Abstract
White matter microstructure is under strong genetic control, yet it is largely unknown how genetic influences change from childhood into adulthood. In one of the largest brain mapping studies ever performed, we determined whether the genetic control over white matter architecture depends on age, sex, socioeconomic status (SES), and intelligence quotient (IQ). We assessed white matter integrity voxelwise using diffusion tensor imaging at high magnetic field (4-Tesla), in 705 twins and their siblings (age range 12–29; 290 M/415 F). White matter integrity was quantified using a widely accepted measure, fractional anisotropy (FA). We fitted gene-environment interaction models pointwise, to visualize brain regions where age, sex, SES and IQ modulate heritability of fiber integrity. We hypothesized that environmental factors would start to outweigh genetic factors during late childhood and adolescence. Genetic influences were greater in adolescence versus adulthood, and greater in males than in females. Socioeconomic status significantly interacted with genes that affect fiber integrity: heritability was higher in those with higher SES. In people with above-average IQ, genetic factors explained over 800% of the observed FA variability in the thalamus, genu, posterior internal capsule, and superior corona radiata. In those with below-average IQ, however, only around 40% FA variability in the same regions was attributable to genetic factors. Genes affect fiber integrity, but their effects vary with age, sex, SES and IQ. Gene-environment interactions are vital to consider in the search for specific genetic polymorphisms that affect brain integrity and connectivity.
Keywords: genetics, cognition, twins, white matter, diffusion imaging, gene-environment interaction
1. Introduction
Since Galton published his first book on heredity and intelligence (Galton, 1869), a long-standing debate has raged over the relative effects of nature versus nurture in influencing human traits. This argument leads to several concrete neuroscientific questions: (1) How heritable are measures of brain structure and function? (2) Does the degree of genetic control remain static throughout life, or do environmental influences start to dominate in late childhood or the teenage years? (3) Does the genetic control of brain integrity vary in different demographic or environmental contexts?
Some known risk genes, such as the apolipoprotein E4 risk allele for Alzheimer's disease, affect the brain in an age-dependent way (Shaw et al., 2007). Likewise, some mental illnesses may be triggered by genetic risk factors interacting with environmental stressors during a certain part of the lifespan, such as late adolescence (Gottesman and Shields, 1967). If we identify situations where the environment modifies genetic effects, we may better understand genetic liability for illness and how to reduce it.
Many brain measures are highly heritable. These include total brain volume (Posthuma et al., 2000), regional gray and white matter volumes (Hulshoff Pol et al., 2006b), cortical thickness (Schmitt et al., 2008; Thompson et al., 2001), and white matter integrity measured with DTI (Chiang et al., 2009b; Pfefferbaum et al., 2001). In functional MRI, genetic factors account for around 80% of the total variation in BOLD response during working memory tasks (Blokland et al., 2008; Karlsgodt et al., 2010; Koten et al., 2009). Measures of default-mode activity observed with resting-state fMRI are also highly heritable (Castellanos et al., 2010; Glahn et al., 2010).
Some studies report age-related changes in the heritability of several traits. One orthodox view of brain plasticity proposes that environmental factors eventually start to outweigh genetic influences. IQ, however, becomes more heritable as we age (Plomin and Spinath, 2004). In children, white but not gray matter volume heritability increases with age (Wallace et al., 2006), perhaps because white matter volumes continue to increase until the late forties (Bartzokis et al., 2001). Cortical gray matter thickness also becomes more heritable with increasing age in late-maturing regions (Lenroot et al., 2009). White matter integrity is influenced by some of the same genes as IQ (Chiang et al., 2009b), yet no study has yet examined how its heritability changes with age.
Here we present the first study of gene-environment interactions (Martin, 2000) on white matter microstructure in a large sample of twins and their siblings (N=705). We used diffusion tensor imaging (DTI) to quantify white matter integrity, by estimating fractional anisotropy (FA) of water diffusion throughout the brain. FA is a widely accepted index of the microstructural integrity of the white matter (Basser and Pierpaoli, 1996; Beaulieu, 2002) and correlates highly with IQ. We mapped where in the brain white matter heritability depends on age, sex, SES, and IQ, by fitting the quantitative gene-environment interaction model (Purcell, 2002) at each point of the brain. We expected genetic influences to change with age, but with a two-sided hypothesis. The existing literature is divided on the direction of the effect. Following Turkheimer's hypothesis that adverse environments deplete the relative contribution of genetic effects (Turkheimer et al., 2003), we expected that white matter integrity would be more heritable in those with higher SES and higher IQ. Any search for modulators of gene effects is vital for understanding mental illnesses with fluctuating genetic liability, and for understanding factors that affect brain integrity and connectivity.
2. Methods
2.1. Participants
705 twins and their non-twin siblings, including 531 healthy adults (aged 18 or older) and 174 adolescents, were recruited from 358 different families. Of the 174 adolescent subjects, 86 were aged 12 and 88 were aged 16. All subjects received high-resolution brain MRI scans and neurocognitive evaluations as part of a 5-year research project evaluating healthy Australian twins and their non-twin siblings. The projected sample size for the adult study is 1150 at completion (de Zubicaray et al., 2008). Subjects' demographic information and the twin/sibling composition of the families are summarized in Table 1. As described previously (Chiang et al., 2009b), zygosity was established objectively by typing nine independent DNA microsatellite polymorphisms (polymorphism information content > 0.7), using standard polymerase chain reaction (PCR) methods and genotyping. Results were cross-checked with blood group (ABO, MNS and Rh), and phenotypic data (hair, skin and eye color), giving an overall probability of correct zygosity assignment greater than 99.99%. All subjects were screened to exclude cases of pathology known to affect brain structure, a history of significant head injury, a neurological or psychiatric illness, substance abuse or dependence, or a psychiatric disorder in any first-degree relative.
Table 1.
Demographic and IQ data, and family composition.
| Adult (n = 531) | Adolescent (n = 174) | |
|---|---|---|
| Age, years | 23.7±2.1 | 12 or 16* |
| Sex (M/F)§ | 217/314 | 73/101 |
| Full-scale IQ† | 113.7±12.4 | 115.1±12.4 |
| MZ pairs | 93 | 26 |
| MZ pair plus one non-twin sibling | 10 | 0 |
| DZ pairs | 93 | 59 |
| DZ trizygotic triplets | 4 | 1 |
| DZ pair plus one non-twin sibling | 12 | 0 |
| DZ pair plus two non-twin siblings | 1 | 0 |
| DTI data available in one co-twin only | ||
| One co-twin with no siblings | 33 | 1 |
| One co-twin and one non-twin sibling | 17 | 0 |
| One co-twin and two non-twin siblings | 1 | 0 |
| Single participants without siblings | 7 | 0 |
MZ: monozygotic, DZ: dizygotic twins. The numbers of families with each type of twin and sibling composition are listed here. Values are displayed as Mean±SD.
The adolescent group consists of 86 subjects of age 12, and 88 subjects of age 16.
No significant difference in sex distribution was found between the adult and adolescent groups.
IQ tests were performed only when subjects were age 16 or older, and IQ data were available in 513 adult subjects and in all 88 subjects of age 16. There was no difference in full-scale IQ between the two groups.
