Abstract
Background
Short-term exposure to air pollution may affect ventricular repolarization, but there is limited information on how long-term exposures might affect the surface ventricular electrocardiographic (ECG) abnormalities associated with cardiovascular events. We carried out a study to determine whether long-term air pollution exposure is associated with abnormalities of ventricular repolarization and conduction in adults without known cardiovascular disease.
Methods
A total of 4783 participants free of clinical cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis underwent 12-lead ECG examinations, cardiac-computed tomography and calcium scoring, as well as estimation of air pollution exposure using a finely resolved spatio-temporal model to determine long-term average individual exposure to fine particulate matter (PM2.5) and proximity to major roadways. We assessed ventricular electrical abnormalities including presence of QT prolongation (Rautaharju QTrr criteria) and intraventricular conduction delay (QRS duration > 120 msec). We used logistic regression to determine the adjusted relationship between air pollution exposures and ECG abnormalities.
Results
A 10 µg/m3-increase in estimated residential PM2.5 was associated with an increased odds of prevalent QT prolongation (adjusted odds ratio [OR]= 1.6 [95% confidence interval (CI)= 1.2 to 2.2]) and intraventricular conduction delay (OR 1.7, 95% CI: 1.0 to 2.6, independent of coronary-artery calcium score. Living near major roadways was not associated with ventricular electrical abnormalities. No significant evidence of effect modification by traditional risk factors or study site was observed.
Conclusions
This study demonstrates an association between long-term exposure to air pollution and ventricular repolarization and conduction abnormalities in adults without clinical cardiovascular disease, independent of subclinical coronary arterial calcification.
The 2010 American Heart Association consensus statement on air pollution and cardiovascular disease1 states that there is strong evidence that air pollution exposure contributes to a substantial public health burden of cardiovascular disease and death, although the specific mechanisms for these effects are poorly defined. One potential mechanism that has gained increasing support is an effect of air pollution on ventricular electrical activity, leading to cardiovascular morbidity and mortality. Ventricular electrical abnormalities – including QT interval prolongation, a measure of abnormal ventricular repolarization, and QRS widening, a measure of prolonged ventricular conduction – predict substantially increased risk of cardiovascular events in persons with and without clinical cardiovascular disease.2–4
Most studies of air pollution exposures and ventricular electrical activity have focused on short-term (hours to days) air pollution exposures,5–8 with relatively simple characterization of air pollution exposure at one monitoring site per study area. Despite this focus on short-term air pollution exposures and simple estimates of urban air pollution at single sites, epidemiologic cohorts suggest that long-term exposures that vary spatially within cities affect cardiovascular morbidity and mortality to a large extent,9 with risk estimates from such studies higher than those seen in large short-term time-series analyses.10 Although prior studies of air pollution-related effects have also focused primarily on repolarization using continuous measures of QT interval, measures of the presence of QT prolongation or QRS widening beyond specific thresholds that are associated with higher risk of cardiovascular events may represent changes more relevant to longer-term exposures than incremental increases in QT duration or QRS interval within the normal range.
Because abnormalities of ventricular repolarization and conduction are associated with atherosclerosis, and because there is a growing body of evidence that long-term air pollution exposures are linked to atherosclerosis,11 an association between air pollution and cardiac electrical abnormalities may be mediated by the development of underlying atherosclerotic disease. Understanding how long-term air pollution exposure relates to cardiac electrical abnormalities, and whether such relationships appear to be mediated by atherosclerotic disease, may provide insight into the underlying mechanisms responsible for the associations.
We hypothesized that long-term exposure to air pollution is associated with surface electrocardiogram (ECG) signs of abnormal ventricular electrical activity, independent of subclinical atherosclerosis. We aimed to test this hypothesis by examining long-term residential fine particulate matter exposure (less than 2.5 microns in mean aerodynamic diameter, PM2.5) measured at multiple locations and times in each study city, and its relation with prevalent ECG characteristics of QRS widening and QT prolongation. To determine whether any observed relationships might be mediated by subclinical atherosclerosis, we additionally aimed to evaluate the impact of adjusting for the presence of prevalent coronary artery calcium measured by cardiac computed tomography (CT). We also explored associations between such ECG findings and residential proximity to major roadways (a measure of exposure to traffic-related pollution). A large, multi-ethnic cohort (the Multi-Ethnic Study of Atherosclerosis, MESA) in six United States cities provided the foundation for testing these hypotheses. Participants were free of cardiovascular disease at entry, and with excellent measures of both exposure to particulate matter air pollution and subclinical atherosclerotic disease.
Methods
The MESA cohort was initiated to study the determinants of cardiovascular disease in a multi-ethnic group of adults without clinical cardiovascular disease at baseline. Details of its design have been previously published.12 Briefly, 6814 men and women aged 45 to 84 years who were free of clinically apparent cardiovascular disease were recruited between July 2000 and August 2002 from 6 US metropolitan areas: Baltimore MD; Chicago IL; Winston-Salem NC; Los Angeles CA; New York NY; and St. Paul MN. Of the participants, 6765 had ECG and cardiac CT scanning performed as described below. Of those with ECG information, 4970 (73%) consented to geocoding and had complete covariate information. 187 (4%) were excluded due to lead connection interchange or poor ECG quality, leaving 4783 participants for this analysis. Institutional review boards at all participating centers approved the study, and all participants gave informed consent.
