Abstract
Compared to the better-known mu opioid receptor, delta opioid receptors have been relatively understudied. However, the development of highly selective delta opioid agonists, and the availability of genetic mouse models have extended our knowledge of delta opioid receptors in vivo. Here we review recent developments in the characterization of delta opioid receptor biology, and aspects of delta opioid receptor function that have potential for therapeutic targeting. Preclinical data have confirmed that delta opioid receptor activation reduces persistent pain and improves negative emotional states; clinical trials have been initiated to assess the effectiveness of delta opioid agonists in chronic pain and depression. Further, a possible role for these receptors in neuroprotection is being investigated. The usefulness of targeting delta opioid receptors in drug abuse remains open, and there is an emerging role of these receptors in impulse control disorders. Finally, the recent demonstration of biased agonism at the delta opioid receptor in vivo opens novel perspectives towards targeting specific therapeutic effects through drug design.
The opioid system
Discovery of the opioid system stems from the use and abuse of opium in ancient history. Opium has strong pain-relieving properties, and also produces euphoria. Morphine, considered the prototypic opiate, is the most active constituent of opium, and remains the most widely used pain killer in contemporary medicine despite strong adverse effects [1, 2]. Heroin, a diacetylated morphine derivative, is a major drug of abuse with strong societal impact. These powerful substances act on nervous system receptors, whose existence was established in 1973 and molecular characterization achieved in the late 1990s. These receptors are activated by a family of endogenous opioid peptides whose first members were isolated in 1975, and genes identified in the late 1970’s. Altogether, the opioid receptor family consists of three receptors, mu, delta, and kappa, encoded by the Oprm1, Oprd1, and Oprk1 genes respectively; and neuropeptides processed from large precursor proteins, encoded by Penk, Pdyn and Pomc genes. Due to high sequence homology, the Oprl1 gene, encoding the Nociceptin/OrphaninFQ receptor, is classified as a member of the opioid receptor gene family (http://www.iuphar-db.org/DATABASE/FamilyIntroductionForward?familyId=50). However this receptor, qualified as “opioid-like receptor”, does not respond to opioids pharmacologically, and will not be further discussed in this review. Opioid receptors and peptides are broadly expressed throughout the peripheral and central nervous systems and have been the subject of intense investigation for several decades (for recent review see [1]).
The opioid system plays a central role in pain control, and is a key player in hedonic homeostasis, mood and well-being. This system also regulates responses to stress, and a number of peripheral physiological functions including respiratory, gastrointestinal, endocrine and immune systems. In the past two decades, refinement of pharmacological tools and availability of genetic approaches have clarified the specific role of each opioid receptor in many aspects of opioid-related behaviors, physiology and disorders. At present, the notion that mu opioid receptors mediate both analgesic and addictive properties of clinically useful and abused opiates is well accepted. Mu opioid receptor activation strongly inhibits severe acute pain, and is a major target for post-operative and cancer pain management [2]. Mu opioid receptors are also central for reward processing [3], representing a main factor in the initiation of addictive behaviors. The activation of kappa opioid receptors also produces antinociception in preclinical models [4]. These receptors, however, oppose mu receptors in the regulation of hedonic homeostasis. Kappa agonists are strongly aversive and potentially hallucinogenic, which limits the therapeutic potential of centrally-acting kappa opioid agonists in pain treatment. Recently, mounting preclinical evidence supports the notion that kappa receptor blockade may beneficially alleviate stress responses, reduce drug craving and remediate depressive states [5-7].
Delta opioid receptors (also known as delta opioid receptors, δ receptors, DORs, or DOP receptors in the IUPHAR nomenclature) appear increasingly attractive, both from the perspective of receptor function in vivo and therapeutic potential. Along with the development of highly selective delta opioid agonists and rapid progress in mouse mutagenesis approaches targeting the Oprd1 gene, the previously reported roles of delta opioid receptors have been clarified and novel functions have emerged. Here we focus on recent advances in delta opioid receptor biology, and the potential of delta opioid agonists in the treatment of neurological and psychiatric disorders. Rather than being exhaustive, this review highlights selected aspects of delta opioid receptor function at cellular and behavioral levels, which have evolved recently and hold promise for biomedical research.
Molecular and cellular aspects of the delta opioid receptor
Opioid receptors are seven-transmembrane proteins which belong to the G protein-coupled receptor (GPCR) superfamily. Upon binding of exogenous opiates or endogenous opioid peptides, opioid receptors are activated and convey extracellular stimulation to multiple intracellular effectors, including ion channels and second messengers that ultimately decrease neuronal activity [8]. The three opioid receptors show highly homologous protein sequences, and in vitro mutagenesis studies have identified a common opioid receptor binding pocket within the helical transmembrane core for mu, delta and kappa receptors. The analysis of mutant receptors in vitro has further demonstrated the importance of extracellular domains for delta opioid receptor selectivity [9], and identified helical domain-mediated mechanisms for delta opioid receptor activation [10]. More recently, delta opioid receptors have been proposed to act as heterodimers with opioid receptors or other GPCRs in cellular models, and the possibility that delta opioid agonists may act at delta receptor-GPCR heteromers with distinct pharmacological properties in vivo is being explored [11-13].
As for most GPCRs, stimulation of the delta opioid receptor triggers intracellular signaling via G protein-dependent or independent pathways. Receptor signaling is typically accompanied by desensitization, a complex feedback regulatory process whereby receptor responsiveness decreases upon continued agonist stimulation. GPCR internalization, trafficking and redistribution is considered one of the key mechanisms underlying this regulatory response [14] and major progress has been achieved recently regarding the dynamics and implications of delta opioid receptor internalization in vivo. Delta opioid receptor internalization has been observed in vitro for several ligands, including endogenous opioids [15,16]. Studies in cellular models indicate that internalized GPCRs may either recycle to the cell surface, or be further processed along several distinct endocytic pathways (see [17] and references therein). Unlike the mu opioid receptor, which rapidly recycles to the cell surface, delta opioid receptors are targeted for lysosomal degradation ([15] and refs therein) via the Endosomal Sorting Complex Required for Transport (ESCRT) machinery using ubiquitination-dependent or independent mechanisms [18]. Other factors upstream of the ESCRT pathway also contribute to delta opioid receptor proteolysis, including G protein coupled receptor Associated Sorting Proteins (GASPs) that were shown to interact directly with delta opioid receptor, as well as other GPCRs [19,20].
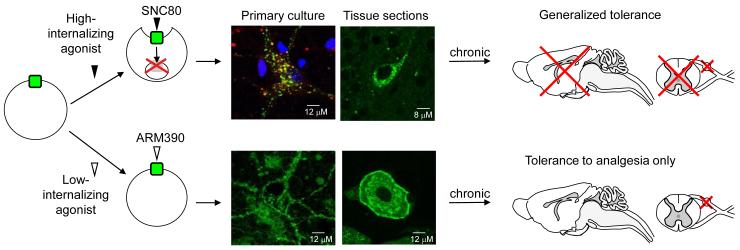
Until recently it was difficult to study the consequences of agonist-induced delta opioid receptor internalization and degradation in vivo. The advent of a novel knock-in mouse model in which endogenous delta opioid receptors are replaced by fluorescently-tagged delta opioid receptors (DOR-eGFP, [21]) has allowed correlating ligand-induced receptor internalization in live neurons with receptor function in vivo. DOR-eGFP mice express fully functional delta opioid receptors, which are directly visible in vivo. In these mice the prototypic delta opioid agonist, SNC80 [22], produced robust receptor internalization at behaviorally active concentrations [15,21]. In a model of inflammatory pain, acute SNC80 induced strong analgesia, together with receptor internalization and G-protein uncoupling, resulting in transient acute behavioral desensitization [15]. Chronic SNC80 ultimately produced widespread receptor degradation, as was anticipated from cellular studies, leading to generalized behavioral tolerance to all agonist effects ([23], Figure 1).