2.2. Evaluation of psychometric intelligence and socioeconomic status
General intellectual ability was assessed at age 16 (as in (Luciano et al., 2003)) using the Multidimensional Aptitude Battery (MAB) (Jackson, 1984), a measure highly correlated with the Wechsler Adult Intelligence Scale. The MAB is designed for assessment of adults and adolescents aged 16 and older. In this study, we examined three verbal (information, arithmetic, and vocabulary) and two performance (spatial and object assembly) sub-tests. Each subtest gave a raw score, and verbal (VIQ), performance (PIQ), and full-scale (FIQ) intelligence quotient standardized scores were derived from these sub-tests. IQ data were available for 513 adult (age 18 years) subjects and in all 88 subjects of age 16, although not in subjects of age 12 (see Table 1).
Subjects' socioeconomic status was evaluated using the Australian Socioeconomic Index 2006 (AUSEI06; (McMillan et al., 2009)). The socioeconomic index (SEI) is determined using a 0–100 scale based on a person's occupational category, which may be associated, to some extent, with educational level and income. In our study, SEI was assessed in the adults only, and defined as the higher SEI of their two parents. SEI data were available in 250 families, for a total of 499 subjects, and the median family SEI was 67.5 (25th percentile = 39.7; 75th percentile = 83.8).
2.3. Image processing and registration
All MR images were collected using a 4 Tesla Bruker Medspec MRI scanner (Bruker Medical, Ettingen, Germany), with a transverse electromagnetic (TEM) headcoil, at the Center for Magnetic Resonance (University of Queensland, Australia). An identical scanning protocol was used for both children and adults. In (Chiang et al., 2009b), we reported on DTI findings in the first 92 adult twins (23 MZ and 23 same-sex DZ pairs), scanned with the protocol reported there; the current study also includes children and a greatly increased sample size sufficient to model age effects (705 versus 92 subjects in the initial report).
High angular resolution diffusion-weighted scans were acquired using single-shot echo planar imaging with a twice-refocused spin echo sequence, to reduce eddy-current induced distortions. Imaging parameters were: 21 axial slices (5 mm thick), FOV = 23 cm, TR/TE 6090/91.7 ms, 0.5 mm gap, with a 128 100 acquisition matrix. 30 images were acquired: 3 with no diffusion sensitization (i.e., T2-weighted images) and 27 diffusion-weighted images (b = 1145.7 s/mm2) with gradient directions evenly distributed on an imaginary hemisphere. The reconstruction matrix was 128 128, yielding a 1.8 × 1.8 mm2 in-plane resolution. Total scan time was 3.05 minutes. We used the FMRIB software library (FSL, http://www.fmrib.ox.ac.uk/fsl/) for pre-processing and linear alignment of the diffusion images. For each subject, motion artifacts were corrected by linearly registering all the T2-weighted and diffusion-weighted images to one of the T2-weighted images (the “eddy_correct” command). Then the three T2-weighted images were averaged and stripped of non-brain tissues to yield a binary brain extraction mask (cerebellum included), using the Brain Extraction Tool (BET; (Smith, 2002)), followed by expert manual editing, if necessary. The masked T2-weighted image was then registered to a standardized high-resolution brain MRI template defined in the International Consortium for Brain Mapping space (ICBM) (Holmes et al., 1998) with a 9-parameter linear transformation using the software FLIRT (Jenkinson and Smith, 2001). The resulting transformation parameters were used to rotationally reorient the diffusion tensors (computed from diffusion-weighted images using the “DTIFIT” command) at each voxel (Alexander et al., 2001). The tensor-valued images were linearly realigned based on trilinear interpolation of the log-transformed tensors (Arsigny et al., 2005), and resampled to isotropic voxel resolution (with dimensions: 128 128 93 voxels, resolution: 1.7 × 1.7 × 1.7 mm3). The FA image derived from the affine-registered DT image (Basser and Pierpaoli, 1996) was then registered to a mean FA image computed for the first 258 subjects scanned (a subset of the study sample in this paper; (Chiang et al., 2009a)). For this alignment step, we used a validated fluid registration algorithm that maximizes the Jensen-Rényi divergence of the joint intensity histogram of the two FA images (Chiang et al., 2007).
We averaged the fluidly-registered FA images across all subjects (N=705) and restricted subsequent data analysis to regions with average FA > 0.2, as recommended by Smith et al. (2006), to focus our regions of interest on major white matter fiber structures. Each participant's FA map was smoothed using an isotropic Gaussian filter with full width at half maximum (FWHM) = 6 mm (Smith et al., 2006).
2.4. Statistical analysis
In the classical twin design, genetic or environmental contributions to the observed variance in a trait are estimated by first computing the covariances of the measure (e.g., FA here) for MZ and DZ pairs (Chiang et al., 2009b; Neale et al., 1992). In our sample, where non-twin siblings are also included, we adopted the extended twin design (Lenroot et al., 2009; Posthuma et al., 2000) where the covariance matrix of FA was modeled for each family based on known genetic similarity between relatives. We then modeled the observed variation of FA using a standard structural equation model, widely used in twin studies. This partitions the observed variance into components due to additive genetic factors (A), shared environment (C) and unique environment (E), or into components due to additive genetic factors (A), genetic dominance (D), and unique environmental factors (E). The parameters of these structural equation models were estimated using a maximum likelihood scheme to maximize their fit (Neale et al., 1992). The extended twin design increases the power to detect genetic effect by combining information from twins and their non-twin siblings. It is also more flexible than traditional twin designs, as it allows samples with extended families where various degrees of kinship exist (van Leeuwen et al., 2008).
We started by fitting the full models involving three variance components - the ACE and the ADE models - to the observed covariance of FA at each voxel. Neither the genetic dominance term (D component) nor the shared environment term (C component) had a significant fit after correcting for multiple comparisons across voxels using the false discovery rate method (FDR; see below). Therefore, we only included the additive genetic (A) and unique environmental (E) components in subsequent analyses to increase the power for detecting genetic influences on FA.
We then evaluated the effects of a moderator variable (denoted by M; e.g., age) on heritability of FA by adding linear interaction terms with respect to M to the variance components models (Purcell, 2002). Adding this to the extended twin design above, we may express the expected FA value at each voxel for subject j in family i (yij) as:
Here Mij is the value of the moderator for that subject, and xij is a vector of nuisance covariates. βM and vector βN are the corresponding regression coefficients. The covariance matrix of FA for family i, denoted by Φi, may be written in terms of the different sources of variance:
Here a is the path coefficient, or relative contribution, of additive genetic factors to the variation of FA in the absence of moderator M, and e is the relative contribution from environmental factors that are unique for each individual. α = 1 when siblings j and k are MZ pairs, and 0.5 when they are DZ pairs, a co-twin and a non-twin sibling, or two non-twin siblings. Figure 1 shows the path diagram for the above model. We assumed that the modulatory effects on these path cofficients are linear, where βa and βe respectively indicate the effect of moderator M on additive genetic and unique environmental variance components. The unique environmental variance (e + βeMij)2 also includes random noise or experimental measurement error s. The above moderator model was fitted using the maximum-likelihood method (Posthuma et al., 2000).
Fig. 1.
