Abstract
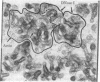
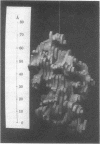
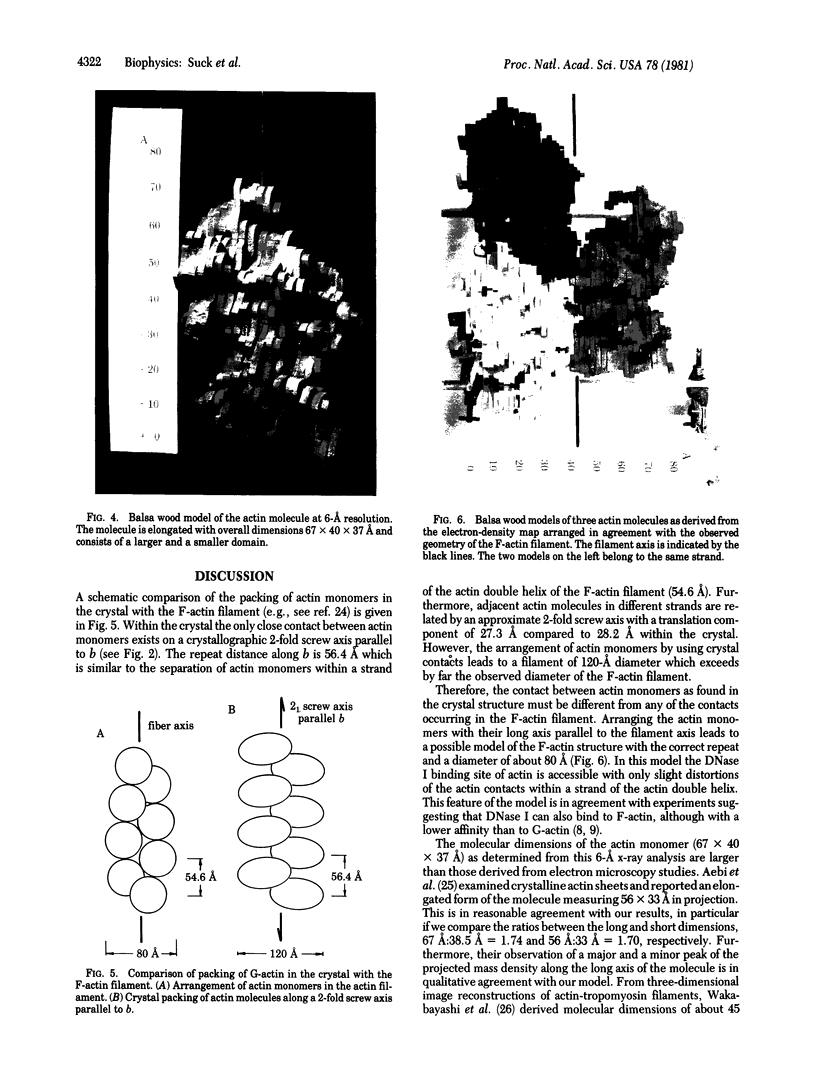
The structure of rabbit skeletal muscle actin complexed with bovine pancreatic DNase I has been determined by x-ray crystallographic methods at 6-A resolution. The analysis was based on a new orthorhombic crystal form, space group P212121, with one complex in the asymmetric unit. Six isomorphous heavy-atom derivatives yielding an overall figure of merit of 0.72 have been used to calculate the electron-density map. Molecular models for actin and DNase I derived from this map have dimensions 67 X 40 X 37 A and 50 X 50 X 40 A, respectively. The actin molecule is elongated and consists of a larger and a smaller domain, each containing density regions resembling a central beta-pleated sheet surrounded by alpha-helices. The highest electron-density peak is found in the cleft between the two domains, perhaps indicating the bound ATP. Observed crystal contacts between actin molecules and a model for the F-actin filament are discussed. Two high-affinity Ca2+-binding sites which also bind Ba2+ have been located at the surface of the DNase I molecule.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., Smith P. R., Isenberg G., Pollard T. D. Structure of crystalline actin sheets. Nature. 1980 Nov 20;288(5788):296–298. doi: 10.1038/288296a0. [DOI] [PubMed] [Google Scholar]
- Burgoyne L. A., Wagar M. A., Atkinson M. R. Calcium-dependent priming of DNA synthesis in isolated rat liver nuclei. Biochem Biophys Res Commun. 1970 Apr 24;39(2):254–259. doi: 10.1016/0006-291x(70)90786-2. [DOI] [PubMed] [Google Scholar]
- Carlsson L., Nyström L. E., Lindberg U., Kannan K. K., Cid-Dresdner H., Lövgren S. Crystallization of a non-muscle actin. J Mol Biol. 1976 Aug 15;105(3):353–366. doi: 10.1016/0022-2836(76)90098-x. [DOI] [PubMed] [Google Scholar]
- Clarke M., Spudich J. A. Nonmuscle contractile proteins: the role of actin and myosin in cell motility and shape determination. Annu Rev Biochem. 1977;46:797–822. doi: 10.1146/annurev.bi.46.070177.004053. [DOI] [PubMed] [Google Scholar]
- Elzinga M., Collins J. H., Kuehl W. M., Adelstein R. S. Complete amino-acid sequence of actin of rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2687–2691. doi: 10.1073/pnas.70.9.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock S. E., Carisson L., Lindberg U. Depolymerization of F-actin by deoxyribonuclease I. Cell. 1976 Apr;7(4):531–542. doi: 10.1016/0092-8674(76)90203-8. [DOI] [PubMed] [Google Scholar]
- Kabsch W., Gast W. H., Schulz G. E., Leberman R. Low resolution structure of partially trypsin-degraded polypeptide elongation factor, EF-TU, from Escherichia coli. J Mol Biol. 1977 Dec 25;117(4):999–1012. doi: 10.1016/s0022-2836(77)80009-0. [DOI] [PubMed] [Google Scholar]
- Kasai M., Oosawa F. The exchangeability of actin-bound calcium with various divalent cations. Biochim Biophys Acta. 1968 Apr 9;154(3):520–528. doi: 10.1016/0005-2795(68)90012-3. [DOI] [PubMed] [Google Scholar]