Figure 1. In vivo consequences of biased agonist-induced trafficking at the delta opioid receptor: two mechanisms towards tolerance.
SNC80 binding to the delta opioid receptor results in massive receptor internalization in primary neurons from DOR-eGFP mice (representative neuron from a hippocampal primary culture, top left panel), as well as throughout central and peripheral nervous systems (representative image from a hippocampal section, top right panel). Following internalization, delta opioid receptors are targeted towards degradation (top left panel), as shown by co-localization of DOR-eGFP fluorescence (green) with the lysosomal marker, lysotracker (red). In contrast, ARM390 does not produce significant internalization of DOR-eGFP, either in primary neurons from DOR-eGFP mice (hippocampal primary culture, bottom left panel) or in central and peripheral nervous systems (dorsal root ganglia section bottom right panel). In vivo, a single injection of the high-internalizing, but not the low-internalizing agonist, produces acute behavioral desensitization [15]. However, chronic treatment with both agonists produces analgesic tolerance, regardless of internalization potency. The high-internalizing agonist down-regulates delta opioid receptors throughout the nervous system leading to generalized tolerance. In contrast, the low-internalizing agonist only affects delta responses at the level of the dorsal root ganglia, resulting in a pain-specific tolerance. In this case, non-analgesic delta opioid agonist effects (anxiolytic effects, for example) remain intact after chronic treatment [23]. Drug design for low-internalizing delta opioid agonists may therefore be helpful in therapeutic strategies targeting chronic psychiatric disorders.
In contrast to SNC80, treatment with the non-internalizing delta opioid agonist ARM390 produced remarkably different effects in vivo. Acute treatment with ARM390 did not induce behavioral desensitization, and delta opioid receptors remained G protein-coupled on the cell surface [15]. Chronic treatment nevertheless produced analgesic tolerance, even though no obvious receptor change was detectable at the cellular level, and other behavioral effects of delta opioid agonists were maintained [23]. In this case, analgesic tolerance resulted from receptor internalization-independent mechanisms that occurred specifically in pain pathways ([23] and Figure 1). In other tolerance studies, SNC80 induced differential tolerance for convulsant, locomotor, and antidepressant responses [24], and the novel delta opioid agonists SB-235863 and JNJ-20788560 did not induce analgesic tolerance in animal models of chronic pain [25,26]. In the future, the exact relationship between agonist-induced internalization, behavioral effects of agonists and the distinct forms of tolerance should be determined for a number of different delta opioid agonists (see Table 1), in order to establish whether in vivo internalization has any predictive value for drug efficacy in clinically relevant experimental settings.
Table 1.
Tools to Study the Delta Opioid Receptor
| Small Molecule Agonists (a) |
Chemical Structure |
Summary | Reference For in vivo data |
|---|---|---|---|
| SNC80 |
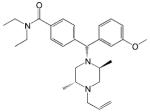
|
- Classic non-peptide delta opioid receptor agonist - Analgesic, antidepressant and anxiolytic effects - Induces receptor internalization in vivo |
[15, 22, 48, 69, 73] |
| AR-M100390 |
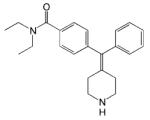
|
- Analgesic effect - No receptor internalization in vivo |
[15] |
| JNJ-20788560 |
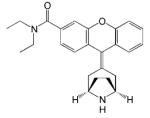
|
- Analgesic effect | [25] |
| Compound 8e |
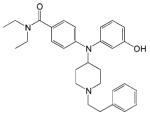
|
- Analgesic effect | [55] |
| ADL5747 |
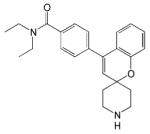
|
- Analgesic effect - Clinical trial phase II ongoing |
[56] |
| ADL5859 |
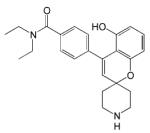
|
- Analgesic and antidepressant effects - Clinical trial phase II completed |
[57] |
| SB-235863 |
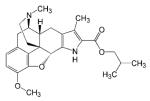
|
- Analgesic effect | [26] |
| NIH 11082 |
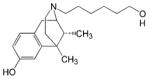
|
- Analgesic and antidepressant effects | [54, 77] |
| UFP-512 |
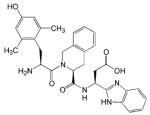
|
- Antidepressant and anxiolytic effects | [74] |
| DVL2DAL5LanEnk |
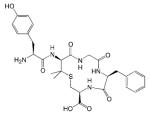
|
- Analgesic effect | [58,59] |
| Genetic Tools | Summary | Reference |
|---|---|---|
| Delta receptor knockout mice | - Conventional delta opioid receptor knockout mouse - Anxiety and depressive-like behavior - Enhanced hyperalgesia under chronic pain state - SNC80 analgesia abolished |
[48-51] |
| Delta receptor conditional knockout mice |
- Deletion of delta opioid receptors in Nav1.8+ sensory neurons - Enhanced hyperalgesia under chronic pain state - SNC80 analgesia reduced |
[60] |
| DOR-eGFP knock-In mice | -Functional delta opioid receptor in fusion with Green Fluorescent Protein in place of native receptor - Receptor visible in vivo at subcellular level |
[15, 21] |
(a) Prototypic (SNC80) and novel delta opioid receptor agonists. These ligands have been selected based on (1) high in vivo delta selectivity determined by blockade with the prototypic delta receptor antagonist naltrindole, or using delta receptor knockout mice, (2) small molecular weight (<600), (3) systemic activity in vivo.
The differential tolerance induced by high- and low-internalizing delta opioid agonists provides an interesting illustration of biased agonism in vivo. This concept, also referred to as functional selectivity, stems from the observation that distinct agonists acting at the same receptor can engage different active receptor conformations, leading to agonist-dependent signaling or regulatory responses [27,28]. Biased agonism has profound implications in understanding the complexity of GPCR pharmacology and facilitating drug development [29-32]. Evidence for this phenomenon is primarily based on in vitro experiments using recombinant cell systems, and a current challenge in GPCR research is to demonstrate the physiological relevance of agonist-biased responses in vivo. Only a few studies have demonstrated behavioral consequences of biased agonism, and a prime example is the finding that hallucinogenic properties of some, but not all 5HT2A agonists result from ligand-biased activation of the Gi/o-Src pathway in cortical neurons [33]. In opioid receptor research, kappa [34] and mu [35] agonist-biased activation of the c-jun cascade was shown to engage specific desensitization mechanisms that had differential impacts on opioid analgesia and tolerance. Ligand-directed trafficking at the mu receptor was also proposed to predict opioid tolerance and dependence [36]. Altogether in vivo biased agonism has now been demonstrated for all three opioid receptors, opening the way to innovative therapeutic strategies based on differential signaling/trafficking properties of opioid drugs. In the case of delta opioid receptors, agonist-biased research may lead to the discovery of drugs with targeted efficacy in either pain, mood enhancement or potentially other disorders discussed further in this review. Additionally, biased agonism may offer a strategy to avoid adverse properties of delta opioid agonists, such as potential convulsions at high doses (see below). The characterization of biased activities of delta opioid agonists is just beginning.
