Abstract
Cancer cells synthesize de novo large amounts of fatty acids and cholesterol, irrespective of the circulating lipid levels and benefit from this increased lipid synthesis in terms of growth advantage, self-survival and drug resistance. Key lipogenic alterations that commonly occur in prostate cancer include over-expression of the enzyme fatty acid synthase (FASN) and deregulation of the 5-AMP-activated protein kinase (AMPK). FASN is a key metabolic enzyme that catalyses the synthesis of palmitate from the condensation of malonyl-CoA and acetyl-CoA de novo and plays a central role in energy homeostasis, by converting excess carbon intake into fatty acids for storage. AMPK functions as a central metabolic switch that governs glucose and lipid metabolism. Recent interest has focused on the potential of targeting metabolic pathways that may be altered during prostate tumorigenesis and progression. Several small molecule inhibitors of FASN have now been described or in development for therapeutic use; in addition, drugs that directly or indirectly induce AMPK activation have potential benefit in prostate cancer prevention and treatment.
Keywords: prostate cancer, lipogenesis, fatty acid synthase, AMPK, inhibitors
Introduction
Prostate cancer (PCa) is the most commonly diagnosed malignancy in men and the second leading cause of cancer-related death in industrialized countries. The main risk factors for this disease are age [1], black race [2], saturated fat-enriched diet [3], and family history [4]. Gleason score and stage at the time of diagnosis remain the gold standards in helping to predict prognosis [5]. Although the advent of PSA screening has led to an increase in the number of early diagnoses per year and to a decrease in PCa mortality, some patients still experience disease progression after receiving primary treatment. In addition, an estimated 4% of patients present with metastatic disease at the time of diagnosis. Currently, the standard systemic treatment for metastatic PCa is based on androgen deprivation (oral anti-androgens in combination with LHRH agonists) while the disease is hormone-sensitive, and on docetaxel plus prednisone in the late hormone-refractory stage. This latter combination therapy only slightly improves survival (median survival 18 months) [6] compared to the previous standard treatment, and overall no alternative therapies are available for patients with hormone-refractory PCa.
With increasing knowledge in cancer genomics and proteomics, many clinical trials have been conducted to test molecular therapies in PCa on advanced metastatic disease or on the primary tumour (neoadjuvant and surveillance trials). Currently, intense research efforts have focused on the potential of targeting metabolic pathways that may be altered during prostate tumorigenesis and PCa progression, often in association with systemic metabolic diseases or specific diet behaviours. Several studies support a significant association between dietary fat, particularly saturated fats, and PCa risk [7]. Thus, increased body mass index (BMI) and an excess of energy intake are associated with the risk of developing PCa and with reduced overall survival [8]; however, the mechanism for this relationship is not fully understood. For the purposes of this review we focus on altered lipogenic pathways in PCa and discuss related targeted therapies.
Altered lipogenic pathways in PCa
An effective strategy in molecular cancer therapeutics is to selectively target distinguishing features of the cancer cell. Three main distinctive features characterize cancer metabolism. The first has been recognized since the 1920s, from Otto Warburg’s observation that cancer cells avidly consume glucose and produce lactic acid even in the presence of ample oxygen (the Warburg effect) [9]. This apparent paradox can be explained by the fact that glycolysis, although being less efficient for energy supply than aerobic respiration, produces ATP 100 times faster than mitochondrial oxidation. This can help sustain the high energy requirements for tumorigenesis through provision of intermediate metabolites for amino acid and pentose phosphate production (which are fundamental for sustaining protein and DNA synthesis), leading in turn to a selective growth advantage for highly proliferating cancer cells in the tumour microenvironment [10–12]. The second feature of cancer cells is the high rate of energy consumption driving increased protein synthesis [13] and more active DNA synthesis [14]. Finally, the third hallmark of cancer cells, functionally linked to the glycolytic pathway, is an increased de novo fatty acid (FA) synthesis [15]. Elevated glucose catabolism produces an excess of the glycolytic end-product pyruvate; most of the pyruvate is converted to lactate, whereas part of it is converted by the enzyme ATP citrate lyase (ACLY) to acetyl-CoA, which, in turn, is used in de novo fatty acid/cholesterol synthesis.
Normal human tissues preferentially use dietary (exogenous) lipid for the synthesis of new structural lipids, whereas de novo (endogenous) FA synthesis is usually suppressed, and the expression of lipogenic enzymes is maintained at low levels. By contrast, cancer cells synthesize de novo large amounts of FAs and cholesterol, irrespective of the circulating lipid levels, and benefit from this increased lipid synthesis in terms of growth advantage, self-survival and drug resistance. Numerous studies have shown that inactivation of most lipogenic enzymes, such as ACLY, fatty acid synthase (FASN), acetyl-CoA carboxylase (ACC), and 3-hydroxy-3-methylglutaryl CoA reductase (HMG-CoA reductase) results in either cell death or growth suppression in tumour cells [16,17]. Highly proliferating cancer cells need to synthesize FAs de novo to continually provide lipids for membrane production, the predominant ones being phospholipids; however, in most clinical PCas only a fraction of cancer cells are at any time point actively proliferating. Nearly all PCas express high levels of the enzyme FASN, suggesting that exacerbated lipogenesis affects multiple key aspects of tumour cell biology. Indeed, the newly synthesized phospholipids (enriched in saturated and in mono-unsaturated fatty acyl chains), together with cholesterol, tend to partition into detergent-resistant membrane microdomains called lipid rafts [18]. These lipid rafts serve as membrane platforms for signal transduction in various cellular pathways, including the phosphoinositide 3-kinase (PI3K) pathway [19]. Moreover, FASN may also be required for post-transcriptional regulation of key signal transduction proteins through post-translational modifications such as palmitoylation and myristoylation [20–23].
In PCa, an increase in aerobic glycolysis has been found only in advanced disease; de-novo FA and sterol synthesis (due to over-expression of key enzymes ACLY, ACC, FASN and HMG-CoA reductase) and increased protein synthesis [due to hyperactivation of mammalian target of rapamycin (mTOR)] are instead common features of both primary and advanced PCa [24–27]. These alterations are induced by androgens and by the PTEN–PI3K–Akt–mTOR pathway, which is one of the most deregulated pathways in human cancers, including PCa. Indeed, deletions/mutations in the tumour suppressor phosphatase and tensin homologue (PTEN ) are found in 30% of primary PCas and in 63% of metastatic PCas [28]. Hence, inhibitors of mTOR and PI3K, alone or in combination with chemotherapeutic agents, are being tested in hormone-refractory PCa [29]. Moreover, the observation of increased de novo FA and sterol synthesis prompted the development of inhibitors of these metabolic pathways.
Fatty acid synthase
FASN is a key metabolic enzyme that catalyses the synthesis of palmitate from the condensation of malonyl-CoA and acetyl-CoA de novo and plays a central role in energy homeostasis by converting excess carbon intake into fatty acids for storage (Figure 1) [30]. Its main enzymatic product, palmitic acid, is responsible for the acylation of key regulatory switches in most signal transduction pathways. FASN expression and its activity are tightly regulated by growth factors, hormones and diet. The enzyme is expressed at low levels in normal cells (except liver and adipose tissue) (reviewed in [31]), whereas it is highly expressed in many cancers, notably prostate, ovarian and breast cancer (reviewed in [15,31–36]) and in some benign and pre-invasive lesions of the prostate [15,37–40]. Elevated expression of FASN has been linked with poor prognosis and reduced disease-free survival in PCa [26,41–44]. In addition, several studies have demonstrated that FASN plays a central role in tumour cell development and survival, with inhibition of FASN resulting in cell death of PCa cells and a reduction in PCa tumour volume in xenograft mouse models, respectively [45–48]. Over-expression of FASN in immortalized prostate epithelial cells transforms them into invasive tumours in immunodeficient mice, while transgenic mice expressing FASN in the prostate develop prostatic intraepithelial neoplasia [30].