Participants underwent an extensive baseline evaluation, including an initial standardized questionnaire. Total and high-density lipoprotein cholesterol and glucose levels were measured from blood samples obtained after a 12-hour fast. Low-density lipoprotein cholesterol was calculated with the Friedewald equation.13 Physical examination included height, weight and blood pressure. Resting blood pressure (BP) was taken three times in the seated position after a five-minute rest using a Dinamap model Pro 100 automated oscillometric sphygmomanometer (Critikon, Tampa, Florida), with the average of the last two measurements recorded and verified.
ECG interpretation
12-lead ECG recordings were obtained using a Marquette MAC-PC instrument (Marquette Electronics, Milwaukee, Wisconsin) and read using Minnesota Code14 and Novacode15 criteria. Readings were performed centrally at the Epidemiological Cardiology Research Center, Wake Forest University (Wake Forest, North Carolina). QT interval was corrected for heart rate (QTrr) using race-specific adjustment factors previously described.16 ECG abnormalities potentially representing chronic changes in ventricular electrical activity related to long-term air pollution exposure were defined by the following criteria: prolonged QT interval, classified by QTrr greater than race-specific cutoffs for 95th percentile16 and intraventricular conduction delay (prolonged QRS duration >120 msec, including left and right bundle branch block, Novacode criteria 3.1, 3.2, or 3.3).
Cardiac Computed Tomography and Calcium Scoring
Computed tomography scanning was performed on all participants either with ECG-triggered scan acquisition (50% of the RR interval) with a multidetector computed tomography system taking 4 simultaneous 2.5-mm slices per cardiac cycle in an axial or sequential scan mode (Baltimore, Forsyth County, and St. Paul field centers; Lightspeed, General Electric or Siemens, Volume Zoom) or with an ECG-triggered (80% of the RR interval) electron-beam computed tomography scanner (Chicago, Los Angeles, and New York field centers; Imatron C-150, Imatron). Each participant was scanned twice. Scans were read at a single center to identify and quantify coronary calcification. Measures of agreement for calcium score are high, with an intraclass correlation coefficient for Agatston score17 between readers of 0.99. Calcium scores between participants and among scanning centers were adjusted with a calcium phantom scanned with the participant. The average phantom-adjusted Agatston score18 was used in all analyses.
Air Pollution Exposures
Long-term exposure to ambient PM2.5 was estimated using a hierarchical spatio-temporal model fit with a pragmatic estimation procedure described elsewhere.19,20 To derive these predictions, we conducted an extensive exposure assessment program to characterize long-term average concentrations of ambient-generated PM2.5, using both two-week average concentrations of PM2.5 collected over the course of several years by regulatory monitoring stations from the U.S. Environmental Protection Agency’s Air Quality System, and multiple supplemental monitoring stations added to better characterize the exposures in MESA participants.21 Briefly, the hierarchical model decomposed the space-time field of concentrations into three pieces: (1) spatially varying long-term averages, (2) spatially varying seasonal and long-term trends, and (3) spatially correlated but temporally independent residuals. A multi-step procedure for model estimation and prediction allowed the incorporation of a large suite of spatial covariates into the model, including traffic sources and local land use, to predict average PM2.5 concentrations at each subject’s home location over the year preceding the participant’s exam date. Model selection and cross-validation were performed, with city-specific cross-validated root mean square errors for predicting long-term average concentrations at MESA Air monitoring locations between 0.34 and 0.94 µg/m3.
To estimate exposure to traffic-related air pollution (and for comparison with prior studies using this metric), we also measured residential proximity to roadways using a street database (Teleatlas, Inc), classifying participants as near major roadways if their home at the time of their ECG examination was within 100 meters of an Interstate or US highway, or within 50 meters of a state or county highway. This classification approach has been commonly used.22,23
To assign exposures, we geocoded participants using a commercially available geodatabase (Teleatlas, Inc) in conjunction with residential-address-history information during the year prior to examination. We validated participant and all monitor addresses using several methods, including GPS unit confirmation by technicians conducting the home monitoring campaign and satellite photography verification.21
Statistical Analysis
We used logistic regression to evaluate the associations between long-term exposure to PM2.5 or roadway proximity and individual prevalent ventricular electrical abnormalities (odds ratio [OR] and 95% confidence interval [CI] for each abnormality associated with a 10 µg/m3 elevation in PM2.5 over the year prior to examination or with residential proximity to major roadways).
We fitted iterative models with increasing numbers of covariates selected a priori . Model 1 included race, sex, age, body mass index (BMI), income, education, cigarette smoking, systolic and diastolic BP, hypertensive status by the Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure criteria, diabetes status by 2003 American Diabetes Association fasting blood glucose criteria (normal, impaired fasting glucose, treated diabetes, untreated diabetes), low-density and high-density lipoprotein (LDL and HDL) cholesterol, and alcohol use. Model 2 included covariates from Model 1 plus site of examination. Model 3 included covariates from Model 2, plus use of beta blockers, calcium channel blockers, alpha blockers, anti-arrhythmics, digoxin, erectile dysfunction drugs, anticholinergics, sympathomimetics, tricyclic antidepressants, and other medications that potentially affect QT interval.24 To investigate whether any observed associations may be mediated by subclinical atherosclerotic disease, we additionally fitted a model including coronary artery calcium (Model 4), modeled as an indicator of nonzero Agatston score.
To evaluate the presence of interactions with race, sex, site, and diabetes status – for which associations were seen in the full model – we explored models including product terms of these variables and pollution exposure. Subgroup effects in all interaction models were calculated using linear combinations of predictors in each model (“lincom” command in Stata).
