Abstract
The synthesis and characterization of two 18-crown-6 functionalized analogues of an extensively studied gadolinium texaphyrin derivative, motexafin gadolinium (1, MGd), are reported. These are the monomeric and dimeric species, compounds 2 and 3, respectively. Both crown ether functionalized species proved to be stable at physiological pH and revealed distinct shifts in the UV spectrum when treated with sodium-, potassium-, ammonium- or zinc(II)-salts. Zinc(II) is believed to play a major role regulating apoptosis mechanisms in cancerous cells. Therefore, cytotoxicity studies of 2 and 3 were carried out using Ramos cell lines in the presence and absence of zinc(II).
Keywords: Expanded Porphyrins, Radiation Sensitizer, Gadolinium Texaphyrin, crown ethers
INTRODUCTION
Texaphyrins, first discovered in 1987, are pentaaza, Schiff-base macrocycles that bear a strong, but “expanded” relationship to the porphyrins and other naturally occurring tetrapyrrolic pigments [1-3]. Represented by the prototypic complex motexafin gadolinium (1, MGd) the texaphyrins have an ability to form stable, nonlabile 1:1 complexes with a range of metal cations. Although recent efforts have demonstrated an ability to chelate metal cations of the first transition series, the texaphyrins, as prototypical expanded porphyrins, were initially characterized in terms of their ability to coordinate “large cations”, including specifically those of the trivalent lanthanide series [1, 4, 5]. This success was rationalized in terms of the fact that the central core of texaphyrins is roughly 20% larger than that of the porphyrins [6]. It also led to explorations of solubilized texaphyrins for various biomedical applications.
As the result of extensive biological testing, it is now appreciated that certain texaphyrins will localize to, or be retained selectively in, rapidly growing tissues, including cancerous lesions [7]. Among the various texaphyrins produced to date, MGd 1 (cf. Fig. 1) has been studied in considerable detail [8]. In particular, it has been shown to be taken up selectively by B cell non-Hodgkins lymphoma, chronic lymphocytic leukemia and myeloma cells [9], as well as non-small cell lung cancer and breast cancer tissues. The ratio of MGd in tumor cells to that in surrounding normal cells is reported to be up to 9:1 [10]; this increases to 50:1 in the case of metastatic brain tumors [11]. While these ratios make texaphyrins of interest as possible targeting agents (e.g., for the delivery of other cytotoxic speces), we believe that the utility of texaphyrins could be further increased were the degree to localization to be enhanced. With such a goal in mind, we set out to prepare deriva- tives of 1 that incorporate crown ether moities. Specifically, we report the synthesis and initial testing of the mono-crown derivative 2, as well as the crown ether-bridged texaphyrin dimer 3.
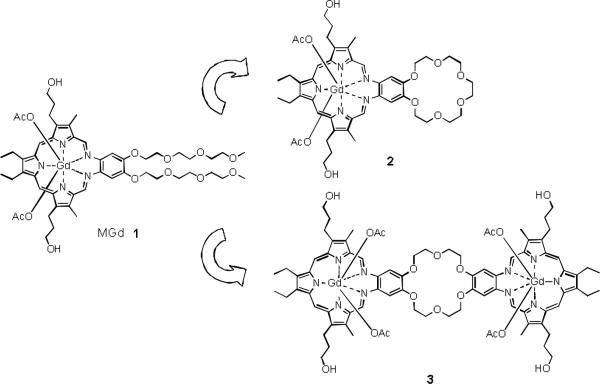
Fig. 1.
MGd 1 and the crown ether functionalized analogs 2 and 3.
Crown ethers are well recognized for their special complexing properties. This has inspired several applications of crown ethers in the area of drug delivery, even though these systems are not known to possess salutary medicinal properties on their own [12, 13]. For instance, crown ethers have found use in vivo as targeting functionalities incorporated inter alia into modified DNA-binding agents [14, 15]. It was shown that the positive charge of the cation-crown ether complexes increased the ability of crown ether linked compounds to interact with the polyanionic phosphate backbone of DNA [14, 16]. Presumably, the in vitro or in vivo formation of cationic crown ether complexes with ions that are abundant in cells, such as sodium (intracellular concentration = 5-15 mM) or potassium (intracellular concentration = 140 mM) leads to an increased interaction with the DNA target. We also appreciate that similar cation capture effects could serve to modulate the membrane diffusion properties. For instance, extracellular complexation of the Na+ cation by a crown ether-functionalized compound might allow for the mediated cell uptake driven by the transmembrane sodium gradient (extracellular Na+ concentration = 145 mM). Taken in concert, these considerations provided an incentive to combine an 18-crown-6 subunit with a texaphyrin core. Operationally, it was considered likely that the resulting systems, compounds 2 and 3, respectively, would display anticancer properties that differed from those of 1. A primary goal of this study was thus to test this hypothesis.
