Abstract
Background
Alzheimer disease (AD) is the most common form of dementia worldwide. Mild cognitive impairment (MCI) is the recent terminology for patients with cognitive deficiencies in the absence of functional decline. Most patients with MCI harbor the pathologic changes of AD and demonstrate transition to dementia at a rate of 10% to 15% per year. Patients with AD and MCI experience progressive brain atrophy.
Objective
To analyze the structural magnetic resonance imaging data for 24 patients with amnestic MCI and 25 patients with mild AD using an advanced 3-dimensional cortical mapping technique.
Design
Cross-sectional cohort design.
Patients/Methods
We analyzed the structural magnetic resonance imaging data of 24 amnestic MCI (mean MMSE, 28.1; SD, 1.7) and 25 mild AD patients (all MMSE scores, >18; mean MMSE, 23.7; SD, 2.9) using an advanced 3-dimensional cortical mapping technique.
Results
We observed significantly greater cortical atrophy in patients with mild AD. The entorhinal cortex, right more than left lateral temporal cortex, right parietal cortex, and bilateral precuneus showed 15% more atrophy and the remainder of the cortex primarily exhibited 10% to 15% more atrophy in patients with mild AD than in patients with amnestic MCI.
Conclusion
There are striking cortical differences between mild AD and the immediately preceding cognitive state of amnestic MCI. Cortical areas affected earlier in the disease process are more severely affected than those that are affected late. Our method may prove to be a reliable in vivo disease-tracking technique that can also be used for evaluating disease-modifying therapies in the future.
Alzheimer disease (AD) is the most common neurodegenerative disease in the elderly population. It results from the abnormal accumulation of misfolded amyloid and tau proteins in neurons and the extracellular space, ultimately leading to cell death and progressive cognitive decline. Pathologic features of AD often are noted in patients with mild cognitive impairment (MCI) with a more restricted anatomical distribution.1 Spread of neuritic plaques and neurofibrillary tangles through the brain is highly systematic. The first amyloid plaques form in the temporooccipital association cortices,2,3 then in the perirhinal or entorhinal area and the parietal cortex, and later in the frontal neocortex.4 Neocortical amyloid deposits ordinarily precede neocortical neurofibrillary tangles.2 Neurofibrillary tangles initially accumulate in the entorhinal cortex and later the hippocampus,2,5 then in the lateral temporal, parietal, and frontal association cortices.2,3
Mild cognitive impairment is a recently introduced term that includes those patients who perform substantially worse than their peers on neuropsychological tests, yet are functionally intact and capable of living independently. Most patients with MCI harbor the pathologic changes of AD. The incidence of AD in the cohort with MCI is as high as 10% to 15% per year.6
Global brain atrophy is highly predictive of imminent progression of MCI to AD.7 Using voxel-based morphometry, several research groups have reported atrophy of the temporal,8,9 posterior cingulate,8,10,11 and precuneal cortices8,10,12 in AD compared with cognitively normal control subjects. In a recent voxel-based morphometry study, significantly greater atrophy was found in the parietal, anterior, and posterior cingulate structures in mild to severe AD compared with MCI.13 In the present study, we used 3-dimensional (3-D) computational gray matter mapping and surface-based cortical modeling techniques to compare amnestic MCI and mild AD. This approach has been successfully used in several neurodegenerative, developmental, and psychiatric disorders, as well as in normal brain development.14
METHODS
PATIENTS
We analyzed the imaging data for 24 patients with amnestic MCI and 25 patients with mild AD (Mini-Mental State Examination [MMSE] score > 18) from the UCLA (University of California at Los Angeles) Alzheimer Disease Research Center database. All subjects provided informed consent according to the Declaration of Helsinki and the restrictions and policies of the UCLA institutional review board. Demographic and cognitive data are given in Table 1 and Table 2. The diagnostic workup consisted of physician interview, general and neurologic examinations, and detailed neuropsychological evaluation.15 Diagnostic decisions were reached by consensus among neurologists, psychiatrists, and neuropsychologists and were based on the NINCDS/ADRDA (National Institute of Neurological and Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association) criteria for AD16 and the Petersen criteria for MCI.6 Additional inclusion criteria were age 55 to 90 years, no evidence of concurrent general medical condition of sufficient severity to affect cognition, no history of drug or alcohol abuse, no concurrent psychiatric or other neurologic illness, and an MMSE score higher than 18 in the group with mild AD.