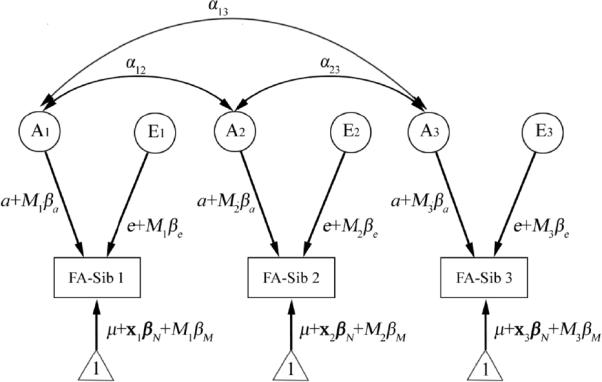
The path diagram for the “genetics plus moderator” model for an example family - in this case, with three siblings. Each sibling's phenotypic measure (e.g., FA in this study) is assumed to be determined by an additive genetic component (A), and an environmental component unique for each individual (denoted by E). Random noise, or experimental measurement error, is also included in the E component. Correlations between the genetic factors between siblings are indicated by double arrows. αij indicates the correlation coefficient, and is equal to 1 when siblings i and j are monozygotic pairs, and 0.5 when they are dizygotic pairs, a co-twin and a non-twin sibling, or two non-twin siblings. E components are assumed to be independent between siblings (no correlation). Interactions between genes and some specific demographic or environmental measure are modeled by including a moderator variable (denoted by M), e.g. age. Here, the contribution of component A or E to the phenotype, weighted by its path coefficient a or e, is assumed to be modified by the linear effect of moderator M, with the value of M in sibling i denoted by Mi. We also modeled the mean value of the phenotype, with a constant 1 (indicated by the triangles), modulated by the effects of the grand mean of the phenotype (μ), the nuisance covariates (x), and the moderator (the main effect of the moderator). Here variable x and its regression coefficient βN are bold-faced to denote vectors that allow more than one nuisance covariate. Modeling the means helps to remove the bias due to a possible correlation between siblings' phenotype and the moderator (Purcell, 2002), as we are more interested in the effect of the moderator on the genetic (A) and the environmental (E) influences. For simplification, all path coefficients, (a and e, and βa, βe, βN, and βM) are assumed to be the same between siblings.
The modulatory effect of M detected above may be biased by the correlation between genetic components that affect moderator M and that affect white matter integrity, if M is simply estimated by fitting the covariance matrix of FA under different values of M. This is why we included the βM term, or the main effect of the moderator, in the regression equation to eliminate this confounding effect, such that any genetic correlation between moderator M and white matter FA was appropriately de-trended (Purcell, 2002). On the other hand, estimating the main effect of M in the context of the moderator model may also help to more accurately evaluate the association between FA and M, as the influences of M on the variances in FA are considered simultaneously. We displayed the main effect of M by the marginal percentage difference of FA caused by the main effect of M, given by [ M (difference in values of M)] ′ (mean FA across the entire sample).
As is standard, the significance of the model parameters was determined based on the difference between the log-likelihood of the full model that included all the parameters and the restricted model where the parameters to be tested were excluded, denoted by logLf for the full model and logLr for the restricted model. Minus two times this difference, or −2(logLr – logLf), is asymptotically distributed approximately as a chi-squared distribution with degrees of freedom equal to the difference in the number of parameters between the two models (Cardon and Abecasis, 2000; Marlow et al., 2003).
We compared the difference in heritability of white matter integrity between adolescents (age < 18 years) vs. adults (age ≥ 18 years), males vs. females, and between the lower IQ group (FIQ < 114, which is the mean value of all the subjects whose IQ data were available) vs. the higher IQ group (FIQ ≥ 114) by setting the values of the moderator (age, sex, FIQ) to 1 for one group and 0 for the other group in the above moderator models. Subjects' SEI was treated as a continuous moderator. Heritability of FA, which is the percentage of FA variability accounted for by genetic influences, for subject j in family i then depends on the value of moderator Mij:
When the value of Mij is dichotomized to take values of 0 or 1, the heritability of FA becomes:
Note that the moderator model allows siblings in the same family to belong to different moderator groups, e.g., the sex of one co-twin may be different from that of the other co-twin or siblings, so long as each individual is assigned an appropriate moderator value (e.g., 0 for male and 1 for female). We also note that h02 and h12 may differ merely due to heteroscedasticity, i.e., differences in variance of FA, between the two moderator groups, without any genuine genetic × moderator interaction (Boomsma et al., 1999; Purcell, 2002; Turkheimer et al., 2003). Therefore, we considered the heritability of FA to be different only when βa was significantly different from zero, while other parameters, especially βe, were freely varied and estimated. This guaranteed that difference in estimated heritability between the two moderator groups was attributable to genetic effects, while heteroscedasticity was modeled in the unique variances as the difference between e2 and (e + βe)2.
All statistical maps in this paper were further assessed using the false discovery rate method (FDR; (Benjamini and Hochberg, 1995)) to correct for multiple comparisons. FDR is now a standard approach in neuroimaging, and is defined as the expected proportion of false positive findings out of all rejected tests. As is conventional in brain mapping studies, statistical maps with an FDR value below 5% were considered to reach overall significance, which means that no more than 5% of the voxels declared as significant are likely to be false positive findings. In this paper, only voxels that pass the FDR ≤ 0.05 threshold are displayed.
3. Results
3.1. White matter integrity and heritability as a function of age, sex, and socioeconomic status
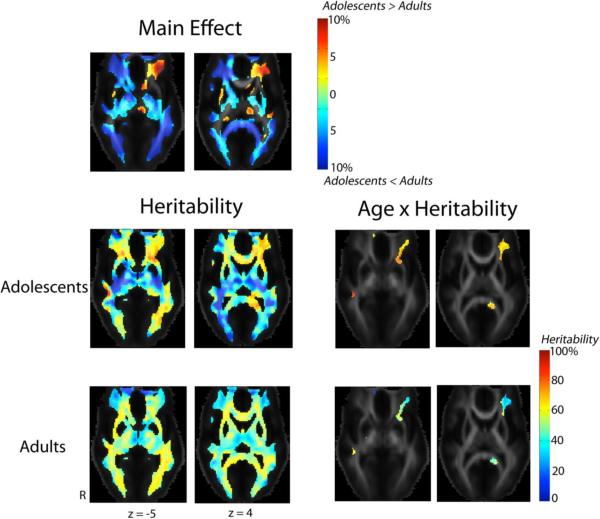
Figure 2 shows that between adolescence and adulthood, fiber organization and coherence, measured by FA, increased by up to 10% in most of the white matter (subjects' age was treated as a categorical variable; inter-subject differences in FA were also adjusted for sex). We also detected significant age × heritability interaction. White matter integrity in the left inferior and middle frontal gyri, the splenium of the corpus callosum on the left, and the right inferior longitudinal fasciculus (ILF)/inferior fronto-occipital fasciculus (IFO), was significantly more heritable in the adolescents than adults. In adolescents, around 70–80% of the variation in FA was attributable to genetic factors, although in adults, only 30–40% of the variation in FA was attributable to genetic factors.
Fig. 2. Changes in white matter integrity during brain development.
These maps show the percentage differences in FA (the main effect of age, adjusted for sex differences) and the proportion of genetic contributions to the overall variance in FA (heritability) between the adult (n = 531) and adolescent (n = 174) groups. Differences in heritability (age × heritability interaction) between the two groups are compared in the same slices. Colored regions indicate voxels that survive the threshold that controls the overall false discovery rate at 5%. The Montreal Neurological Institute (MNI) coordinate (expressed in mm) for each of the slices is indicated, in each column. Adult subjects had higher white matter integrity, measured by FA, than the adolescents in most white matter regions. However, in the left inferior and middle frontal gyri, the adolescent subjects had up to 10% higher FA than the adults. Genetic influences on FA in this area, along with the splenium of the corpus callosum on the left, and the right inferior longitudinal fasciculus (ILF)/inferior fronto-occipital fasciculus (IFO), were significantly higher in the adolescents, where around 70–80% of the variation in FA for the adolescents, while only 30–40% of the variation in FA for the adults, was attributable to genetic factors. Abbreviation - R: right.