The potential for delta opioid agonists for the treatment of chronic pain
Mu opioid receptor agonists are the most commonly used drugs for the treatment of pain. However, there are several severe drawbacks with the use of mu agonists, such as respiratory depression, sedation, constipation and abuse liability. In addition, mu agonists show variable efficacy in the treatment of chronic pain, partly due to the development of tolerance [37], and there is a debate on whether opioid drugs are useful for chronic non-cancer pain [38]. In contrast to agonists of mu receptors, delta opioid agonists weakly modulate acute nociception [4]. Remarkably however, delta opioid agonists are effective under experimental conditions involving persistent pain [39-42], stress [43] or chronic morphine treatment [44-47]. Mechanisms underlying increased delta opioid agonist efficacy under those specific conditions include higher receptor number and externalization to the cell surface, or better receptor coupling to signaling effectors [39].
Genetic approaches have corroborated the importance of delta opioid receptors in chronic pain rather than acute nociception. Delta opioid receptor knockout mice showed no, or only subtle, modification of pain thresholds in models of acute pain [48-51], but displayed increased neuropathic [51] and inflammatory pain [48], demonstrating the existence of an endogenous delta opioid receptor activity that reduces chronic pain. Notably, knockout mice were also insensitive to nortriptyline, a tricyclic antidepressant drug that fully reverses mechanical allodynia in an animal model of neuropathic pain [52]. The latter data suggest that delta opioid receptors reduce chronic pain downstream of aminergic systems.
Altogether therefore, pharmacological and genetic data highlight delta opioid agonists as a promising alternative to mu analgesics in the treatment of chronic pain (for reviews see [39,41,53]. More recently, the identification of several novel systemically active delta opioid agonists has confirmed the potential of delta opioid receptors as a useful target for chronic pain (see [4,22,25,26,54-59] and Table 1) and some of these drugs are under clinical trials (http://clinicaltrials.gov/ct2/results?term=“delta+opioid”).
Opioid receptors exert their antinociceptive activities at several levels of pain pathways, including the periphery, spinal cord, brain stem and higher-order brain centers ([60], and refs therein). Receptor binding and in situ hybridization studies, as well as the analysis of DOR-eGFP knock-in mice (see above), show delta opioid receptor expression in dorsal root ganglia (DRGs) and spinal cord, as well as limbic forebrain structures ([3,21,61] and Figure 2), suggesting a role for these receptors in primary pain processing as well as emotional and cognitive aspects of pain. Contrasting with mu receptors, delta opioid receptor density is extremely low in nociceptive circuits of the mid-brain (eg. periaqueductal grey) and brainstem (eg. raphe magnus). In the mouse peripheral nervous system, delta opioid receptors are expressed in all DRG cell types, including Aδand C nociceptive neurons, where limited co-expression with mu receptors was described [62], and possible functional interaction with these receptors is currently being debated [12,63]. Notably there are species differences and, in contrast to rodents, delta opioid receptor expression is limited to small diameter neurons in human DRGs [64].
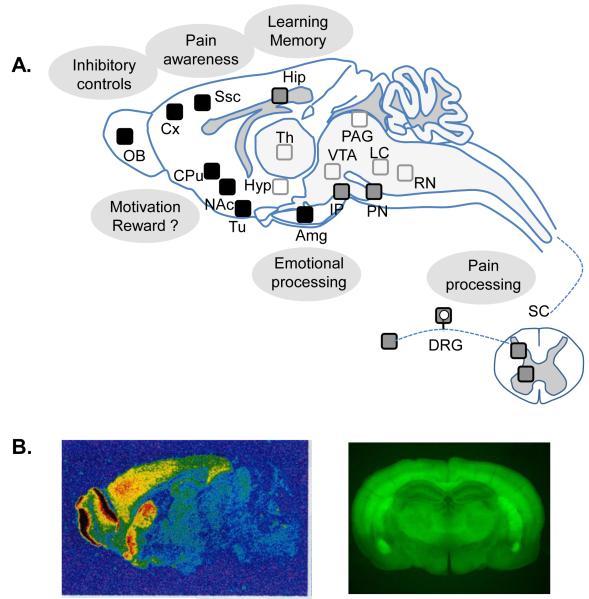
Figure 2. Delta opioid receptors in the central and peripheral nervous systems as potential targets for neurologic and psychiatric disorders.
A. Schematic representation of delta opioid receptor binding sites. Delta opioid receptors are particularly abundant (black squares) in the olfactory bulb, cortex, amygdala and striatum (caudate putamen and nucleus accumbens). Delta opioid receptors are also expressed at moderate level in the interpeduncular and pontine nuclei, hippocampus as well as spinal cord and dorsal root ganglia (grey squares), and at a much lower level in hypothalamus, thalamus, mesencephalon, and brain stem (open squares) (Adapted from [3]). Brain areas of high delta opioid receptor expression are involved in several neural processes whose dysfunction may lead to neurological or psychiatric conditions, including pain (pain processing and awareness), anxiety and depression (emotional processing), addictive and impulse disorders (motivation and reward, learning and memory, inhibitory controls). There is a high expression of delta opioid receptors in dopaminergic terminals (striatum), and the role of delta opioid receptors in reward and motivation is complex. B. Delta opioid receptor binding sites visualized by ligand autoradiography ([3H]deltorphin I, sagittal section left, courtesy of Ian Kitchen) or in knockin fluorescent delta opioid receptor reporter mice (coronal section, [21]). Abbreviations: Amg: amygdala; Cpu: Caudate putamen; Cx, cortex; DRG: dorsal root ganglia; FC: frontal cortex; Hip: hippocampus; Hyp: hypothalamus; IP, interpeduncular nucleus; LC: locus coeruleus; NAc: nucleus accumbens; OB: olfactory bulb; PN, pontine nucleus; PAG: periaqueductal gray; RN: raphe nucleus; SC: spinal cord; Ssc: somatosensorial cortex; Th: thalamus; Tu: olfactory tubercle; VTA: ventral tegmental area.
Understanding the role of delta opioid receptors in peripheral neurons has gained importance [65,66], and the respective contribution of peripheral versus central receptors has been addressed recently. Delta opioid receptors were eliminated genetically in Nav1.8+ sensory neurons that include unmyelinated C and thinly myelinated Aδ nociceptive neurons. Conditional mutant mice showed increased mechanical sensitivity and strongly reduced analgesic responses to delta opioid agonist, in both models of chronic inflammatory and neuropathic pain [60]. These findings confirm the reported role of peripheral delta opioid receptors in the tonic inhibition of mechanical allodynia [62], and demonstrate that peripheral delta opioid receptors are essential to mediate analgesic effects of delta opioid agonists. The development of peripherally-acting delta drugs, therefore, is a feasible strategy for treating nociceptive hypersensitivity associated with chronic pain while avoiding drug-induced central effects.
