Abstract
Fatty acid synthase (FASN) is a key enzyme involved in neoplastic lipogenesis. Overexpression of FASN is common in many cancers, and accumulating evidence suggests that it is a metabolic oncogene with an important role in tumor growth and survival, making it an attractive target for cancer therapy. Early small-molecule FASN inhibitors such as cerulenin, C75 and orlistat have been shown to induce apoptosis in several cancer cell lines and to induce tumor growth delay in several cancer xenograft models but their mechanism is still not well understood. These molecules suffer from pharmacological limitations and weight loss as a side effect that prevent their development as systemic drugs. Several potent inhibitors have recently been reported that may help to unravel and exploit the full potential of FASN as a target for cancer therapy in the near future. Furthermore, novel sources of FASN inhibitors, such as green tea and dietary soy, make both dietary manipulation and chemoprevention potential alternative modes of therapy in the future.
Keywords: C75, cancer, ERBB-2, fatty acid synthase, MAPK, PI3K/AKT, SREBP-1
Fatty acid synthase (FASN) is a key bio synthetic enzyme involved in lipogenesis and the production of long-chain fatty acids from acetyl-coenzyme A (CoA) and malonyl-CoA. Uptake of glucose into cancer cells leads to the production of pyruvate via the glycolytic pathway. Pyruvate is utilized to produce ATP via the Krebs cycle in the mitochondria; in turn, acetyl-CoA, one of the products, acts as a substrate for neoplastic lipogenesis (Figure 1). Normal cells (except liver and adipose tissue) have low levels of expression and activity of FASN, which is tightly regulated by diet, hormones and growth factors (reviewed in [1]). However, in rapidly proliferating cancer cells, fatty acids can be synthesized de novo in order to provide lipids for membrane formation and energy production via β-oxidation and lipid modification of proteins. As such, FASN is highly expressed in many cancers, including prostate, ovarian, breast, endometrial, thyroid, colorectal, bladder, lung, thyroid, oral, tongue, esophageal, hepatocellular, pancreatic and gastric carcinomas, as well as malignant melanoma, mesothelioma, nephroblastoma and retinoblastoma, soft tissue sarcoma (reviewed in [1–7]), gastrointestinal stromal tumor [8], Paget’s disease of the vulva [9] and multiple myeloma [10]. Interestingly, increased FASN expression has also been observed in some benign and pre-invasive lesions of prostate, breast, lung, stomach, colon (aberrant crypt foci) and cutaneous nevi [2,11–14].
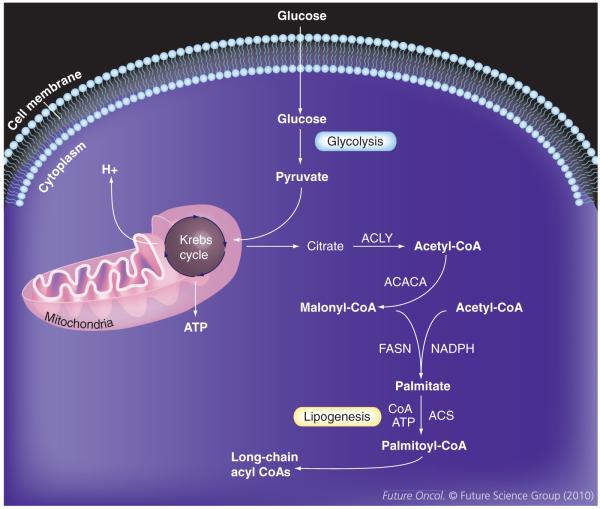
Figure 1. Fatty acid biosynthesis in malignancy.
Glucose is taken up into cells and is converted into pyruvate via anaerobic glycolysis. Pyruvate in turn is converted into citrate in the mitochondria via Krebs cycle to generate ATP. Excess citrate is metabolized to acetyl-CoA, which enters the lipogenesis pathway, ultimately leading to production of long-chain acyl-CoA.
ACACA: Acetyl co-enzyme A carboxylase; ACLY: ATP citrate lyase; ACS: Acyl co-enzyme A synthetase; CoA: Co-enzyme A; FASN: Fatty acid synthase; NADPH: Nicotinamide adenine dinucleotide phosphate.