In sensitivity analyses, we also explored linear models evaluating the relationship between the two air pollution exposure variables and continuous measures of ventricular depolarization and repolarization (QTrr and QRS duration). In addition, we adjusted for roadway proximity in PM models and vice-versa, adjusted for interactions between race and site, evaluated nonlinear forms of QTrr and QRS duration in generalized additive models examining the relationship between PM2.5 and these measures, and examined the effect of adjustment for second-hand smoke exposure and pack-years of smoking in the subset of participants (n = 4232 and n = 4732, respectively) with data on these variables. We also tested adjustment using various forms of Agatston score, including a log-transformed continuous measure and modeled as indicator variables for decile categories of the score.
Because of the importance of site as a potential confounder or effect modifier, in addition to the effect-modification and site-adjustment approaches described, we constructed individual fully adjusted regression models stratified by site, and then pooled the estimates using random effects meta-analysis. Heterogeneity of effect estimates was assessed with a chi2 test with 5 degrees of freedom.
All analyses were performed using R 2.10 (R Foundation for Statistical Computing, Vienna, Austria) and Stata 10 (StataCorp LP, College Station, Texas).
Results
Study participants
Comparing participants in this analysis with those who were excluded (who met exclusion criteria or did not consent to geocoding), participants were older, more likely to be women and to be white (and less likely to be African American), more likely to be from Chicago, New York, and St. Paul and less likely to be from Winston-Salem, Baltimore, and Los Angeles, with higher income and education, and lower levels of hypertension and diabetes. Participant characteristics by quartile of PM2.5 exposure and proximity to a major roadway are shown in Table 1. More than 40% of the population was classified as hypertensive (JNC VI criteria, based upon seated BP), and nearly one quarter had impaired fasting glucose or diabetes. We found that 48% had a non-zero Agatston score; 11% had prolonged QTrr and 4% had intraventricular conduction delay. PM2.5 exposures were highest in Los Angeles and lowest in St. Paul, while roadway proximity exposure was highest in New York and lowest in Winston-Salem (Figure 1).
Table 1.
Baseline characteristicsa of study participants, by quartile of PM2.5 exposure and residential proximity to major roadways.
| PM2.5 exposure (µg/m3) |
||||||
|---|---|---|---|---|---|---|
| 7.4 – 13.5 (n=1225) |
13.6 – 16.2 (n=1204) |
16.3 – 18.7 (n=1141) |
18.8 – 39.2 (n=1213) |
Near to roadway (n=1357) |
All (n=4783) |
|
| Age (years); mean (SD) | 61.9 (10.1) | 61.9 (10.0) | 60.7 (9.8) | 61.3 (9.9) | 61.8 (10.2) | 61.5 (10.0) |
| Men | 49 | 44 | 45 | 47 | 45 | 46 |
| Race | ||||||
| African American | 21 | 34 | 27 | 22 | 27 | 26 |
| White | 53 | 42 | 42 | 24 | 37 | 40 |
| Chinese | 4 | 7 | 10 | 28 | 11 | 12 |
| Hispanic | 22 | 17 | 21 | 27 | 25 | 22 |
| Study site | ||||||
| St. Paul, MN | 49 | 6 | 9 | 2 | 13 | 17 |
| Winston-Salem, NC | 25 | 11 | 12 | 9 | 8 | 14 |
| Baltimore, MD | 15 | 24 | 12 | 5 | 10 | 14 |
| New York, NY | 3 | 31 | 28 | 10 | 36 | 18 |
| Chicago, IL | 9 | 27 | 25 | 16 | 20 | 19 |
| Los Angeles, CA | 0 | 2 | 14 | 58 | 14 | 19 |
| BMI (kg/m2); mean (SD) | 29.2 (5.3) | 28.4 (5.4) | 28.3 (5.5) | 27.4 (5.4) | 28.4 (5.7) | 28.3 (5.4) |
| Hypertension | 42 | 45 | 43 | 41 | 44 | 42 |
| Diabetes | ||||||
| Normal | 76 | 77 | 77 | 73 | 75 | 76 |
| Impaired fasting blood glucose | 14 | 13 | 12 | 14 | 14 | 13 |
| Untreated diabetes | 2 | 2 | 2 | 2 | 2 | 2 |
| Treated diabetes | 8 | 8 | 8 | 11 | 10 | 9 |
| LDL (mg/dl); mean (SD) | 118.2 (30.3) | 117.1 (31.6) | 117.8 (31.2) | 115.7 (31.1) | 116.8 (30.9) | 117.2 (31.0) |
| Cigarette use | ||||||
| Never | 47 | 51 | 50 | 59 | 49 | 52 |
| Former | 41 | 37 | 38 | 31 | 38 | 37 |
| Current | 12 | 13 | 12 | 11 | 13 | 12 |
| Income | ||||||
| <$20,000 | 17 | 19 | 19 | 29 | 23 | 21 |
| $20,000-49,999 | 28 | 25 | 25 | 28 | 27 | 26 |
| >=$50,000 | 55 | 56 | 56 | 44 | 50 | 53 |
| Education | ||||||
| Not high school graduate | 11 | 12 | 16 | 22 | 17 | 15 |
| High school graduate or GED | 21 | 17 | 16 | 17 | 16 | 18 |
| Some college, associate degree, or technical certificate | 34 | 31 | 26 | 26 | 31 | 29 |
| Bachelor’s degree or higher | 35 | 40 | 42 | 35 | 37 | 38 |
| Agatston score > 0 | 52 | 46 | 46 | 48 | 45 | 48 |
| Electrocardiogram Abnormalities | ||||||
| Prolonged QTrr | 11 | 12 | 10 | 10 | 11 | 11 |
| Intraventricular conduction delay | 4 | 5 | 5 | 4 | 5 | 4 |
| Near to major roadway | 20 | 34 | 33 | 27 | -- | 28 |
Percent, unless otherwise indicated.