In conclusion, chronic pain was identified as the first therapeutic area of interest for delta opioid agonists and, although only briefly summarized here, has been heavily documented. Most efforts to develop novel delta compounds stem from pain research. These novel drugs together with recent genetic tools (Table 1), have allowed the expansion of delta opioid receptor research to other disease areas that are discussed below.
The potential of delta opioid agonists for the treatment of emotional disorders
Genetic deletion of the delta opioid receptor [50] or preproenkephalin [67,68] was shown to increase levels of anxiety and depressive-like behavior. These emotional alterations were specifically associated to delta opioid receptors, because kappa and mu receptor knockout mice tested in parallel did not show this phenotype [50]. In accordance, pharmacological blockade of delta opioid receptors by naltrindole, the prototypic delta antagonist, also increased anxiety [69], together with blood corticosterone levels; and this effect was reversed by the agonist SNC80 [70]. Data from receptor ablation or blockade, therefore, concur to indicate that endogenous delta opioid receptor activity improves emotional states.
Accordingly, activation of the delta opioid receptor, either endogenously or by agonist treatment, improved emotional responses. The enkephalinase inhibitor RB101, which increases endogenous levels of opioid peptides, reduced anxiety levels and depressive-like responses even in the absence of mu receptors, and in a naltrindole-reversible manner [71,72]. Focusing on anxiety, several authors showed that systemic SNC80 [73] and UFP-512 [74] decreased levels of anxiety comparable to the prototypic anxiolytic drug diazepam. Microinjections of DPDPE into the amygdala produced a comparable response, which was blocked by naltrindole [75]. The latter observation suggests that delta opioid receptors modulate anxiety at the level of amygdala circuits, a main area for emotional processing and a major site of delta opioid receptor expression ([21] and Figure 2). Other studies have specifically shown the potential of delta opioid agonists as antidepressant drugs. SNC80 [24,69,73,76], NIH11082 [77], UFP512 [74] and ADL5859 [57] inhibited depressive-like behavior, and these effects were comparable to that of prototypic antidepressant drugs, including selective serotonin reuptake inhibitors and tricyclic antidepressants [73,77].Interestingly, acute delta opioid agonist treatment increased BDNF expression in the frontal cortex, similar to classic antidepressants [78], suggesting a common mechanism for the two classes of drugs.
The potential for delta opioid agonists for the treatment of anxiety and depression has raised substantial interest (see Table 1), and clinical trials in this area are ongoing. At the forefront of this effort is AstraZeneca, with one compound (AZD2327) which has successfully completed phase I studies and is currently being assessed in a phase II trial on patients with anxious major depressive disorder (ClinicalTrials.gov ID: NCT00759395).
In the future, the ability of delta opioid receptors to enhance mood in emotional disorders may be considered in the context of pathological pain conditions, known to induce depressive states. The dual activity of delta opioid receptors in both reducing pain and improving mood is particularly attractive in terms of setting novel therapeutic strategies. This research field is still in its infancy, and the finding that delta opioid receptors are down-regulated in the frontal cingulate cortex and amygdala of animals with peripheral neuropathy [79,80] provides a first indication that targeting delta opioid receptors may efficiently contribute to the management of pain-induced emotional disorders.
The potential for delta opioid agonists in neurological disorders
The notion that delta opioid receptors have neuroprotective activity is currently being examined [1]. Deprivation of oxygen and blood supply induces neuronal death, and delta opioid receptor activation appears beneficial in situations of ischemia or hypoxia (review [81]). Hypoxia decreased delta opioid receptor expression, and delta opioid receptor activation counteracted ischemia-induced disruption of ionic homeostasis (review [82]). One strategy to protect neurons from harmful oxidative stress is preconditioning, which consists of exposing cells to sub-lethal amounts of stress leading to enhanced cellular resistance to subsequent stress or injury. Delta opioid receptor blockade inhibited hypoxic preconditioning-induced protection (see reviews [81,82]). Furthermore, delta activation also prevented neurodegeneration in the retina induced by elevation of intraocular pressure [83]. Delta opioid receptors may finally be involved in neuroprotective mechanisms in the context of Alzheimer’s [84] and Parkinson’s diseases [85].
The role of delta opioid receptors in reward and addiction
Delta opioid receptors are heavily expressed in corticolimbic areas of the brain (Figure 2), and the role of delta opioid receptors in reward processes and in the development of addictive behaviors is complex and debated (for recent reviews see [86-88]). Recent data have clarified a number of issues, and the conclusions are summarized here.
Drug addiction originates from the recreational use of drugs, which possess rewarding (or euphoria-producing) properties. The disorder develops upon repeated brain exposure to the drug and excessive stimulation of reward systems. In rodent models, drug reward is classically evaluated using drug-induced conditioned place preference (CPP) or drug self-administration (SA). The switch from drug use to drug abuse is reflected in these models, where adaptive behavioral modifications become detectable following chronic drug exposure. For example, sensitization to drug-induced CPP or extinction of drug SA reflects increased or decreased drug-seeking, respectively. Delta opioid agonists, antagonists, and knockout mice have been tested in these behavioral models and interactions between delta opioid receptors and several drugs of abuse have been investigated.
Whether direct delta opioid receptor activation produces reward is equivocal from pharmacological evidence. Several delta opioid agonists or enkephalinase inhibitors elicit reward, whereas others are ineffective ([89-91] and for review see [86]). Hence, the abuse liability of delta opioid agonists remains controversial. However, several pharmacological studies suggest that delta opioid receptors regulate rewarding or addictive properties of drugs acting at mu opioid receptors or other non-opioid receptor targets. First, delta opioid agonists enhance, while delta antagonists block morphine reward, as well as sensitization to morphine-CPP or heroin-SA ([90,92] and ref therein; [93,94]). Further, pharmacological blockade of delta opioid receptor reduces the rewarding properties of cocaine, methamphetamine, and MDMA (see in [93,95,96] and ref therein). Brain site-specific infusion of delta opioid agonists, antagonists or enkephalinase inhibitor also modify cocaine-SA or cocaine-seeking behavior [97,98]. Therefore, although a number of reports failed to observe any effect of delta antagonists on rewarding properties of cocaine or nicotine [86,99], there is evidence to suggest that delta opioid receptors influence rewarding and addictive effects of psychostimulants. Delta opioid receptors also modulate behavioral effects of alcohol. Opposite results on ethanol intake were described using delta opioid agonists either systemically (decrease) or locally administered in the brain (increase) ([89,100] and ref therein). Site-specific delta antagonist injection disrupted reward properties of ethanol [101] and the two delta antagonists naltriben and BNTX oppositely modified ethanol intake, suggesting differential activity of the two ligands at the receptor [89]. Delta opioid receptor blockade finally reduced cue- or context-induced alcohol seeking [86,102].