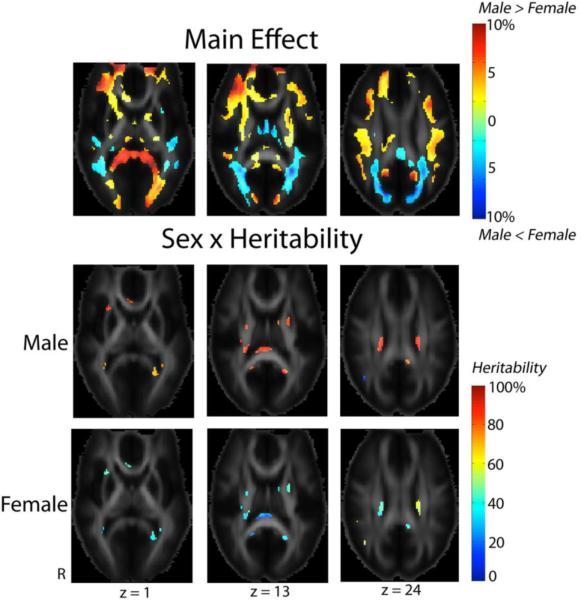
Figure 3 shows regional differences in white matter integrity between male and female subjects. After adjusting for age as a nuisance covariate, male subjects had higher FA in the frontal white matter bilaterally, in the splenium of the corpus callosum, and in the optic radiations bilaterally (more on the left). White matter FA was higher in females in the middle and superior occipital gyri that are parts of the dorsal stream of the visual pathways. Even so, there are small regions where genetic influence was higher in males and accounted for around 80% of variation in FA. Among these regions were the genu and splenium of the corpus callosum, the external capsule and posterior limbs of the internal capsule, and the superior fronto-occipital fasciculus bilaterally.
Fig. 3. Sex differences in white matter microstructure.
These maps demonstrate regional differences in the values (main effect of sex) and heritability (modulatory effect of sex) in FA between male and female subjects. Males had higher FA (adjusted for age) in frontal white matter bilaterally, the splenium of the corpus callosum and the optic radiation bilaterally (more on the left) (MNI z coordinate = 1 mm). Females had higher FA in the middle and superior occipital gyri (z = 13 and 24). Showing evidence for a sex × heritability interaction, genetic influences were much higher in males and accounted for around 80% of the observed variation in FA in the external capsule and the genu and splenium of the corpus callosum (z = 1), the posterior limbs of the internal capsule (z = 13), and the superior fronto-occipital fasciculus, bilaterally (z = 24).
We found no significant associations between the adult subjects' socioeconomic status, measured by SEI, and the value of white matter FA. However, socioeconomic status significantly interacted with genes that affect white matter integrity, after adjusting for subjects' age and sex. Fig. 4 shows that higher socioeconomic status was associated with higher heritability of FA in the thalamus (which was included in the regions where FA > 0.2), the middle temporal gyrus on the left, and the callosal splenium, although in some smaller regions in the anterior corona radiata, heritability in FA was higher in subjects with lower socioeconomic status.
Fig. 4. Interactions between socioeconomic status and genetic components that affect white matter integrity.
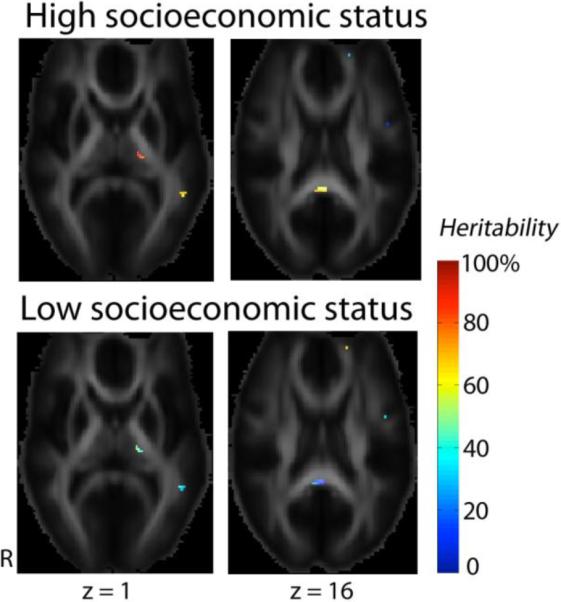
Socioeconomic status was evaluated using the socioeconomic index (SEI, range = 0–100). To show the contrast in FA heritability between subjects with higher versus lower socioecnomic status, we used the 75th percentile (SEI = 83.8) and the 25th percentile of SEI (SEI = 39.7) to represent the higher and the lower socioecnomic groups. In the thalamus, and the middle temporal gyrus on the left (z = 1), and the callosal splenium (z = 16), genetic contributions to the overall variation of FA were higher in the higher socioeconomic group. However, for some small regions in the anterior corona radiata (z = 16), the genetic variance proportion is higher in the lower socioeconomic group. The main effect of SEI on FA was not significant.
3.2. Mapping the linkage between heritability and intellectual performance
IQ data were available in 601 subjects (513 adults and all 88 subjects of age 16). FIQ score was higher in the males, however the difference was slight (male: 116.6±12.8, female: 112.3±11.9, P = 2.8×10−5). FIQ was also associated with a higher socioeconomic index (adult subjects only; Pearson correlation r = 0.21, P = 5×10−5). There was no significant correlation between subjects' age and their FIQ score.
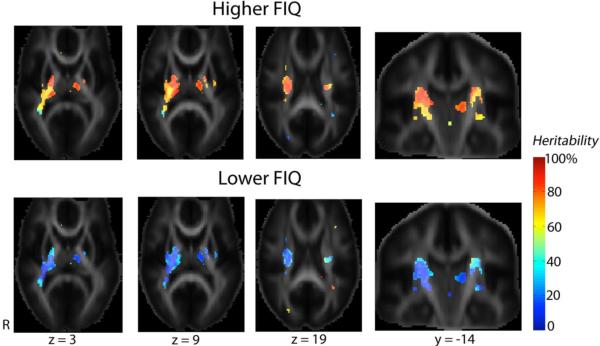
To study variations in white matter heritability with respect to intellectual performance, we divided the subjects into a higher IQ group (FIQ≥114; n = 327) and a lower IQ group (FIQ < 114; n = 274). We did not split these groups at 100, as the twins had an average IQ well above 100. The higher IQ group had a slightly younger age (though the difference was only trend-level; higher IQ group: 22.3±3.4, lower IQ group: 22.8±3.3, P = 0.06), a greater proportion of males (higher IQ group: 153 M/174 F, 46.8% of the subjects were males; lower IQ group: 92 M/182 F, 33.6% were males; P = 0.001), and higher socioeconomic status (adult subjects only; SEI: 65.3±22.9 for the higher IQ group, 57.8±24.0 for the lower IQ group, P < 0.001). By including subjects' age, sex, and SEI (adult subjects) as covariates, we found that higher intellectual performance was associated with higher white matter heritability, especially in the corticobulbar and corticospinal tracts. Fig. 5 shows this — in the higher FIQ group, more than 80% of FA variability was genetically determined, compared to around 40% in the lower IQ group, in the thalamus, the genu and posterior limbs of the internal capsule, and the superior corona radiata. FA was not different between the two IQ groups when the FIQ score was modeled as the main effect.