Elevated expression of FASN has been linked to poor prognosis and reduced disease-free survival in many cancer types [15–19]. In addition, several reports have demonstrated that FASN plays an important role in tumor cell development and survival, with siRNA knockdown or pharmacological inhibition of FASN resulting in apoptosis of cancer cells and prolonged survival of xenograft tumors [20–23]. Overexpression studies in immortalized non-transformed human prostate epithelial cells and in transgenic mice have demonstrated that FASN is a bona fide oncogene in prostate cancer [24], and similarly in breast cancer, fatty acid biosynthesis induces a cancer-like phenotype in noncancerous epithelial cells that is dependent on HER1/HER2 signaling [25]. A potential mechanism of FASN onco genicity may involve cytoplasmic stabilization of β-catenin with palmitoylation of Wnt-1 and subsequent activation of the WNT/β-catenin pathway [26]. In this article, we focus on the mechanisms of FASN regulation in cancer and discuss recent updates on the potential of FASN as a therapeutic target in cancer treatment.
Regulation of FASN in cancer
The regulation of FASN expression in cancer is complex and involves transcriptional and post-translational control acting in concert with several microenvironmental influences (reviewed in [1,3,27]; Figure 2). Growth factor receptors, such as ERBB-2 and EGF receptor, interact and activate downstream PI3K/AKT and MAPK signaling pathways with subsequent transcriptional activation of FASN expression (loss of PTEN in prostate cancer tissue may also activate AKT thereby indirectly regulating FASN levels) [28]. Similarly, aberrant activation of AKT and MAPK can occur in hormonally sensitive organs (breast, endometrium, ovary and prostate) through activation of sex hormone receptors by estrogen, progesterone and androgen. Mutual crosstalk between upstream regulators: growth factors, sex hormones and their corresponding receptors, may also occur, amplifying FASN overexpression [27]. FASN, in turn, may activate the tyrosine kinase growth factor receptor as evidenced in human breast epithelial cells [25], thereby setting up an auto-regulatory loop. Ultimately, both the AKT and MAPK transduction pathways regulate FASN expression through the modulation of expression of sterol regulatory element-binding protein (SREBP)-1c, which binds to regulatory elements in the FASN promoter. Proto-oncogene FBI-1 (Pokemon), a transcription factor of the bric-à-brac tramtrack broad complex/pox viruses and zinc fingers (BTB/POZ) domain family, interacts directly with SREBP-1c through its DNA-binding domain to synergistically activate the transcription of FASN (Figure 2) [29]. This is accomplished by acting on the proximal GC box and SRE/E box.
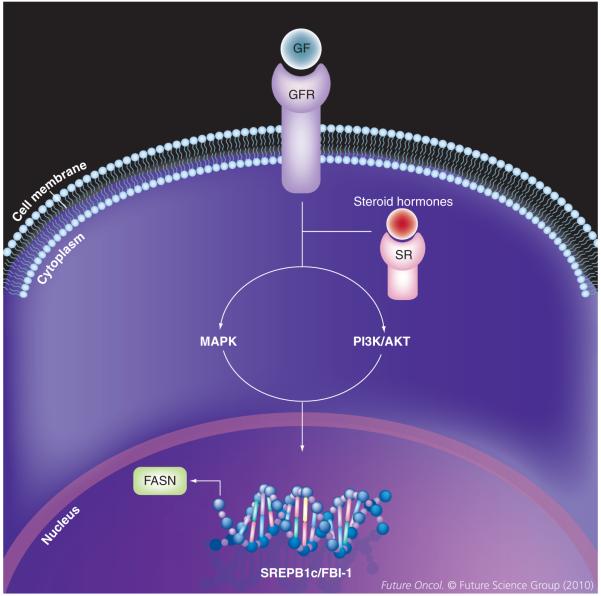
Figure 2. Regulation of fatty acid synthase expression in malignancy.
Once growth factor or steroid hormone receptors are activated by their corresponding ligand this leads to downstream activation of the PI3K/AKT or MAPK pathways. Both transduction pathways regulate FASN expression through modulation of expression of SREBP-1c and FBI-1, which binds to regulatory elements in the FASN promoter.
FASN: Fatty acid synthase; FBI-1: Pokemon; GF: Growth factor; GFR: Growth factor receptor; SR: Steroid Hormone receptor; SREBP-1c: Sterol regulatory element-binding protein 1c.