Figure 1.
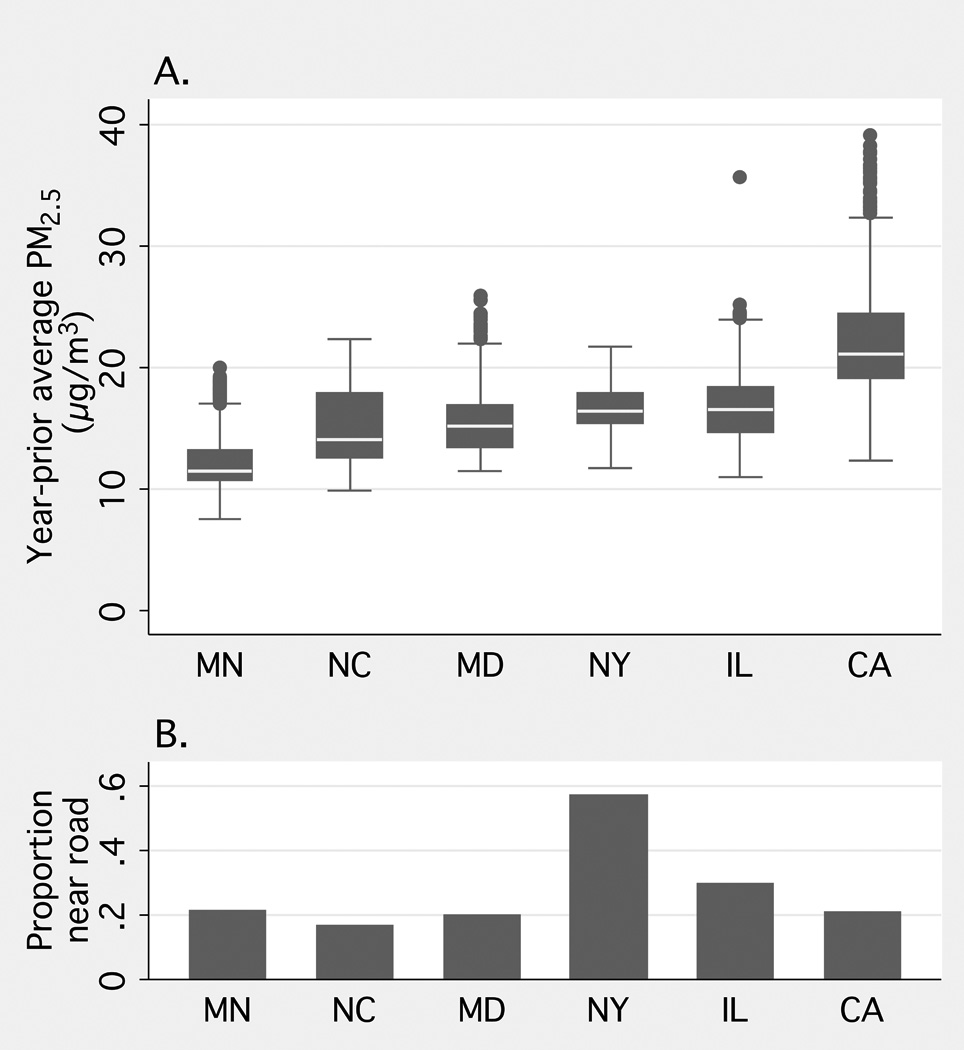
Participant estimates of A. year-prior average fine particulate matter (PM2.5) and B. major roadway proximity exposure, by study site. MN indicates St Paul, Minnesota; NC, Winston-Salem, North Carolina; MD, Baltimore, Maryland; NY, New York, New York; IL, Chicago, Illinois; and CA, Los Angeles, California .
Bivariate Analyses
Based on bivariate analyses, participants in the lowest quartile of PM2.5 exposures were older, with a higher proportion of men, former smokers, higher BMI, higher income, high school or some college educational attainment, and white race. Income and education generally were associated negatively with PM2.5, exposure, as expected. Participants in the lowest quartile also were less likely to be Chinese or African American. Participants who were Hispanic or African American resided nearer to major roadways than other racial groups, as did people with lower income. Persons with Agatston score greater than zero overall lived farther from major roadways and had lower estimated PM2.5 exposures. Adjusting for age, sex, race / ethnicity, and socioeconomic status, however, there was no relationship of Agatston score with either PM2.5 or proximity to major roadways. The unexpected inverse bivariate association between PM2.5 and calcium score appeared most likely related to confounding by age, given that older people tend to live in regions with higher PM2.5, as described, and that adjustment for age eliminated this association. Bivariate relationships between ECG abnormalities and covariates selected a priori as potential confounders were generally strong and in the expected direction (ventricular electrical abnormalities were positively associated with increased age, male sex, diabetes, smoking, LDL cholesterol, and BMI; data not shown). Nonzero Agatston score was associated with increased prevalence of intraventricular conduction delay and prolonged QT on bivariate analysis, although not after adjustment for age, sex, race / ethnicity, and socioeconomic status.
