Abstract
Structural deficits in the frontotemporal network have been shown in individuals with psychopathy and are posited to contribute to neuropsychological impairments such as response perseveration. However, no study to date has examined structural correlates of response perseveration in individuals with psychopathy. In this structural MRI study, the authors found higher correlations between increased response perseveration and reduced cortical thickness in the orbitofrontal and anterior temporal regions in individuals with psychopathy than in healthy-comparison subjects. The findings provide preliminary evidence suggesting potential contributions of frontotemporal structural deficits in neurocognitive impairment with perseveration in individuals with psychopathy.
Psychopathy, as defined in Hare’s Psychopathy Checklist Revised (PCL–R), is a clinical condition composed of emotional deficits, social dysfunction, deviant lifestyle, and antisocial behavior.1 The majority of individuals with psychopathy meet the criteria for antisocial personality disorder.2 One remarkable behavioral profile of individuals with psychopathy is the selective impairment in executive function, namely response perseveration,3,4 a behavioral pattern involving the inappropriate repetition of a particular response despite the absence or cessation of reward. It was theorized that this core feature of psychopathy may originate from attentional deficits to inhibitory cues that are extraneous to the dominant response set.5,6 In an experimental setting, perseveration may be measured using neuropsychological tasks such as the Wisconsin Card-Sorting Test (WCST),7 and studies using the WCST in psychopathic populations have reported increased perseverative response to be correlated with increased features of psychopathy.8,9
Neuroimaging studies have revealed several prefrontal and temporal regions critical for WCST performance,10,11 overlapping with regions found to be impaired in individuals with psychopathy.12–14 If neural mechanisms leading to structural abnormalities in the frontal and temporal structures are one of the main neurobiological causes of psychopathy, structural deficits should contribute to response perseveration in individuals with psychopathy. Thus, it is hypothesized that there will be exaggerated negative correlations between cortical thickness of the dorsolateral prefrontal cortex (PFC), orbitofrontal cortex (OFC), and the anterior temporal cortex and WCST perseverative error scores in individuals with psychopathy relative to healthy-comparison subjects. By examining structural MRI data from a community sample, we can test this hypothesis and provide preliminary evidence for a neurological basis for response perseveration in individuals with psychopathy that may potentially have forensic implications for psychopathy-related disorders.
METHOD
Eighty-six participants were recruited from five temporary-employment agencies in Los Angeles.12,15 Psychopathy was assessed using the PCL-R1 and supplemented by five sources of collateral data15 to evaluate 20 distinct psychopathic characteristics (i.e., glibness/superficial charm, grandiose sense of self-worth, pathological lying, conning/manipulation, lack of remorse/guilt, shallow affect, callousness/lack of empathy, failure to accept responsibility for own actions, need for stimulation/proneness to boredom, parasitic lifestyle, poor behavioral control, promiscuous sexual behavior, lack of realistic long-term goals, impulsivity, irresponsibility, juvenile delinquency, early behavior problems, revocation of conditional release, many short-term marital relationships, criminal versatility). The collateral data included 1) the Interpersonal Measure of Psychopathy; 2) self-reported crime and violence assessed using an adult extension of the National Youth Survey self-report delinquency measure; 3) criminal history transcripts obtained from the Department of Justice; 4) data derived from, and behavioral observations made during, the Structured Clinical Interview for Axis I DSM-IV Disorders and Axis II Personality Disorders; and 5) independent IM-P ratings made by two different laboratory assistants during separate phases of testing.15 Individuals diagnosed with a DSM-IV disorder (e.g., schizophrenia) other than antisocial personality disorder were excluded. A cutoff of 23 (high) and 15 (low) on the total PCL-R score was used to define psychopathy, resulting in a total of 27 individuals with psychopathy (PCL-R range: 23–40) and 32 comparison subjects (PCL-R range: 5–14). Additional demographic, diagnostic, and cognitive characteristics were assessed, including past/current substance dependence using DSM–IV and full-scale IQ (WAIS–Revised; WAIS–R).15 The two groups did not differ in age, gender, ethnicity, handedness, or substance dependence.12 This cutoff was chosen to be consistent with our previous research on this sample,9,12,15 and is similar to the optimal cutoff suggested by taxometric analyses of the PCL-R.16 Full informed, written consent was obtained from all subjects in accordance with Institutional Review Board procedures at University of Southern California.12
For each participant, structural MRI data were collected on a 1.5T Philips S15/ACS scanner (repetition time/echo time: 34 msec/12.4 msec; voxel size: 0.9×0.9×1.7 mm) and processed using a previously detailed cortical pattern-matching analysis.12 Response perseveration was assessed using a computerized version of the WCST,7 and standardized scores were computed for perseverative and nonperseverative errors.9 We conducted a partial correlation analysis, examining the group difference in the performance scores of the WCST, and findings revealed that increased total PCL–R score correlated significantly with increased WCST perseverative (r=0.2; p=0.027) but not nonperseverative error scores (r=0.12, p=0.12) across the entire sample, while controlling for age and full-scale IQ.
