Abstract
Sex, handedness, and disease processes in schizophrenia may affect the magnitude and/or direction of structural brain asymmetries. Using MRI data from 67 healthy (30 men, 10 nondextral) and 84 schizophrenia patients (60 men, 16 nondextral), cortical thickness asymmetries were compared at high spatial resolution. Within-group asymmetries were observed in sensorimotor, perisylvian, and parahippocampal cortices (leftward) and in anterior mesial frontal cortices (rightward). Asymmetry patterns were similar across diagnosis and sex, although some regional asymmetry increases were observed in men. Hand preference (dextrality) significantly influenced regional asymmetries in parietal association and dorsomedial frontal cortices (false discovery rate-corrected), where medial-frontal regions showed diagnosis by dextrality effects (uncorrected). Thus, dextrality relates to cortical thickness asymmetries, although schizophrenia may differentially affect asymmetry patterns across handedness.
Keywords: cortical thickness, laterality, magnetic resonance imaging, schizophrenia
Introduction
Earlier imaging and postmortem studies have identified asymmetries in the structural morphology of the brain [1,2]. Asymmetries are reported in Sylvian fissure, planum parietale, and parietal operculum anatomy. Leftward shape and volume asymmetries are also frequently reported in language-related cortices such as the planum temporale, a component of Wernicke’s area [3]. Factors such as handedness, sex, and disease processes associated with schizophrenia have been suggested to modulate the structural lateralization of the cerebral hemispheres. Sex has been shown to affect structural and functional asymmetries with greater asymmetries found in men in both the planum temporale and the planum parietale [4]. Furthermore, pathological processes in schizophrenia are reported to exert effects on lateralization, where it has been hypothesized that disturbances in hemispheric specialization for language may be the basis for the disease [5]. Handedness also appears to be associated with structural and functional asymmetries in language regions [4]. For example, studies have shown asymmetries related to handedness in the primary somatosensory cortex and motor cortex, in which the cortical representation for the right hand was larger than that of the left-hand for right handers, and vice versa for left handers [6]. Voxel-based studies examining tissue density, however, report negative findings for handedness [7,8]. To our knowledge, no studies have explicitly examined the effects of hand preference or schizophrenia on cortical thickness asymmetries. Thus, this study investigated whether cortical thickness asymmetries are influenced by handedness, sex, and schizophrenia.
Methods
Participants
Participants included 67 healthy controls (30 men, seven of whom were nondextral; 37 women, three of whom were nondextral) and 84 patients experiencing their first episode of schizophrenia (60 men, 12 of whom were nondextral; 24 women, four of whom were nondextral) similar in age [controls (mean±SD): 29.3±7.1 years; patients: 24.26±4.6 years]. Handedness was determined using a modified 20-item Edinburgh Handedness Inventory [9]. Laterality quotients (LQs) were obtained using the following formula [2]:
LQ scores have a distribution of −1 (extremely sinistral) to + 1 (extremely dextral). LQ scores of more than 0.70 were used to separate participants who were dextral from those who were nondextral [10]. Participants in this study were identical to those in our earlier study on asymmetries in cortical shape [2]. The diagnostic status of patients was confirmed using the Schedule for Affective Disorders and Schizophrenia [11] and the Structured Clinical Interview for Axis I Diagnostic and statistical manual of mental disorders-IV (DSM-IV) [12].
Healthy comparison participants were recruited from local newspaper advertisements and community word of mouth. Control participants were required to have no history of psychiatric illness as assessed by clinical interview using the SCID-NP [13]. Exclusion criteria included serious neurological or endocrine disorders, any medical condition or treatment known to affect the brain, or meeting DSM-IV criteria for mental retardation. The North Shore–Long Island Jewish Health System Institutional Review Board approved all procedures and informed written consent was obtained from all participants. Additional approval for image processing and analysis was received from the UCLA Institutional Review Board.