Adjusted Associations of Air Pollution with ECG Abnormality
Figure 2 and eTable 3 (http://links.lww.com) show the adjusted associations of ventricular electrical abnormalities with PM2.5 exposure and traffic. For PM2.5, the minimally adjusted model (Model 1) showed a modest association between PM2.5 and intraventricular conduction delay (OR= 1.3 [95% CI= 0.9 to 1.9]). Adjustment for site (Model 2) resulted in a relatively large change in effect estimates that was not altered by the addition of medication usage (Model 3). After adjustment for all potential confounders (Model 3), a 10-µg/m3 increase in PM2.5 was associated with increased odds of intraventricular conduction delay (1.7 [1.0 to 2.6]) and QT prolongation (1.6 [1.2 to 2.2]). Additionally including calcium score as a potential mediator of the association (Model 4) produced no change in estimates of association. There was no evidence of an association between roadway proximity and ventricular electrical abnormalities.
Figure 2.
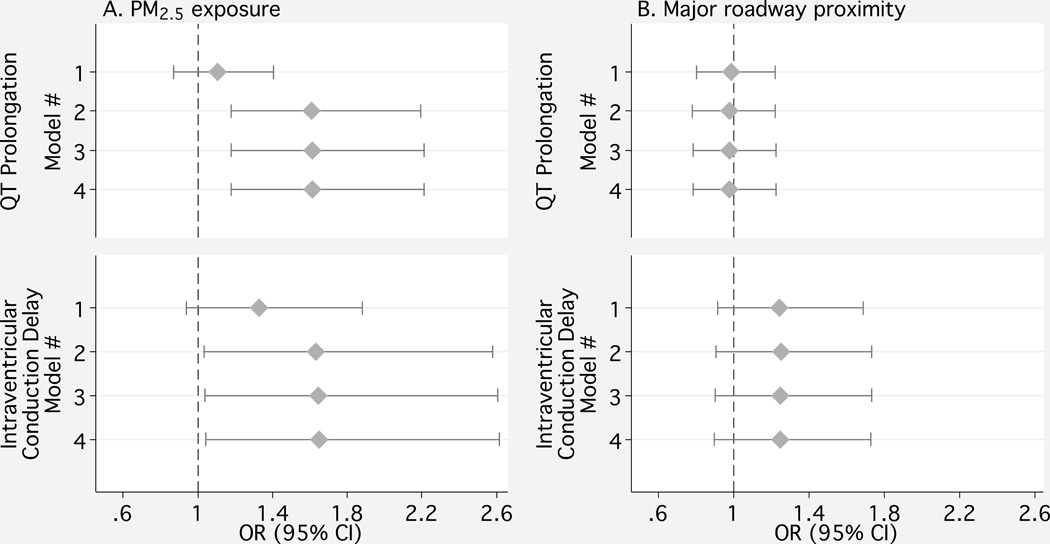
Associations between ventricular electrical abnormalities and A) fine particulate matter (PM2.5) exposure per 10 µg/m3 increment and B) residential major roadway proximity. Model 1 includes race, sex, age, and body mass index, income, education, cigarette smoking, systolic and diastolic blood pressure, hypertensive status by JNC VI criteria, diabetes status by 2003 American Diabetes Association fasting blood glucose criteria, LDL and HDL cholesterol, and alcohol use. Model 2 additionally includes study site. Model 3 additionally includes medications known to impact ventricular conduction, and Model 4 includes presence of nonzero Agatston calcium score.
Sensitivity analyses
In interaction analyses using the fully adjusted model (Model 3), we found no evidence of interactions of PM2.5 with sex, age, race, diabetes status, or study site (eFigure 1; http://links.lww.com), although power to detect such interactions was limited. In random-effects meta-analysis of site-specific fully adjusted regression models, we found no evidence of heterogeneity of effect by site for either outcome, with a pooled estimate of effect nearly identical to our primary, full-model results (eFigures 2 and 3; http://links.lww.com). Additionally adjusting for roadway proximity in PM2.5 models and for PM2.5 in roadway proximity models, adjusting for race-by-site interactions, and adjusting for second-hand smoking and pack-years history of smoking (in participants with these data) produced no change in estimates of association. Using different adjustment forms of Agatston score, including log(Agatston score + 1) and decile categories of calcium score, as indicator variables did not alter the results. Adjusting for QT prolongation in the ventricular conduction analyses modestly attenuated the QRS prolongation results (OR= 1.4 [CI= 0.9 to 2.2]). Adjusting for ventricular conduction delay in the repolarization analyses had little impact on associations between PM2.5 and QT prolongation (1.5 [1.1 to 2.1]).
Using linear terms for QT interval (QTrr) and QRS duration instead of dichotomous measures of QT prolongation and intraventricular conduction delay, in fully adjusted linear regression models, we found no relationship between PM2.5 and these linear measures across the entire range. After stratifying by quintiles of QTrr and QRS duration and then performing fully adjusted linear regression in each strata, there was weak evidence of a linear relationship between PM2.5 and the intervals, limited to the highest quintiles of QTrr and QRS duration (eFigure 4; http://links.lww.com). Using logistic generalized additive models with cubic penalized regression splines for long-term PM2.5 demonstrated a linear relationship between PM2.5 and dichotomous prolongation of QT and QRS (with 1 degree of freedom for the smooth PM2.5 term).