A further rationale for the study of 2 and 3 is the recent finding that MGd 1 potentiates zinc(II) in in vitro bioactivity assays [17, 18]. Intracellular free zinc concentrations are normally extremely low (around 1 nM); however, elevated zinc levels are closely connected to prostate and pancreatic cancer [19, 20]. Moreover, under the conditions of oxidative stress, as would be true in the case of radiotherapy (a modality in which 1 has been tested), the concentration of free zinc is expected to be significantly increased. In fact, the antiproliferative activity of 1 was found to be greatly enhanced in the presence of zinc(II) (with, e.g., a reduction to about 40% viability being seen at 50 μM zinc(II) and 5 μM 1 in Ramos cells). While the origins of this increase in antiproliferative activity are still being explored, one hypothesis is that the polyethylene glycol chains present in 1 are serving to chelate the zinc(II) cation and thus facilitate its into-cell uptake. While there is little evidence in the literature that aza-free polyethers complex zinc(II) to an appreciable extent, it was considered likely that any such putative cation chelation and through-membrane transport activity would be enhanced in the case of a crown containig derivative. We thus sought to examine the two modified texaphyrins of this study in combination with zinc(II).
RESULTS AND DISCUSSION
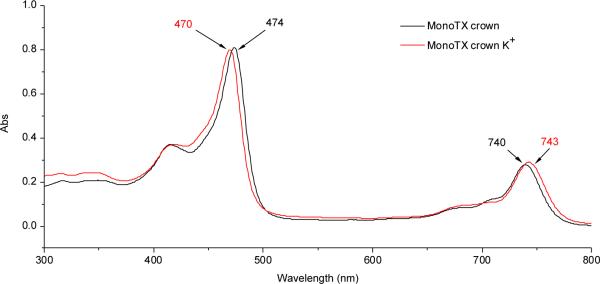
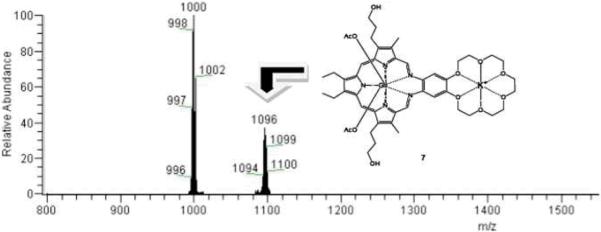
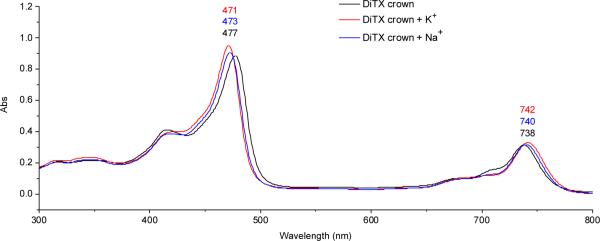
Since gadolinium(III) is a strongly paramagnetic lanthanide cation, standard NMR spectroscopic characterization is not practical. Hence, UV-Vis spectroscopic analyses of 2 were performed; these revealed the typical absorption bands expected for gadolinium(III) texaphyrins, specifically a Soret-like band at 474 nm and a Q-type band at 740 nm. Incubation of the MGd-crown system 2 with a concentration of potassium corresponding to that typically found in cells (140 mM, as potassium acetate) over the course of one hour at 37 °C resulted in a shift of the absorption bands, with the Soret-like band moving to the blue by 4 nm and the Q-type band by 3 mm to the red (Fig. 2). In addition to UV-Vis data, the texaphyrin potassium-crown ether complex was also characterized by ESI-mass spectrometry. This revealed two major gadolinium cluster peaks (isotope patterns), one at m/z = 1000 (M – AcO–) and one at m/z = 1096 (M + K+, structure 7), as can be seen by an inspection of Fig. 3. Corresponding mass spectrometric and UV-Vis spectroscopic changes were not seen when analogous experiments were carried out with 1. Taken in concernt, these findings provide support for the assumption that potassium complexation is taking place and that the crown ether subunit is responsible for the the underlying recognition process. It is noteworthy that this complexation takes place even though the gadolinium(III) texaphyrin portion of this binucleating system bears two positive charges, at least in the absence of the axial ligands.