Image acquisition and preprocessing
High-resolution 3D SPGR MR images were obtained on a 1.5-T scanner (Milwaukee, Wisconsin, USA) as a series of 124 contiguous 1.5 mm coronal brain slices (2562 matrix, 0.86 mm2 in-plane resolution). Image volumes were prepared for analysis by removing nonbrain tissue (interrater reliability, rI=0.99), correcting for intensity nonuniformity due to magnetic field inhomogeneities [14], and by reorienting each volume into the standard position of the International Consortium for Brain Mapping-305 average brain [15], using a six-parameter rigid body transformation with no scaling [16] to correct for head tilt and alignment. Hemispheric surfaces comprised of 65 536 surface points were extracted [17] after manually separating the right and left hemispheres.
Cortical thickness analysis
Cortical pattern-matching algorithms were applied to spatially relate homologous cortical surface locations between individuals so that anatomically comparable measures of cortical thickness could be obtained and compared across hemispheres [2]. Cortical thickness was measured by referencing tissue-classified brain volumes using an implementation of the 3D Eikonal equation [18]. The thickness of the cortex was defined as the shortest distance in 3D, without crossing CSF voxels, from the cortical white–gray matter boundary to the outer gray-CSF hemispheric surface at all hemispheric surface points. A smoothing filter of 8 mm was applied [2].
Statistical analyses
To identify within-group cortical thickness asymmetries, paired t-tests were used to compare thickness measures obtained at thousands of spatially equivalent surface points between the left and the right hemispheres. Results were mapped within groups defined by handedness, sex, and diagnosis. As nondextral study groups were small, hemispheric effects were examined by pooling participants across diagnosis and separately, across sex. To examine regional differences of thickness asymmetries between groups, an asymmetry index (L−R)/[0.5(L + R)], was computed at corresponding hemispheric locations. The General Linear Model was then used to examine effects of schizophrenia, sex, and handedness while controlling for age and all other factors in the model. Two-way interactions between sex or diagnosis and handedness were also examined. Cell sizes were considered too small to meaningfully interpret three-way interactions. As cortical thickness asymmetries were compared at thousands of hemispheric locations across groups and measurements obtained from adjacent data points may be correlated, a false discovery rate (FDR) of P≤0.01 was used to control for multiple spatial comparisons [19].
Results
Within-group effects
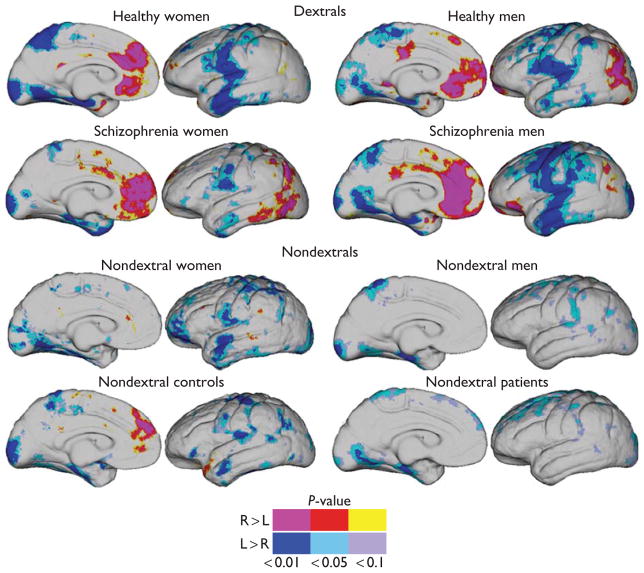
Significant cortical thickness asymmetries were observed in motor, perisylvian, lateral temporal, parahippocampal, and surrounding cortices (leftward) and in anterior mesial frontal and posterior parietal cortices (rightward) within dextral patient and control groups (Fig. 1). Localized leftward thickness asymmetries appeared less pronounced within nondextral participants pooled across diagnosis and/or sex. Rightward asymmetries were largely absent in participants who were nondextral with the exception of rightward thickness asymmetries in mesial frontal cortices in nondextral controls.
Fig. 1.