Discussion
Our data suggest that long-term PM2.5 exposure may affect ECG measures of ventricular electrical activity associated with cardiovascular morbidity and mortality (including QT prolongation and ventricular conduction delay) independent of underlying subclinical atherosclerotic disease.
Prior studies have demonstrated associations between short-term PM exposures and small changes in QT interval. In older men in Boston8 and adults with coronary artery disease in Germany,5 traffic-related particles in the 24 hours preceding ECG measurement were associated with short-term increases in QT interval.8 Short-term total PM2.5 levels (2–3 hours to 24 hours prior to ECG ascertainment) have also been variably associated with small changes in QT interval.6,7Ours is the first study to show a relationship between long-term exposure to air pollution and ventricular electrical activity changes beyond a normal threshold. Furthermore, we use a well-validated measure of atherosclerotic disease burden (Agatston score) to evaluate whether this relationship may be mediated by subclinical atherosclerosis.
Relevance of ventricular conduction abnormalities to clinical events, and mechanistic implications
In contrast to air pollution effects on QT interval across the normal range, ECG abnormalities such as QT prolongation and intraventricular conduction delay are more important. QT prolongation is associated with a 2–3 times higher risk of arrhythmia and cardiac death (and as much as 8 times higher risk in those less than 68 years old).3,25–28 Left- and right-bundle-branch-block, including QRS prolongation, are also strongly associated with cardiovascular morbidity and mortality.29–34 Although it has been hypothesized that such ECG abnormalities (and their concomitant risk of cardiovascular events) may result from underlying subclinical atherosclerotic disease,35 in this study population, ECG abnormalities have not been associated with subclinical atherosclerosis quantified by calcium score.36 The observation in this study that adjustment for coronary arterial calcification (a well-established marker of atherosclerosis37) did not alter effect estimates suggests that the association between pollutants and ventricular electrical abnormalities is not mediated by atherosclerotic disease. This finding supports the hypothesis that long-term exposure to particulate matter air pollution has an impact on cardiac electrical activity independent of an effect on atherosclerosis. Studies specifically designed to address such mechanistic questions are still needed.
Effect of site adjustment on primary results
With the important exception of study site, our primary findings were insensitive to adjustment for potential confounders. Including study site in PM2.5 models generally strengthened association estimates. Such strengthened estimates after accounting for study site most likely represent confounding by unmeasured characteristics related to site – similar to our prior analysis of the relationship between PM2.5 exposure and left ventricular mass in this study population.38 These observations, indicating smaller associations when using between- and within-city gradients in PM2.5 compared with using within-city gradients in PM2.5 alone, are also consistent with prior observations in the Women’s Health Initiative study.9 In that study, within-city differences in PM2.5 were more strongly associated with cardiovascular events than between-city differences.
Shape of the relationship between PM2.5 and ECG abnormalities
Despite the relationship between PM2.5 and the ECG abnormalities of QT prolongation and intraventricular conduction delay (wide QRS), there was no apparent linear relationship with continuous measures of QT interval or QRS duration. This suggests that the relationship between long-term air pollution exposures and these intervals is nonlinear. Our stratified analyses, investigating the associations between continuous measures of intervals and PM2.5 in quintile strata of interval duration, although exploratory in nature, suggest that persons with abnormal QT intervals and ventricular conduction in the highest quintile of complex duration show stronger and more positive associations with PM2.5 than do persons with normal duration complexes. It is possible that people with genetic or other risk factors for conduction or repolarization abnormalities are more susceptible to the effects of pollutants, producing this apparently stronger association at the upper range of these intervals. Such a possibility warrants further investigation, perhaps taking advantage of recent evidence for common genetic contributions to these abnormalities.39,40
Study strengths and limitations
The primary strength of this study is its very high quality long-term PM2.5 exposure assessment, which involved an intensive pollutant monitoring campaign in addition to traditional geographic predictors and advanced statistical modeling methods. In addition, the study had a relatively large and multi-ethnic population, free of cardiovascular disease, with well-characterized ECG examinations, and measurement of coronary-artery calcium on each participant. Limitations include the study’s cross-sectional design (which makes causal inference more challenging due to difficulty in ascertaining the time order of events) its focus on residential exposures only, and the analysis of single 12-lead ECGs at a single point in time. Although there were important differences between the full cohort and the sample included in this analysis, the results of our interaction analyses (showing no differences in effects comparing relevant subgroups) suggest that such differences would not be expected to lead to bias. Results from several epidemiologic studies suggest that people with low socioeconomic status (SES), diabetes and hypertension may have enhanced susceptibility to the effects of air pollutants,41–43 suggesting that our results (from a subpopulation with relatively high SES and low levels of diabetes and hypertension) may underestimate true associations between air pollution exposures and ventricular electrical abnormalities.
Another potential limitation of this study is the inability to determine acute (daily or hourly) spatially resolved association estimates for PM2.5. We cannot fully exclude the possibility of confounding between short- and long-term associations, although the relatively much larger magnitude of effect seen in this chronic exposure study compared with prior acute work discussed (which has been focused on small incremental changes in ECG intervals within the range of normal rather than prolongation of such intervals beyond a threshold) suggests that chronic exposure may be at least as important as acute exposure.
This study was designed to capture variation in within-city measures of chronic PM2.5, an important pollutant that is a major target of regulatory attention, rather than traffic-related pollutants near roadways. It is possible that the lack of association between roadway proximity and ECG abnormalities is related more to misclassification of traffic-related pollutant exposure than to the absence of a relationship between traffic-related pollutants and ventricular electrical abnormalities.