Fig.2.
UV-Vis spectrum of 2 [4.7 μM] and upon incubation with K+; incubated for one hour at 37 °C in aqueous buffered solution (pH = 7.4, [K+] = 140 mM).
Fig. 3.
ESI-MS spectrum of 2 observed following incubation with potassium acetate for one hour at 37 °C in buffered aqueous solution (pH = 7.4, [K+] = 140 mM).
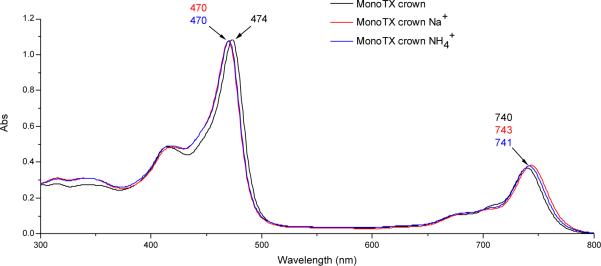
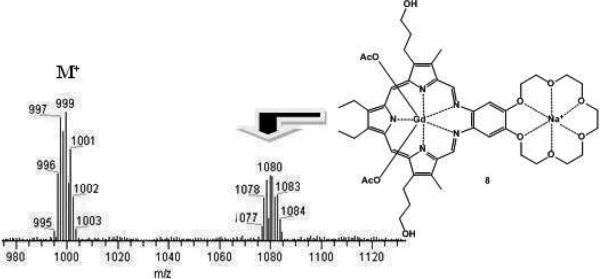
Analogous experiments were also conducted with aqueous sodium acetate (300 mM) and ammonium acetate (500 mM). As would be expected for complex formation, comparable blue- and red shifts were seen in the Soret-like and Q-type bands, respectively, in the UV-Vis spectrum of compound 2 in the presence of these two cations (Fig. 4). Although no further proof of the interaction between 2 and the ammonium cation could be obtained, ESI-MS analyses revealed peaks for the corresponding sodium complex 8 that displayed the expected gadolinium isotope pattern (Fig. 5). However, neither these ESI-MS signals nor the shifts in the UV-Vis spectrum were observed for 2 at physiological sodium concentrations (5-15 mM). Furthermore, no shifts in the UV-Vis spectrum and no ESI-signal (low resolution and high resolution) was observed for 2, when lithium acetate (various concentrations ranging from 5-500 mM) was used as alkali metal salt. This is consistent with the reasonable expectation that the lithium cation is too small to interact with the 18-crown-6 subunit present in 2.
Fig. 4.
UV-Vis spectrum of 2 [4.7 μM] and upon incubation with Na+ and NH4+; both incubated for one hour at 37 °C in aqueous buffered solution (pH = 7.4, [Na+] = 300 mM, pH = 7.4, [NH4+] = 500 mM).
Fig. 5.
ESI-MS spectrum of 2 recorded after incubat with sodium acetate at 37 °C for one hour in buffered aqueous media (pH = 7.4, [Na+] = 300 mM).