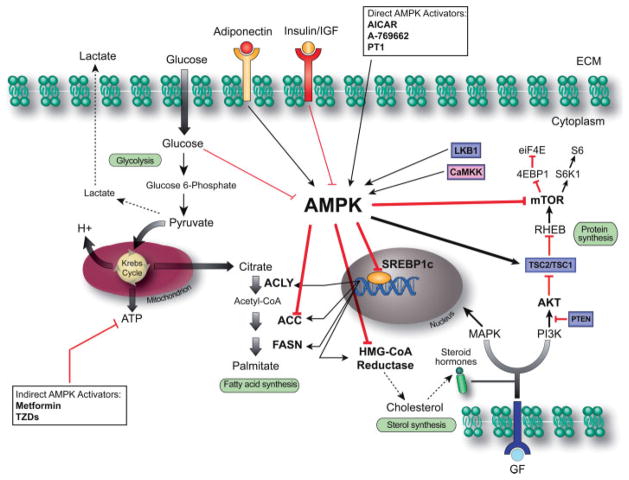
Figure 1.
AMPK controls the main metabolic pathways in PCa cells. PCa cells are characterized by exacerbation of lipogenesis associated with hyperactivation of the mTOR pathway. Physiological and pharmacological activation of AMPK by LKB1/CaMKK and AMPK activators, respectively, can inhibit these pathways by direct phosphorylation of key lipogenic enzymes (ACC, in particular isoform 1, HMG-CoA reductase) and key kinases (the complex TSC1/TSC2 and the mTOR-associated factor Raptor) or by regulating transcription through SREBP1c. Black and red arrows indicate activation and inhibition, respectively. Tumour suppressor genes are represented in violet boxes. AMPK, AMP-activated protein kinase; CaMKK, calmodulin-dependent protein kinase kinase; SREBP1c, sterol regulatory element binding protein-1c; ACLY, ATP citrate lyase; ACC, acetyl-CoA carboxylase; FASN, fatty acid synthase; HMG-CoA reductase, 3-hydroxy-3-methyl-glutaryl-CoA reductase; MAPK, mitogen-activated protein kinase; PI3K, phosphatidylinositol-3-kinase; PTEN, phosphatase and tensin homologue; TSC2/TSC1, tuberous sclerosis complex 1/2; RHEB, Ras homologue enriched in brain; mTOR, mammalian target of rapamycin; 4EBP1, 4E-binding protein 1; S6K1, S6 kinase 1; eiF4E, eukaryotic translation initiation factor 4E.
The proposed mechanisms of FASN oncogenicity include inhibition of the intrinsic/mitochondrial pathway of apoptosis; protection from endoplasmic reticulum stress, cytoplasmic stabilization of β-catenin through palmitoylation of Wnt-1 and the structural needs of synthesized lipids [30,49–50]. FASN regulation is complex and occurs at both transcriptional and post-transcriptional levels. This may involve regulation from steroid hormones/growth factors and from deubiquitinating enzymes (USP2a), respectively. Microenviornmental stresses such as hypoxia may also have a regulatory influence [51]. The ubiquitin-specific pro-tease 2a (USP2a) is a pre-proteosomal, androgen-regulated, deubiquitinating enzyme over-expressed in approximately 40% of PCas [52]. It has been shown to play a role in PCa cell survival through stabilization of FASN protein [46]. PCa cells undergo apoptosis following USP2a silencing, a process which is reversed by induced FASN over-expression, suggesting that potential therapeutic inhibition of FASN can be achieved either by directly targeting the metabolic enzyme, or indirectly through USP2a [53].
5′-AMP-activated protein kinase (AMPK)
AMPK is an energy-sensing serine/threonine kinase that is well conserved in all eukaryote species, indicating its fundamental role in cellular regulation. The enzyme is a heterotrimeric complex consisting of an α-catalytic subunit and regulatory β- and γ-subunits with multiple genes encoding each subunit. γ-Subunits (the binding sites for AMP) have different alternative splicing forms, resulting in different structures of the AMPK complex with different tissue distributions and cellular localizations [54]. At the cellular level, AMPK is activated under conditions of metabolic stress (such as glucose deprivation, hypoxia, exercise) that deplete intracellular ATP and increase AMP [54]. At the level of the organism, the enzyme activity is also under the control of hormones and cytokines, such as adiponectin and leptin [54]. The increase in AMP levels controls the enzyme activity by allosteric activation and renders AMPK less susceptible to dephosphorylation by protein phosphatases [54]. However, for the full activation of the enzyme, phosphorylation on Thr172 in the AMPK α-subunit is required. The two most well-documented upstream kinases are LKB1 and calcium/calmodulin-dependent protein kinase kinase-β (CaMKKβ). LKB1 is constitutively active and transduces signals generated by changes in cellular status [54], whereas CaMKKβ is switched on by increased Ca2+ levels and functions in the absence of changes in cellular AMP. Other upstream kinases have been identified, such as transforming growth factor β1-activated kinase 1; however, their physiological relevance remains still unclear [54].
Once activated, AMPK reduces plasma insulin levels, suppresses ATP-consuming metabolic functions (such as synthesis of FAs, sterols, glycogen and proteins) and increases ATP-producing activities (such as glucose uptake, FA oxidation and mitochondrial biogenesis) to restore energy homeostasis. Thus, AMPK functions as a central metabolic switch that governs glucose and lipid metabolism (see Figure 2). Links between AMPK and cancer can be made at both the level of the organism and the molecular level. Decreased AMPK activation is implicated in human metabolic disorders associated with increased cancer risk. Prominent examples include obesity and the metabolic syndrome (MS) [55], which have been shown to be associated with an increased PCa risk [56,57]. Moreover, a recent study revealed an association between polymorphisms in the PRKAA2 gene (encoding the α2 subunit of AMPK, which is responsible for the MS phenotype) [58] and susceptibility to insulin resistance and diabetes in the Japanese population [59]. Interestingly, the same locus correlates with PCa risk [60], suggesting that AMPK dysregulation may provide a mechanistic link between MS and PCa. Although the contribution of AMPK to the aetiology of this disorder is unclear, drugs that ameliorate MS conditions through AMPK activation (Table 1) may be beneficial for PCa prevention and treatment.
Figure 2.
The central role of FASN in PCa fatty acid metabolism. FASN catalyses the synthesis of palmitate from the condensation of malonyl-CoA and acetyl-CoA de novo and plays a central role in energy homeostasis by converting excess carbon intake into fatty acids for storage. Dysregulation of lipogenesis is associated with over-expression of FASN in PCa cells. ACoA, acetyl CoA; ACC, acetyl-CoA carboxylase; ATP, adenosine triphosphate; FASN, fatty acid synthase; MCoA, malonyl-CoA; SREBP1c, sterol regulatory element binding protein-1c.
Table 1.
Current lipogenesis inhibitors and AMPK activators
| Compound | Mechanism of action |
|---|---|
| HMG-CoA reductase inhibitors | |
| Statins | Decreased activation of Ras and Rho oncoproteins, cholesterol depletion of intracellular lipid rafts and anti-inflammatory effects |
| ACC inhibitors | |
| Soraphen A | Blockage of FA synthesis and stimulates FA oxidation with reduction in intracellular phospholipids and resulting apoptosis or autophagy |
| FASN inhibitors | |
| Orlistat | Formation of covalent adduct with the active serine of FASN TE domain leading to cell cycle effects, activation of apoptotic cascade |
| Cerulenin | Reaction of cerulenin’s epoxy group, with KS domain of FASN interfering with DNA replication, p53 accumulation and mitochondrial-mediated apoptosis |
| C75 | Interaction with TE, ER and KS domains of FASN, inducing caspase-mediated apoptosis |
| C93 | Structure not known; may be mediated through AKT signalling pathway or AMPK |
| C247 | Structure not known; mechanism not fully clarified |
| FAS31 | Structure not known; mechanism not fully clarified |
| GSK837149A | Interacts with KR domain of FASN |
| Astra Zeneca bisamide scaffold | n.d. |
| Platensimycin | n.d. |
| Merck 3-aryl-4-hydroxyquinolin-2(1H)-one scaffold | n.d. |
| AMPK activators | |
| Metformin | Indirect; increases AMP/ATP by inhibition of complex 1 of mitochondrial respiratory chain |
| TZDs | Indirect; increases PPARγ-mediated release of adiponectin, which consequently activates AMPK |
| Deguelin | Indirect; not fully clarified; decrease of ATP |
| Epigallocatechin-3-gallate | Indirect; activation of the AMPK activator CaMKK |
| Barberin | Indirect; increase of AMP/ATP by inhibition of mitochondrial function |
| α-Lipoic acid | Indirect; n.d. |
| Resveratrol | Indirect; not fully clarified; possible activation of SIRT1 and consequent deacetylation of the AMPK activator LKB1 |
| AICAR | Direct; AMP mimetic |
| A-769662 | Direct; allosteric binding of β1 AMPK subunit |
| PT1 | Direct; allosteric binding of α1 AMPK subunit |
FA, fatty acid; TE, thioesterase; ER, enoyl reductase; KS, β-ketoacyl synthase; KR, β-ketoacyl reductase; n.d., not determined; TZDs, thiazolidinediones; CAMKK, calmodulin-dependent protein kinase kinase; SIRT1, sirtuin 1; AICAR, 5-aminoimidazole-4-carboxamide-1-β-riboside.