Supplementary Material
Acknowledgments
Financial Support:
National Heart, Lung, and Blood Institute contracts N01-HC-95159 through N01-HC-95165 and N01-HC-95169; U.S. Environmental Protection Agency STAR Grant RD831697; National Institute of Environmental Health Sciences grant K24ES013195 (J.D.K.); and National Institute of Environmental Health Sciences grant K23ES019575 (V.C.V.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SDC Supplemental digital content is available through direct URL citations in the HTML and PDF versions of this article (www.epidem.com).
References
- 1.Brook RD, Rajagopalan S, Pope CA, et al. Particulate Matter Air Pollution and Cardiovascular Disease. An Update to the Scientific Statement From the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 2.Desai AD, Yaw TS, Yamazaki T, Kaykha A, Chun S, Froelicher VF. Prognostic Significance of Quantitative QRS Duration. Am J Med. 2006;119:600–606. doi: 10.1016/j.amjmed.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 3.Dekker JM, Crow RS, Hannan PJ, Schouten EG, Folsom AR ARIC Study. Heart rate-corrected QT interval prolongation predicts risk of coronary heart disease in black and white middle-aged men and women: the ARIC study. J Am Coll Cardiol. 2004;43:565–571. doi: 10.1016/j.jacc.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 4.Schouten EG, Dekker JM, Meppelink P, Kok FJ, Vandenbroucke JP, Pool J. QT interval prolongation predicts cardiovascular mortality in an apparently healthy population. Circulation. 1991;84:1516–1523. doi: 10.1161/01.cir.84.4.1516. [DOI] [PubMed] [Google Scholar]
- 5.Henneberger A, Zareba W, Ibald-Mulli A, et al. Repolarization changes induced by air pollution in ischemic heart disease patients. Environ Health Perspect. 2005;113:440–446. doi: 10.1289/ehp.7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lux RL, Pope CA. Air pollution effects on ventricular repolarization. Res Rep Health Eff Inst. 2009:3–28. Research Report 141. [PubMed]
- 7.Liao D, Shaffer ML, Rodriguez-Colon S, et al. Acute Adverse Effects of Fine Particulate Air Pollution on Ventricular Repolarization. Environ Health Perspect. 2010;118:1010–1015. doi: 10.1289/ehp.0901648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baja ES, Schwartz JD, Wellenius GA, et al. Traffic-related air pollution and QT interval: modification by diabetes, obesity, and oxidative stress gene polymorphisms in the normative aging study. Environ Health Perspect. 2010;118:840–846. doi: 10.1289/ehp.0901396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller KA, Siscovick DS, Sheppard L, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 10.Dominici F, McDermott A, Daniels M, Zeger SL, Samet JM. Revised analyses of the National Morbidity, Mortality, and Air Pollution Study: mortality among residents of 90 cities. J Toxicol Environ Health A. 2005;68:1071–1092. doi: 10.1080/15287390590935932. [DOI] [PubMed] [Google Scholar]
- 11.Brook RD, Rajagopalan S. Particulate matter air pollution and atherosclerosis. Curr Atheroscler Rep. 2010;12:291–300. doi: 10.1007/s11883-010-0122-7. [DOI] [PubMed] [Google Scholar]
- 12.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 13.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 14.Prineas RJ, Crow RS, Zhang Z. Minnesota Code Manual of Electrocardiographic Findings. London: Springer Verlag; 2009. [Google Scholar]
- 15.Rautaharju PM, Park LP, Chaitman BR, Rautaharju F, Zhang ZM. The Novacode criteria for classification of ECG abnormalities and their clinically significant progression and regression. J Electrocardiol. 1998;31:157–187. [PubMed] [Google Scholar]
- 16.Rautaharju P, Rautaharju F. Investigative electrocardiography in epidemiological studies and clinical trials. London: Springer; 2007. [Google Scholar]
- 17.Carr JJ, Crouse JR, Goff DC, D'Agostino RB, Peterson NP, Burke GL. Evaluation of subsecond gated helical CT for quantification of coronary artery calcium and comparison with electron beam CT. AJR Am J Roentgenol. 2000;174:915–921. doi: 10.2214/ajr.174.4.1740915. [DOI] [PubMed] [Google Scholar]
- 18.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 19.Szpiro AA, Sampson PD, Sheppard L, Lumley T, Adar SD, Kaufman JD. Predicting intra-urban variation in air pollution concentrations with complex spatio-temporal dependencies. Environmetrics. 2009 doi: 10.1002/env.1014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sampson PD, Szpiro AA, Sheppard L, Lindström J, Kaufman JD. Pragmatic Estimation of a Spatio-Temporal Air Quality Model With Irregular Monitoring Data. UW Biostatistics Working Paper Series. 2009 Working Paper 353.
- 21.Cohen MA, Adar SD, Allen RW, et al. Approach to estimating participant pollutant exposures in the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) Environ Sci Technol. 2009;43:4687–4693. doi: 10.1021/es8030837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brauer M, Hoek G, van Vliet P, et al. Estimating long-term average particulate air pollution concentrations: application of traffic indicators and geographic information systems. Epidemiology. 2003;14:228–239. doi: 10.1097/01.EDE.0000041910.49046.9B. [DOI] [PubMed] [Google Scholar]
- 23.Allen RW, Criqui MH, Diez Roux AV, et al. Fine particulate matter air pollution, proximity to traffic, and aortic atherosclerosis. Epidemiology. 2009;20:254–264. doi: 10.1097/EDE.0b013e31819644cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woosley RL. Drugs That Prolong the QT Interval and/or Induce Torsades de Pointes. 2010 http://www.azcert.org/medical-pros/drug-lists/printable-drug-list.cfm.