Table 1.
Demographic Characteristicsa
| Variable | AD | MCI | Statistical Test and Score | P Value |
|---|---|---|---|---|
| Age, y | 73.1 (9.5) | 74.6 (7.1) | t Test, 0.54 | .54 |
| Sex, M/F | 12/13 | 14/10 | χ2 Test, 0.53 | .47 |
| Educational achievement, y | 14.4 (2.6) | 16.3 (2.8) | t Test, −2.34 | .02 |
| Race/ethnicity (W/AA/A) | 22/1/2 | 20/2/2 | χ2 Test, 0.41 | .82 |
| MMSE score | 23.7 (2.9) | 28.1 (1.7) | t Test, 6.48 | <.001 |
Abbreviations: A, Asian; AA, African American; AD, Alzheimer disease; F, female; M, male; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; W, white.
Values are given as mean (SD) unless otherwise indicated.
Table 2.
Selected Neuropsychological Variablesa
| Variable | No. of Patients in Each Group, AD/MCI | Mean (SD) Raw Score
|
P Value | Mean (SD) z Score
|
P Value | ||
|---|---|---|---|---|---|---|---|
| AD | MCI | AD | MCI | ||||
| FSIQ | 17/24 | 89.4 (13.1) | 106.6 (12.8) | <.001 | NA | NA | NA |
| VIQ | 17/24 | 94.7 (18.1) | 106.5 (10.4) | .01 | NA | NA | NA |
| PIQ | 17/24 | 86.7 (10.4) | 105.1 (15.0) | <.001 | NA | NA | NA |
| BNT | 17/24 | 40.1 (14.0) | 51.6 (6.6) | .001 | −3.6 (3.5) | −0.6 (1.4) | <.001 |
| Animal fluency | 18/24 | 9.6 (4.1) | 14.7 (4.1) | <.001 | −1.8 (1.0) | −0.7 (1.0) | <.001 |
| FAS test | 18/24 | 29.8 (14.1) | 35.8 (12.7) | .16 | −0.8 (1.2) | −0.3 (1.0) | .22 |
| WAIS LM II del rec | 17/22 | 3.2 (3.5) | 15.2 (7.2) | <.001 | −1.9 (0.8) | −0.2 (0.9) | <.001 |
| CVLT del rec | 15/24 | 1.6 (1.9) | 5.5 (3.6) | <.001 | −2.5 (0.8) | −1.1 (1.1) | <.001 |
| WAIS BD | 13/20 | 14.2 (10.7) | 26.4 (14.4) | .01 | −0.9 (0.9) | 0.1 (1.2) | .01 |
| ReyO Copy | 17/24 | 21.5 (8.8) | 30.3 (4.4) | <.001 | −2.4 (2.2) | −0.4 (0.7) | <.001 |
| ReyO DR | 17/24 | 4.5 (5.3) | 9.4 (6.0) | .01 | −1.8 (1.5) | −0.7 (1.4) | .01 |
| Trails A | 16/24 | 66.4 (32.7) | 46.5 (15.1) | .01 | −1.7 (2.3) | −0.3 (1.1) | .01 |
| Trails B | 9/23 | 231.3 (127.6) | 124.8 (73.5) | .006 | −10.8 (15.6) | −2.0 (2.6) | .01 |
| Stroop Color-Word Test | 8/22 | 255.8 (99.2) | 162.1 (46.6) | .001 | −4.1 (3.9) | −0.18 (1.0) | <.001 |
Abbreviations: AD, Alzheimer disease; BNT, Boston Naming Test; CVLT del rec, California Verbal Learning Test, delayed recognition; FSIQ, full-scale intelligence quotient; MCI, mild cognitive impairment; NA, data not available; PIQ, Performance Intelligence Quotient; ReyO Copy and ReyO DR, Rey-Osterreith Complex Figure Copy and Delayed Recall tests, respectively; Trails A and B, Trail Making Test, Part A and Part B, respectively; VIQ, Verbal Intelligence Quotient; WAIS BD, Wechsler Adult Intelligence Scale, Block Design; WAIS LM-II, Wechsler Adult Intelligence Scale, Logical Memory II Recall.
