Abstract
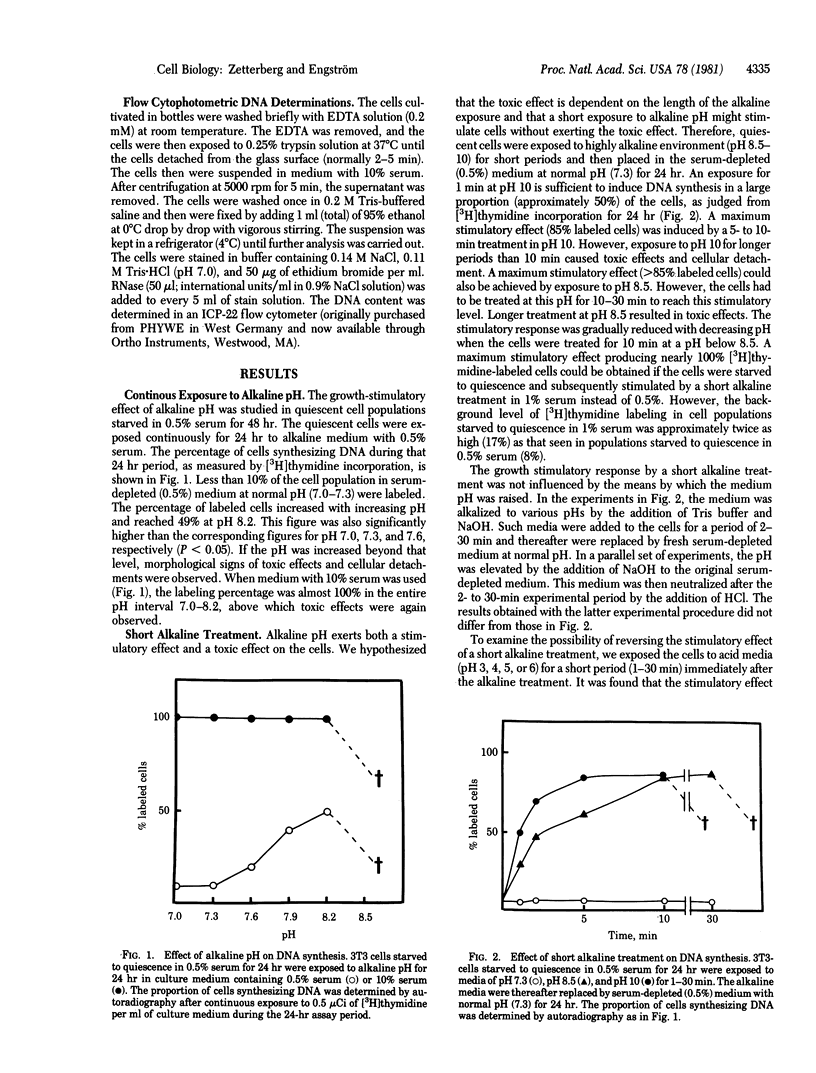
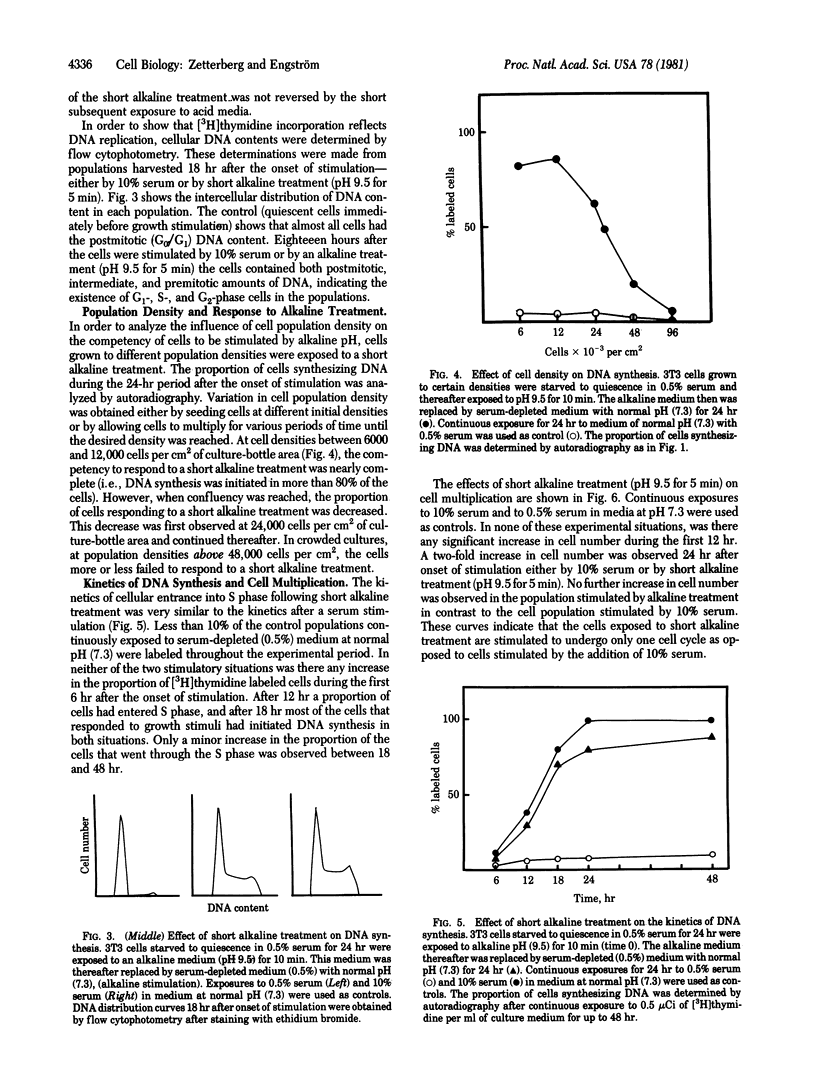
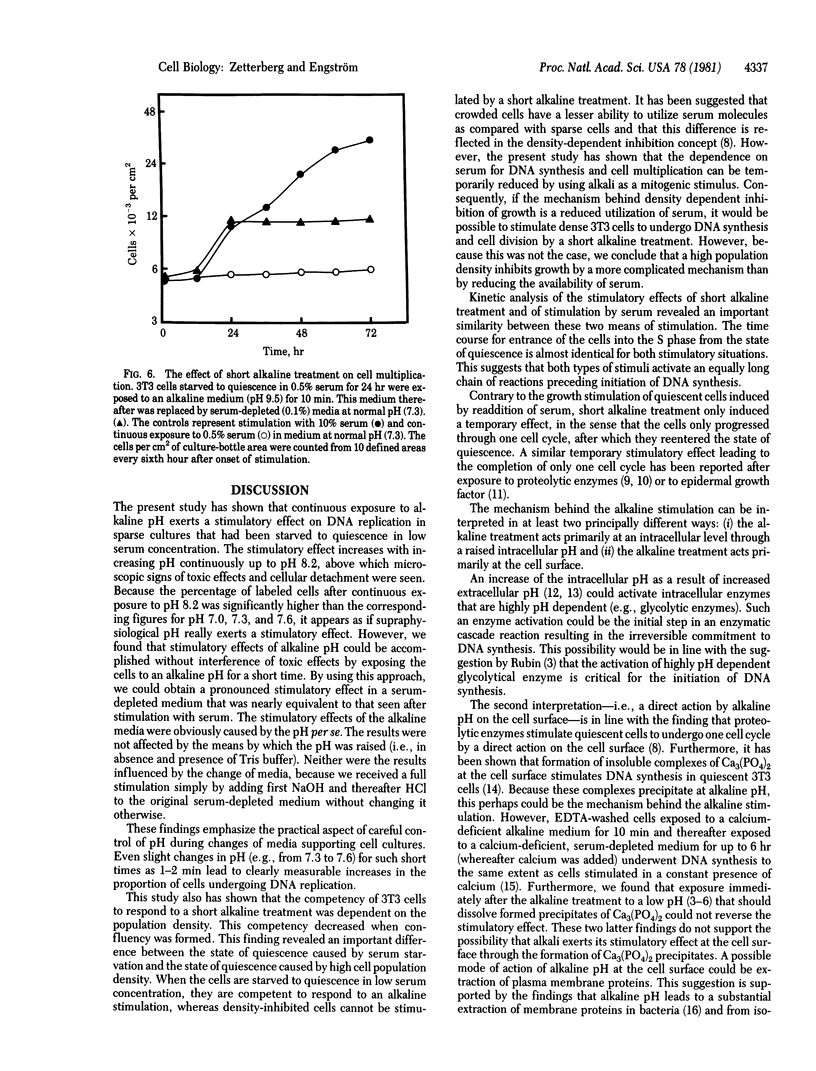
The effect of environmental pH on the proliferative activity of sparse 3T3 cell cultures was investigated. When quiescent (serum-starved) cells were transferred to a serum-depleted medium in which pH had been elevated, cell proliferation was stimulated during the first 24 hr after the medium change, as reflected by an increase in cell number and in the proportion of cells synthesizing DNA (measured by autoradiography and flow cytophotometry). The growth-stimulatory effect was greater with increasing pH. At pH 8.2 the effect was approximately 50% of that seen in serum-stimulated cells (i.e., upon the addition of 10% serum). Above pH 8.2 toxic effects and cell death were observed. However, the stimulatory effect could be performed without interference of toxic effects by exposing the cells to the high alkaline environment for a short period. A maximum stimulatory effect on the quiescent cells--without any observed toxic effects--was seen after exposure to an alkaline pulse with a duration of 2-10 min at a pH between 8.5 and 10. More than 80% of the cells synthesized DNA during the first 24 hr after the alkaline-pulse stimulation, which is an almost similar response to that seen after stimulation with serum. The growth-stimulatory effect of the alkaline-pulse treatment could not be prevented by a subsequent treatment at acid pH. Kinetic analysis revealed that DNA synthesis was not initiated until 9-12 hr after the alkaline-pulse treatment. This lag period was of the same length as that seen after stimulation with serum. Alkaline-pulse treatment only resulted in stimulation of DNA synthesis and mitosis during one cell cycle. When the alkaline treatment was repeated, only a small proportion of cells could proceed through a second cell cycle.