- 25.Straus SM, Kors JA, De Bruin ML, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–367. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 26.Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83:1888–1894. doi: 10.1161/01.cir.83.6.1888. [DOI] [PubMed] [Google Scholar]
- 27.de Bruyne MC, Hoes AW, Kors JA, Hofman A, van Bemmel JH, Grobbee DE. Prolonged QT interval predicts cardiac and all-cause mortality in the elderly. The Rotterdam Study. Eur Heart J. 1999;20:278–284. doi: 10.1053/euhj.1998.1276. [DOI] [PubMed] [Google Scholar]
- 28.Dekker JM, Schouten EG, Klootwijk P, Pool J, Kromhout D. Association between QT interval and coronary heart disease in middle-aged and elderly men. The Zutphen Study. Circulation. 1994;90:779–785. doi: 10.1161/01.cir.90.2.779. [DOI] [PubMed] [Google Scholar]
- 29.Elhendy A, Hammill SC, Mahoney DW, Pellikka PA. Relation of QRS duration on the surface 12-lead electrocardiogram with mortality in patients with known or suspected coronary artery disease. Am J Cardiol. 2005;96:1082–1088. doi: 10.1016/j.amjcard.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 30.Kashani A, Barold SS. Significance of QRS complex duration in patients with heart failure. J Am Coll Cardiol. 2005;46:2183–2192. doi: 10.1016/j.jacc.2005.01.071. [DOI] [PubMed] [Google Scholar]
- 31.Newby KH, Pisanó E, Krucoff MW, Green C, Natale A. Incidence and clinical relevance of the occurrence of bundle-branch block in patients treated with thrombolytic therapy. Circulation. 1996;94:2424–2428. doi: 10.1161/01.cir.94.10.2424. [DOI] [PubMed] [Google Scholar]
- 32.Melgarejo-Moreno A, Galcerá-Tomás J, Garciá-Alberola A, et al. Incidence, clinical characteristics, and prognostic significance of right bundle-branch block in acute myocardial infarction: a study in the thrombolytic era. Circulation. 1997;96:1139–1144. doi: 10.1161/01.cir.96.4.1139. [DOI] [PubMed] [Google Scholar]
- 33.Lee SJ, McCulloch C, Mangat I, Foster E, De Marco T, Saxon LA. Isolated bundle branch block and left ventricular dysfunction. J Card Fail. 2003;9:87–92. doi: 10.1054/jcaf.2003.19. [DOI] [PubMed] [Google Scholar]
- 34.Hesse B, Diaz LA, Snader CE, Blackstone EH, Lauer MS. Complete bundle branch block as an independent predictor of all-cause mortality: report of 7,073 patients referred for nuclear exercise testing. Am J Med. 2001;110:253–259. doi: 10.1016/s0002-9343(00)00713-0. [DOI] [PubMed] [Google Scholar]
- 35.Kannel WB. Common electrocardiographic markers for subsequent clinical coronary events. Circulation. 1987;75:II25–II27. [PubMed] [Google Scholar]
- 36.Lloyd-Jones DM, Walsh JA, Prineas RJ, et al. Association of electrocardiographic abnormalities with coronary artery calcium and carotid artery intima-media thickness in individuals without clinical coronary heart disease (from the Multi-Ethnic Study of Atherosclerosis [MESA]) Am J Cardiol. 2009;104:1086–1091. doi: 10.1016/j.amjcard.2009.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenland P, Bonow RO, Brundage BH, et al. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA Writing Committee to Update the 2000 Expert Consensus Document on Electron Beam Computed Tomography) developed in collaboration with the Society of Atherosclerosis Imaging and Prevention and the Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2007;49:378–402. doi: 10.1016/j.jacc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Van Hee VC, Adar SD, Szpiro AA, et al. Exposure to traffic and left ventricular mass and function: the Multi-Ethnic Study of Atherosclerosis. Am J Respir Crit Care Med. 2009;179:827–834. doi: 10.1164/rccm.200808-1344OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holm H, Gudbjartsson DF, Arnar DO, et al. Several common variants modulate heart rate, PR interval and QRS duration. Nat Genet. 2010;42:117–122. doi: 10.1038/ng.511. [DOI] [PubMed] [Google Scholar]
- 40.Sotoodehnia N, Isaacs A, de Bakker PI, et al. Common variants in 22 loci are associated with QRS duration and cardiac ventricular conduction. Nat Genet. 2010;42:1068–1076. doi: 10.1038/ng.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forastiere F, Stafoggia M, Tasco C, et al. Socioeconomic status, particulate air pollution, and daily mortality: differential exposure or differential susceptibility. Am J Ind Med. 2007;50:208–216. doi: 10.1002/ajim.20368. [DOI] [PubMed] [Google Scholar]
- 42.Dubowsky SD, Suh H, Schwartz J, Coull BA, Gold DR. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect. 2006;114:992–998. doi: 10.1289/ehp.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Neill MS, Veves A, Zanobetti A, et al. Diabetes enhances vulnerability to particulate air pollution-associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111:2913–2920. doi: 10.1161/CIRCULATIONAHA.104.517110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