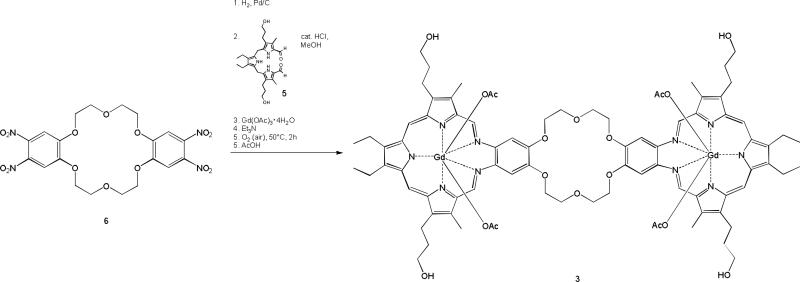
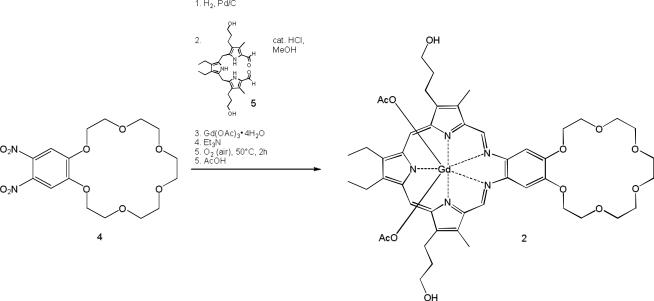
An 18-crown-6 moiety bearing two texaphyrin units (complex 3) was also prepared in an effort to probe more fully the effect crown ether incorporation might have on the anticancer activity of the basic texaphyrin core. The initial steps in the synthesis of 3 bear analogy to those used to prepare the texaphyrin system 2 (see Scheme 2). Interestingly, in the case of 3, the yield of the final complexation step with gadolinium acetate was found to depend strongly on the concentration, with the reaction only proving efficient when carried out at concentrations around 2 mM. Higher concentrations led to formation of significant by-products, while lower concentration led to an increase in the reaction time without any corresponding improvements in yield. Compound 3 proved to be very soluble in methanol, as well as soluble in both water and dimethylsulfoxide. However, it proved insoluble in dichloromethane, toluene and diethyl ether.
Scheme 2.
Synthesis of 3.
With a view to studying the binding of potassium and sodium cations (as acetate salts) to 3, UV-Vis and MS studies were carried out according to the experimental conditions described previously for 2. As true for 2, the UV-Vis absorption spectra of the samples featured significant shifts in the Soret-like and Q-type bands (cf. Fig. 6). Specifically, the spectra recorded after the addition of these three metal cations and incubation at 37 °C for one hour proved to be distinct from those seen in the case of the starting complex 3. The most dramatic shift in the UV-Vis spectrum was observed in the case of potassium acetate; here, the Soret-like band shows a red-shift of ca. 6 nm, whereas the Q-type band was blue shifted by ca. 4 nm. Furthermore, no significant changes in the UV-Vis spectrum were observed when ammonium acetate or lithium acetate were added to 3 in various concentrations ranging from 5 to 500 mM. No evidence of zinc(II) complexation was seen by ESI-MS. However, a slight increase in absorbtivity was seen upon addition of zinc(II) acetate (200 μM). We ascribe this to an aggregation effect.
Fig. 6.
UV-Vis spectrum of 3 [2.7 mM] and upon incubation with K+, Na+ and Zn2+; incubated for one hour at 37 °C in aqueous buffered solution (pH = 7.4, [K+] = 140 mM, [Na+] = 300 mM).
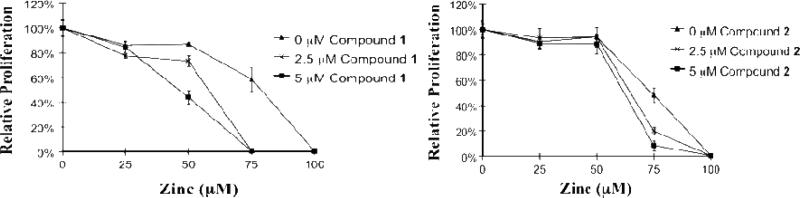
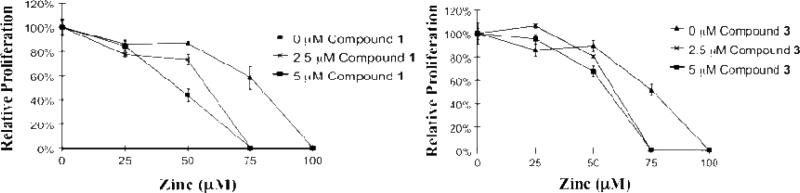
Texaphyrins 2 and 3, as well as MGd, were tested for anticancer activity using the Ramos cell line in the presence and absence of zinc(II) ions (as Zn(OAc)2). In these studies, both complexes 2 and 3 were quantified using the extinction coefficient for MGd 1 (ε at 474 nm in methanol = 122,395 M-1cm-1) using sterile filtered solutions formulated in 5% mannitol. As can be seen from an inspection of Figures 7 and 8, the 18-crown-6 functionalized texaphyrins 2 and 3 potentiate zinc in Ramos. As true for MGd 1, little antiproliferative activity was seen in the absence of zinc (II) even at the 5 μM level. Good activity was seen in the presence of zinc(II) ions. However, in the case of 2 the original, crown-free texaphyrin MGd 1 is as good or better on a molar basis, as judged by potency in this in vitro assay. A significant drop in Ramos cell survival is seen at zinc(II) concentrations above 50 μM, whereas MGd shows significant activity at and below zinc(II) concentrations of 50 μM at an equal texaphyrin concentration.