Fig. 5. Intellectual performance is associated with the degree of genetic control on brain microstructure.
These maps show that higher intellectual performance was associated with higher heritability in FA (n = 601, including 513 adults and 88 subjects of age 16). In subjects with higher FIQ, more than 80% of FA variability was genetically determined, in contrast to only around 40% for the lower IQ group, in the thalamus, the genu and posterior limbs of the internal capsule, and the superior corona radiata. These areas carry the corticobulbar and corticospinal tracts, and the coronal slices show the directions of motor tracts more clearly. The main effect of FIQ is not significant, i.e. no difference in FA between the two IQ groups was detected.
4. Discussion
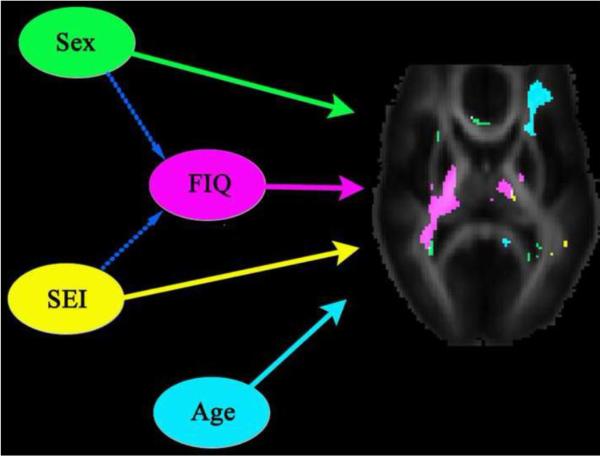
We found moderate but significant modulatory effects of age, sex, intellectual performance (measured by FIQ), and socioeconomic status (SES), on the heritability of white matter integrity measured by FA. Higher white matter heritability was associated with younger age (adolescents), male sex, higher FIQ, and higher socioeconomic status. We did not expect the modulatory effects of age, sex, IQ, or SES on the heritability of FA to be strong everywhere in the white matter, and it would be surprising if they were detectable in the majority of white matter – interaction effects are notoriously hard to detect as they are second-order (indirect) effect. To avoid false positives in the interaction maps, we deliberately enforced a very stringent criterion to define the significance in differences in heritability between two comparison groups, to avoid any spurious effects that could arise from possible heteroscedasticity in FA. As such, only a few regions show detectable interaction effects, with modest but robust interaction effects for age, sex, and socioeconomic status. Fig. 6 summarizes the modulatory effects of these demographic factors in one representative slice, showing that each individual factor may affect genetic influence preferentially in specific brain regions.
Fig. 6. Summary diagram showing factors that affect white matter integrity.
Solid arrows indicate the modulatory effect on white matter heritability of age, sex, intellectual performance (measured by FIQ), and socioeconomic index (SEI). Brain regions where the heritability of FA was modulated by an individual variable were displayed using a specific color for each variable. Higher white matter heritability was associated with younger age (adolescents), male sex, higher FIQ, and higher socioeconomic status, as shown in the previous figures. Broken arrows indicate linear association between two variables; here, higher FIQ is associated with male sex and higher SEI. Among these variables, the effect of intellectual performance on FA heritability is the most obvious and involves the thalamus and the posterior limbs of the internal capsule, where various sensory inputs are relayed and motor signals are conveyed. Although subjects' intellectual performance is affected by their sex and socioeconomic status, it is less likely that FIQ is a variable that is merely tracking sex and SEI, as the effect size of FIQ is greater than that of either sex or SEI. Alternatively, the modulatory effect of FIQ on heritability of FA may reflect the influences of some unknown environmental, family, or socioeconomic factors not yet explored in this study, or it may indicate that genetic components that underlie intellectual performance may facilitate the expression of genes that affect microstrucural integrity in certain brain regions.
In most white matter regions, FA in adults was up to 10% greater than that in adolescents. This agrees with prior findings that white matter FA increases with age until the early 30s (Hasan et al., 2009; Kochunov et al., 2010). On the other hand, heritability of white matter integrity in the left frontal lobe, the callosal splenium, and the right inferior longitudinal fasciculus decreased as subjects aged. This may indicate that environmental influences, e.g., learning, education, life experiences, diet, and exercise, start to dominate and increasingly determine brain fiber networks as one matures into adulthood. To our knowledge, our study is the first to investigate changes in the heritability of white matter integrity from adolescence to adulthood. Our sample does not include subjects in early childhood so we cannot determine whether heritability decreases from early childhood or peaks in adolescence before it decreases – the latter appears to be the case for the heritability of cortical thickness (Lenroot et al., 2009).
The effect size for the observed interaction between age and genetic effects on white matter integrity is somewhat smaller than that reported previously for cortical thickness (Lenroot et al., 2009). This may be because our sample includes three age categories (ages 12, 16, and adults) and not a continuous sampling of age. Therefore, we may have been less sensitive to differences in FA heritability between adolescence and adulthood because of (1) insufficient sampling of the youngest age group, and (2) we had to adopt a more stringent criterion in which differences in heritability between the two groups were significant only when βa was not zero, to avoid the bias from possible heteroscedasticity in FA between adolescents and adults.
We also found that males had a higher FA than females in the frontal white matter and the callosal splenium. This agrees with prior studies (Kochunov et al., 2010). FA in most frontal white matter regions is reported to be higher in boys than girls aged 5–18 (Schmithorst et al., 2008). Moreover, in young adults, men have higher relative anisotropy in the genu and the posterior corpus callosum (Westerhausen et al., 2004). Interestingly, we also found higher FA in females versus males in the middle and superior occipital white matter. Higher white matter integrity in these regions may be related to better visuospatial ability (Chiang et al., 2009b), but if that is true, our results would seem to contradict the orthodox view that men have marginally better visuospatial function than women, on average (Kimura, 1999; Neisser et al., 1996). Nevertheless, some researchers attributed this difference to the use of different visual cues by men and women to solve spatial problems, and women outperformed men in some spatial tasks, such as object location (Jones and Healy, 2006; Montello et al., 1999). The significant gene-sex interaction found in the corpus callosum, the external and the internal capsule, and the superior fronto-occipital fasciculus provides the first imaging evidence that sex differences may modify genetic influences on brain structure. Sex may be regarded in some respects as a microenvironmental factor modifying the brain, in part, via hormonal effects — testosterone, for example, affects gene expression in avian brains (Absil et al., 2003). In humans, androgen treatment in female-to-male transsexuals tends to increase the total brain and hippocampal volumes (Hulshoff Pol et al., 2006a). On the other hand, sexual dimorphism in white matter integrity could be attributed to differential magnitude of gene-trait linkages between men and women, as was reported for other human traits in a genome-wide linkage study (Weiss et al., 2006).
Socioeconomic status (SES) also affected the relative degree of genetic control over white matter integrity. Previous studies found that SES influences brain structure and function. Children from families with higher SES intellectually outperformed those with lower SES, especially in language and executive tasks (Hackman and Farah, 2009). Lower SES was associated with lower gray matter volume in the cingulate cortex, measured using voxel-based morphometry (VBM; (Gianaros et al., 2007)). In a mouse study, mRNA levels of genes involved in neuronal growth were up-regulated when mice were exposed to an enriched learning environment (Rampon et al., 2000). Nonetheless, the causal mechanisms for our findings are unclear. One possible explanation is based on the linkage between SES and IQ. Given that IQ and white matter FA were influenced by an overlapping set of genes (Chiang et al., 2009b), and higher SEI was associated with higher heritability of IQ (Turkheimer et al., 2003), it might be reasonable to expect that SES positively modulates the heritability of FA. As Turkheimer argues, each individual is more likely to achieve their genetic potential, when adverse environmental factors are reduced as far as possible.