At the molecular level, the upstream AMPK activator LKB1 is a well known tumour suppressor. Germ-line LKB1 mutations are associated with Peutz–Jegher syndrome [61], which predisposes carriers to benign hamartomas and a variety of malignant epithelial tumours [62]. Moreover, 83% of LKB1 knock-out mice develop prostate intraepithelial neoplasia (PIN) [63]. Thus, the LKB1–AMPK pathway may represent the potential link between cancer and energy homeostasis. Indeed, when activated, AMPK acts in a tumour suppressor-like fashion. It inhibits key lipogenic enzymes by direct phosphorylation (ACC, HMG-CoA reductase) or by transcriptional regulation (ACLY, FASN) through the suppression of the transcriptional factor sterol regulator element binding protein 1 (SREBP-1). In addition, AMPK inhibits the mTOR pathway through direct phosphorylation of tuberous sclerosis complex 2 protein tuberin and the mTOR-associated factor Raptor (Figure 2). Finally, it induces cell cycle arrest or apoptosis through phosphorylation of p53 and FOXO3a [64]. Thus, activated AMPK can switch off multiple oncogenic pathways at once. In particular, it may simultaneously inhibit the two major pathways (lipogenic and PI3K/mTOR pathways) that drive PCa carcinogenesis, antagonizing the activity of Akt at multiple levels (eg SREBP-1, TSC-2 and mTOR complex 1 level). Consequently, AMPK activators, overcoming the feedback activation loop of Akt following long-term mTORC1 inhibition (likely responsible for the clinical failure of mTORC1 inhibitor rapamycin) [65], might be effective in metastatic PCa harbouring PTEN deletions. Hence, targeting AMPK may represent a promising therapeutic option as discussed below.
Targeting lipogenic pathways for the treatment of PCa
Inhibitors of FASN
FASN is selectively over-expressed in many types of cancer and natural sense and pharmacological inhibition experiments have shown that multiple cancer cell lines depend on FASN for proliferation and survival. As such, several small molecule inhibitors of FASN have now been described. Table 1 lists the FASN inhibitors outlined in this review.
Orlistat, which was originally developed as an anti-obesity drug, is a potent irreversible inhibitor of FASN with modest anti-cancer activity; it has many pharmacological limitations, however, including poor oral bioavailability and metabolic stability [66], low solubility, low cell permeability and lack of selectivity (several analogues have now been developed in an attempt to improve on these limitations [67–71]). Orlistat acts through formation of a covalent adduct with the thioesterase domain of the FASN molecule [43]. It was first identified as a novel inhibitor of FASN with anti-tumour activity in PCa following a proteomic screen for PCa-specific enzymes [47]. The growth inhibitory effects of orlistat have been demonstrated in animal models of both prostate and gastric cancer and in metastatic melanoma and breast cancer cell lines [47,72–74].
Small molecule FASN inhibitors such as cerulenin and C75 (and potent analogues of C75) have demonstrated significant antitumour activity [75]. Cerulenin contains an epoxy group that reacts with the ketoacyl synthase domain of FASN [76]. FASN inhibition with Cerulenin induces apoptosis and delays disease progression in breast cancer cell lines and in ovarian cancer xenografts, respectively; cytotoxic effect are dependent on the level of FASN activity [77,78]. C75, is a relatively inefficient synthetic inhibitor of FASN that inactivates the β-ketoacyl synthase enoyl reductase and thioesterase partial activities of FASN [79]. However, it has demonstrated to possess both anti-tumour and anti-obesity properties. Subcutaneous xenografts of MCF7 breast cancer cells in nude mice treated with C75 showed fatty acid synthesis inhibition, apoptosis and inhibition of tumour growth to less than one-eighth of control volumes, without comparable toxicity in normal tissues [23]. In addition, treatment of the neu-N transgenic mouse model of mammary cancer with C75 was chemopreventive, significantly delaying tumour progression [80]. The systemic side effects of anorexia coupled to rapid weight loss limits the potential use of both cerulenin and C75 in cancer therapeutics [81]; these effects may be induced centrally through inhibition of expression of neuropepetide Y within the hypothalamus and peripherally through an increase in mitochondrial fatty acid oxidation via stimulation of CPT-1 [51,81–83].
In order to overcome the lack of potency and side-effects of the aforementioned FASN inhibitors, such as C75, naturally occurring thiolactomycins were used as a template to develop a new class of type I FASN inhibitors (C93 and C247) [84]. A non-toxic FASN inhibitor with oral bioavailability, FAS31, has also been developed [85]. C93 is an effective inhibitor of FASN, significantly inhibiting the growth of non-small cell lung and ovarian cancer xenograft tumours [73,86]. Indeed, inhibiting FASN with C93 has been proposed as an effective strategy in preventing and retarding the growth of lung tumours that have high expression of the enzyme [74]. Similar tumour growth inhibition has been observed in a transgenic model of breast cancer and in ovarian cancer xenograft models using C247 and FAS31, respectively [49,71,75]. Recently, newer, more potent FASN inhibitors, including bisamide derivatives, 3-aryl-4-hydroxyquinolin-2(1H)-one derivatives, dibenzenesulphonamide urea GSK837149A and platensimycin [87–89], have been identified through medicinal chemistry programmes and high-throughput screening. Many of these compounds have activities in the low nanomolar range but there are no data regarding their mechanism of action or cellular/in vivo activity [90,91].
Other inhibitors of lipogenesis
Although most attention on the role of endogenous FA metabolism in PCa has been directed towards the molecular and cellular consequences of FASN hyper-activation, in the past years other key lipid enzymes have been pointed out as potential targets for PCa. Thus, HMG-CoA reductase inhibitors (statins) and ACC inhibitors (such as soraphen A) have shown promising preclinical results both in vitro and in vivo [92–93]. Moreover, recent data showed a reduced incidence of PCa among statin users in the Finnish Prostate Cancer Screening Trial, associated with lower PSA levels [94]. This evidence suggests that interfering with lipid metabolism represents an important direction to pursue. At present, two clinical trials are ongoing to investigate the effect of statin therapy prior to prostatectomy (NCT00572468) or during external beam radiation therapy (NCT00580970).
AMPK activators
Indirect AMPK activators
Metformin
Metformin is a derivative of guanidine. Together with the related biguanide, phenphormin (which was withdrawn in 1994 due to the emergence of lactic acidosis as a serious side-effect), it is a widely prescribed oral drug used as first-line therapy for diabetes mellitus type 2. The primary actions of metformin are inhibition of hepatic glucose production and reduction of insulin resistance in peripheral tissue, leading to enhanced glucose uptake and utilization in skeletal muscle. This reduces the levels of circulating glucose and decreases the plasma insulin levels, improving long-term glycaemic control and reducing the incidence of diabetes-related complications. Metformin is an inexpensive and safe drug, with minor gastrointestinal upset being the most common toxicity. Recent evidence indicates that: (a) diabetics under treatment with metformin show a reduced cancer incidence [95] and cancer-related mortality, compared to patients exposed to sulphonylureas or insulin [96]; (b) metformin use is associated with a 44% risk reduction in PCa cases compared to controls in Caucasian men [97]; (c) breast cancer patients treated with neoadjuvant chemotherapy and metformin have significantly higher complete pathological responses than patients not taking metformin (retrospective study) [98]. Thus, the potential of metformin as an anti-cancer drug is currently under investigation. Early promise has been reinforced by in vitro and xenograft mouse model studies highlighting: (a) metformin’s antitumour activity on PCa cells [99,100]; and (b) its ability to selectively kill cancer stem cells from four genetically distinct breast cancer lines [101].