Delta opioid receptor gene knockout in mice represent another approach to probe receptor function in drug reward and addiction-related behaviors. Morphine CPP was decreased in mutant animals [92,95], suggesting a role for delta opioid receptors in either morphine reward or conditioned contextual learning. Interestingly, the same [95] and another [103] study showed preserved morphine-SA in mutant mice indicating that rewarding properties of morphine, and motivation to obtain the drug are intact in these mice. These data, together with the observation that both morphine CPP (appetitive stimulus) and lithium conditioned place aversion (aversive stimulus) are impaired in delta opioid receptor knockout mice [95], suggested that delta opioid receptors do not contribute to morphine reward, but are involved and may facilitate contextual learning. The CPP deficit was not evident in other place conditioning studies, showing unchanged CPP to 3,4-methylenedioxymetamphetamine (ecstasy) or D9-tetrahydrocannabinol ([104], and ref in [3]).
Delta opioid receptor knockout mice also showed enhanced voluntary ethanol consumption ([89], and refs in [3]), and innate anxiety levels of mutant mice were reduced after alcohol self-administration [3] providing support for delta opioid receptors as a vulnerability factor in alcohol abuse. Within this line, delta opioid agonists may be effective in preventing relapse by reducing emotional alterations that emerge during withdrawal periods. Accordingly, delta opioid agonists diminished cocaine withdrawal-induced anxiety [105,106]. Also, the delta opioid agonist TAN-67, although not effective in decreasing anxiety-like behavior in naive mice, showed anxiolytic effects in ethanol-withdrawn mice [107].
In conclusion, data from both pharmacological and genetic approaches indicate that delta opioid receptor activity modulates rewarding activities of a number of drugs of abuse, and influences several aspects of addictive behaviors, including drug-seeking, emotional responses and learning processes. Intriguing is the recent finding that delta opioid receptors play a key role in motor impulsivity [108,109], suggesting a possible implication of these receptors in yet another facet of addiction, (i. e. the loss of control over drug intake involving alteration of inhibitory controls) (See Figure 2 and [110,111]). Delta drugs may therefore target several distinct components of this complex relapsing disorder, and the question of whether delta opioid agonists or antagonists may be therapeutically useful in the treatment of drug abuse remains open.
Proconvulsant effects of delta opioid agonists
Altogether, delta opioid receptor activation appears beneficial in several disease conditions that include chronic pain, emotional disorders and perhaps some aspects of addiction. Worth mentioning, however, is the fact that delta opioid receptor activation may lead to convulsions. Delta opioid agonist-induced seizures are mild, closer to a model of absence seizures rather than grand mal, and are prevented by drugs that attenuate absence seizure in humans [112]. Convulsant effects are dependent on intact delta opioid receptor function [113], and were originally considered a caveat to the development for delta drugs. Interestingly however, convulsive effects of SNC80 can be separated from other effects [76]. In addition, tolerance has been shown to develop rapidly to the convulsant effects of SNC80 [24], and many of the recently developed delta opioid agonists presented in Table 1 showed no convulsant effects at analgesic doses. Therefore, the proconvulsant activity of delta opioid agonists appears to be avoidable or surmountable.
Concluding remarks
Delta opioid receptor pharmacology has recently progressed and many novel highly selective delta opioid agonists with potent in vivo activities are now available (Table 1). Beneficial effects of delta opioid agonists have been clearly established in areas of chronic pain and emotional disorders, while the usefulness of delta drugs (agonists or antagonists) in neuroprotection, as well as addictive or impulse disorders, remains to be clarified. Moreover other areas such as learning and memory, in which delta opioid receptors may play a role, are currently being investigated. Importantly, the demonstration that biased agonism at the delta opioid receptor allows the discrimination of delta opioid agonist efficacies in stimulant, analgesic and anxiolytic responses offers novel perspectives for targeted therapies. Altogether, new discoveries in the field of delta opioid receptor biology at the cellular and behavioral level, combined with advanced medicinal chemistry will doubtless open new avenues in several disease areas.
Acknowledgments
The Kieffer lab was supported by the CNRS, INSERM, the Université de Strasbourg, ANR IMOP and LYMPHOPIOID, NIH-NIDA #DA05010 and NIH-NIAAA# AA016658. AP was supported by INSERM-FRSQ and NIH-NIDA K99DA031243. CN was supported by the Fondation pour la Recherche Médicale (FRM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sauriyal DS, et al. Extending pharmacological spectrum of opioids beyond analgesia: Multifunctional aspects in different pathophysiological states. Neuropeptides. 2011 doi: 10.1016/j.npep.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Zollner C, Stein C. Opioids. Handb Exp Pharmacol. 2007:31–63. doi: 10.1007/978-3-540-33823-9_2. [DOI] [PubMed] [Google Scholar]
- 3.Le Merrer J, et al. Reward processing by the opioid system in the brain. Physiol Rev. 2009;89:1379–1412. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanderah TW. Delta and kappa opioid receptors as suitable drug targets for pain. Clin J Pain. 2010;26(Suppl 10):S10–S15. doi: 10.1097/AJP.0b013e3181c49e3a. [DOI] [PubMed] [Google Scholar]
- 5.Shippenberg TS. The dynorphin/kappa opioid receptor system: a new target for the treatment of addiction and affective disorders? Neuropsychopharmacology. 2009;34:247. doi: 10.1038/npp.2008.165. [DOI] [PubMed] [Google Scholar]
- 6.Knoll AT, Carlezon WA., Jr. Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavkin C. The therapeutic potential of kappa-opioids for treatment of pain and addiction. Neuropsychopharmacology. 2011;36:369–370. doi: 10.1038/npp.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams JT, et al. Cellular and synaptic adaptations mediating opioid dependence. Physiol Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- 9.Kane BE, et al. Molecular recognition of opioid receptor ligands. AAPS J. 2006;8:E126–E137. doi: 10.1208/aapsj080115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Decaillot FM, et al. Opioid receptor random mutagenesis reveals a mechanism for G protein-coupled receptor activation. Nat Struct Biol. 2003;10:629–636. doi: 10.1038/nsb950. [DOI] [PubMed] [Google Scholar]
