M, Alpert R, Wang GJ, Volkow N. Effects of crack cocaine on neurocognitive function. Psychiatry Res. 1996;60:167–176. doi: 10.1016/0165-1781(96)02758-8. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Heaton RK, Ellis RJ, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. Am J Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. Neuroimage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999;399:148–151. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol Psychiatry. 2002;51:890–895. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, Shinn AK, Miotto K, Learn J, Dong Y, Matochik JA, Kurian V, Newton T, Woods R, Rawson R, Ling W. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Spaniol J, Costello MC, Bucur B, White LE, Cabeza R, Davis SW, Dennis NA, Provenzale JM, Huettel SA. Cerebral white matter integrity mediates adult age differences in cognitive performance. J Cogn Neurosci. 2009;21:289–302. doi: 10.1162/jocn.2009.21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manschreck TC, Schneyer ML, Weisstein CC, Laughery J, Rosenthal J, Celada T, Berner J. Freebase cocaine and memory. Compr Psychiatry. 1990;31:369–375. doi: 10.1016/0010-440x(90)90045-t. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- Milton WJ, Atlas SW, Lexa FJ, Mozley PD, Gur RE. Deep gray matter hypointensity patterns with aging in healthy adults: MR imaging at 1.5 T. Radiology. 1991;181:715–719. doi: 10.1148/radiology.181.3.1947087. [DOI] [PubMed] [Google Scholar]
- Nielen MM, Heslenfeld DJ, Heinen K, Van Strien JW, Witter MP, Jonker C, Veltman DJ. Distinct brain systems underlie the processing of valence and arousal of affective pictures. Brain Cogn. 2009;71:387–396. doi: 10.1016/j.bandc.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res. 1995;19:1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch Gen Psychiatry. 1998;55:905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- Prohovnik I, Skudlarski P, Fulbright RK, Gore JC, Wexler BE. Functional MRI changes before and after onset of reported emotions. Psychiatry Res. 2004;132:239–250. doi: 10.1016/j.pscychresns.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Sim ME, Lyoo IK, Streeter CC, Covell J, Sarid-Segal O, Ciraulo DA, Kim MJ, Kaufman MJ, Yurgelun-Todd DA, Renshaw PF. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 2007;32:2229–2237. doi: 10.1038/sj.npp.1301346. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory (STAI) Palo Alto: Consulting Psychologists Press; 1983. [Google Scholar]
- Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, Banich M. Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatry. 2009;65:160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex Mar. 2000;10(3):318–325. doi: 10.1093/cercor/10.3.318. Review. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11:184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21(23):9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weddington WW, Brown BS, Haertzen CA, Cone EJ, Dax EM, Herning RI, Michaelson BS. Changes in mood, craving, and sleep during short-term abstinence reported by male cocaine addicts. A controlled, residential study. Arch Gen Psychiatry. 1990;47:861–868. doi: 10.1001/archpsyc.1990.01810210069010. [DOI] [PubMed] [Google Scholar]
- Woicik PA, Moeller SJ, Alia-Klein N, Maloney T, Lukasik TM, Yeliosof O, Wang GJ, VolkowND GRZ. The neuropsychology of cocaine addiction: recent cocaine use masks impairment. Neuropsychopharmacology. 2009 Apr;34(5):1112–1122. doi: 10.1038/npp.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]