The mechanism of action for metformin’s anti-tumour effect is not completely understood and has been ascribed to both direct and indirect effects. Metformin’s direct effects on tumour have been partly attributed to AMPK activation [102]. Thus, metformin inhibits complex I of the respiratory chain, resulting in an increased AMP : ATP ratio and secondary activation of the AMPK pathway [103]. This results in inhibition of mTOR and p70S6kinase 1 (S6K1) activity and decreased translational efficiency in PCa cell lines [99]. However, silencing of AMPK expression did not prevent the antiproliferative effect of metformin in PCa cell lines, suggesting the involvement of AMPK-independent mechanisms. Thus, the induction of G0/G1 cell cycle arrest, accompanied by a strong decrease in cyclin D1 protein level, pRb phosphorylation and an increase in p27kip protein expression, has been claimed [99]. Moreover, in vitro studies have also shown that metformin may inhibit tumour growth by preventing p53-induced autophagy [104] and its treatment has an inhibitory effect on nuclear factor-κB (NF-κB) and extracellular regulated-signal kinase (Erk) 1/2 and 5 activation by an AMPK-independent mechanism [105]. Very recently, the inhibition of mTOR complex 1 (mTORC1) by metformin in a GTPase-dependent but AMPK-independent manner has also been shown, highlighting the plurality of energy charge-response kinases [106].
Metformin’s indirect effect on tumour proliferation can be explained via inhibition of hepatic gluconeogenesis and increased glucose uptake in skeletal muscle, thereby decreasing circulating glucose, insulin and IGF-1 levels, and resultant signalling through the insulin/IGF-1 pathway that promote cell proliferation [107]. Determining how biguanides mediate their anti-cancer effect is critical before initiating clinical trials for advanced disease. If its anti-tumour effects are mainly mediated by the AMPK pathway, PCa patients with low AMPK activation might be enrolled in clinical trials with biguanides. Alternatively, if the anti-tumour effects of biguanides are predominantly by an indirect mechanism, PCa patients most likely to be suitable might be those with concomitant insulin resistance. Therefore, although metformin is very safe and remarkably inexpensive, it is critical to understand the feasibility of its use in PCa before starting trials in metastatic disease. A phase II clinical trial is currently ongoing to study the effect of neoadjuvant metformin therapy in PCa patients prior to radical prostatectomy (NCT00881725). This trial has been designed to test whether metformin has any effect on signalling pathways within the tumours, rather than whether there is any effect on long-term outcome. However, if the results are promising it seems likely that additional trials to test the latter will follow. A randomized phase II surveillance trial to test the combinatorial effect of the 5-α-reductase inhibitor dutasteride and metformin in low risk PCa patients, not previously treated, has also been recently planned at Dana Farber Cancer Institute, Boston. The value of (metabolic) tumour imaging is therefore becoming critical. As highlighted, metformin treatment mediates changes in tumour metabolism and therefore positron emission tomography (PET) imaging may be used to determine tumour response to this drug. Unfortunately, we cannot ignore that activation of AMPK in tumour cells might also be a double-edged sword, in that while AMPK inhibits their growth, it may also promote cell survival under low-energy conditions. In support of this idea, cells lacking LKB1 (which have low AMPK activity) were shown to be hypersensitive to apoptosis in response to energy stress [108]. In addition, activation of AMPK in response to hypoxia induces the expression of vascular endothelial growth factor (VEGF), a key regulator of angiogenesis, essential for tumour growth and metastasis [109]. Because of these potential tumour-promoting properties of AMPK activation, it is essential that caution be exercised in the design of trials testing AMPK activators as treatments for cancer, including PCa.
Thiazolidinediones
Like metformin, thiazolidinediones (TZDs) are used clinically to treat type 2 diabetes. They activate AMPK likely via inhibition of complex I of the respiratory chain [110]. Currently, their activity as anticancer agents is under evaluation. Indeed, the TZD derivative CGP 52 608 has been shown to have a strong cytostatic activity in LNCaP cells by reducing cell proliferation and by affecting cell cycle distribution through the modulation of the expression of cell cycle-related genes [111]. Recently, AMPK has been implicated in TZDs mechanism of action [112].
Natural products of plants
Tumour prevention by natural products such as berberine and deguelin has been more directly linked to AMPK. Berberine, a plant alkaloid, activates AMPK by an indirect mechanism similar to that of metformin and TZDs [113]. The derivative of rotenone, deguelin, has been shown to decrease ATP, activate AMPK and suppress AKT activation and mTOR/survivin signalling [114]. A number of other well-known chemopreventives are competent to activate AMPK. Examples include resveratrol, epigallocatechin-3-gallate genistein and selenium. However, the contribution of AMPK to their preventive actions is still unknown at present [115].
Direct AMPK activators
AICAR
The nucleoside 5-aminoimidazole-4-carboxamide-1-β-ribofuranoside (AICAR) was the first compound reported to activate AMPK in intact cells and in vivo, and has been widely used to investigate the downstream effects of AMPK activation in animals. AICAR is taken up into cells by adenosine transporters [116] and is then converted by adenosine kinase to the monophosphorylated derivative ZMP, which is an analogue of 5′-AMP and thus mimics several of its cellular effects. AICAR inhibits the growth of established tumour cells in vitro and in vivo. In particular, it has recently been shown to inhibit PCa cell proliferation [117] and tumour growth in PCa xenograft models [99]. However, AICAR is not entirely specific for AMPK, exerting AMPK-independent effects mainly on AMP-regulated enzymes and mitochondrial oxidative phosphorylation (OXPHOS) [118]. Moreover, it has limited oral bioavailability [119], making it a poor candidate for long-term use in humans. Hence, the design of novel small-molecule AMPK activators with reduced off-target effects is actively sought and was greatly enhanced by the publication of the crystal structure of AMPK subunits [120].
Small molecule AMPK activators: A-769 662 and PT1
Abbott laboratories have pioneered the area of developing subunit-specific AMPK activators and identified thienopyridone A-769 662 in a screening of a chemical library of over 700 000 compounds, using partially purified AMPK αβγ complex [121]. Similar to AMP, A-769 662 has two effects in cell-free assays; it activates AMPK via an allosteric mechanism and by inhibiting dephosphorylation at Thr172 [122]. Activation of AMPK by A-769 662 does not appear to require the AMP-binding sites in the γ-subunit, although it does require autophosphorylation of the Ser108 site within the β-subunit [123], particularly in the β1 isoform, which is selectively activated by the compound. A-769 662 lacks the off-target effects of AICAR [118,121] because it is not an AMP mimetic. In cultured cells, it can activate AMPK in the absence of the upstream kinase LKB1 [122,123], which may indicate that it is acting primarily via the allosteric mechanism. This is supported by observations that increased phosphorylation of the AMPK substrate, ACC, is not always associated with a substantial increase in Thr172 phosphorylation [122]. Importantly, treatment of ob/ob mice with A-769 662 decreased plasma glucose and triglyceride levels, and reduced hepatic gluconeogenic and lipogenic gene expression [121]. Importantly, A-769 662 has been shown to delay tumour development and decrease tumour incidence in PTEN +/− mice with a hypomorphic LKB1 allele [124], suggesting its potential anticancer properties. Unfortunately, although it has beneficial effects in vivo, the promise of A-769 662 as a lead compound for drug development is tempered by its poor oral absorption [121] and some recently reported AMPK-independent effects [125]. Thus, intense efforts have been lately directed to develop more specific small-molecule AMPK activators. Recently, Pang and colleagues [126] identified PT1 as a novel direct activator of AMPK. PT1 appears to increase AMPK activity by interacting with the auto-inhibitory domain found in the α-subunits, relieving inhibition of kinase activity by this domain. Treatment of cultured cells with PT1 activates AMPK via increased phosphorylation of Thr172 without altering the intracellular AMP : ATP ratio, and can occur in LKB1-deficient cells, where it requires CaMKKβ. However, the effects of PT1 in vivo and its potential use as an anticancer agent are still to be investigated.