Fig. 7.
Graphs showing the %survival of Ramos cells when exposed to MGd 1 (left side) and compound 2 (right side) in the presence of different Zn(II) concentrations.
Fig. 8.
Graphs showing the %survival of Ramos cells when exposed to MGd 1 (left side) and compound 3 (right side) in the presence of different Zn(II) concentrations.
The biological activity of 3 (Ramos cells) was also studied in the presence of zinc(II). In analogy to what proved true for 2, the crown ether bridged texaphyrin dimer 3 was also seen to potentiate zinc(II) in this cell line (Fig. 8). However, no increase in activity was noted relative to the monotexaphyrin systems 1 or 2.
EXPERIMENTAL
Preparation of 2
4,5-Dinitrobenzo-18-crown-6 (compound 4, 400 mg, 1 mmol, synthesized as published previously [21]) was placed into a hydrogenation vessel along with 1 g of platinum on activated carbon (5%) and 100 ml ethanol under argon. The mixture was allowed to react with hydrogen gas at 40 psi with agitation for 18 hours. The solution was filtered under Schlenk conditions through a minimal pad of celite and the clear solution was added to tripyrrane 5 (481.63 mg, 1 mmol) and hydochloric acid (2M, 2 ml) were added. The solution was heated under reflux for two hours at 75 °C under argon. Gd(OAc)3·4H2O (503.9 mg 1.2 mmol) and triethylamine (2.18 ml, 146 mmol) were added and the reaction mixture was stirred in an open flask for another two hours at 50°C. Acetic acid (12M, 2 ml) was added and the solvent was evaporated. The brown viscous residue was washed with toluene, hexane and chloroform twice. The brownish-green viscuous material was subjected to column chromatography (silica gel, using first 94% CH2Cl2, 5% MeOH, 1% AcOH (12M) and then 100% MeOH as the eluents). After evaporating the solvent and drying in vacuo, a deep green solid was obtained. Yield 256.9 mg (25%), UV-vis (MeOH, 25°C): λmax, nm 474 (Soret-like band), 740 (Q-type band); MS (ESI): 999 (M+ - AcO); High Resolution MS (ESI): calculated for C46H59GdN5O10 = 999.3503; found: 999.3498 (C46H59GdN5O10, M+ - AcO);
Preparation of 3
4,4′,5,5′-Tetranitrodibenzo-18-crown-6 (540 mg, 1 mmol, synthesized as published previously [22]) was placed into a hydrogenation vessel along with 1 g of platinum on activated carbon (5%) and 100 ml ethanol under argon. The mixture was allowed to react with hydrogen gas at 40 psi with agitation for 18 hours. The solution was filtered under Schlenk conditions through a minimal pad of celite and the clear solution was added to 5 (963 mg, 2 mmol) and hydochloric acid (2M, 2 ml) were added. The solution was heated at reflux for two hours at 75°C under argon. Activated charcoal (0.7 g) was then added and the solution was stirred for an additional 15 min. After cooling to room temperature, the solution was filtered through a bed of celite. The resulting deep red solution was placed into a 2 l two neck flask equipped with a reflux condenser and an argon inlet and diluted with 1000 ml methanol. Gd(OAc)3·4H2O (1.63 g, 4 mmol) and triethylamine (9 ml, 64.7 mmol) were added and air was bubbled through the solution for 10 minutes. This gave rise to a dark brown solution that was then heated at reflux for 4 hours at 80 °C. Acetic acid (12N, 10 ml) was added and the reaction mixture was cooled to room temperature. After filtration through a bed of celite, the filtrate