- 11.Kabli N, et al. Agonists at the delta-opioid receptor modify the binding of micro-receptor agonists to the micro-delta receptor hetero-oligomer. Br J Pharmacol. 2010;161:1122–1136. doi: 10.1111/j.1476-5381.2010.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta A, et al. Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci Signal. 2010;3:ra54. doi: 10.1126/scisignal.2000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bushlin I, et al. Cannabinoid-opioid interactions during neuropathic pain and analgesia. Curr Opin Pharmacol. 2010;10:80–86. doi: 10.1016/j.coph.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernard V, et al. Intraneuronal trafficking of G-protein-coupled receptors in vivo. Trends Neurosci. 2006;29:140–147. doi: 10.1016/j.tins.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Pradhan AA, et al. In vivo delta opioid receptor internalization controls behavioral effects of agonists. PLoS One. 2009;4:e5425. doi: 10.1371/journal.pone.0005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bradbury FA, et al. G protein independent phosphorylation and internalization of the delta-opioid receptor. J Neurochem. 2009;109:1526–1535. doi: 10.1111/j.1471-4159.2009.06082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puthenveedu MA, et al. Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell. 2010;143:761–773. doi: 10.1016/j.cell.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry AG, et al. The role of ubiquitination in lysosomal trafficking of delta-opioid receptors. Traffic. 2011;12:170–184. doi: 10.1111/j.1600-0854.2010.01145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abu-Helo A, Simonin F. Identification and biological significance of G protein-coupled receptor associated sorting proteins (GASPs) Pharmacol Ther. 2010;126:244–250. doi: 10.1016/j.pharmthera.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Moser E, et al. G protein-coupled receptor-associated sorting protein 1 regulates the postendocytic sorting of seven-transmembrane-spanning G protein-coupled receptors. Pharmacology. 2010;86:22–29. doi: 10.1159/000314161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scherrer G, et al. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci U S A. 2006;103:9691–9696. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bilsky EJ, et al. SNC 80, a selective, nonpeptidic and systemically active opioid delta opioid agonist. J Pharmacol Exp Ther. 1995;273:359–366. [PubMed] [Google Scholar]
- 23.Pradhan AA, et al. Ligand-directed trafficking of the delta-opioid receptor in vivo: two paths toward analgesic tolerance. J Neurosci. 2010;30:16459–16468. doi: 10.1523/JNEUROSCI.3748-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jutkiewicz EM, et al. Differential behavioral tolerance to the delta-opioid agonist SNC80 ([(+)-4-[(alphaR)-alpha-[(2S,5R)-2,5-dimethyl-4-(2-propenyl)-1-piperazinyl]-(3-methoxyphenyl)methyl]-N,N-diethylbenzamide) in Sprague-Dawley rats. J Pharmacol Exp Ther. 2005;315:414–422. doi: 10.1124/jpet.105.088831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Codd EE, et al. JNJ-20788560 [9-(8-azabicyclo[3.2.1]oct-3-ylidene)-9H-xanthene-3-carboxylic acid diethylamide], a selective delta opioid receptor agonist, is a potent and efficacious antihyperalgesic agent that does not produce respiratory depression, pharmacologic tolerance, or physical dependence. J Pharmacol Exp Ther. 2009;329:241–251. doi: 10.1124/jpet.108.146969. [DOI] [PubMed] [Google Scholar]
- 26.Petrillo P, et al. Evidence for a selective role of the delta-opioid agonist [8R-(4bS*,8aalpha,8abeta, 12bbeta)]7,10-Dimethyl-1-methoxy-11-(2-methylpropyl)oxycarbonyl 5,6,7,8,12,12b-hexahydro-(9H)-4,8-methanobenzofuro[3,2-e]pyrrolo[2,3-g]iso quinoline hydrochloride (SB-235863) in blocking hyperalgesia associated with inflammatory and neuropathic pain responses. J Pharmacol Exp Ther. 2003;307:1079–1089. doi: 10.1124/jpet.103.055590. [DOI] [PubMed] [Google Scholar]
- 27.Kobilka BK, Deupi X. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:397–406. doi: 10.1016/j.tips.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Vaidehi N, Kenakin T. The role of conformational ensembles of seven transmembrane receptors in functional selectivity. Curr Opin Pharmacol. 2010;10:775–781. doi: 10.1016/j.coph.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Kenakin T, Miller LJ. Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol Rev. 2010;62:265–304. doi: 10.1124/pr.108.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bosier B, Hermans E. Versatility of GPCR recognition by drugs: from biological implications to therapeutic relevance. Trends Pharmacol Sci. 2007;28:438–446. doi: 10.1016/j.tips.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Galandrin S, et al. The evasive nature of drug efficacy: implications for drug discovery. Trends Pharmacol Sci. 2007;28:423–430. doi: 10.1016/j.tips.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Mailman RB. GPCR functional selectivity has therapeutic impact. Trends Pharmacol Sci. 2007;28:390–396. doi: 10.1016/j.tips.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez-Maeso J, et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 34.Bruchas MR, et al. Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase. J Biol Chem. 2007;282:29803–29811. doi: 10.1074/jbc.M705540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melief EJ, et al. Ligand-directed c-Jun N-terminal kinase activation disrupts opioid receptor signaling. Proc Natl Acad Sci U S A. 2010;107:11608–11613. doi: 10.1073/pnas.1000751107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JA, et al. Morphine-induced receptor endocytosis in a novel knockin mouse reduces tolerance and dependence. Curr Biol. 2008;18:129–135. doi: 10.1016/j.cub.2007.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oertel B, Lotsch J. Genetic mutations that prevent pain: implications for future pain medication. Pharmacogenomics. 2008;9:179–194. doi: 10.2217/14622416.9.2.179. [DOI] [PubMed] [Google Scholar]
- 38.Crofford LJ. Adverse effects of chronic opioid therapy for chronic musculoskeletal pain. Nat Rev Rheumatol. 2010;6:191–197. doi: 10.1038/nrrheum.2010.24. [DOI] [PubMed] [Google Scholar]
- 39.Cahill CM, et al. Trafficking of delta-opioid receptors and other G-protein-coupled receptors: implications for pain and analgesia. Trends Pharmacol Sci. 2007;28:23–31. doi: 10.1016/j.tips.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Kabli N, Cahill CM. Anti-allodynic effects of peripheral delta opioid receptors in neuropathic pain. Pain. 2007;127:84–93. doi: 10.1016/j.pain.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Bie B, Pan ZZ. Trafficking of central opioid receptors and descending pain inhibition. Mol Pain. 2007;3:37. doi: 10.1186/1744-8069-3-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rowan MP, et al. Peripheral delta opioid receptors require priming for functional competence in vivo. Eur J Pharmacol. 2009;602:283–287. doi: 10.1016/j.ejphar.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Commons KG. Translocation of presynaptic delta opioid receptors in the ventrolateral periaqueductal gray after swim stress. J Comp Neurol. 2003;464:197–207. doi: 10.1002/cne.10788. [DOI] [PubMed] [Google Scholar]
- 44.Morgan MM, et al. Behavioral consequences of delta-opioid receptor activation in the periaqueductal gray of morphine tolerant rats. Neural Plast. 2009;2009:516328. doi: 10.1155/2009/516328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bie B, et al. Rewarding morphine-induced synaptic function of delta-opioid receptors on central glutamate synapses. J Pharmacol Exp Ther. 2009;329:290–296. doi: 10.1124/jpet.108.148908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schramm CL, Honda CN. Co-administration of delta- and mu-opioid receptor agonists promotes peripheral opioid receptor function. Pain. 2010;151:763–770. doi: 10.1016/j.pain.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Negus SS, et al. Modulation of delta opioid agonist-induced antinociception by repeated morphine pretreatment in rhesus monkeys. Life Sci. 2010;86:385–392. doi: 10.1016/j.lfs.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaveriaux-Ruff C, et al. Inflammatory pain is enhanced in delta opioid receptor-knockout mice. Eur J Neurosci. 2008;27:2558–2567. doi: 10.1111/j.1460-9568.2008.06223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Y, et al. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 50.Filliol D, et al. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- 51.Nadal X, et al. Neuropathic pain is enhanced in delta-opioid receptor knockout mice. Eur J Neurosci. 2006;23:830–834. doi: 10.1111/j.1460-9568.2006.04569.x. [DOI] [PubMed] [Google Scholar]