Exploiting metabolic alterations for prostate cancer diagnosis and prognosis
In the post-PSA era, PCa metabolic imaging is playing a central role in the evolution of a patient-specific diagnostic approach, as it directly provides information on the presence and extent of disease [127]. Several clinical trials have shown that metabolic imaging can significantly impact patient management by improving tumour staging, restaging, treatment planning and monitoring of tumour response to therapy. However, the use of imaging to assess PCa remains a challenge, owing to the inherent heterogeneity of the disease. PET and PET-computed tomography (PET–CT) with the glucose analogue 18F-FDG has become a routine clinical test for staging and restaging of most solid tumours [128]. However, PCa is not as glycolytic as the majority of other cancers and increased glycolysis is found mainly in the advanced stages of the disease. Thus, the rather low uptake in low-grade cancers and rapid excretion to the urine renders FDG quite unfavourable for staging prostate cancers. On the contrary, an increase in fatty acid synthesis seems to be early event in PCa tumorigenesis and is correlated with the progression of the disease. Thus, imaging with PET tracers such as 11C acetate, 18F fluoroacetate, 11C choline or 18F choline has been evaluated to monitor the lipogenic phenotype. Both 11C acetate and 11C choline have shown increased sensitivity in the detection of primary PCa, metastasis and recurrent disease [129]. However, both of the tracers fail to detect small metastatic sites [130] and 11C acetate has low sensitivity for disease detection at PSA levels < 3 ng/ml [129]. Thus, further studies in a large population of patients are still necessary to establish their final clinical role in PCa [131].
Conclusion
It is now evident that alterations in cellular metabolism are strongly linked with oncogene activation and silencing of tumour suppressor genes and hence are required for neoplastic transformation in PCa. These alterations are currently being targeted in both the diagnostic and therapeutic settings to help improve patient management. Current interest has focused on the potential of targeting key lipogenic enzymes, such as FASN or the energy sensor AMPK, in PCa treatment. The development of metabolic profiling technologies will, in the future, allow a more accurate stratification of patients eligible for lipogenic pathway-inhibiting therapies. Moreover, the new PET-based metabolic imaging techniques will allow a safer and more accurate approach to monitoring tumour response to therapy.
Acknowledgments
We thank Michael Cichanowski for his graphic assistance. This work was supported by the Prostate Cancer Foundation, the National Cancer Institute (Grant Nos R01CA131945, P01CA89021 and P50CA90381) and the Linda and Arthur Gelb Center for Translational Research.
Footnotes
No conflicts of interest were declared.
Author contributions
Richard Flavin and Giorgia Zadra wrote the manuscript and prepared the pictures. Massimo Loda reviewed the final draft of the manuscript.
Teaching Materials
PowerPoint slides of the figures from this review are supplied as supporting information in the online version of this article.
References
- 1.Yin M, Bastacky S, Chandran U, et al. Prevalence of incidental prostate cancer in the general population: a study of healthy organ donors. J Urol. 2008;179:892–895. doi: 10.1016/j.juro.2007.10.057. discussion, 895. [DOI] [PubMed] [Google Scholar]
- 2.Platz EA, Rimm EB, Willett WC, et al. Racial variation in prostate cancer incidence and in hormonal system markers among male health professionals. J Natl Cancer Inst. 2000;92:2009–2017. doi: 10.1093/jnci/92.24.2009. [DOI] [PubMed] [Google Scholar]
- 3.Bairati I, Meyer F, Fradet Y, et al. Dietary fat and advanced prostate cancer. J Urol. 1998;159:1271–1275. [PubMed] [Google Scholar]
- 4.Johns LE, Houlston RS. A systematic review and meta-analysis of familial prostate cancer risk. BJU Int. 2003;91:789–794. doi: 10.1046/j.1464-410x.2003.04232.x. [DOI] [PubMed] [Google Scholar]
- 5.Stark JR, Perner S, Stampfer MJ, et al. Gleason score and lethal prostate cancer: does 3 + 4 = 4 + 3? J Clin Oncol. 2009;27:3459–3464. doi: 10.1200/JCO.2008.20.4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 7.Fleshner N, Bagnell PS, Klotz L, et al. Dietary fat and prostate cancer. J Urol. 2004;171:S19–24. doi: 10.1097/01.ju.0000107838.33623.19. [DOI] [PubMed] [Google Scholar]
- 8.Wright ME, Chang SC, Schatzkin A, et al. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 2007;109:675–684. doi: 10.1002/cncr.22443. [DOI] [PubMed] [Google Scholar]
- 9.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 10.Bui T, Thompson CB. Cancer’s sweet tooth. Cancer Cell. 2006;9:419–420. doi: 10.1016/j.ccr.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Garber K. Energy boost: the Warburg effect returns in a new theory of cancer. J Natl Cancer Inst. 2004;96:1805–1806. doi: 10.1093/jnci/96.24.1805. [DOI] [PubMed] [Google Scholar]
- 12.Shaw RJ. Glucose metabolism and cancer. Curr Opin Cell Biol. 2006;18:598–608. doi: 10.1016/j.ceb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Clemens MJ. Targets and mechanisms for the regulation of translation in malignant transformation. Oncogene. 2004;23:3180–3188. doi: 10.1038/sj.onc.1207544. [DOI] [PubMed] [Google Scholar]
- 14.Voeller D, Rahman L, Zajac-Kaye M. Elevated levels of thymidylate synthase linked to neoplastic transformation of mammalian cells. Cell Cycle. 2004;3:1005–1007. [PubMed] [Google Scholar]
- 15.Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16:202–208. doi: 10.1016/s0899-9007(99)00266-x. [DOI] [PubMed] [Google Scholar]
- 16.Migita T, Narita T, Nomura K, et al. ATP citrate lyase: activation and therapeutic implications in non-small cell lung cancer. Cancer Res. 2008;68:8547–8554. doi: 10.1158/0008-5472.CAN-08-1235. [DOI] [PubMed] [Google Scholar]
- 17.Sivaprasad U, Abbas T, Dutta A. Differential efficacy of 3-hydroxy-3-methylglutaryl CoA reductase inhibitors on the cell cycle of prostate cancer cells. Mol Cancer Ther. 2006;5:2310–2316. doi: 10.1158/1535-7163.MCT-06-0175. [DOI] [PubMed] [Google Scholar]
- 18.Swinnen JV, Van Veldhoven PP, Timmermans L, et al. Fatty acid synthase drives the synthesis of phospholipids partitioning into detergent-resistant membrane microdomains. Biochem Biophys Res Commun. 2003;302:898–903. doi: 10.1016/s0006-291x(03)00265-1. [DOI] [PubMed] [Google Scholar]
- 19.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 20.Komekado H, Yamamoto H, Chiba T, et al. Glycosylation and palmitoylation of Wnt-3a are coupled to produce an active form of Wnt-3a. Genes Cells. 2007;12:521–534. doi: 10.1111/j.1365-2443.2007.01068.x. [DOI] [PubMed] [Google Scholar]
- 21.Kurayoshi M, Yamamoto H, Izumi S, et al. Post-translational palmitoylation and glycosylation of Wnt-5a are necessary for its signalling. Biochem J. 2007;402:515–523. doi: 10.1042/BJ20061476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laude AJ, Prior IA. Palmitoylation and localisation of RAS isoforms are modulated by the hypervariable linker domain. J Cell Sci. 2008;121:421–427. doi: 10.1242/jcs.020107. [DOI] [PubMed] [Google Scholar]
- 23.Wright MH, Heal WP, Mann DJ, Tate EW. Protein myristoylation in health and disease. J Chem Biol. 2010;3:19–35. doi: 10.1007/s12154-009-0032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauer DE, Hatzivassiliou G, Zhao F, et al. ATP citrate lyase is an important component of cell growth and transformation. Oncogene. 2005;24:6314–6322. doi: 10.1038/sj.onc.1208773. [DOI] [PubMed] [Google Scholar]