White matter integrity was more heritable in subjects with better intellectual performance, in the white matter of the thalamus, and in the genu and posterior limbs of the internal capsule, that carry corticobulbar and corticospinal tracts. The thalamus is not merely a relay station for sensory input and motor control, it is also crucially involved in the interconnections between higher-order somatosensory cortices (Theyel et al., 2010). Neurons in the corticospinal system control movements that require the greatest skill and flexibility (Martin, 2005), and higher FA in the corticospinal tract is associated with higher IQ (Yu et al., 2008). Even though our subjects' intellectual performance was associated with their sex and socioeconomic status, FIQ was not merely an intervening variable for sex and SEI, as the effect size to the FIQ × heritability interaction for white matter was greater than that of either sex or SEI. More likely, levels of intelligence per se may influence heritability of brain structures, and several mechanisms may contribute to this. First, learning and education may promote intellectual performance and also affect gene expression in the brain. For example, learning upregulated the mRNA expression of the brain-derived neurotrophic factor, BDNF, a growth factor that is essential for neuronal growth and cognitive function (Kesslak et al., 1998). Second, since intellectual performance is highly heritable (Chiang et al., 2009b; Wright et al., 2001), our findings may reflect epistasis between genes that influence intelligence and those controlling white matter integrity. Future genetic association studies may be able to test this hypothesis by identifying genes that show epistatic effects on FA and IQ. Genetic epistasis was implicated in a recent fMRI study, where epistasis between dopamine transporter (DAT) and catechol-O-methyltransferase (COMT) genes, both crucial in neurocognitive function, were found to influence brain activation during verbal cognitive tasks (Prata et al., 2009). A third explanation for IQ effects on white matter heritability is gene-environment correlation, whereby genetic effects play an active role in modifying physical and social environments (Kendler and Baker, 2007). Specifically, genes that influence brain white matter integrity may speed axonal conduction in the thalamus and corticospinal tract, and this may indirectly help those with higher IQ to take greater advantage of educational experiences that promote higher IQ. Lastly, assortative mating (Plomin and Spinath, 2004) may result in an FIQ × heritability interaction for FA. In general, people tend to choose a partner of a comparable level of intelligence (Mascie-Taylor, 1989). As some of the positive correlation between intelligence and white matter microstructure is accounted for by partially overlapping sets of genes (Chiang et al., 2009b), the proportion of high-IQ subjects who have these shared genes that affect both IQ and FA may be increased due to some degree of assortative mating within the higher-IQ subgroup of the population.
We found that higher FIQ was associated with higher heritability of FA in the thalamus, and this may raise questions as to whether thalamic FA measures reflect differences in gray rather than white matter, or some mixture of both. The thalamus is commonly considered as one of the deep gray matter structures, but it contains abundant myelinated fibers (Rinvik and Grofova, 1974), and therefore FA in the thalamus may still reflect integrity and coherence of white matter fibers (Pfefferbaum et al., 2010; Qiu et al., 2009). Moreover, the FA > 0.2 threshold we adopted here also helped to include thalamic regions that contain more white matter than gray matter tissues into analysis. Nevertheless, the thalamus is not a “pure” white matter structure and its aggregate FA may reflect some attenuation due to partial voluming of signals with those from gray matter components.
This study has some limitations. First, some regions with significant sex × heritability or SES × heritability interaction effects were detected at the edge of the white matter (e.g., at the border of the corpus callosum; see Figs. 3 and 4). As such our results should be interpreted carefully, as they may be attributable not solely to differences in fiber microstructure, but also to possible differences in callosal size that have not been entirely removed by the nonlinear registration transformation. This is an issue shared by all brain mapping studies that attempt to spatially normalize homologous anatomy to a template, and is somewhat alleviated but not completely eliminated by using high-dimensional transformations. Second, the voxel dimensions of our DT images are somewhat anisotropic, and their in-plane resolution is greater than their out of plane resolution (1.8 × 1.8 × 5 mm3). Because of this, before we derived maps of FA, the DT images were resampled to an isotropic voxel resolution, based on log-transformation to preserve the correct shape of the interpolated tensors (Arsigny et al., 2005). Even so, we still cannot rule out that estimation of diffusion parameters might be biased by the partial volume effect. This may limit the degree to which comparisons can be made between tracts, in different brain regions, that are oriented along different axes (e.g., the corpus callosum vs. the corticospinal tract).
We treated age and intellectual performance (measured by FIQ) as categorical variables because even with our large sample size, the effect size (regions where modulatory effects of age on the heritability of white matter were significant) was much greater when age or FIQ was considered as a categorical variable than as a continuous one. As noted by Purcell (2002), some moderators can be measured on a continuous scale, but it is also entirely reasonable that they act in a more discrete manner – in the case of IQ, some threshold or plateau effect of IQ may be observed, such that effects of a 10 point difference in IQ may differ depending on whether the IQ is very high or very low. Age and FIQ may be such moderators that their interaction effects on the heritability of white matter may be easier to detect using binary groupings. In some of our other studies of pediatric development, we used age and age squared as covariates, but it is not clear that this leads to a tenable model for people in their twenties, as the development processes occurring in late childhood and adolescence are not necessarily just faster versions of the processes that occur later in the twenties (and we know the growth and cortical thinning occur in different regions at these ages). As such, we preferred a less strongly parameterized model that differentiated adolescents versus adults using conventional definitions.
In conclusion, we reported the first maps to demonstrate influences of age, sex, SES and IQ on the heritability of brain fiber architecture. Although our findings were derived from a large sample with N = 705 subjects, we must still interpret the findings cautiously, until they are replicated in independent large-sample studies. Even so, our findings may help to understand how genetics and environmental context both affect brain structure. Knowledge that genetic control is context dependent is also useful to guide the search for genetic polymorphisms that influence white matter integrity, realizing that their causal role may vary with environmental factors and with age.