Statistical comparisons were made using independent-sample t tests.
MAGNETIC RESONANCE IMAGING DATA ACQUISITION AND ANALYSIS
Magnetic resonance images were obtained with a 1.5-T Signa scanner (GE Medical Systems, Milwaukee, Wisconsin) with the following protocol: spoiled gradient echo; gapless coronal acquisition; repetition time, 28 milliseconds; echo time, 6 milliseconds; field of view, 220 mm; acquisition matrix, 256×192 voxels; and section thickness, 1.5 mm. We used a 9-parameter linear transformation17 to spatially normalize and scale the individual magnetic resonance images to the International Consortium for Brain Mapping 53 (ICBM53) average brain imaging template and a regularized tricubic B-spline approach for image intensity nonuniformity correction.18 The scalp and other extracerebral tissues were automatically removed. All volumes were visually inspected and mislabeled brain and non-brain tissues were manually corrected. After automated 3-D hemispheric reconstruction, 38 sulci per hemisphere were traced following a detailed and extensively validated protocol.19 Individual sulcal maps were averaged to create a common average sulcal map for all of the subjects in the study. The individual cortical surfaces were parameterized, flattened, and warped so that all individual sulci were aligned with the respective average sulcal representation. This step ensures explicit matching of homologous gyri, insofar as possible, before averaging of data on gray matter distribution across subjects. Image voxels were classified using a partial volume classifier18 that considers the fact that more than 1 tissue type may be found in a given voxel and assigns each voxel to the most representative tissue class (ie, gray matter, white matter, cerebrospinal fluid, and a background class). Gray matter volumes were extracted18 and mapped onto the corresponding parametric hemispheric model in exact spatial correspondence (Figure 1).
Figure 1.
Schema of the cortical pattern matching method. ICBM indicates International Consortium for Brain Mapping.
As in many previous studies from our group and others, a commonly used measure of regional gray matter volume, known as gray matter density, was defined as the proportion of tissue segmenting as gray matter in a small spherical region (10-mm radius) around each point on each subject’s cortical surface model. Ashburner and Friston20 have used and described similar measures. To ensure that our findings were not biased by global brain scaling to ICBM space, we reverted the individual parametric hemispheric models back to native space (ie, scanner space), then computed a native space segmented gray matter volume, thereby creating a second set of native space individual cortical density maps. Average 3-D gray matter density maps in native and ICBM space were created for each group. Correlation maps of the linkage between structural differences and clinical diagnosis and their statistical significance were created both in native space maps (Figure 2) and ICBM space maps. The gray matter differences were quantitatively examined (Figure 3). The maps were corrected for multiple comparisons with permutation analysis, which assessed the fraction of the cortical surface area with statistics exceeding a given fixed threshold (P=.001), and compared with the null distribution constructed empirically by randomly assigning subjects to groups.
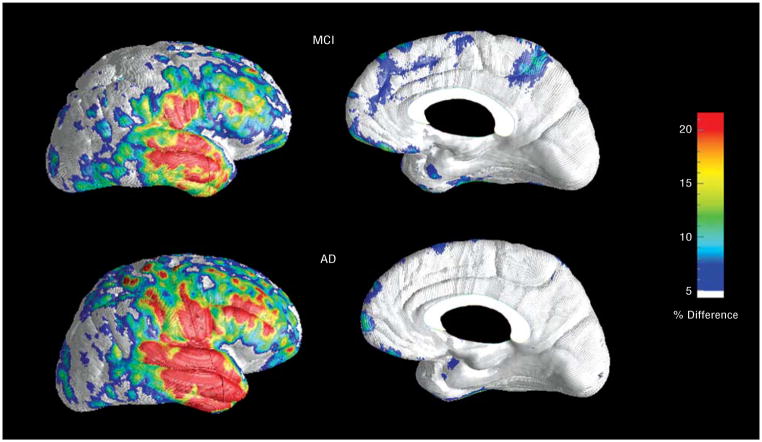
Figure 2.