- 25.Ettinger SL, Sobel R, Whitmore TG, et al. Dysregulation of sterol response element-binding proteins and downstream effectors in prostate cancer during progression to androgen independence. Cancer Res. 2004;64:2212–2221. doi: 10.1158/0008-5472.can-2148-2. [DOI] [PubMed] [Google Scholar]
- 26.Rossi S, Graner E, Febbo P, et al. Fatty acid synthase expression defines distinct molecular signatures in prostate cancer. Mol Cancer Res. 2003;1:707–715. [PubMed] [Google Scholar]
- 27.Swinnen JV, Vanderhoydonc F, Elgamal AA, et al. Selective activation of the fatty acid synthesis pathway in human prostate cancer. Int J Cancer. 2000;88:176–179. doi: 10.1002/1097-0215(20001015)88:2<176::aid-ijc5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 28.Sellers W, Sawyers C. Somatic Genetics of Prostate Cancer: Oncogenes and Tumor Suppressors. Lippincott Williams & Wilkins; Philadelphia, PA: 2002. [Google Scholar]
- 29.Sarker D, Reid AH, Yap TA, et al. Targeting the PI3K/AKT pathway for the treatment of prostate cancer. Clin Cancer Res. 2009;15:4799–4805. doi: 10.1158/1078-0432.CCR-08-0125. [DOI] [PubMed] [Google Scholar]
- 30.Migita T, Ruiz S, Fornari A, et al. Fatty acid synthase: a metabolic enzyme and candidate oncogene in prostate cancer. J Natl Cancer Inst. 2009;101:519–532. doi: 10.1093/jnci/djp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 32.Kuhajda FP. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res. 2006;66:5977–5980. doi: 10.1158/0008-5472.CAN-05-4673. [DOI] [PubMed] [Google Scholar]
- 33.Menendez JA, Lupu R. Fatty acid synthase-catalyzed de novo fatty acid biosynthesis: from anabolic energy-storage pathway in normal tissues to jack-of-all-trades in cancer cells. Arch Immunol Ther Exp (Warsz) 2004;52:414–426. [PubMed] [Google Scholar]
- 34.Menendez JA, Lupu R. Oncogenic properties of the endogenous fatty acid metabolism: molecular pathology of fatty acid synthase in cancer cells. Curr Opin Clin Nutr Metab Care. 2006;9:346–357. doi: 10.1097/01.mco.0000232893.21050.15. [DOI] [PubMed] [Google Scholar]
- 35.Menendez JA, Lupu R, Colomer R. Targeting fatty acid synthase: potential for therapeutic intervention in her-2/neu-overexpressing breast cancer. Drug News Perspect. 2005;18:375–385. doi: 10.1358/dnp.2005.18.6.927929. [DOI] [PubMed] [Google Scholar]
- 36.Swinnen JV, Brusselmans K, Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care. 2006;9:358–365. doi: 10.1097/01.mco.0000232894.28674.30. [DOI] [PubMed] [Google Scholar]
- 37.Innocenzi D, Alo PL, Balzani A, et al. Fatty acid synthase expression in melanoma. J Cutan Pathol. 2003;30:23–28. doi: 10.1034/j.1600-0560.2003.300104.x. [DOI] [PubMed] [Google Scholar]
- 38.Kusakabe T, Nashimoto A, Honma K, et al. Fatty acid synthase is highly expressed in carcinoma, adenoma and in regenerative epithelium and intestinal metaplasia of the stomach. Histopathology. 2002;40:71–79. doi: 10.1046/j.1365-2559.2002.01289.x. [DOI] [PubMed] [Google Scholar]
- 39.Milgraum LZ, Witters LA, Pasternack GR, et al. Enzymes of the fatty acid synthesis pathway are highly expressed in in situ breast carcinoma. Clin Cancer Res. 1997;3:2115–2120. [PubMed] [Google Scholar]
- 40.Piyathilake CJ, Frost AR, Manne U, et al. The expression of fatty acid synthase (FASE) is an early event in the development and progression of squamous cell carcinoma of the lung. Hum Pathol. 2000;31:1068–1073. doi: 10.1053/hupa.2000.9842. [DOI] [PubMed] [Google Scholar]
- 41.Ogino S, Nosho K, Meyerhardt JA, et al. Cohort study of fatty acid synthase expression and patient survival in colon cancer. J Clin Oncol. 2008;26:5713–5720. doi: 10.1200/JCO.2008.18.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shurbaji MS, Kalbfleisch JH, Thurmond TS. Immunohistochemical detection of a fatty acid synthase (OA-519) as a predictor of progression of prostate cancer. Hum Pathol. 1996;27:917–921. doi: 10.1016/s0046-8177(96)90218-x. [DOI] [PubMed] [Google Scholar]
- 43.Takahiro T, Shinichi K, Toshimitsu S. Expression of fatty acid synthase as a prognostic indicator in soft tissue sarcomas. Clin Cancer Res. 2003;9:2204–2212. [PubMed] [Google Scholar]
- 44.Visca P, Sebastiani V, Botti C, et al. Fatty acid synthase (FAS) is a marker of increased risk of recurrence in lung carcinoma. Anticancer Res. 2004;24:4169–4173. [PubMed] [Google Scholar]
- 45.De Schrijver E, Brusselmans K, Heyns W, et al. RNA interference-mediated silencing of the fatty acid synthase gene attenuates growth and induces morphological changes and apoptosis of LNCaP prostate cancer cells. Cancer Res. 2003;63:3799–3804. [PubMed] [Google Scholar]
- 46.Graner E, Tang D, Rossi S, et al. The isopeptidase USP2a regulates the stability of fatty acid synthase in prostate cancer. Cancer Cell. 2004;5:253–261. doi: 10.1016/s1535-6108(04)00055-8. [DOI] [PubMed] [Google Scholar]
- 47.Kridel SJ, Axelrod F, Rozenkrantz N, et al. Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Res. 2004;64:2070–2075. doi: 10.1158/0008-5472.can-03-3645. [DOI] [PubMed] [Google Scholar]
- 48.Pizer ES, Thupari J, Han WF, et al. Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced by fatty-acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res. 2000;60:213–218. [PubMed] [Google Scholar]
- 49.Fiorentino M, Zadra G, Palescandolo E, et al. Overexpression of fatty acid synthase is associated with palmitoylation of Wnt1 and cytoplasmic stabilization of β-catenin in prostate cancer. Lab Invest. 2008;88:1340–1348. doi: 10.1038/labinvest.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Little JL, Wheeler FB, Fels DR, et al. Inhibition of fatty acid synthase induces endoplasmic reticulum stress in tumor cells. Cancer Res. 2007;67:1262–1269. doi: 10.1158/0008-5472.CAN-06-1794. [DOI] [PubMed] [Google Scholar]
- 51.Furuta E, Pai SK, Zhan R, et al. Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res. 2008;68:1003–1011. doi: 10.1158/0008-5472.CAN-07-2489. [DOI] [PubMed] [Google Scholar]
- 52.Priolo C, Tang D, Brahamandan M, et al. The isopeptidase USP2a protects human prostate cancer from apoptosis. Cancer Res. 2006;66:8625–8632. doi: 10.1158/0008-5472.CAN-06-1374. [DOI] [PubMed] [Google Scholar]
- 53.Benedettini E. The pathogenesis of prostate cancer: from molecular to metabolic alterations. Diagn Histopathol. 2008;14:195–201. doi: 10.1016/j.mpdhp.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 55.Luo Z, Saha AK, Xiang X, et al. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci. 2005;26:69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 56.Freedland SJ. Obesity and prostate cancer: a growing problem. Clin Cancer Res. 2005;11:6763–6766. doi: 10.1158/1078-0432.CCR-05-1305. [DOI] [PubMed] [Google Scholar]
- 57.Grundmark B, Garmo H, Loda M, et al. The metabolic syndrome and the risk of prostate cancer under competing risks of death from other causes. Cancer Epidemiol Biomarkers Prev. 2010;19:2088–2096. doi: 10.1158/1055-9965.EPI-10-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Viollet B, Andreelli F, Jorgensen SB, et al. Physiological role of AMP-activated protein kinase (AMPK): insights from knockout mouse models. Biochem Soc Trans. 2003;31:216–219. doi: 10.1042/bst0310216. [DOI] [PubMed] [Google Scholar]