Research Highlights
Between age 12 and adulthood, FA increases by up to 10% in most of the white matter
Males have higher FA than females in most white matter structures
High WM heritability is related to young age, male, high FIQ/socioeconomic status
Acknowledgments
This study was supported by grant number RO1 HD050735 from the National Institute of Child Health and Human Development, USA, and Project Grant 496682 from the National Health and Medical Research Council, Australia. The collection of IQ data and zygosity typing was supported by the Australian Research Council (A7960034, A79906588, A79801419, DP0212016). Additional support for algorithm development was provided by the NIA, NIBIB, and the National Center for Research Resources (AG016570, EB01651, RR019771 to PT). We are also grateful to the twins for their willingness to participate in our studies, and research nurses, Marlene Grace and Ann Eldridge, Queensland Institute of Medical Research, for twin recruitment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Absil P, Pinxten R, Balthazart J, Eens M. Effects of testosterone on Reelin expression in the brain of male European starlings. Cell Tissue Res. 2003;312:81–93. doi: 10.1007/s00441-003-0701-9. [DOI] [PubMed] [Google Scholar]
- Alexander DC, Pierpaoli C, Basser PJ, Gee JC. Spatial transformations of diffusion tensor magnetic resonance. IEEE Trans Med Imaging. 2001;20:1131–1139. doi: 10.1109/42.963816. [DOI] [PubMed] [Google Scholar]
- Arsigny V, Fillard P, Pennec X, Ayache N. Fast and simple calculus on tensors in the log-Euclidean framework. Int Conf Med Image Comput Comput Assist Interv (MICCAI) 2005;8:115–122. doi: 10.1007/11566465_15. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- Blokland GA, McMahon KL, Hoffman J, Zhu G, Meredith M, Martin NG, Thompson PM, de Zubicaray GI, Wright MJ. Quantifying the heritability of task-related brain activation and performance during the N-back working memory task: a twin fMRI study. Biol Psychol. 2008;79:70–79. doi: 10.1016/j.biopsycho.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boomsma DI, de Geus EJ, van Baal GC, Koopmans JR. A religious upbringing reduces the influence of genetic factors on disinhibition: evidence for interaction between genotype and environment on personality. Twin Res. 1999;2:115–125. doi: 10.1375/136905299320565988. [DOI] [PubMed] [Google Scholar]
- Cardon LR, Abecasis GR. Some properties of a variance components model for fine-mapping quantitative trait loci. Behav Genet. 2000;30:235–243. doi: 10.1023/a:1001970425822. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Zuo X-N, Williams K, Bangaru S, Kelly C, Mennes M, Fair D, Biswal BB, Wright M, Martin N, de Zubicaray G, McMahon K, Hickie I, Milham M. Genetic analyses of resting-state studies in adolescent twins: preliminary results. 16th Annual Meeting of the Organization of Human Brain Mapping; Barcelona, Spain. Jun 6–10, 2010.2010. [Google Scholar]
- Chiang M-C, Avedissian C, Barysheva M, Toga AW, McMahon K, de Zubicaray GI, Wright MJ, Thompson PM. Extending genetic linkage analysis to diffusion tensor images to map single gene effects on brain fiber architecture. Med Image Comput Comput Assist Interv (MICCAI) 2009a;12:506–513. doi: 10.1007/978-3-642-04271-3_62. [DOI] [PubMed] [Google Scholar]
- Chiang MC, Barysheva M, Shattuck DW, Lee AD, Madsen SK, Avedissian C, Klunder AD, Toga AW, McMahon KL, de Zubicaray GI, Wright MJ, Srivastava A, Balov N, Thompson PM. Genetics of brain fiber architecture and intellectual performance. J Neurosci. 2009b;29:2212–2224. doi: 10.1523/JNEUROSCI.4184-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, Dutton RA, Hayashi KM, Lopez OL, Aizenstein HJ, Toga AW, Becker JT, Thompson PM. 3D pattern of brain atrophy in HIV/AIDS visualized using tensor-based morphometry. Neuroimage. 2007;34:44–60. doi: 10.1016/j.neuroimage.2006.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Zubicaray G, Chiang M-C, McMahon K, Shattuck D, Toga A, Martin N, Wright M, Thompson P. Meeting the challenges of neuroimaging genetics. Brain Imaging Behav. 2008;2:258–263. doi: 10.1007/s11682-008-9029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galton F. Heredity, genius: An enquiry into its laws and consequences. Macmillan; London: 1869. [Google Scholar]
- Gianaros PJ, Horenstein JA, Cohen S, Matthews KA, Brown SM, Flory JD, Critchley HD, Manuck SB, Hariri AR. Perigenual anterior cingulate morphology covaries with perceived social standing. Soc Cogn Affect Neurosci. 2007;2:161–173. doi: 10.1093/scan/nsm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Winkler AM, Kochunov P, Almasy L, Duggirala R, Carless MA, Curran JC, Olvera RL, Laird AR, Smith SM, Beckmann CF, Fox PT, Blangero J. Genetic control over the resting brain. Proc Natl Acad Sci U S A. 2010;107:1223–1228. doi: 10.1073/pnas.0909969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman, Shields J. A polygenic theory of schizophrenia. Proc Natl Acad Sci U S A. 1967;58:199–205. doi: 10.1073/pnas.58.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends Cogn Sci. 2009;13:65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Kamali A, Iftikhar A, Kramer LA, Papanicolaou AC, Fletcher JM, Ewing-Cobbs L. Diffusion tensor tractography quantification of the human corpus callosum fiber pathways across the lifespan. Brain Res. 2009;1249:91–100. doi: 10.1016/j.brainres.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Collins L, Woods R, Toga AW, Evans AC. Enhancement of MR images using registration for signal averaging. J Comput Assist Tomogr. 1998;22:324–333. doi: 10.1097/00004728-199803000-00032. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Cohen-Kettenis PT, Van Haren NEM, Peper JS, Brans RGH, Cahn W, Schnack HG, Gooren LJG, Kahn RS. Changing your sex changes your brain: influences of testosterone and estrogen on adult human brain structure. Eur J Endocrinol. 2006a;155:S107–S114. [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Posthuma D, Mandl RC, Baare WF, van Oel C, van Haren NE, Collins DL, Evans AC, Amunts K, Burgel U, Zilles K, de Geus E, Boomsma DI, Kahn RS. Genetic contributions to human brain morphology and intelligence. J Neurosci. 2006b;26:10235–10242. doi: 10.1523/JNEUROSCI.1312-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DN. MAB, multidimensional aptitude battery: manual. Research Psychologists Press; Port Hurton, Mich.: 1984. [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jones CM, Healy SD. Differences in cue use and spatial memory in men and women. Proc Biol Sci. 2006;273:2241–2247. doi: 10.1098/rspb.2006.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, Kochunov P, Winkler AM, Laird AR, Almasy L, Duggirala R, Olvera RL, Fox PT, Blangero J, Glahn DC. A multimodal assessment of the genetic control over working memory. J Neurosci. 2010;30:8197–8202. doi: 10.1523/JNEUROSCI.0359-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Baker JH. Genetic influences on measures of the environment: a systematic review. Psychol Med. 2007;37:615–626. doi: 10.1017/S0033291706009524. [DOI] [PubMed] [Google Scholar]
- Kesslak JP, So V, Choi J, Cotman CW, Gomez-Pinilla F. Learning upregulates brain-derived neurotrophic factor messenger ribonucleic acid: a mechanism to facilitate encoding and circuit maintenance? Behav Neurosci. 1998;112:1012–1019. doi: 10.1037//0735-7044.112.4.1012. [DOI] [PubMed] [Google Scholar]
- Kimura D. Sex and cognition. MIT Press; Cambridge, Mass.: 1999. [Google Scholar]