Statistical (top) and correlation maps (bottom) of the cortical gray matter differences between patients with amnestic mild cognitive impairment (MCI) and mild Alzheimer disease (AD). The significance maps were created using 3-dimensional surface-based linear regression analysis and show regions in which gray matter atrophy is significantly associated with diagnosis of mild AD (as opposed to MCI). The correlation maps show the regional strength of the association between gray matter atrophy and clinical diagnosis, with positive correlations showing an association with a diagnosis of AD.
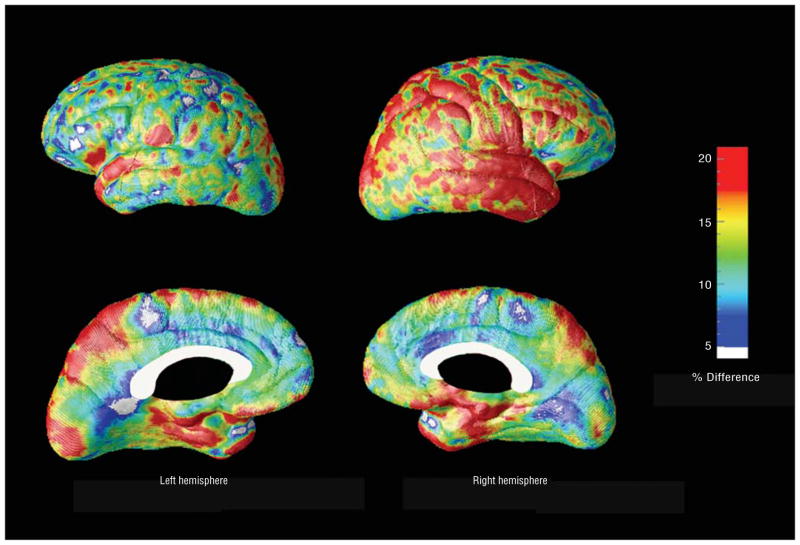
Figure 3.
Ratio maps show the areas of the cortex with greater gray matter atrophy (percentage) in Alzheimer disease compared with amnestic mild cognitive impairment.
RESULTS
The differences between the groups with amnestic MCI and mild AD on the native and ICBM space hemispheric maps were highly significant after controlling for multiple comparisons at a stringent voxel level threshold (permutation threshold, P=.001; left hemisphere map, P<.001; right hemisphere map, P<.001). The ICBM space maps were similar to the native space maps and, therefore, are not further reported here. Regionally pronounced and highly significant (P<.0001) gray matter atrophy in mild AD vs amnestic MCI was seen throughout the cortex in both brain hemispheres (Figure 2, top). The strongest correlations between clinical diagnosis of AD and gray matter loss were found bilaterally in the entorhinal, parahippocampal, fusiform, precuneus, posterior cingulate, lateral temporal, and medial orbitofrontal cortices (r>0.5), the right temporo-occipital and parieto-occipital areas (r>0.5), followed by the left parietal, bilateral medial and lateral frontal, bilateral medial occipital, and left lateral occipital association cortices (r=0.3–0.5; Figure 2, bottom). The least significant correlations were seen in the primary sensory and motor cortices (r<0.3).
A quantitative analysis of the gray matter differences between the 2 groups is shown in Figure 3. The bilateral entorhinal, the right more than left lateral temporal, right parietal cortex, and bilateral precuneus showed more than 15% greater atrophy and the remainder of the cortex showed mostly 10% to 15% greater atrophy in patients with mild AD compared with patients with amnestic MCI.
The 3-D statistical maps showed greater effect sizes in the right hemisphere. However, variability across subjects for the left and right hemispheres was comparable in both groups. Next we compared the left and right group-average gray matter density maps in each group. There was less cortical gray matter in the right hemisphere than the left hemisphere in both groups. The greatest differences were found in the lateral temporal and inferior frontal areas (Figure 4).
Figure 4.