- 52.Benbouzid M, et al. Delta-opioid receptors are critical for tricyclic antidepressant treatment of neuropathic allodynia. Biol Psychiatry. 2008;63:633–636. doi: 10.1016/j.biopsych.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X, et al. Role of delivery and trafficking of delta-opioid peptide receptors in opioid analgesia and tolerance. Trends Pharmacol Sci. 2006;27:324–329. doi: 10.1016/j.tips.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 54.Aceto MD, et al. Pharmacological studies with a nonpeptidic, delta-opioid (−)-(1R,5R,9R)-5,9-dimethyl-2′-hydroxy-2-(6-hydroxyhexyl)-6,7-benzomorphan hydrochloride ((−)-NIH 11082) Eur J Pharmacol. 2007;566:88–93. doi: 10.1016/j.ejphar.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jones P, et al. N,N-Diethyl-4-[(3-hydroxyphenyl)(piperidin-4-yl)amino] benzamide derivatives: the development of diaryl amino piperidines as potent delta opioid receptor agonists with in vivo antinociceptive activity in rodent models. Bioorg Med Chem Lett. 2009;19:5994–5998. doi: 10.1016/j.bmcl.2009.09.072. [DOI] [PubMed] [Google Scholar]
- 56.Le Bourdonnec B, et al. Spirocyclic delta opioid receptor agonists for the treatment of pain: discovery of N,N-diethyl-3-hydroxy-4-(spiro[chromene-2,4′-piperidine]-4-yl) benzamide (ADL5747) J Med Chem. 2009;52:5685–5702. doi: 10.1021/jm900773n. [DOI] [PubMed] [Google Scholar]
- 57.Le Bourdonnec B, et al. Potent, orally bioavailable delta opioid receptor agonists for the treatment of pain: discovery of N,N-diethyl-4-(5-hydroxyspiro[chromene-2,4′-piperidine]-4-yl)benzamide (ADL5859) J Med Chem. 2008;51:5893–5896. doi: 10.1021/jm8008986. [DOI] [PubMed] [Google Scholar]
- 58.Svensson CI, et al. Systemic and spinal analgesic activity of a delta-opioid-selective lanthionine enkephalin analog. J Pharmacol Exp Ther. 2003;304:827–832. doi: 10.1124/jpet.102.039750. [DOI] [PubMed] [Google Scholar]
- 59.Brainin-Mattos J, et al. Cancer-related bone pain is attenuated by a systemically available delta-opioid receptor agonist. Pain. 2006;122:174–181. doi: 10.1016/j.pain.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 60.Gaveriaux-Ruff C, et al. Genetic ablation of delta opioid receptors in nociceptive sensory neurons increases chronic pain and abolishes opioid analgesia. Pain. 2011 doi: 10.1016/j.pain.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 61.Mansour A, et al. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- 62.Scherrer G, et al. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang HB, et al. Coexpression of delta- and mu-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci U S A. 2010;107:13117–13122. doi: 10.1073/pnas.1008382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mennicken F, et al. Phylogenetic changes in the expression of delta opioid receptors in spinal cord and dorsal root ganglia. J Comp Neurol. 2003;465:349–360. doi: 10.1002/cne.10839. [DOI] [PubMed] [Google Scholar]
- 65.Stein C, et al. Peripheral mechanisms of pain and analgesia. Brain Res Rev. 2009;60:90–113. doi: 10.1016/j.brainresrev.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hua S, Cabot PJ. Mechanisms of peripheral immune-cell-mediated analgesia in inflammation: clinical and therapeutic implications. Trends Pharmacol Sci. 2010;31:427–433. doi: 10.1016/j.tips.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 67.Konig M, et al. Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature. 1996;383:535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- 68.Ragnauth A, et al. Female preproenkephalin-knockout mice display altered emotional responses. Proc Natl Acad Sci U S A. 2001;98:1958–1963. doi: 10.1073/pnas.041598498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Perrine SA, et al. Delta opioid receptor ligands modulate anxiety-like behaviors in the rat. Br J Pharmacol. 2006;147:864–872. doi: 10.1038/sj.bjp.0706686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saitoh A, et al. Role of delta-opioid receptor subtypes in anxiety-related behaviors in the elevated plus-maze in rats. Psychopharmacology (Berl) 2005;182:327–334. doi: 10.1007/s00213-005-0112-6. [DOI] [PubMed] [Google Scholar]
- 71.Nieto MM, et al. Physiological control of emotion-related behaviors by endogenous enkephalins involves essentially the delta opioid receptors. Neuroscience. 2005;135:305–313. doi: 10.1016/j.neuroscience.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 72.Jutkiewicz EM, et al. Behavioral and neurobiological effects of the enkephalinase inhibitor RB101 relative to its antidepressant effects. Eur J Pharmacol. 2006;531:151–159. doi: 10.1016/j.ejphar.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saitoh A, et al. Potential anxiolytic and antidepressant-like activities of SNC80, a selective delta-opioid agonist, in behavioral models in rodents. J Pharmacol Sci. 2004;95:374–380. doi: 10.1254/jphs.fpj04014x. [DOI] [PubMed] [Google Scholar]
- 74.Vergura R, et al. Anxiolytic- and antidepressant-like activities of H-Dmt-Tic-NH-CH(CH2-COOH)-Bid (UFP-512), a novel selective delta opioid receptor agonist. Peptides. 2008;29:93–103. doi: 10.1016/j.peptides.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 75.Randall-Thompson JF, et al. A role for delta opioid receptors in the central nucleus of the amygdala in anxiety-like behaviors. Psychopharmacology (Berl) 2010;212:585–595. doi: 10.1007/s00213-010-1980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jutkiewicz EM, et al. Separation of the convulsions and antidepressant-like effects produced by the delta-opioid agonist SNC80 in rats. Psychopharmacology (Berl) 2005;182:588–596. doi: 10.1007/s00213-005-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Naidu PS, et al. NIH 11082 produces anti-depressant-like activity in the mouse tail-suspension test through a delta-opioid receptor mechanism of action. Eur J Pharmacol. 2007;566:132–136. doi: 10.1016/j.ejphar.2007.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Torregrossa MM, et al. Peptidic delta opioid receptor agonists produce antidepressant-like effects in the forced swim test and regulate BDNF mRNA expression in rats. Brain Res. 2006;1069:172–181. doi: 10.1016/j.brainres.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Narita M, et al. Chronic pain-induced emotional dysfunction is associated with astrogliosis due to cortical delta-opioid receptor dysfunction. J Neurochem. 2006;97:1369–1378. doi: 10.1111/j.1471-4159.2006.03824.x. [DOI] [PubMed] [Google Scholar]
- 80.Narita M, et al. Chronic pain induces anxiety with concomitant changes in opioidergic function in the amygdala. Neuropsychopharmacology. 2006;31:739–750. doi: 10.1038/sj.npp.1300858. [DOI] [PubMed] [Google Scholar]
- 81.Johnson SM, Turner SM. Protecting motor networks during perinatal ischemia: the case for delta-opioid receptors. Ann N Y Acad Sci. 2010;1198:260–270. doi: 10.1111/j.1749-6632.2010.05434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chao D, Xia Y. Ionic storm in hypoxic/ischemic stress: can opioid receptors subside it? Prog Neurobiol. 2010;90:439–470. doi: 10.1016/j.pneurobio.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peng PH, et al. Novel role for the delta-opioid receptor in hypoxic preconditioning in rat retinas. J Neurochem. 2009;108:741–754. doi: 10.1111/j.1471-4159.2008.05807.x. [DOI] [PubMed] [Google Scholar]