The General Linear Model, implemented with R (http://www.r-project.org/), was used to determine whether associations between cortical thickness and WCST perseverative error scores differ between individuals with psychopathy and healthy-comparison subjects. Significant interaction effects were followed up by examining correlations between the WCST perseverative error scores and cortical thickness within groups separately while controlling for inter-individual variation in whole brain volume. Uncorrected two-tailed probability (p) or correlation (r) values obtained from statistical tests conducted for each cortical surface point were color-coded and displayed on the averaged cortical surface representations of the entire group to allow initial visualization of group differences. We performed permutation analyses, using masks of frontal and temporal regions of interest generated from an atlas12 to control for multiple comparisons conducted on thousands of surface points, and corrected p values (two-tailed) were then reported. Given our previous hypothesis, findings outside of the prefrontal and temporal cortex were treated as exploratory.
RESULTS
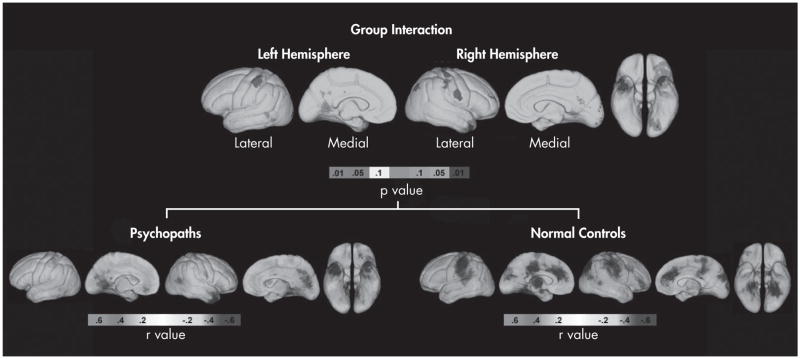
Significant regional group interactions between cortical thickness and WCST perseverative errors were observed in the orbitofrontal and temporal regions (Figure 1), especially in the left and right anterior temporal cortex (permutation-corrected p=0.042, p=0.034, respectively). Follow-up analyses of relationships within each group separately showed increased WCST perseverative errors to be significantly associated with decreased cortical gray-matter thickness in the left OFC and bilateral anterior temporal cortex in individuals with psychopathy (all permutation-corrected p<0.027), whereas healthy-comparison subjects show significant negative correlations in the bilateral ACC, right dorsolateral PFC and right OFC (all permutation-corrected p<0.008; Figure 1). No significant interaction effects were observed for WCST nonperseverative errors (p>0.31 in all cases).
FIGURE 1. Uncorrected Statistical Mapping Results.
The figure shows the results of the group interaction effect (top panel) and within-group correlations (bottom panels) between cortical thickness and WCST perseverative errors in individuals with psychopathy and healthy comparison subjects.