- Liao T. H., Salnikow J., Moore S., Stein W. H. Bovine pancreatic deoxyribonuclease A. Isolation of cyanogen bromide peptides; complete covalent structure of the polypeptide chain. J Biol Chem. 1973 Feb 25;248(4):1489–1495. [PubMed] [Google Scholar]
- Mannherz H. G., Goody R. S., Konrad M., Nowak E. The interaction of bovine pancreatic deoxyribonuclease I and skeletal muscle actin. Eur J Biochem. 1980 Mar;104(2):367–379. doi: 10.1111/j.1432-1033.1980.tb04437.x. [DOI] [PubMed] [Google Scholar]
- Mannherz H. G., Goody R. S. Proteins of contractile systems. Annu Rev Biochem. 1976;45:427–465. doi: 10.1146/annurev.bi.45.070176.002235. [DOI] [PubMed] [Google Scholar]
- Mannherz H. G., Kabsch W., Leverman R. Crystals of skeletal muscle actin: pancreatic DNAase I complex. FEBS Lett. 1977 Feb 1;73(2):141–143. doi: 10.1016/0014-5793(77)80966-6. [DOI] [PubMed] [Google Scholar]
- Mannherz H. G., Leigh J. B., Leberman R., Pfrang H. A specific 1:1 G-actin:DNAase i complex formed by the action of DNAase I on F-actin. FEBS Lett. 1975 Dec 1;60(1):34–38. doi: 10.1016/0014-5793(75)80412-1. [DOI] [PubMed] [Google Scholar]
- Moore P. B., Huxley H. E., DeRosier D. J. Three-dimensional reconstruction of F-actin, thin filaments and decorated thin filaments. J Mol Biol. 1970 Jun 14;50(2):279–295. doi: 10.1016/0022-2836(70)90192-0. [DOI] [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Price P. A. The essential role of Ca2+ in the activity of bovine pancreatic deoxyribonuclease. J Biol Chem. 1975 Mar 25;250(6):1981–1986. [PubMed] [Google Scholar]
- Rohr G., Mannherz H. G. Isolation and characterization of secretory actin . DNAase I complex from rat pancreatic juice. Eur J Biochem. 1978 Aug 15;89(1):151–157. doi: 10.1111/j.1432-1033.1978.tb20907.x. [DOI] [PubMed] [Google Scholar]
- Salnikow J., Moore S., Stein W. H. Comparison of the multiple forms of bovine pancreatic deoxyribonuclease. J Biol Chem. 1970 Nov 10;245(21):5685–5690. [PubMed] [Google Scholar]
- Strzelecka-Gołaszewska H., Próchniewicz E., Nowak E., Zmorzyński S., Drabikowski W. Chicken-gizzard actin: polymerization and stability. Eur J Biochem. 1980 Feb;104(1):41–52. doi: 10.1111/j.1432-1033.1980.tb04397.x. [DOI] [PubMed] [Google Scholar]
- Suck D., Kabsch W. X-ray determination of the GDP-binding site of Escherichia coli elongation factor Tu by substitution with ppGpp. FEBS Lett. 1981 Apr 6;126(1):120–122. doi: 10.1016/0014-5793(81)81048-4. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. The amino acid sequence of actin from chicken skeletal muscle actin and chicken gizzard smooth muscle actin. FEBS Lett. 1979 Jun 15;102(2):219–222. doi: 10.1016/0014-5793(79)80004-6. [DOI] [PubMed] [Google Scholar]
- Wakabayashi T., Huxley H. E., Amos L. A., Klug A. Three-dimensional image reconstruction of actin-tropomyosin complex and actin-tropomyosin-troponin T-troponin I complex. J Mol Biol. 1975 Apr 25;93(4):477–497. doi: 10.1016/0022-2836(75)90241-7. [DOI] [PubMed] [Google Scholar]