S14 is a lipogenesis-related nuclear protein that is overexpressed in most breast cancers. A recent study demonstrated that SREBP-1c drives S14 gene expression in breast cancer cells, and progesterone magnifies that effect via an indirect mechanism. This supports the prediction, based on S14 gene amplification and overexpression in breast tumors, that S14 augments breast cancer cell growth and survival [30]. These effects are mediated through FASN expression. p53 transcription family proteins may also have a role in regulating FASN expression. D’Erchia et al. report that the FASN gene is a conserved target of the p53 family throughout evolution; CEP-1, the Caenorhabditis elegans p53 homolog, is able to bind the two p53 family responsive elements identified in the worm fasn-1 gene. Moreover, by comparing wild-type and CEP-1 knockout worms, they demonstrated that fasn-1 expression is modulated by CEP-1 in vivo [31].
Overexpression and copy number gain of the FASN gene has been previously demonstrated in prostate cancer and related to distinct molecular signatures [16,32]. Using immunohistochemistry and FISH ana lysis in paraffin-embedded tissue microarrays, Shah et al. observed gene copy gain in 24% of all prostate adenocarcinoma specimens examined, with concurrent increased FASN protein expression. These findings suggest that FASN gene copy number increases may be involved in the resultant increase in FASN protein expression observed and indicate that potentially alternate post-translational mechanisms of FASN regulation exist in cancer [32]. Similarly, ubiquitin-specific protease 2a (USP2a), a pre-proteosomal deubinquinating enzyme, may interact with and stabilize FASN through the removal of ubiquitin [21]. USP2a is androgen regulated and overexpressed in prostate cancer; its functional inactivation results in decreased FASN protein and enhanced apoptosis. Thus, the isopeptidase USP2a plays a critical role in prostate cancer cell survival through FASN stabilization. An alternate mechanism of regulation may occur via mTOR-mediated translational induction in breast cancer cells overexpressing HER2. Yoon et al. found that SK-BR-3 and BT-474 breast cancer cells that overexpress HER2 also express higher levels of FASN, compared with MCF-7 and MDA-MB-231 breast cancer cells, in which HER2 expression is low. The induction of FASN in BT-474 cells was not mediated by the activation of SREBP-1c. Exogenous HER2 expression in MDA-MB-231 cells induced the expression of FASN, and the HER2-mediated increase in FASN was inhibited by both LY294002 (a PI3K inhibitor) and rapamycin (a mTOR inhibitor). In addition, the activation of mTOR by the overexpression of Ras homolog enriched in brain (RHEB) in MDA-MB-231 cells increased the synthetic rates of FASN. On the other hand, FASN was reduced in BT-474 cells by blockade of the mTOR signaling pathway [33].
As stated earlier, microenvironmental stresses also have a role to play in regulating FASN expression. Oliveras-Ferraros et al. found that extracellular levels of FASN are dependent on the metabolic state of the cell, whereby AMPK, in response to increasing AMP:ATP ratios, leads to extracellular FASN release and a restoration of the cellular energy state [34]. Aminoimidazole carboxamide ribonucleotide (AICAR), an AMPK-activating drug, by stimulating an elevation of the AMP:ATP ratio in breast cancer cells, leads to a dose- and time-dependent augmentation of extracellular FASN levels. Conversely, siRNA blockade of AMPK attenuated the release of FASN. Furuta et al. demonstrated that FASN is significantly upregulated by hypoxia in human breast cancer cell lines [35]. They also found that hypoxia significantly upregulated SREBP-1c via phosphorylation of AKT followed by activation of HIF1. Moreover, results of reporter assay and chromatin immunoprecipitation analysis indicate that SREBP-1c is strongly bound to the SREBP-binding site/E-box sequence on the FASN promoter under hypoxia. In their xenograft mouse model, FASN was strongly expressed in the hypoxic regions of the tumor. In addition, immunohistochemical analyses of human breast tumor specimens indicated that the expressions of both FASN and SREBP-1c were co-localized within hypoxic regions. Furthermore, they found that hypoxia-induced chemoresistance to cyclophosphamide was partially blocked by a combination of FASN inhibitor and cyclophosphamide, which has obvious therapeutic implications [35]. Separately, transient transfection studies performed using a 178-bp FASN promoter fragment harboring a complex SREBP-binding site was used to demonstrate that extracellular acidosis may act in an epigenetic fashion to induce changes in the transcriptional activation of FASN gene in breast cancer cells [36]. Interestingly, this stimulatory effect is equally mimicked by well-characterized oncogenic stimuli such as Her2/neu [36].