DISCUSSION
Findings provide the first neuroanatomical evidence showing abnormal structural correlates in response perseveration in individuals with psychopathy. Specifically, individuals with psychopathy showed significantly greater negative correlations between increased perseverative errors and decreased cortical thickness in the OFC and anterior temporal cortex relative to healthy-comparison subjects. These findings suggest that cortical thickness-reductions in frontal and temporal regions in individuals with psychopathy, as previously reported,12 may contribute to the neuropsychological disturbances of increased perseverative errors in the WCST. Lesion studies have consistently reported that damage to the OFC is linked to disinhibition and impulsivity;11 thus, structural deficits in the OFC in individuals with psychopathy may contribute to their inability to inhibit responses that were no longer being rewarded. Although highly speculative, findings of exaggerated correlations in the anterior temporal cortex, a region crucial for memory, may reflect the difficulty of individuals with psychopathy in retrieving crucial information from past failed trials so as to form new strategies in order to achieve the most beneficial outcome.
The lack of significant correlation between the dorsolateral PFC and perseveration in individuals with psychopathy, however, was surprising, as the structural deficits in this region have been reported in several independent samples of individuals with psychopathy.17 It is possible that, although reflecting a potentially disturbed neuroanatomical architecture, cortical thickness may not be as sensitive a measure of functional performance as gross regional volume. Furthermore, the assessment of substance dependence in the population was based largely on self-reported information. Substance dependence has been linked to reduced morphometry in the dorsolateral PFC18 and poor WCST performance.19 Thus, it remains a possibility that substance dependence may have contributed to the lack of findings for the dorsolateral PFC. Also, evidence to-date supporting a significant association between structure and function has come largely from studies examining regional gray-matter volumes,20 whereas evidence of such correlations for cortical thickness is lacking. Therefore, it is possible that increased perseverative errors in individuals with psychopathy would correlate with reduced gray-matter volume in the dorsolateral PFC, despite the lack of findings for cortical thickness in this study. Studies using volumetric and other morphological measures to examine functional correlates of perseveration in the WCST as well as other neuropsychological measures (e.g., go/nogo task, reversal learning task) are needed to clarify these issues. Although preliminary, these findings support a neurological basis to behavioral problems, specifically, response perseveration, in individuals with psychopathy that may render them more prone to impulsive criminal acts. If neurobiological factors beyond their control give rise to a relative inability to modify their behavior, the question arises as to whether individuals with psychopathy are fully responsible for their actions. This in turn has potential legal implications with respect to criminal responsibility, diminished capacity, and also mitigating factors in the punishment phases of trials involving individuals with psychopathy.
CONCLUSION
Although the precise neuropathological mechanism underlying perseveration in individuals with psychopathy awaits further investigation, findings of this study provide initial evidence suggesting that structural deficits in the frontotemporal regions may contribute to increased response perseveration in individuals with psychopathy.
Acknowledgments
This study was supported by a grant to the first author from the NIMH (1F31MH079592) and a grant to the second author from the National Institute of Child Health and Development (I RO1 HD42259). Research grants from the National Center for Research Resources, the NIH through the NIH Roadmap for Medical Research supported contributions of the UCLA co-authors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or the NIH.