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burger M. M. Proteolytic enzymes initiating cell division and escape from contact inhibition of growth. Nature. 1970 Jul 11;227(5254):170–171. doi: 10.1038/227170a0. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceccarini C., Eagle H. Induction and reversal of contact inhibition of growth by pH modification. Nat New Biol. 1971 Oct 27;233(43):271–273. doi: 10.1038/newbio233271a0. [DOI] [PubMed] [Google Scholar]
- Ceccarini C., Eagle H. pH as a determinant of cellular growth and contact inhibition. Proc Natl Acad Sci U S A. 1971 Jan;68(1):229–233. doi: 10.1073/pnas.68.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle H. The effect of environmental pH on the growth of normal and malignant cells. J Cell Physiol. 1973 Aug;82(1):1–8. doi: 10.1002/jcp.1040820102. [DOI] [PubMed] [Google Scholar]
- Engström W. Calcium requirements for mitogenic stimulation of 3T3-cells in low and high serum concentration. Cell Biol Int Rep. 1981 May;5(5):509–516. doi: 10.1016/0309-1651(81)90178-8. [DOI] [PubMed] [Google Scholar]
- Gillies R. J., Deamer D. W. Intracellular pH changes during the cell cycle in Tetrahymena. J Cell Physiol. 1979 Jul;100(1):23–31. doi: 10.1002/jcp.1041000103. [DOI] [PubMed] [Google Scholar]
- Glaumann H. Studies on the synthesis and transport of albumin in microsomal subfractions from rat liver. Biochim Biophys Acta. 1970 Nov 12;224(1):206–218. doi: 10.1016/0005-2787(70)90634-9. [DOI] [PubMed] [Google Scholar]
- Hutter O. F., Warner A. E. The pH sensitivity of the chloride conductance of frog skeletal muscle. J Physiol. 1967 Apr;189(3):403–425. doi: 10.1113/jphysiol.1967.sp008176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natraj C. V., Datta P. Control of DNA synthesis in growing BALB/c 3T3 mouse cells by a fibroblast growth regulatory factor. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6115–6119. doi: 10.1073/pnas.75.12.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H., Sanui H. Complexes of inorganic pyrophosphate, orthophosphate, and calcium as stimulants of 3T3 cell multiplication. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5026–5030. doi: 10.1073/pnas.74.11.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. pH and population density in the regulation of animal cell multiplication. J Cell Biol. 1971 Dec;51(3):686–702. doi: 10.1083/jcb.51.3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin H. pH, serum and Zn++ in the regulation of DNA synthesis in cultures of chick embryo cells. J Cell Physiol. 1973 Oct;82(2):231–238. doi: 10.1002/jcp.1040820211. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Rubin H. Release from density dependent growth inhibition by proteolytic enzymes. Nature. 1970 Aug 22;227(5260):843–845. doi: 10.1038/227843a0. [DOI] [PubMed] [Google Scholar]
- Stoker M. G. Role of diffusion boundary layer in contact inhibition of growth. Nature. 1973 Nov 23;246(5430):200–203. doi: 10.1038/246200a0. [DOI] [PubMed] [Google Scholar]
- Tasaki I., Singer I., Takenaka T. Effects of internal and external ionic environment on excitability of squid giant axon. A macromolecular approach. J Gen Physiol. 1965 Jul;48(6):1095–1123. doi: 10.1085/jgp.48.6.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt M., Dulbecco R. VIRUS-CELL INTERACTION WITH A TUMOR-PRODUCING VIRUS. Proc Natl Acad Sci U S A. 1960 Mar;46(3):365–370. doi: 10.1073/pnas.46.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg A., Killander D. Quantitative cytophotometric and autoradiographic studies on the rate of protein synthesis during interphase in mouse fibroblasts in vitro. Exp Cell Res. 1965 Oct;40(1):1–11. doi: 10.1016/0014-4827(65)90284-3. [DOI] [PubMed] [Google Scholar]