- Kochunov P, Williamson DE, Lancaster J, Fox P, Cornell J, Blangero J, Glahn DC. Fractional anisotropy of water diffusion in cerebral white matter across the lifespan. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.01.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koten JW, Jr., Wood G, Hagoort P, Goebel R, Propping P, Willmes K, Boomsma DI. Genetic contribution to variation in cognitive function: an FMRI study in twins. Science. 2009;323:1737–1740. doi: 10.1126/science.1167371. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, Kendler KS, Evans AC, Giedd JN. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Hum Brain Mapp. 2009;30:163–174. doi: 10.1002/hbm.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano M, Wright MJ, Geffen GM, Geffen LB, Smith GA, Evans DM, Martin NG. A genetic two-factor model of the covariation among a subset of Multidimensional Aptitude Battery and Wechsler Adult Intelligence Scale--Revised subtests. Intelligence. 2003;31:589–605. [Google Scholar]
- Marlow AJ, Fisher SE, Francks C, MacPhie IL, Cherny SS, Richardson AJ, Talcott JB, Stein JF, Monaco AP, Cardon LR. Use of multivariate linkage analysis for dissection of a complex cognitive trait. Am J Hum Genet. 2003;72:561–570. doi: 10.1086/368201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JH. The corticospinal system: from development to motor control. Neuroscientist. 2005;11:161–173. doi: 10.1177/1073858404270843. [DOI] [PubMed] [Google Scholar]
- Martin N. Gene-environment interaction and twin studies. In: Spector TD, Snieder H, MacGregor AJ, editors. Advances in Twin and Sib-pair Analysis. Greenwich Medical Media; Cambridge, United Kingdom: 2000. pp. 144–150. [Google Scholar]
- Mascie-Taylor CG. Spouse similarity for IQ and personality and convergence. Behav Genet. 1989;19:223–227. doi: 10.1007/BF01065906. [DOI] [PubMed] [Google Scholar]
- McMillan J, Beavis A, Jones FL. The AUSEI06 - a new socioeconomic index for Australia. J Sociol. 2009;45:123–149. [Google Scholar]
- Montello DR, Lovelace KL, Golledge RG, Self CM. Sex-related differences and similarities in geographic and environmental spatial abilities. Ann Assoc Am Geogr. 1999;89:515–534. [Google Scholar]
- Neale MC, Cardon LR, The NATO Scientific Affairs Division . Methodology for genetic studies of twins and families. Kluwer Academic Publishers; Dordrecht; Boston: 1992. [Google Scholar]
- Neisser U, Boodoo G, Bouchard TJ, Jr., Boykin AW, Brody N, Ceci SJ, Halpern DF, Loehlin JC, Perloff R, Sternberg RJ, Urbina S. Intelligence: Knowns and Unknowns. Am Psychol. 1996;51:77–101. [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Rohlfing T, Sullivan EV. Diffusion tensor imaging of deep gray matter brain structures: effects of age and iron concentration. Neurobiol Aging. 2010;31:482–493. doi: 10.1016/j.neurobiolaging.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Carmelli D. Genetic regulation of regional microstructure of the corpus callosum in late life. Neuroreport. 2001;12:1677–1681. doi: 10.1097/00001756-200106130-00032. [DOI] [PubMed] [Google Scholar]
- Plomin R, Spinath FM. Intelligence: genetics, genes, and genomics. J Pers Soc Psychol. 2004;86:112–129. doi: 10.1037/0022-3514.86.1.112. [DOI] [PubMed] [Google Scholar]
- Posthuma D, de Geus EJ, Neale MC, Hulshoff Pol HE, Baare WEC, Kahn RS, Boomsma D. Multivariate genetic analysis of brain structure in an extended twin design. Behav Genet. 2000;30:311–319. doi: 10.1023/a:1026501501434. [DOI] [PubMed] [Google Scholar]
- Prata DP, Mechelli A, Fu CH, Picchioni M, Toulopoulou T, Bramon E, Walshe M, Murray RM, Collier DA, McGuire P. Epistasis between the DAT 3' UTR VNTR and the COMT Val158Met SNP on cortical function in healthy subjects and patients with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:13600–13605. doi: 10.1073/pnas.0903007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Res. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Qiu A, Zhong J, Graham S, Chia MY, Sim K. Combined analyses of thalamic volume, shape and white matter integrity in first-episode schizophrenia. Neuroimage. 2009;47:1163–1171. doi: 10.1016/j.neuroimage.2009.04.027. [DOI] [PubMed] [Google Scholar]
- Rampon C, Jiang CH, Dong H, Tang YP, Lockhart DJ, Schultz PG, Tsien JZ, Hu Y. Effects of environmental enrichment on gene expression in the brain. Proc Natl Acad Sci U S A. 2000;97:12880–12884. doi: 10.1073/pnas.97.23.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinvik E, Grofova I. Light and electron microscopical studies of the normal nuclei ventralis lateralis and ventralis anterior thalami in the cat. Anat Embryol (Berl) 1974;146:57–93. doi: 10.1007/BF00341383. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Holland SK, Dardzinski BJ. Developmental differences in white matter architecture between boys and girls. Hum Brain Mapp. 2008;29:696–710. doi: 10.1002/hbm.20431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt JE, Lenroot RK, Wallace GL, Ordaz S, Taylor KN, Kabani N, Greenstein D, Lerch JP, Kendler KS, Neale MC, Giedd JN. Identification of genetically mediated cortical networks: a multivariate study of pediatric twins and siblings. Cereb Cortex. 2008;18:1737–1747. doi: 10.1093/cercor/bhm211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Lerch JP, Pruessner JC, Taylor KN, Rose AB, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN. Cortical morphology in children and adolescents with different apolipoprotein E gene polymorphisms: an observational study. Lancet Neurol. 2007;6:494–500. doi: 10.1016/S1474-4422(07)70106-0. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Theyel BB, Llano DA, Sherman SM. The corticothalamocortical circuit drives higher-order cortex in the mouse. Nat Neurosci. 2010;13:84–88. doi: 10.1038/nn.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, Lonnqvist J, Standertskjold-Nordenstam CG, Kaprio J, Khaledy M, Dail R, Zoumalan CI, Toga AW. Genetic influences on brain structure. Nat Neurosci. 2001;4:1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Turkheimer E, Haley A, Waldron M, D'Onofrio B, Gottesman Socioeconomic status modifies heritability of IQ in young children. Psychol Sci. 2003;14:623–628. doi: 10.1046/j.0956-7976.2003.psci_1475.x. [DOI] [PubMed] [Google Scholar]
- van Leeuwen M, van den Berg SM, Boomsma DI. A twin-family study of general IQ. Learn Individ Differ. 2008;18:76–88. [Google Scholar]
- Wallace GL, Eric Schmitt J, Lenroot R, Viding E, Ordaz S, Rosenthal MA, Molloy EA, Clasen LS, Kendler KS, Neale MC, Giedd JN. A pediatric twin study of brain morphometry. J Child Psychol Psychiatry. 2006;47:987–993. doi: 10.1111/j.1469-7610.2006.01676.x. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Pan L, Abney M, Ober C. The sex-specific genetic architecture of quantitative traits in humans. Nat Genet. 2006;38:218–222. doi: 10.1038/ng1726. [DOI] [PubMed] [Google Scholar]
- Westerhausen R, Kreuder F, Dos Santos Sequeira S, Walter C, Woerner W, Wittling RA, Schweiger E, Wittling W. Effects of handedness and gender on macro- and microstructure of the corpus callosum and its subregions: a combined high-resolution and diffusion-tensor MRI study. Brain Res Cogn Brain Res. 2004;21:418–426. doi: 10.1016/j.cogbrainres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Wright M, De Geus E, Ando J, Luciano M, Posthuma D, Ono Y, Hansell N, Van Baal C, Hiraishi K, Hasegawa T, Smith G, Geffen G, Geffen L, Kanba S, Miyake A, Martin N, Boomsma D. Genetics of cognition: outline of a collaborative twin study. Twin Res. 2001;4:48–56. doi: 10.1375/1369052012146. [DOI] [PubMed] [Google Scholar]
- Yu C, Li J, Liu Y, Qin W, Li Y, Shu N, Jiang T, Li K. White matter tract integrity and intelligence in patients with mental retardation and healthy adults. Neuroimage. 2008;40:1533–1541. doi: 10.1016/j.neuroimage.2008.01.063. [DOI] [PubMed] [Google Scholar]