Left-right asymmetry maps in mild cognitive impairment (MCI) and Alzheimer disease (AD). The right hemisphere shows more gray matter atrophy in both groups. The effect is most pronounced in the lateral temporal and inferior frontal cortices.
COMMENT
In the present study, we used a state-of-the-art method for 3-D analyses of gray matter atrophy that has proved to be both sensitive and reliable.21 Imaging techniques that rely on computational anatomy are increasingly used to study neurodegenerative disorders because they allow for quantification of changes in regional gray matter volume and for detection of subtle anatomical disturbances.
Using an advanced computational anatomy technique, we compared findings in patients who had amnestic MCI with patients who had mild AD. Despite the relatively small cognitive differences between our 2 groups (mean MMSE score difference, 4.4), we found strikingly different levels of gray matter atrophy. The most pronounced differences between the 2 groups were seen in the mesial and inferior temporal, posterior cingulate, temporal, and parietal association cortices, and the least differences in the primary sensory and motor cortices. These data agree with the well-documented progression of AD pathology in the brain where the amyloid and neurofibrillary tangle burden are most pronounced in the temporal area, followed by the parietal and, finally, the frontal areas, with relative sparing of the sensorimotor and primary visual cortices.2,3
Pathologic features of AD do not spread symmetrically through the brain.22,23 Using the same technique, our group analyzed the progression of cortical atrophy in patients with moderate AD (baseline mean±SD MMSE score, 17.7±1.9). We observed more severe gray matter atrophy in the left hemisphere both at baseline and at follow-up.24 In the present study, we observed more severe atrophy of the right hemisphere both in the amnestic MCI and the mild AD groups. Although these results may seem to conflict, we can offer 2 plausible explanations. First, these 2 studies included patients with different stages of AD and the observed discrepancy may represent a stage-specific lateralization of the disease process. The second hypothesis is based on the well-documented variability of AD features. Decades of research on brain-behavior relationships have established the left hemisphere as the primary site for language faculties in right-handed persons. Thus, we might postulate that patients with AD with predominantly left-sided pathologic features would have a more profound language impairment and, consequently, poorer performance on neuropsychological tests and the MMSE compared with patients with AD with predominantly right-sided pathologic features. The present study included functionally impaired patients with AD who had high MMSE scores, potentially overrepresenting right-predominant AD pathologic features. Conversely, the study by Thompson et al24 may have included patients with left-predominant AD pathologic features, who score relatively low on the MMSE but who were not profoundly impaired so that they would not be able to tolerate the follow-up magnetic resonance imaging. In addition, our group recently demonstrated that in young persons with normal cognition, the left lateral temporal neocortex was 15% thicker than the right lateral temporal neocortex.25
To our knowledge, this is only the second comparative magnetic resonance imaging study of amnestic MCI and mild AD. Using the voxel-based morphometry technique, Chételat et al26 found significantly greater atrophy in the left precuneus, left parietal lobe, left superior and middle temporal gyri, and the right middle temporal gyrus in patients with mild AD vs amnestic MCI. Two other studies compared the gray matter atrophy pattern between patients with MCI and AD with mild to severe disease (MMSE score, 4–28)13 and mild to moderate disease (mean±SD MMSE score, 19.8±4.1).27 In Chételat et al,26 the reported differences in the lateral parietal, posterior cingulate, posterior temporal, and occipital cortices between mild AD and MCI did not survive stringent multiple comparison corrections. Bozzali et al27 reported subtle differences between patients with MCI that progressed to AD and patients with mild to moderate AD located in the superior frontal, precuneus, and inferior temporal cortices (P<.001, uncorrected for multiple comparisons) and extensive differences between patients with MCI who remained cognitively stable and patients with mild to moderate AD located the superior, middle, inferior frontal, middle and inferior temporal, and anterior and posterior cingulate (P<.001, uncorrected for multiple comparisons) but not the precuneus and the lateral parietal cortices. By removing confounding anatomical variance, our approach of matching cortical surfaces and sulcal patterns24 increases the statistical power to detect atrophy and provides better anatomical localization than