Significant cortical thickness asymmetries mapped within dextral healthy male and female participants (top); dextral male and female schizophrenia patients (second row); nondextral male and female participants collapsed across diagnosis (third row); and nondextral patients and controls collapsed across sex (bottom). Significance and direction of regional cortical thickness asymmetries are indexed by the color bar.
Between-group effects of sex, diagnosis, and dextrality
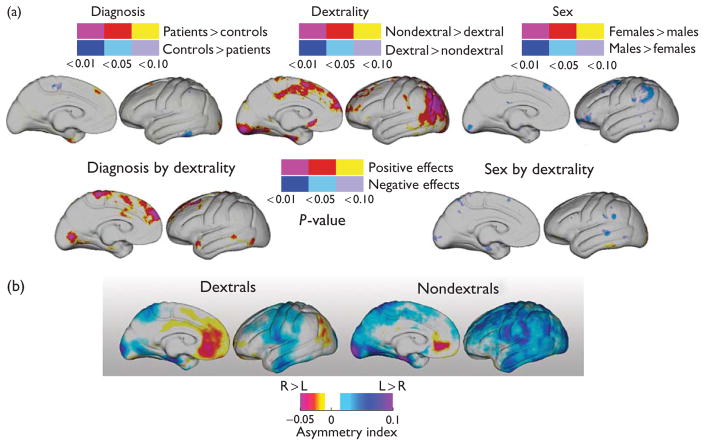
Between-group comparisons of thickness asymmetries showed little evidence for differences in asymmetry patterns between groups defined by sex or diagnosis (FDR>0.99 for P<0.01), although men exhibited some small, localized asymmetry increases compared with women in the uncorrected data (Fig. 2a).
Fig. 2.
(a) Statistical maps (uncorrected) show significant diagnosis (top left), dextrality (top center), and sex (top right) effects for cortical thickness asymmetry indices compared at thousands of hemispheric surface locations. Regional interactions between diagnosis and dextrality (left) and sex and dextrality (right) are mapped in the second row. The color bars encode the probability and direction of effects. (b) Average asymmetry indices mapped within dextral and nondextral participants. Cool colors indicate leftward (positive) asymmetries; hot colors indicate rightward (negative) asymmetries.
Nondextral participants showed significant shifts of thickness asymmetries in mesial frontal and parietal cortices including angular gyrus and bordering supramarginal gyrus (Brodmann areas 22 and 39; Fig. 2a; FDR<0.044 for P<0.01). These alterations in asymmetry may be attributed to regional reductions of rightward asymmetries exhibited by dextral participants, as evident in the asymmetry indices averaged within groups defined by dextrality (Fig. 2b). These average maps of asymmetry indices for dextral and nondextral participants, pooled across sex and diagnosis, indicate more spatially diffuse leftward asymmetries in nondextral participants.
Significant regional diagnosis by dextrality group interactions indicated that altered thickness asymmetries in mesial cortices were schizophrenia related, but effects did not survive FDR correction (FDR>0.99 for P<0.01). The sex by dextrality interaction did not survive FDR correction (FDR>0.99 for P<0.01).
Discussion
Structural and functional asymmetries are ubiquitous in the human brain, where developmental, hereditary, and pathological factors may influence the magnitude and direction of asymmetries [4]. We report significant leftward cortical thickness asymmetries in motor, perisylvian, lateral temporal, and parahippocampal regions that are largely consistent with earlier findings, at least for the lateral aspects of the brain [1]. Leftward asymmetries, however, appeared less pronounced in nondextral participants, where localized leftward asymmetry patterns in dextral participants were more spatially diffuse in those who were nondextral (Fig. 2b). Furthermore, rightward asymmetries shown in parietal association regions in dextral groups were observed in the opposite direction in nondextral groups. Thus, our findings support that dextrality relates to both the pattern and direction of regional cortical thickness asymmetries. Trends in our results may also indicate that disease processes in schizophrenia mediate rightward asymmetry patterns in the dorsomedial prefrontal cortices of both dextral and nondextral participants, though effects did not survive FDR correction.