Structure of FASN
Recently, the crystal structure and catalytically active sites of FASN have been delineated. FASN is made up of a paired multifunctional poly peptide with seven catalytic domains that include an acyl-carrier protein (ACP). These domains (in linear order from the carboxy terminus) are: thioesterase, ACP, β-ketoacyl reductase, enoyl reductase, β-hydroxyacyl dehydratase, acetyl/malonyl-CoA transferase and β-ketoacyl synthase. There are two additional non enzymatic domains: a pseudoketoreductase; and a peripheral pseudomethyltransferase, which is probably a remnant of an ancestral methyltransferase domain maintained in some related polyketide synthases [37]. Initial work by Maier et al. resolved the 4.5-Å crystal structure of intact porcine FASN [38]; while, later, the crystal structure of mammalian FASN at 3.2-Å resolution, covering five catalytic domains, was determined (however, the flexibly tethered terminal ACP and thioesterase domains remain unresolved) [37]. Earlier work helped identify the active sites and the inter-relationships of the domain sites by biochemical methods [39–42]. A significant step forward was the determination of the crystal structure of the thioesterase domain from human FASN in complex with the orlistat ligand [43]. Importantly, natural product inhibitors of the ketoreductase domain and small-molecule inhibitors of the β-ketoacyl synthase and thioesterase domains have been described as having anti-oncogenic properties.
FASN as a potential drug target in cancer therapy
Fatty acid synthase is an attractive potential target for cancer therapy. As described in the previous sections, FASN is selectively overexpressed in many types of cancer, and these elevated levels have been linked to poor prognosis. RNAi knockdown experiments have shown that multiple cancer cell lines depend on FASN for proliferation and survival. To date, several compounds are known to inhibit FASN. These include cerulenin, C75, orlistat, C93 and naturally occurring polyphenols. Figure 3 outlines the structures of reported FASN inhibitors, and Box 1 is a summary of all compounds that inhibit FASN as outlined in this article.
Figure 3. Fatty acid synthase inhibitors.
(A) Cerulenin. (B) C75. (C) Orlistat. (D) GSK837149A. (E) AstraZeneca bisamide scaffold. (F) Merck hydroxyquinolin-2(1H)-one scaffold. (G) Platensimycin. (H) Epigallocatechin gallate analog.
Cerulenin and C75, both early small-molecule FASN inhibitors, have demonstrated significant antitumor activity. Cerulenin was isolated from Cephalosporium caerulens; it contains an epoxy group that reacts with the ketoacyl synthase domain of FASN [44]. It was one of the first compounds to be found to inhibit FASN in breast cancer cell lines, inducing programmed cell death, and to delay disease progression in a xenograft model of ovarian cancer; its cytotoxic effects are dependent on the level of FASN activity [45,46]. C75 was designed after cerulenin to overcome its chemical instability [47]. C75 is a weak, irreversible inhibitor of FASN that interacts with the β-ketoacyl synthase, the enoyl reductase and the thioesterase domains [48]. C75 showed tumor growth inhibition in a xenograft breast cancer model [23] and chemopreventive activity for mammary cancer in neu-N transgenic mice [49]. Recently, more potent analogs of C75 have been designed as FASN inhibitors [50]. Both cerulenin and C75 have been shown to cause profound effects on food intake and bodyweight in mice that could be limiting in the development of cancer therapy [51]. Weight loss seems to occur through the activation of mitochondrial fatty acid oxidation via the stimulation of carnitine palmitoyltransferase I and, furthermore, through the inducement of anorexia via the inhibition of production of neuropeptide Y within the hypothalamus [51,52]. This effect has been proposed to be mediated by brain FASN, which, with PPARα, would consti would constitute an integrative sensory module controlling energy balance and feeding behavior [53]. As a result of these effects, FASN has also been considered as a potential target for the treatment of obesity [54].
Several natural plant-derived polyphenols have been shown to inhibit FASN, including epigallocatechin-3-gallate (EGCG) and the flavonoids luteolin, taxifolin, kaempferol, quercetin and apigenin [55–57]. One of the best characterized polyphenol FASN inhibitors is EGCG, a natural component of green tea. EGCG is a high micromolar time-dependent inhibitor of FASN ketoacyl reductase domain [58]. Although EGCG is a promiscuous inhibitor targeting multiple signaling pathways [59], its apoptosis-inducing effect seems to correlate with its activity at FASN [60]. Another compound, luteolin, has the greatest effect on lipogenesis of the polyphenols and inhibits FASN directly. It has structural homology to PI3K inhibitors and has strong antioxidant activity [56]. Recently, more potent analogs of EGCG have been developed and have been shown to inhibit tumor growth in a breast cancer xenograft model [61].