Contributor Information
Dr. Yaling Yang, Laboratory of NeuroImaging, Department of Neurology, at the David Geffen School of Medicine at University of California, Los Angeles
Dr. Adrian Raine, Department of Criminology, Psychiatry, and Psychology at the University of Pennsylvania
Dr. Patrick Colletti, Department of Radiology at University of Southern California School of Medicine
Dr. Arthur W. Toga, Laboratory of NeuroImaging, Department of Neurology, at the David Geffen School of Medicine at University of California, Los Angeles
Dr. Katherine L. Narr, Laboratory of NeuroImaging, Department of Neurology, at the David Geffen School of Medicine at University of California, Los Angeles
References
- 1.Hare RD. Manual for the Revised Psychopathy Checklist. 2. Toronto, Ontario, Canada: Multi-Health Systems; 2003. [Google Scholar]
- 2.Edens JF, Petrila J. Legal and ethical issues in the assessment and treatment of psychopathy. In: Patrick CJ, editor. Handbook of Psychopathy. New York: Guilford; 2004. [Google Scholar]
- 3.Morgan AB, Lilienfeld SO. A meta-analytic review of the relation between antisocial behavior and neuropsychological measures of executive function. Clin Psychol Rev. 2000;20:113–156. doi: 10.1016/s0272-7358(98)00096-8. [DOI] [PubMed] [Google Scholar]
- 4.Newman JP, Patterson CM, Kosson DS. Response perseveration in psychopaths. J Abnorm Psychol. 1987;96:145–148. doi: 10.1037//0021-843x.96.2.145. [DOI] [PubMed] [Google Scholar]
- 5.Zeier JD, Maxwell JS, Newman JP. Attention moderates the processing of inhibitory information in primary psychopathy. J Abnorm Psychol. 2009;118:554–563. doi: 10.1037/a0016480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadeh N, Verona E. Psychopathic personality traits associated with abnormal selective attention and impaired cognitive control. Neuropsychology. 2008;22:669–680. doi: 10.1037/a0012692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heaton RK, Chelune GJ, Talley JL, et al. Wisconsin Card Sorting Test Manual: Revised and Expanded. Odessa, Fla: Psychological Assessment Resources; 1993. [Google Scholar]
- 8.Sellbom M, Verona E. Neuropsychological correlates of psychopathic traits in a non-incarcerated sample. J Res Personality. 2006;41:276–294. [Google Scholar]
- 9.Ishikawa SS, Raine A, Lencz T, et al. Autonomic stress reactivity and executive functions in successful and unsuccessful criminal psychopaths from the community. J Abnor Psychol. 2001;110:423–432. doi: 10.1037//0021-843x.110.3.423. [DOI] [PubMed] [Google Scholar]
- 10.Buchsbaum BR, Greer S, Chag WL, et al. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Raine A, Narr KL, et al. Abnormal temporal and prefrontal cortical gray matter thinning in psychopaths. Mol Psychiatry. 2009;14:561–562. doi: 10.1038/mp.2009.12. [DOI] [PubMed] [Google Scholar]
- 13.de Oliveirahyphen Souza R, Hare RD, Bramati IE, et al. Psychopathy as a disorder of the moral brain: fronto-temporo-limbic grey matter reductions demonstrated by voxel-based morphometry. Neuroimage. 2008;40:1202–1213. doi: 10.1016/j.neuroimage.2007.12.054. [DOI] [PubMed] [Google Scholar]
- 14.Müller JL, Gänssbauer S, Sommer M, et al. Gray matter changes in right superior temporal gyrus in criminal psychopaths: evidence from voxel-based morphometry. Psychiatry Res. 2008;163:213–222. doi: 10.1016/j.pscychresns.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Raine A, Lencz T, Bihrle S, et al. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Arch Gen Psychiatry. 2000;57:119–127. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- 16.Harris GT, Rice ME, Quinsey VL. Psychopathy as a taxon: evidence that psychopaths are a discrete class. J Consult Clin Psychol. 1994;62:387–397. doi: 10.1037//0022-006x.62.2.387. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Glenn AL, Raine A. Brain abnormalities in antisocial individuals: implications for the law. Behav Sci Law. 2008;26:65–83. doi: 10.1002/bsl.788. [DOI] [PubMed] [Google Scholar]
- 18.Taki Y, Kinomura S, Sato K, et al. Both global gray matter volume and regional gray matter volume negatively correlate with lifetime alcohol intake in non-alcohol-dependent Japanese men: a volumetric analysis and a voxel-based morphometry. Alcohol Clin Exp Res. 2006;30:1045–1050. doi: 10.1111/j.1530-0277.2006.00118.x. [DOI] [PubMed] [Google Scholar]
- 19.Dolan SL, Bechara A, Nathan PE. Executive dysfunction as a risk marker for substance abuse: the role of impulsive personality traits. Behav Sci Law. 2008;26:799–822. doi: 10.1002/bsl.845. [DOI] [PubMed] [Google Scholar]
- 20.Antonova E, Sharma T, Morris R, et al. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr Res. 2004:117–145. doi: 10.1016/j.schres.2003.12.002. [DOI] [PubMed] [Google Scholar]