- 59.Horikoshi M, Hara K, Ohashi J, et al. A polymorphism in the AMPKα2 subunit gene is associated with insulin resistance and type 2 diabetes in the Japanese population. Diabetes. 2006;55:919–923. doi: 10.2337/diabetes.55.04.06.db05-0727. [DOI] [PubMed] [Google Scholar]
- 60.Matsui H, Suzuki K, Ohtake N, et al. Genome-wide linkage analysis of familial prostate cancer in the Japanese population. J Hum Genet. 2004;49:9–15. doi: 10.1007/s10038-003-0099-y. [DOI] [PubMed] [Google Scholar]
- 61.Jenne DE, Reimann H, Nezu J, et al. Peutz–Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet. 1998;18:38–43. doi: 10.1038/ng0198-38. [DOI] [PubMed] [Google Scholar]
- 62.Sanchez-Cespedes M. A role for LKB1 gene in human cancer beyond the Peutz–Jeghers syndrome. Oncogene. 2007;26:7825–7832. doi: 10.1038/sj.onc.1210594. [DOI] [PubMed] [Google Scholar]
- 63.Pearson HB, McCarthy A, Collins CM, et al. Lkb1 deficiency causes prostate neoplasia in the mouse. Cancer Res. 2008;68:2223–2232. doi: 10.1158/0008-5472.CAN-07-5169. [DOI] [PubMed] [Google Scholar]
- 64.Shackelford DB, Shaw RJ. The LKB1–AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wan X, Harkavy B, Shen N, et al. Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene. 2007;26:1932–1940. doi: 10.1038/sj.onc.1209990. [DOI] [PubMed] [Google Scholar]
- 66.Zhi J, Melia AT, Funk C, et al. Metabolic profiles of minimally absorbed orlistat in obese/overweight volunteers. J Clin Pharmacol. 1996;36:1006–1011. doi: 10.1177/009127009603601104. [DOI] [PubMed] [Google Scholar]
- 67.Ma G, Zancanella M, Oyola Y, et al. Total synthesis and comparative analysis of orlistat, valilactone, and a transposed orlistat derivative: Inhibitors of fatty acid synthase. Org Lett. 2006;8:4497–4500. doi: 10.1021/ol061651o. [DOI] [PubMed] [Google Scholar]
- 68.Purohit VC, Richardson RD, Smith JW, et al. Practical, catalytic, asymmetric synthesis of β-lactones via a sequential ketene dimerization/hydrogenation process: inhibitors of the thioesterase domain of fatty acid synthase. J Org Chem. 2006;71:4549–4558. doi: 10.1021/jo060392d. [DOI] [PubMed] [Google Scholar]
- 69.Richardson RD, Ma G, Oyola Y, et al. Synthesis of novel β-lactone inhibitors of fatty acid synthase. J Med Chem. 2008;51:5285–5296. doi: 10.1021/jm800321h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang W, Richardson RD, Chamni S, et al. β-Lactam congeners of orlistat as inhibitors of fatty acid synthase. Bioorg Med Chem Lett. 2008;18:2491–2494. doi: 10.1016/j.bmcl.2008.02.043. [DOI] [PubMed] [Google Scholar]
- 71.Hoover HS, Blankman JL, Niessen S, et al. Selectivity of inhibitors of endocannabinoid biosynthesis evaluated by activity-based protein profiling. Bioorg Med Chem Lett. 2008;18:5838–5841. doi: 10.1016/j.bmcl.2008.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Menendez JA, Vellon L, Lupu R. Antitumoral actions of the anti-obesity drug orlistat (Xenical™) in breast cancer cells: blockade of cell cycle progression, promotion of apoptotic cell death and PEA3-mediated transcriptional repression of Her2/neu (erbB-2 ) oncogene. Ann Oncol. 2005;16:1253–1267. doi: 10.1093/annonc/mdi239. [DOI] [PubMed] [Google Scholar]
- 73.Carvalho MA, Zecchin KG, Seguin F, et al. Fatty acid synthase inhibition with orlistat promotes apoptosis and reduces cell growth and lymph node metastasis in a mouse melanoma model. Int J Cancer. 2008;123:2557–2565. doi: 10.1002/ijc.23835. [DOI] [PubMed] [Google Scholar]
- 74.Dowling S, Cox J, Cenedella RJ. Inhibition of fatty acid synthase by orlistat accelerates gastric tumor cell apoptosis in culture and increases survival rates in gastric tumor bearing mice in vivo. Lipids. 2009;44:489–498. doi: 10.1007/s11745-009-3298-2. [DOI] [PubMed] [Google Scholar]
- 75.Wang X, Lin J, Chen Y, et al. Novel fatty acid synthase (FAS) inhibitors: design, synthesis, biological evaluation, and molecular docking studies. Bioorg Med Chem. 2009;17:1898–1904. doi: 10.1016/j.bmc.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 76.Funabashi H, Kawaguchi A, Tomoda H, et al. Binding site of cerulenin in fatty acid synthetase. J Biochem. 1989;105:751–755. doi: 10.1093/oxfordjournals.jbchem.a122739. [DOI] [PubMed] [Google Scholar]
- 77.Pizer ES, Jackisch C, Wood FD, et al. Inhibition of fatty acid synthesis induces programmed cell death in human breast cancer cells. Cancer Res. 1996;56:2745–2747. [PubMed] [Google Scholar]
- 78.Pizer ES, Wood FD, Heine HS, et al. Inhibition of fatty acid synthesis delays disease progression in a xenograft model of ovarian cancer. Cancer Res. 1996;56:1189–1193. [PubMed] [Google Scholar]
- 79.Rendina AR, Cheng D. Characterization of the inactivation of rat fatty acid synthase by C75: inhibition of partial reactions and protection by substrates. Biochem J. 2005;388:895–903. doi: 10.1042/BJ20041963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alli PM, Pinn ML, Jaffee EM, et al. Fatty acid synthase inhibitors are chemopreventive for mammary cancer in neu-N transgenic mice. Oncogene. 2005;24:39–46. doi: 10.1038/sj.onc.1208174. [DOI] [PubMed] [Google Scholar]
- 81.Loftus TM, Jaworsky DE, Frehywot GL, et al. Reduced food intake and body weight in mice treated with fatty acid synthase inhibitors. Science. 2000;288:2379–2381. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- 82.Chakravarthy MV, Zhu Y, Lopez M, et al. Brain fatty acid synthase activates PPARα to maintain energy homeostasis. J Clin Invest. 2007;117:2539–2552. doi: 10.1172/JCI31183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thupari JN, Landree LE, Ronnett GV, et al. C75 increases peripheral energy utilization and fatty acid oxidation in diet-induced obesity. Proc Natl Acad Sci USA. 2002;99:9498–9502. doi: 10.1073/pnas.132128899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McFadden JM, Medghalchi SM, Thupari JN, et al. Application of a flexible synthesis of (5R)-thiolactomycin to develop new inhibitors of type I fatty acid synthase. J Med Chem. 2005;48:946–961. doi: 10.1021/jm049389h. [DOI] [PubMed] [Google Scholar]
- 85.el Meskini R, Medghalchi SM, Vadlamudi A, et al. Fatty acid synthase inhibition for ovarian cancer. Poster No. 5667, AACR Meeting; San Diego, CA. 12–16 April 2008. [Google Scholar]
- 86.Orita H, Coulter J, Lemmon C, et al. Selective inhibition of fatty acid synthase for lung cancer treatment. Clin Cancer Res. 2007;13:7139–7145. doi: 10.1158/1078-0432.CCR-07-1186. [DOI] [PubMed] [Google Scholar]
- 87.Rivkin A, Kim YR, Goulet MT, et al. 3-Aryl-4-hydroxyquinolin-2(1H)-one derivatives as type I fatty acid synthase inhibitors. Bioorg Med Chem Lett. 2006;16:4620–4623. doi: 10.1016/j.bmcl.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 88.Bostrom J, Brickmann K, Johannesson P, et al. Bisamide derivatives and use thereof as fatty acid synthase inhibitors. http://www.sumobrain.com/patents/wipo/Bisamlde-derivatives-use-thereof-as/WO2008059214.html.
- 89.Wang J, Soisson SM, Young K, et al. Platensimycin is a selective FabF inhibitor with potent antibiotic properties. Nature. 2006;441:358–361. doi: 10.1038/nature04784. [DOI] [PubMed] [Google Scholar]
- 90.Vazquez MJ, Leavens W, Liu R, et al. Discovery of GSK837149A, an inhibitor of human fatty acid synthase targeting the β-ketoacyl reductase reaction. FEBS J. 2008;275:1556–1567. doi: 10.1111/j.1742-4658.2008.06314.x. [DOI] [PubMed] [Google Scholar]
- 91.Singh SB, Tota MR, Wang J. Method of treatment using fatty acid synthesis inhibitors. http://www.wipo.int/pctdb/en/wo.jsp?WO=2008039327.