conventional voxel-based morphometry approaches do. With this approach, we were able to detect widespread differences in cortical atrophy between patients with amnestic MCI and mild AD. Of the 2 voxel-based morphometry studies comparing patients with mild AD and age-matched cognitively normal control subjects, one showed substantial bilateral involvement of the posterior cingulate and precuneus and the left inferior temporal lobe in AD11 and the other showed bilateral middle temporal and frontal cortical, and right inferior temporal cortical and precuneal atrophy.12 Comparative analyses of gray matter integrity in patients with mild to moderate AD compared with elderly persons with normal cognition have yielded reports of extensive cortical atrophy in AD but with a somewhat variable spatial distribution among studies. Areas of involvement include the mesial temporal lobe structures,8 posterior cingulate and precuneus,8,12,28 and the temporoparietal,8,28 lateral temporal,10,12,28–30 inferior parietal,28,29 and fusiform cortices.10,29
Several strengths and limitations of our study should be recognized. We conducted a cross-sectional analysis that shows strikingly greater atrophy in mild AD vs amnestic MCI, conforming to the pattern of spread of AD pathologic changes throughout the brain observed at postmortem analysis. However, a longitudinal design24 would provide stronger evidence that the observed differences reflect pathologic spread of the disease. In both AD and MCI, the clinical findings and focal atrophy pattern can show substantial variability. Because regional variability in gray matter density would lead to reduction in effect sizes, precise alignment of cortical structures, as we did in the present study, is invaluable. Mild cognitive impairment can be caused by disorders other than AD. To minimize this variability, we included only patients with the amnestic subtype of MCI and applied stringent diagnostic criteria for both disorders. In addition, we created group gray matter variability maps and confirmed that both groups had comparable gray matter variability. By limiting our study to the predementia amnestic MCI stage and the milder stages of AD, we may have introduced a selection bias for enrollment of patients with predominantly right-sided pathologic features. However, our 2 patient groups had similar lateralization of gray matter distribution, which does not support the idea that ascertainment bias could account for the observed group differences. In the present study, interindividual variability was also carefully controlled because our technique ensures precise alignment of cortical anatomy, thus fostering our ability to detect disease-induced as opposed to spurious associations due to mis-registration of anatomy, for example. To understand any potential effects of spatial normalization and scaling and ensure preservation of gray matter voxel counts and volume, we inverted the spatial transformation and created the native space maps, which agreed with those obtained after stereotaxic scaling.
CONCLUSION
Mild cognitive impairment is a relatively recent concept. Almost all patients with amnestic MCI develop AD, and this progression occurs at an average rate of 10% to 15% per year. The diagnosis of MCI currently relies on arbitrarily selected cognitive cutoff points in the absence of functional decline. To better understand the MCI state, further exploration is warranted to examine the cognitive and etiologic heterogeneity of MCI and its relation to mild AD.
All approved therapeutic agents for AD have primarily symptomatic effects and have not been conclusively shown to halt or slow pathologic progression of the disease. With several promising disease-modifying candidate compounds under development, being able to discern subtle structural cortical changes between mild AD and the immediately preceding cognitive state of amnestic MCI with anatomical precision raises hopes for our ability to show structural disease-modifying effects.
Acknowledgments
Funding/Support: This study was supported by grant K23 AG026803 from the National Institute on Aging (jointly sponsored by the National Institute on Aging, the American Foundation for Aging Research, the John A. Hartford Foundation, the Atlantic Philanthropies, the Starr Foundation, and an anonymous donor [Dr Apostolova]); the Kassel Parkinson’s Disease Foundation (Dr Apostolova); and by grants P50 AG16570 from the National Institute on Aging (Drs Apostolova, Cummings, and Thompson), EB01651 from the National Institute of Biomedical Imaging and BioEngineering, LM05639 from the National Library of Medicine, RR019771 from the National Center for Research Resources (Dr Thompson), R01 MH071940 from the National Institute on Mental Health, P41 RR013642 from the National Center for Research Resources, and U54 RR021813 from the National Institutes of Health (Drs Toga and Thompson).
Footnotes
Financial Disclosure: None reported.