Orlistat is a US FDA-approved pancreatic lipase inhibitor, originally developed as an anti-obesity drug, and is a potent inhibitor of FASN. Kridel et al. first identified orlistat in a proteomic screen for prostate cancer-specific enzymes as a potent FASN inhibitor showing antiproliferative activity against several prostate cancer cell lines in vitro, as well as tumor growth inhibition in a xenograft prostate cancer model [22]. Orlistat is an irreversible inhibitor forming a covalent adduct with the active serine of FASN thioesterase domain as shown in a recently published co-crystal structure [43]. In addition to the original report, orlistat has shown modest anticancer activity in a few in vivo models. Inhibition of tumor FASN activity by orlistat reduces prostate tumor growth in mice xenografts and, at a high concentration, reduces proliferation and promotes apoptosis in the mouse metastatic melanoma cell line B16-F10 (helping reduce the number of mediastinal lymph node metastases) and HER2-overexpressing breast cancer cell lines [22,62,63]. Further evidence indicates that orlistat can accelerate tumor cell apoptosis in culture at high concentrations and increase survival rates somewhat in gastric tumor-bearing mice in vivo [64]. However, orlistat suffers from several limitations hampering its development as a systemic drug: low cell permeability, low solubility, lack of selectivity [65], poor oral bioavailability and poor metabolic stability [66]. Several orlistat analogs have been developed in an attempt to improve on these limitations [67–70].
C93 (or FAS93), a synthetic FASN inhibitor designed after the bacterial FabB inhibitor thiolactomycin, was recently developed as part of an effort to overcome C75’s lack of potency and side effects [71]. C93 has shown some significant tumor growth delay in non-small-cell lung cancer xenograft models and ovarian cancer xenograft models, as well as some chemopreventive effects in chemically induced lung tumors [72–74]. Importantly, C93 did not cause anorexia and weight loss in treated animals [72]. C247 belongs to the same class of compounds as C93 and has also demonstrated efficacy in a transgenic model of breast cancer with no weight-loss side effects [49,71,75]. Recently, the team that developed FAS93 reported a new orally available FASN inhibitor, FAS31. FAS31 showed tumor reduction in ovarian cancer xenograft models with no effect on bodyweight. In preliminary toxicity studies, FAS31 showed no observable toxicity to normal tissues in the rat or mouse [76]. Unfortunately, C93, C247 and FAS31 structures have not been released yet.
As a testimony to the interest in FASN as a therapeutic target, several recent reports describe new potent FASN inhibitors identified through high-throughput screening or medicinal chemistry programs. For example, a research group at Merck developed a series of 3-aryl-4-hydroxyquinolin-2(1H)-one derivatives while another research group at AstraZeneca developed a series of bisamide derivatives as FASN inhibitors [77,201]. Both groups obtained compounds with activities in the low nanomolar range in their respective FASN biochemical assays but did not report any data regarding cellular or in vivo activity. The dibenzenesulfonamide urea GSK837149A was identified as a low, nanomolar FASN inhibitor by high-throughput screening at GlaxoSmithKline. Biochemical studies showed that GSK837149A is a reversible inhibitor of the FASN β-ketoacyl reductase domain, but its poor cell permeability prevented the study of its mechanism in cells [78]. A systematic screening of 250,000 natural product extracts led to the isolation of platensimycin as a potent inhibitor of bacterial FabF/B with a broad-spectrum Gram-positive antibacterial activity [79]. Platensimycin has also been claimed to potently inhibit mammalian FASN in a biochemical assay, but to our knowledge, no studies in cancer cell lines have been reported [202].