- 92.Beckers A, Organe S, Timmermans L, et al. Chemical inhibition of acetyl-CoA carboxylase induces growth arrest and cytotoxicity selectively in cancer cells. Cancer Res. 2007;67:8180–8187. doi: 10.1158/0008-5472.CAN-07-0389. [DOI] [PubMed] [Google Scholar]
- 93.Murtola TJ, Visakorpi T, Lahtela J, et al. Statins and prostate cancer prevention: where we are now, and future directions. Nat Clin Pract Urol. 2008;5:376–387. doi: 10.1038/ncpuro1146. [DOI] [PubMed] [Google Scholar]
- 94.Murtola TJ, Tammela TL, Maattanen L, et al. Prostate cancer and PSA among statin users in the Finnish prostate cancer screening trial. Int J Cancer. 2010;127:1650–1659. doi: 10.1002/ijc.25165. [DOI] [PubMed] [Google Scholar]
- 95.Evans JM, Donnelly LA, Emslie-Smith AM, et al. Metformin and reduced risk of cancer in diabetic patients. Br Med J. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bowker SL, Majumdar SR, Veugelers P, et al. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–258. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 97.Wright JL, Stanford JL. Metformin use and prostate cancer in Caucasian men: results from a population-based case-control study. Cancer Causes Control. 2009;20:1617–1622. doi: 10.1007/s10552-009-9407-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiralerspong S, Palla SL, Giordano SH, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27:3297–3302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ben Sahra I, Laurent K, Loubat A, et al. The antidiabetic drug metformin exerts an antitumoral effect in vitro and in vivo through a decrease of cyclin D1 level. Oncogene. 2008;27:3576–3586. doi: 10.1038/sj.onc.1211024. [DOI] [PubMed] [Google Scholar]
- 100.Zakikhani M, Dowling RJ, Sonenberg N, et al. The effects of adiponectin and metformin on prostate and colon neoplasia involve activation of AMP-activated protein kinase. Cancer Prev Res (Philadelphia, PA) 2008;1:369–375. doi: 10.1158/1940-6207.CAPR-08-0081. [DOI] [PubMed] [Google Scholar]
- 101.Hirsch HA, Iliopoulos D, Tsichlis PN, et al. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–7511. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hardie DG. Neither LKB1 nor AMPK are the direct targets of metformin. Gastroenterology. 2006;131:973. doi: 10.1053/j.gastro.2006.07.032. author reply, 974–975. [DOI] [PubMed] [Google Scholar]
- 104.Buzzai M, Jones RG, Amaravadi RK, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–6752. doi: 10.1158/0008-5472.CAN-06-4447. [DOI] [PubMed] [Google Scholar]
- 105.Tan BK, Adya R, Chen J, et al. Metformin decreases angiogenesis via NF-κB and Erk1/2/Erk5 pathways by increasing the antiangiogenic thrombospondin-1. Cardiovasc Res. 2009;83:566–574. doi: 10.1093/cvr/cvp131. [DOI] [PubMed] [Google Scholar]
- 106.Kalender A, Selvaraj A, Kim SY, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–928. doi: 10.1038/nrc2536. [DOI] [PubMed] [Google Scholar]
- 108.Shaw RJ, Kosmatka M, Bardeesy N, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ouchi N, Shibata R, Walsh K. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res. 2005;96:838–846. doi: 10.1161/01.RES.0000163633.10240.3b. [DOI] [PubMed] [Google Scholar]
- 110.Hardie D. AMP-activated protein kinase as a drug target. Annu Rev Pharmacol Toxicol. 2007;47:185–210. doi: 10.1146/annurev.pharmtox.47.120505.105304. [DOI] [PubMed] [Google Scholar]
- 111.Moretti RM, Montagnani Marelli M, Motta M, et al. Oncostatic activity of a thiazolidinedione derivative on human androgen-dependent prostate cancer cells. Int J Cancer. 2001;92:733–737. doi: 10.1002/1097-0215(20010601)92:5<733::aid-ijc1254>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 112.He G, Sung YM, Digiovanni J, et al. Thiazolidinediones inhibit insulin-like growth factor-i-induced activation of p70S6 kinase and suppress insulin-like growth factor-I tumor-promoting activity. Cancer Res. 2006;66:1873–1878. doi: 10.1158/0008-5472.CAN-05-3111. [DOI] [PubMed] [Google Scholar]
- 113.Turner N, Li JY, Gosby A, et al. Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes. 2008;57:1414–1418. doi: 10.2337/db07-1552. [DOI] [PubMed] [Google Scholar]
- 114.Jin Q, Feng L, Behrens C, et al. Implication of AMP-activated protein kinase and Akt-regulated survivin in lung cancer chemopreventive activities of deguelin. Cancer Res. 2007;67:11630–11639. doi: 10.1158/0008-5472.CAN-07-2401. [DOI] [PubMed] [Google Scholar]
- 115.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 116.Gadalla AE, Pearson T, Currie AJ, et al. AICA riboside both activates AMP-activated protein kinase and competes with adenosine for the nucleoside transporter in the CA1 region of the rat hippocampus. J Neurochem. 2004;88:1272–1282. doi: 10.1046/j.1471-4159.2003.02253.x. [DOI] [PubMed] [Google Scholar]
- 117.Xiang X, Saha AK, Wen R, et al. AMP-activated protein kinase activators can inhibit the growth of prostate cancer cells by multiple mechanisms. Biochem Biophys Res Commun. 2004;321:161–167. doi: 10.1016/j.bbrc.2004.06.133. [DOI] [PubMed] [Google Scholar]
- 118.Guigas B, Sakamoto K, Taleux N, et al. Beyond AICA riboside: in search of new specific AMP-activated protein kinase activators. IUBMB Life. 2009;61:18–26. doi: 10.1002/iub.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Drew BG, Kingwell BA. Acadesine, an adenosine-regulating agent with the potential for widespread indications. Expert Opin Pharmacother. 2008;9:2137–2144. doi: 10.1517/14656566.9.12.2137. [DOI] [PubMed] [Google Scholar]
- 120.Xiao B, Heath R, Saiu P, et al. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature. 2007;449:496–500. doi: 10.1038/nature06161. [DOI] [PubMed] [Google Scholar]
- 121.Cool B, Zinker B, Chiou W, et al. Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab. 2006;3:403–416. doi: 10.1016/j.cmet.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 122.Goransson O, McBride A, Hawley SA, et al. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J Biol Chem. 2007;282:32549–32560. doi: 10.1074/jbc.M706536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sanders MJ, Ali ZS, Hegarty BD, et al. Defining the mechanism of activation of AMP-activated protein kinase by the small molecule A-769662, a member of the thienopyridone family. J Biol Chem. 2007;282:32539–32548. doi: 10.1074/jbc.M706543200. [DOI] [PubMed] [Google Scholar]
- 124.Huang X, Wullschleger S, Shpiro N, et al. Important role of the LKB1–AMPK pathway in suppressing tumorigenesis in PTEN-deficient mice. Biochem J. 2008;412:211–221. doi: 10.1042/BJ20080557. [DOI] [PubMed] [Google Scholar]
- 125.Moreno D, Knecht E, Viollet B, et al. A769662, a novel activator of AMP-activated protein kinase, inhibits non-proteolytic components of the 26S proteasome by an AMPK-independent mechanism. FEBS Lett. 2008;582:2650–2654. doi: 10.1016/j.febslet.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 126.Pang T, Zhang ZS, Gu M, et al. Small molecule antagonizes autoinhibition and activates AMP-activated protein kinase in cells. J Biol Chem. 2008;283:16051–16060. doi: 10.1074/jbc.M710114200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Benaron DA. The future of cancer imaging. Cancer Metast Rev. 2002;21:45–78. doi: 10.1023/a:1020131208786. [DOI] [PubMed] [Google Scholar]
- 128.Kelloff GJ, Hoffman JM, Johnson B, et al. Progress and promise of FDG–PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res. 2005;11:2785–2808. doi: 10.1158/1078-0432.CCR-04-2626. [DOI] [PubMed] [Google Scholar]
- 129.Czernin J. PET imaging of prostate cancer using 11C-acetate. PET Clinics. 2009;4:163–172. doi: 10.1016/j.cpet.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kotzerke J, Volkmer BG, Glatting G, et al. Intraindividual comparison of 11C-acetate and 11C-choline PET for detection of metastases of prostate cancer. Nuklearmedizin. 2003;42:25–30. [PubMed] [Google Scholar]
- 131.Picchio M, Crivellaro C, Giovacchini G, et al. PET–CT for treatment planning in prostate cancer. Q J Nucl Med Mol Imaging. 2009;53:245–268. [PubMed] [Google Scholar]