AuthorContributions: Study concept and design: Apostolova, Cummings, and Thompson. Analysis and interpretation of data: Apostolova, Steiner, Akopyan, Dutton, Hayashi, Toga, and Thompson. Drafting of the manuscript: Apostolova. Critical revision of the manuscript for important intellectual content: Apostolova, Steiner, Akopyan, Dutton, Hayashi, Toga, Cummings, and Thompson. Statistical analysis: Apostolova and Thompson. Obtained funding: Apostolova, Toga, Cummings, and Thompson. Administrative, technical, and material support: Apostolova, Toga, Cummings, and Thompson. Study supervision: Apostolova, Toga, Cummings, and Thompson.
Additional Contributions: George Bartzokis, MD; Donna Masterman, MD; Michael Mega, MD, PhD; Susan McPerson, PsyD; Michele Carter, RN; Danny Huang, and the UCLA dementia fellows assisted with data and acquisition and storage.
References
- 1.Jicha GA, Parisi JE, Dickson DW, et al. Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch Neurol. 2006;63(5):674–681. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- 2.Duyckaerts C, Dickson DW. Neuropathology of Alzheimer’s disease. In: Dickson DW, editor. Neurodegeneration: The Molecular Pathology of Dementia and Movement Disorders. Basel, Switzerland: ISN Neuropath Press; 2003. pp. 47–65. [Google Scholar]
- 3.Mesulam MM. Aging, Alzheimer’s disease and dementia. In: Mesulam MM, editor. Principles of Behavioral and Cognitive Neurology. 2. Oxford, England: Oxford University Press; 2000. pp. 439–510. [Google Scholar]
- 4.Braak H, Braak E. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18(4):351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 5.Braak H, Del Tredici K, Schultz C, Braak E. Vulnerability of select neuronal types to Alzheimer’s disease. Ann N Y Acad Sci. 2000;924:53–61. doi: 10.1111/j.1749-6632.2000.tb05560.x. [DOI] [PubMed] [Google Scholar]
- 6.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 7.Jack CR, Jr, Shiung MM, Gunter JL, et al. Comparison of different MRI brain atrophy rate measures with clinical disease progression in AD. Neurology. 2004;62(4):591–600. doi: 10.1212/01.wnl.0000110315.26026.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baron JC, Chételat G, Desgranges B, et al. In vivo mapping of gray matter loss with voxel-based morphometry in mild Alzheimer’s disease. Neuroimage. 2001;14(2):298–309. doi: 10.1006/nimg.2001.0848. [DOI] [PubMed] [Google Scholar]
- 9.Kidron D, Black SE, Stanchev P, et al. Quantitative MR volumetry in Alzheimer’s disease: topographic markers and the effects of sex and education. Neurology. 1997;49(6):1504–1512. doi: 10.1212/wnl.49.6.1504. [DOI] [PubMed] [Google Scholar]
- 10.Busatto GF, Garrido GE, Almeida OP, et al. A voxel-based morphometry study of temporal lobe gray matter reductions in Alzheimer’s disease. Neurobiol Aging. 2003;24(2):221–231. doi: 10.1016/s0197-4580(02)00084-2. [DOI] [PubMed] [Google Scholar]
- 11.Scahill RI, Schott JM, Stevens JM, Rossor MN, Fox NC. Mapping the evolution of regional atrophy in Alzheimer’s disease: unbiased analysis of fluid-registered serial MRI. Proc Natl Acad Sci U S A. 2002;99(7):4703–4707. doi: 10.1073/pnas.052587399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frisoni GB, Testa C, Zorzan A, et al. Detection of grey matter loss in mild Alzheimer’s disease with voxel based morphometry. J Neurol Neurosurg Psychiatry. 2002;73(6):657–664. doi: 10.1136/jnnp.73.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karas GB, Scheltens P, Rombouts SA, et al. Global and local gray matter loss in mild cognitive impairment and Alzheimer’s disease. Neuroimage. 2004;23(2):708–716. doi: 10.1016/j.neuroimage.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Apostolova LG, Thompson PM. Brain mapping: a tool to study neurodegeneration [published online ahead of print May 7, 2007] Neurotherapeutics. 2007;4(3):387–400. doi: 10.1016/j.nurt.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apostolova LG, Dinov ID, Dutton RA, et al. 3D comparison of hippocampal atrophy in mild cognitive impairment and Alzheimer’s disease [published online ahead of print October 3, 2006] Brain. 2006;129(pt 11):2867–2873. doi: 10.1093/brain/awl274. [DOI] [PubMed] [Google Scholar]