Mechanism of action of FASN inhibitors
The mode of action of several small-molecule FASN inhibitors is not fully understood. Their study can be hampered by their lack of potency, selectivity or cell permeability [48,59,65,78]. FASN inhibition initiates selective apoptosis of cancer cells both in vivo and in vitro, which may involve accumulation of toxic intermediary metabolite malonyl-CoA with reduction of both membrane synthesis and phospholipid function leading to both cytostatic and cytotoxic effects [23,80]. Indeed, recent evidence suggests that malonyl-CoA decarboxylase inhibition may be a potential novel target for cancer treatment [81]. Inhibition of FASN has been shown to induce endoplasmic reticulum stress in tumor cells [82], and a further mechanism of action may involve cooperation with endoplasmic reticulum stress inducers to enhance apoptosis [83]. In addition, ceramide accumulation following siRNA inhibition of FASN may have cytotoxic effects leading to tumor cell death in breast cancer cells (which can be rescued with inhibition of ceramide synthesis) [84]. Focusing on specific compounds, cerulenin has been shown to interfere with DNA replication and both p53 accumulation and mitochondrial-mediated apoptosis appear to have important roles in tumor-cell death [85–87]. C75 has similar effects with rapid malonyl-CoA and p53 accumulation. Similarly, in a subset of papillary thyroid carcinomas, FASN inhibition with C75 induces growth arrest and promotes apoptosis via activation of the mitochondrial arm of the apoptotic pathway with subsequent activation of the caspase cascade [88]. Orlistat appears to have cell cycle effects, inducing G1/S arrest, leads to dysregulation of Skp2 with resultant p27kip1 accumulation and activation of the retinoblastoma pathway [89]. Furthermore, it prevents endothelial cell proliferation and importantly inhibits human neovascularization in ex vivo assays, suggesting it maybe useful as an antiangiogenic drug. These effects are mediated through prevention of VEGF receptor appearance on the endothelial cell surface [90]. Knowles et al. have shown that inhibition of FASN, by either knockdown with siRNA or inhibition with the small-molecule drug orlistat, leads to activation of the receptor-mediated apoptotic cascade (caspase-8-mediated) and ultimately to cell death. The unique apoptotic effect of FASN inhibition results from negative regulation of the mTOR pathway via stress response gene DDIT4 (DNA damage-inducible transcript 4) [91].
Several oncogenic signaling pathways are affected by FASN inhibition.
Recent evidence suggests interaction between FASN and ErbB systems in ovarian cancer cells and that interference with FASN and ErbB abrogates their oncogenicity [92]. Furthermore, C75 reduces HER2 expression in breast cancer cells [93]. This has the potential to be exploited therapeutically both in breast and ovarian cancer treatment, whereby FASN inhibition with C75 can sensitize tumor cells against anti-ErbB drugs (e.g., pelintinib, canertinib, erlotinib, cetuximab, matuzumab and trastuzumab). It seems that AKT is crucial for ErbB/FASN interaction, and several studies indicate a connection between FASN inhibition and the PI3K pathway. High-level expression of FASN in prostate cancer is linked to activation and nuclear localization of AKT and PI3K inhibition synergizes with FASN siRNA to induce prostate tumor cell apoptosis [28,94]. Furthermore, positive-feedback regulation between AKT activation and FASN expression in ovarian cancer cells has been demonstrated and, indeed, inhibition of the PI3K/AKT pathway sensitizes breast cancer cells to cerulenin-induced cell death [95,96]. Similarly, the mode of action of C93 potentially may be mediated through the AKT signaling pathway or through AMPK [73,74]. Incidentally, FASN inhibition drives the synthesis of phospholipid partioning into detergent-resistant membrane microdomains or lipid rafts that are associated with these signaling complexes and pathways, highlighting a functional link between FASN inhibitors and these signaling pathways [97].
FASN & multidrug resistance
Multidrug resistance is a significant problem in cancer chemotherapy and, importantly, FASN overexpression seems to be a recently identified mechanism of multidrug resistance in cancer. Liu et al. identified that ectopic overexpression of FASN induced drug resistance in breast cancer cell lines MCF7 and MDA-MB-468; use of orlistat sensitized these cells to anticancer therapy. The proposed mechanism is FASN overexpression may lead to a decrease in drug-induced apoptosis due to an overproduction of palmitic acid [98]. This phenomenon of FASN-induced drug resistance may be exploited therapeutically through the use of FASN inhibitors solely or in combination with other chemo-therapeutic agents. For example, FASN blockade can induce a synergistic chemosensitization of breast cancer cells to microtubule-interfering agents, such as docetaxel, paclitaxel and vinorelbine. Furthermore, cerulenin and 5-fluorouracil display a schedule-dependent synergistic interaction, leading to an enhancement of the efficacy of antimetabolite treatment in breast carcinoma cells [99]. Indeed, pharmacological blockade of FASN reverses autoresistance to trastuzumab by transcriptionally inhibiting HER2 ‘super expression’ occurring in high-dose trastuzumab-conditioned SKBR3/Tzb100 breast cancer cells [100]. Interestingly, disruption of crosstalk between the fatty acid synthesis and proteasome pathways in prostate cancer cell lines can enhance unfolded protein response signaling and cell death [101]. FASN inhibition may also have an anti-angiogenic role, as orlistat has been found to suppress endothelial cell proliferation in vivo [90].