- 84.Thathiah A, De Strooper B. The role of G protein-coupled receptors in the pathology of Alzheimer’s disease. Nat Rev Neurosci. 2011;12:73–87. doi: 10.1038/nrn2977. [DOI] [PubMed] [Google Scholar]
- 85.Mabrouk OS, et al. The novel delta opioid receptor agonist UFP-512 dually modulates motor activity in hemiparkinsonian rats via control of the nigro-thalamic pathway. Neuroscience. 2009;164:360–369. doi: 10.1016/j.neuroscience.2009.08.058. [DOI] [PubMed] [Google Scholar]
- 86.Shippenberg TS, et al. Targeting endogenous mu- and delta-opioid receptor systems for the treatment of drug addiction. CNS Neurol Disord Drug Targets. 2008;7:442–453. doi: 10.2174/187152708786927813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mendez M, Morales-Mulia M. Role of mu and delta opioid receptors in alcohol drinking behaviour. Curr Drug Abuse Rev. 2008;1:239–252. doi: 10.2174/1874473710801020239. [DOI] [PubMed] [Google Scholar]
- 88.Rodriguez-Arias M, et al. Preclinical evidence of new opioid modulators for the treatment of addiction. Expert Opin Investig Drugs. 2010;19:977–994. doi: 10.1517/13543784.2010.500612. [DOI] [PubMed] [Google Scholar]
- 89.van Rijn RM, Whistler JL. The delta(1) opioid receptor is a heterodimer that opposes the actions of the delta(2) receptor on alcohol intake. Biol Psychiatry. 2009;66:777–784. doi: 10.1016/j.biopsych.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suzuki T, et al. Effect of the highly selective and nonpeptide delta opioid receptor agonist TAN-67 on the morphine-induced place preference in mice. J Pharmacol Exp Ther. 1996;279:177–185. [PubMed] [Google Scholar]
- 91.Do Carmo GP, et al. The selective non-peptidic delta opioid agonist SNC80 does not facilitate intracranial self-stimulation in rats. Eur J Pharmacol. 2009;604:58–65. doi: 10.1016/j.ejphar.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chefer VI, Shippenberg TS. Augmentation of morphine-induced sensitization but reduction in morphine tolerance and reward in delta-opioid receptor knockout mice. Neuropsychopharmacology. 2009;34:887–898. doi: 10.1038/npp.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shippenberg TS, et al. Delta-opioid receptor antagonists prevent sensitization to the conditioned rewarding effects of morphine. Biol Psychiatry. 2009;65:169–174. doi: 10.1016/j.biopsych.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moron JA, et al. Modulation of opiate-related signaling molecules in morphine-dependent conditioned behavior: conditioned place preference to morphine induces CREB phosphorylation. Neuropsychopharmacology. 2010;35:955–966. doi: 10.1038/npp.2009.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Le Merrer J, et al. Deletion of the delta Opioid Receptor Gene Impairs Place Conditioning But Preserves Morphine Reinforcement. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 96.Belkai E, et al. Modulation of MDMA-induced behavioral and transcriptional effects by the delta opioid antagonist naltrindole in mice. Addict Biol. 2009;14:245–252. doi: 10.1111/j.1369-1600.2009.00156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ward SJ, Roberts DC. Microinjection of the delta-opioid receptor selective antagonist naltrindole 5′-isothiocyanate site specifically affects cocaine self-administration in rats responding under a progressive ratio schedule of reinforcement. Behav Brain Res. 2007;182:140–144. doi: 10.1016/j.bbr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Simmons D, Self DW. Role of mu- and delta-opioid receptors in the nucleus accumbens in cocaine-seeking behavior. Neuropsychopharmacology. 2009;34:1946–1957. doi: 10.1038/npp.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ismayilova N, Shoaib M. Alteration of intravenous nicotine self-administration by opioid receptor agonist and antagonists in rats. Psychopharmacology (Berl) 2010;210:211–220. doi: 10.1007/s00213-010-1845-4. [DOI] [PubMed] [Google Scholar]
- 100.Barson JR, et al. Opioids in the hypothalamic paraventricular nucleus stimulate ethanol intake. Alcohol Clin Exp Res. 2010;34:214–222. doi: 10.1111/j.1530-0277.2009.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gremel CM, et al. Blockade of opioid receptors in anterior cingulate cortex disrupts ethanol-seeking behavior in mice. Behav Brain Res. 2011 doi: 10.1016/j.bbr.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marinelli PW, et al. Roles of opioid receptor subtypes in mediating alcohol-seeking induced by discrete cues and context. Eur J Neurosci. 2009;30:671–678. doi: 10.1111/j.1460-9568.2009.06851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.David V, et al. Brain regional Fos expression elicited by the activation of mu- but not delta-opioid receptors of the ventral tegmental area: evidence for an implication of the ventral thalamus in opiate reward. Neuropsychopharmacology. 2008;33:1746–1759. doi: 10.1038/sj.npp.1301529. [DOI] [PubMed] [Google Scholar]
- 104.Robledo P. Cannabinoids, opioids and MDMA: neuropsychological interactions related to addiction. Curr Drug Targets. 2010;11:429–439. doi: 10.2174/138945010790980330. [DOI] [PubMed] [Google Scholar]
- 105.Ambrose-Lanci LM, et al. Cocaine withdrawal-induced anxiety in females: impact of circulating estrogen and potential use of delta-opioid receptor agonists for treatment. J Neurosci Res. 2010;88:816–824. doi: 10.1002/jnr.22259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Perrine SA, et al. Withdrawal from chronic administration of cocaine decreases delta opioid receptor signaling and increases anxiety- and depression-like behaviors in the rat. Neuropharmacology. 2008;54:355–364. doi: 10.1016/j.neuropharm.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.van Rijn RM, et al. Dual efficacy of delta opioid receptor-selective ligands for ethanol drinking and anxiety. J Pharmacol Exp Ther. 2010;335:133–139. doi: 10.1124/jpet.110.170969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Olmstead MC, et al. Mu and delta opioid receptors oppositely regulate motor impulsivity in the signaled nose poke task. PLoS One. 2009;4:e4410. doi: 10.1371/journal.pone.0004410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Befort K, et al. Effects of delta opioid receptors activation on a response inhibition task in rats. Psychopharmacology (Berl) 2010 doi: 10.1007/s00213-010-2108-0. [DOI] [PubMed] [Google Scholar]
- 110.Pattij T, Vanderschuren LJ. The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci. 2008;29:192–199. doi: 10.1016/j.tips.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 111.Winstanley CA, et al. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res. 2010;34:1306–1318. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jutkiewicz EM, et al. The convulsive and electroencephalographic changes produced by nonpeptidic delta-opioid agonists in rats: comparison with pentylenetetrazol. J Pharmacol Exp Ther. 2006;317:1337–1348. doi: 10.1124/jpet.105.095810. [DOI] [PubMed] [Google Scholar]
- 113.Broom DC, et al. Comparison of receptor mechanisms and efficacy requirements for delta-agonist-induced convulsive activity and antinociception in mice. J Pharmacol Exp Ther. 2002;303:723–729. doi: 10.1124/jpet.102.036525. [DOI] [PubMed] [Google Scholar]