- 16.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 17.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18(2):192–205. [PubMed] [Google Scholar]
- 18.Shattuck DW, Sandor-Leahy SR, Schaper KA, Rottenberg DA, Leahy RM. Magnetic resonance image tissue classification using a partial volume model. Neuroimage. 2001;13(5):856–876. doi: 10.1006/nimg.2000.0730. [DOI] [PubMed] [Google Scholar]
- 19.Sowell ER, Mega MS, Zoumalan CI, Lindshield C, Rex DE. Laboratory of Neuro Imaging. UCLA; [Accessed July 16, 2007]. Gyral pattern delineation in 3D: surface curve protocol. http://www.loni.ucla.edu/~esowell/new_sulcvar.html. [Google Scholar]
- 20.Ashburner J, Friston KJ. Voxel-based morphometry: the methods. Neuroimage. 2000;11(6 pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 21.Thompson PM, Hayashi KM, Sowell ER, et al. Mapping cortical change in Alzheimer’s disease, brain development, and schizophrenia. Neuroimage. 2004;23(suppl 1):S2–S18. doi: 10.1016/j.neuroimage.2004.07.071. [DOI] [PubMed] [Google Scholar]
- 22.Janota I, Mountjoy CQ. Asymmetry of pathology in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1988;51(7):1011–1012. doi: 10.1136/jnnp.51.7.1011-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moossy J, Zubenko GS, Martinez AJ, Rao GR, Kopp U, Hanin I. Lateralization of brain morphologic and cholinergic abnormalities in Alzheimer’s disease. Arch Neurol. 1989;46(6):639–642. doi: 10.1001/archneur.1989.00520420059023. [DOI] [PubMed] [Google Scholar]
- 24.Thompson PM, Hayashi KM, de Zubicaray G, et al. Dynamics of gray matter loss in Alzheimer’s disease. J Neurosci. 2003;23(3):994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luders E, Narr KL, Thompson PM, Rex DE, Jancke L, Toga AW. Hemispheric asymmetries in cortical thickness [published online ahead of print November 2, 2005] Cereb Cortex. 2006;16(8):1232–1238. doi: 10.1093/cercor/bhj064. [DOI] [PubMed] [Google Scholar]
- 26.Chételat G, Desgranges B, De La Sayette V, Viader F, Eustache F, Baron JC. Mapping gray matter loss with voxel-based morphometry in mild cognitive impairment. Neuroreport. 2002;13(15):1939–1943. doi: 10.1097/00001756-200210280-00022. [DOI] [PubMed] [Google Scholar]
- 27.Bozzali M, Filippi M, Magnani G, et al. The contribution of voxel-based morphometry in staging patients with mild cognitive impairment. Neurology. 2006;67 (3):453–460. doi: 10.1212/01.wnl.0000228243.56665.c2. [DOI] [PubMed] [Google Scholar]
- 28.Testa C, Laakso MP, Sabattoli F, et al. A comparison between the accuracy of voxel-based morphometry and hippocampal volumetry in Alzheimer’s disease. J Magn Reson Imaging. 2004;19(3):274–282. doi: 10.1002/jmri.20001. [DOI] [PubMed] [Google Scholar]
- 29.Rombouts SA, Barkhof F, Witter MP, Scheltens P. Unbiased whole-brain analysis of gray matter loss in Alzheimer’s disease. Neurosci Lett. 2000;285(3):231–233. doi: 10.1016/s0304-3940(00)01067-3. [DOI] [PubMed] [Google Scholar]
- 30.Good CD, Scahill RI, Fox NC, et al. Automatic differentiation of anatomical patterns in the human brain: validation with studies of degenerative dementias. Neuroimage. 2002;17(1):29–46. doi: 10.1006/nimg.2002.1202. [DOI] [PubMed] [Google Scholar]