was concentrated in vacuo. Column chromatography (silica gel, 13 × 8.5 cm, 79% CH2Cl2, 20% MeOH, 1% AcOH (12N), eluent) afforded a crude green product. A tC18 reversed phase column (5 × 2.5 cm, 10 g, 65% MeOH, 35% aqueous NH4OAc buffer, pH = 7.4, eluent) provided the product as a deep green solution. After evaporating the solvent and drying in vacuo, a green crystalline solid was obtained. Yield 407 mg (10%), UV-vis (MeOH, 25°C): λmax, nm 477 (Soret-like band); 738 (Q-type band); MS (ESI): 1852 (M+); 1795 (M+ - AcO); 867 (M++ - 2AcO); 853 (M++ - 3AcO + MeOH); 837 (M++ - 3AcO); High Resolution MS (ESI): 837 (Deconvoluted: 1672, C78H88Gd2N10O14, M++ - 3AcO); 867 (Deconvoluted: 1732, C80H92Gd2N10O14, M++ - 2AcO)
Cell Culture Protocol
Ramos B-cell lymphoma line was purchased from the American Type Culture Collection. Unless otherwise indicated, all cell culture reagents were purchased from Invitrogen (Carlsbad, CA). Cells were cultured in RPMI1640 medium supplemented with 10% fetal calf serum in a 5% CO2 incubator at 37 °C at a density between 0.2 and 1 × 106 cells/mL as previously described [23]. Motexafin gadolinium (MGd) was prepared as a 2 mM (2.3 mg/mL) formulation in 5% aqueous mannitol. Compounds 2 and 3 were formulated in 5% mannitol and quantified using their extinction coefficient at 460 nm in methanol (122,395 or 244,790 M-1cm-1, respectively). Zinc acetate (Zn(OAc)2) (Aldrich Chemical, Milwaukee, WI) was used as a 2 mM formulation in 5% aqueous mannitol.
Cellular Proliferation
The proliferation of exponential phase cultures was assessed by colorimetric assay [24]. Briefly, 4 × 105 suspension cells per well were seeded on 96-well V-bottom microtiter plates. Stock solutions of control vehicle, compounds 1, 2, 3, or Zn(OAc)2 in medium were added and plates were incubated at 37 °C under a 5% CO2/95% air atmosphere. After 72 hours, the medium was exchanged with fresh medium (150 μL/well) supplemented with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, 0.5 mg/mL, Sigma Biochemical, St. Louis, MO). Plates were incubated at 37 °C and viable cells measured as described previously [24].
CONCLUSION
In summary, we have detailed the synthesis of two new 18-crown-6 functionalized analogues of the well-studied texaphyrin complex, MGd 1. Both species, complexes 2 and 3, proved stable under normal laboratory conditions and were found to interact with the potassium, sodium and zinc(II) cations, as inferred from UV-Vis and mass spectrometric studies. The ability to interact with zinc(II,) a cation known to be correlated in its free form with oxidative stress within the cell, prompted initial studies of the in vitro activity of complexes 2 and 3 in Ramos cells. These studies revealed that zinc(II) is potentiated, albeit not at a level that is increased relative to what is seen in the case of MGd 1. Accordingly, efforts are under way to create new conjugates of texaphyrin that incorporate other zinc(II) chelating agents.
Scheme 1.
Synthesis of 2.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health (grant CA 68682 to J.L.S.) and The Texas Institute for Drug & Diagnostic Development and the Robert A. Welch Foundation (grant F-1018 to J.L.S.). J.L.S. also thanks WCU (World Class University) program of Korea (R32-2008-000-10217-0).
Footnotes
Dedicated to Karl M. Kadish on the occasion of his 65Th birthday.