Nondextral participants exhibited leftward asymmetries in posterior association cortices including the angular and bordering supramarginal gyrus, regions that showed rightward asymmetries in dextral participants. These supra-modal and heteromodal cortical association regions serve several brain functions including aspects of language and somatosensory processing, with localized lesions to these areas resulting in the disruption of lateralized functions [20]. Thus, the presence of handedness-related cortical thickness asymmetries within these areas is not surprising, although the functional significance of increases versus decreases in hemispheric cortical thickness patterns in relation to dextrality is less obvious. As there is an established left-hemisphere bias for language processing and hand preference, and parietal association regions play a role in integrating language information, these regional asymmetries and their differences across handedness may relate to language dominance. Notably, recently identified fiber density asymmetries in the arcuate fasciculus, which connects parietal and other language-related regions, are suggested to relate to functional asymmetries in language [21]. Although a greater percentage of left handers than right handers, however, are right-lateralized for language, 70% of left handers remain left-lateralized [4]. Dextrality effects for parietal lobule cortical thickness asymmetries may thus also relate to somatosensory functions through lateralized inputs from the left and right hands [6]. Finally, given that nondextral participants exhibit less localized thickness asymmetries than dextral participants (Fig. 2b), our results suggest that cortical thickness asymmetries are less structurally organized in those with nondextral hand preference and, consequently, that those who are nondextral are less lateralized than those who were dextral.
Sex and disease status had little measurable effect on cortical thickness asymmetries. We observed some regional thickness asymmetry increases in men compared with women that are consistent with our earlier studies showing similar sex-related thickness asymmetry trends [1], with reports of increased structural asymmetries in men [4,7], but findings did not survive FDR correction. As earlier research indicates that schizophrenia is a lateralized disease [22,23] with disturbances in both functional laterality as well as increases in left, mixed, and ambiguous handedness [3], we sought to clarify how schizophrenia and handedness interact with regard to cortical thickness asymmetries. Although we found that diagnosis alone had little effect on cortical thickness asymmetry patterns, uncorrected results showed some indication that schizophrenia affects cortical thickness asymmetries differentially in dextral and nondextral, which implies more complex relationships between handedness and disease processes. Our findings do not preclude the existence of disturbances in other structural asymmetries in schizophrenia, but suggest that cortical thickness abnormalities reported in the disorder are similar across hemispheres. This is consistent with earlier reports of bilateral effects in schizophrenia, albeit that asymmetry effects were not tested explicitly [24].
Although our overall sample size was large (N=157), the number of nondextral participants in our study was relatively modest (N=26). Thus, statistical power issues may have influenced our results in which cortical asymmetry patterns examined within nondextral participants included fewer subjects. These issues also rendered it impractical to investigate the presence of more complex interactions among sex, diagnosis, and dextrality. The methods used for defining handedness may also have impacted results. Future studies relating functional and structural asymmetries could help to elucidate the underlying mechanisms responsible for handedness and specifically to determine how the direction of cortical thickness asymmetries relate to hand preference or other behavioral asymmetries.
Conclusion
Our data show that dextrality affects the pattern and direction of cortical thickness asymmetries, particularly in parietal association cortices. Although sex and disease processes are reported to influence shape or volume asymmetries in the brain, neither of these factors appears to have a large influence on cortical thickness asymmetries.
Acknowledgments
This work was generously supported by research grants from the National Center for Research Resources (P41 RR13642), the National Institute of Mental Health (RO1 MH60374), the NIH Roadmap Initiative (P20 RR020750), the National Library of Medicine (RO1 LM05639), the NIH Roadmap for Medical Research, Grant U54 RR021813 entitled Center for Computational Biology (CCB), and a Career Development Award (KO1 MH073990, to K.L.N.).
References
- 1.Luders E, Narr KL, Thompson PM, Rex DE, Jancke L, Toga AW. Hemispheric asymmetries in cortical thickness. Cereb Cortex. 2006;16:1232–1238. doi: 10.1093/cercor/bhj064. [DOI] [PubMed] [Google Scholar]
- 2.Narr KL, Bilder RM, Luders E, Thompson PM, Woods RP, Robinson D, et al. Asymmetries of cortical shape: effects of handedness, sex and schizophrenia. NeuroImage. 2007;34:939–948. doi: 10.1016/j.neuroimage.2006.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hugdahl K, Davidson RJ. The asymmetrical brain. Cambridge, Massachusetts: MIT Press; 2003. [Google Scholar]
- 4.Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- 5.Crow TJ. Schizophrenia as failure of hemispheric dominance for language. Trends Neurosci. 1997;20:339–343. doi: 10.1016/s0166-2236(97)01071-0. [DOI] [PubMed] [Google Scholar]
- 6.Sun T, Walsh CA. Molecular approaches to brain asymmetry and handedness. Nat Rev. 2006;7:655–662. doi: 10.1038/nrn1930. [DOI] [PubMed] [Google Scholar]
- 7.Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. NeuroImage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- 8.Watkins KE, Paus T, Lerch JP, Zijdenbos A, Collins DL, Neelin P, et al. Structural asymmetries in the human brain: a voxel-based statistical analysis of 142 MRI scans. Cereb Cortex. 2001;11:868–877. doi: 10.1093/cercor/11.9.868. [DOI] [PubMed] [Google Scholar]
- 9.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 10.Bilder RM, Wu H, Bogerts B, Degreef G, Ashtari M, Alvir JM, et al. Absence of regional hemispheric volume asymmetries in first-episode schizophrenia. Am J Psychiatry. 1994;151:1437–1447. doi: 10.1176/ajp.151.10.1437. [DOI] [PubMed] [Google Scholar]
- 11.Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- 12.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV axis I disorders-patient edition. New York, New York: State Psychiatric Institute; 1997. [Google Scholar]
- 13.Spitzer RL, Williams JB, Gibbon M. Structured Clinical Interview for DSM-IV; Non-Patient Version (SCID-NP) New York: New York State Psychiatric Institute; 1996. [Google Scholar]
- 14.Sled JG, Pike GB. Standing-wave and RF penetration artifacts caused by elliptic geometry: an electrodynamic analysis of MRI. IEEE Trans Med Imaging. 1998;17:653–662. doi: 10.1109/42.730409. [DOI] [PubMed] [Google Scholar]
- 15.Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philos Trans R Soc Lond B Biol Sci. 2001;356:1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. NeuroImage. 1999;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- 17.MacDonald D, Avis D, Evans AC. Multiple surface identification and matching in magnetic resonance imaging. Proc SPIE. 1994;2359:160–169. [Google Scholar]
- 18.Sapiro G. Geometric partial differential equations and image analysis. Cambridge, UK, New York: Cambridge University Press; 2001. [Google Scholar]
- 19.Storey JD. A direct approach to false discovery rates. J R Stat Soc: Series B (Stat Methodol) 2002;64:479–498. [Google Scholar]
- 20.Parent A, Carpenter MB. Carpenter’s human neuroanatomy. Baltimore: Williams & Wilkins; 1996. [Google Scholar]
- 21.Nucifora PG, Verma R, Melhem ER, Gur RE, Gur RC. Leftward asymmetry in relative fiber density of the arcuate fasciculus. NeuroReport. 2005;16:791–794. doi: 10.1097/00001756-200505310-00002. [DOI] [PubMed] [Google Scholar]
- 22.Annett M. The theory of an agnosic right shift gene in schizophrenia and autism. Schizophr Res. 1999;39:177–182. doi: 10.1016/s0920-9964(99)00072-9. [DOI] [PubMed] [Google Scholar]
- 23.Petty RG. Structural asymmetries of the human brain and their disturbance in schizophrenia. Schizophr Bull. 1999;25:121–139. doi: 10.1093/oxfordjournals.schbul.a033360. [DOI] [PubMed] [Google Scholar]
- 24.Narr KL, Bilder RM, Toga AW, Woods RP, Rex DE, Szeszko PR, et al. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex. 2005;15:708–719. doi: 10.1093/cercor/bhh172. [DOI] [PubMed] [Google Scholar]