REFERENCES
- 1.Sessler JL, Hemmi G, Mody TD, Murai T, Burrell A, Young SW. Acc. Chem. Res. 1994;27:43–50. [Google Scholar]
- 2.Mody TD, Sessler JL. In: Supramolecular Materials and Technologies. Reinhoudt DN, editor. Vol. 4. Chichester; Wiley: 1999. pp. 245–299. [Google Scholar]
- 3.Mody TD, Fu L, Sesser JL. In: Progress Inorganic Chemistry. Karlin KJ, editor. Vol. 49. Chichester; Wiley: 2001. p. 551. [Google Scholar]
- 4.Sessler JL, Mody TD, Hemmi GW, Lynch VM. Inorg. Chem. 1993;32:3175–3187. [Google Scholar]
- 5.Sessler JL, Tvermoes NA, Guldi DM, Mody TD, Allen WE. Phys. Chem. 1999;103:787–794. [Google Scholar]
- 6.Mody TD, Sessler JL. J. Porphyrins Phthalocyanins. 2001;5:134–142. [Google Scholar]
- 7.Sessler JL, Miller RA. Biochem. Pharmacol. 2000;59:733–739. doi: 10.1016/s0006-2952(99)00314-7. [DOI] [PubMed] [Google Scholar]
- 8.Young SW, Quing F, Harriman A, Sessler JL, Dow WC, Mody TD, Hemmi G, Hao Y, Miller RA. Proc. Natl. Acad. Sci. USA. 1996;93:6610–6615. doi: 10.1073/pnas.93.13.6610. [DOI] [PMC free article] [PubMed] [Google Scholar]; Proc. Natl. Acad. Sci. USA. 1999;96:2569. Correction. [Google Scholar]
- 9.Evens AM, Balasubramanian L, Gordon L. Curr. Treat. Options Oncol. 2005;6:289–296. doi: 10.1007/s11864-005-0033-y. [DOI] [PubMed] [Google Scholar]
- 10.Miller RA, Woodburn K, Fan Q, Renschler MF, Sessler JL, Koutcher JA. Int. J. Radiation Oncology Biol. Phys. 1999;45:981–989. doi: 10.1016/s0360-3016(99)00274-6. [DOI] [PubMed] [Google Scholar]
- 11.Mehta MP, Shapiro WR, Glantz MJ, Patchell RA, Weitzner MA, Meyer CA, Schultz CJ, Roa WH, Leibenhout M, Ford J, Curran W, Phan S, Smith JA, Miller RA, Renschler MF. J. Clin. Oncol. 2002;20:3445–3453. doi: 10.1200/JCO.2002.07.500. [DOI] [PubMed] [Google Scholar]
- 12.Darwish IA, Uchegbu IF. Int. J. Pharm. 1997;159:207–213. [Google Scholar]
- 13.Uchegbu IF, Vyas SP. Int. J Pharm. 1998;172:33–70. [Google Scholar]
- 14.Fukuda R, Takenaka S, Takagi M. J. Chem. Soc., Chem. Commun. 1990;15:1028–1030. [Google Scholar]
- 15.Dumont B, Joly JP, Chapleur Y, Marsura A. Bioorg. Med. Chem. Lett. 1994;4:1123–1126. [Google Scholar]
- 16.Moroshkina EB, Zagoruiko NV, Glibin EN. Molecular Biology. 2001;1:98–105. Vol 35. [PubMed] [Google Scholar]
- 17.Lecane PS, Karaman MW, Sirisawad M, Naumovski L, Miller RA, Hacia JG, Magda D. Cancer Research. 2005;65:11676–11688. doi: 10.1158/0008-5472.CAN-05-2754. [DOI] [PubMed] [Google Scholar]
- 18.Magda D, Lecane PS, Miller RA, Lepp C, Miles D, Mesfin M, Biaglow JE, Ho VV, Chawannakul D, Nagpal S, Karaman MW, Hacia JG. Cancer Research. 2005;65:3837–3845. doi: 10.1158/0008-5472.CAN-04-4099. [DOI] [PubMed] [Google Scholar]
- 19.Leitzmann MF, Stampfer MJ, Wu K, Colditz GA, Willett WC, Giovannucci EL. J. Natl. Cancer Inst. 2003;95:1004–1007. doi: 10.1093/jnci/95.13.1004. [DOI] [PubMed] [Google Scholar]
- 20.Liang X, Parkinson JA, Parsons S, Weishaeupl M, Gould RO, Paisey SJ, Park H, Hunter TM, Blindauer CA, Sadler PJ. J. Am. Chem. Soc. 2002;124:9105–9112. doi: 10.1021/ja0260723. [DOI] [PubMed] [Google Scholar]
- 21.Kirkovits GJ, Zimmerman RS, Huggins MT, Lynch VM, Sessler JL. Eur. J. Org. Chem. 2002;22:3768–3778. [Google Scholar]
- 22.Duggan SA, Fallo G, Langford SJ, Lau V-L, Satchell JF, Paddow-Row MN. J. Org. Chem. 2001;66:4419–4426. doi: 10.1021/jo001700p. [DOI] [PubMed] [Google Scholar]
- 23.Magda D, Lecane P, Miller RA, Lepp C, Miles D, Mesfin M, et al. Cancer Res. 2005;65:3837–3845. doi: 10.1158/0008-5472.CAN-04-4099. [DOI] [PubMed] [Google Scholar]
- 24.Mosmann T. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]