Conclusion
Fatty acid synthase appears to play a key role in tumor initiation and propagation for many malignancies and, as such, represents an attractive target for cancer treatment. Further improvements in developing and identifying novel FASN inhibitors, as well as identifying the patients who may benefit most from them, will potentially offer more effective treatment strategies.
Future perspective
Over the last 15 years, FASN has emerged as an attractive target for cancer therapy. Early small-molecule FASN inhibitors like cerulenin, C75 and orlistat have been shown to induce apoptosis in several cancer cell lines and tumor-growth delay in several cancer xenograft models but their mechanism is still not well understood. These molecules suffer from selectivity, metabolic and pharmacologic limitations that hamper their use in preclinical and clinical settings. Several new potent inhibitors recently reported in the scientific and patent literature testify to the activity in the field and may help unravel and exploit the full potential of FASN as a target for cancer therapy in the near future.
Overexpression of FASN in many preinvasive lesions indicates the potential utility of FASN inhibitors for chemoprevention [12–14,102]. Dietary manipulation may become a real possibility: green tea catechin inhibits FASN without stimulating crossactivation of fatty acid oxidation and inducing weight loss [103,104]. In addition, Xiao et al. found that dietary soy protein inhibits DNA damage and cell survival of colon epithelial cells through attenuated expression of FASN and decreasing circulating insulin levels [105]. The anti-oncogenic effects of the main olive oil monounsaturated fatty acid oleic acid (18:1n-9) which is found in the Mediterranean diet has also been demonstrated [106]. Oleic-acid treatment efficiently blocks FASN activity and down regulates protein expression, which directly leads to an accumulation of FASN substrate malonyl-CoA suppressing HER2 expression.
Moving forward, if sufficiently tolerable FASN inhibitors become widely available, further work will be needed to identify patients in whom FASN inhibition is most likely to be beneficial so that they can be selected for FASN inhibitor trials. Recent work from epidemiological studies suggests an interaction between obesity and the impact of FASN, such that FASN’s deleterious effects on survival seem to be most pronounced in obese patients. Specifically, Ogino et al. observed that increased FASN expression in colon cancer tumors was associated with increased mortality in those with a high BMI but not those with a low BMI [15]. Similarly, unpublished data from the Physician’s Health Study and Health Professionals Follow-up Study suggest that increased FASN expression in prostate cancer tumors is associated with increased prostate-cancer-specific mortality in men with a high BMI but not those with a low BMI [Nguyen et al., Unpublished Data]. Taken together, this epidemiological evidence raises the possibility that men who are obese and whose tumors express high levels of FASN may have the most to gain from FASN inhibition, and should be selected first for FASN inhibitor trials as they become available.
Box 1. Potential fatty acid synthase inhibitors.
Small-molecule inhibitors
▪ Cerulenin
▪ C75
▪ Orlistat
▪ C93 (FAS93)
▪ FAS31
▪ C247
▪ GSK837149A
▪ Platensimycin
▪ Merck 3-aryl-4-hydroxyquinolin-2(1H)-one scaffold
▪ AstraZeneca bisamide scaffold
Naturally occuring polyphenols
▪ Epigallocatechin
▪ Luteolin
▪ Taxifolin
▪ Kaempferol
▪ Quercetin
▪ Apigenin
Dietary compounds
▪ Catechin
▪ Soy protein
▪ Monounsaturated fatty acid oleic acid (18:1n-9)
Executive summary.
Regulation of fatty acid synthase in cancer
▪ The regulation of fatty acid synthase (FASN) expression in cancer is complex. This may involve transcriptional control from growth factors or steroid hormones and post-translational control from elements such as deubiquitinating enzymes. Microenvironmental factors such as hypoxia may also exert a regulatory influence.
FASN as a potential drug target in cancer therapy
▪ Over the last 15 years, FASN has emerged as an attractive potential target for cancer therapy.
▪ Early small-molecule inhibitors of FASN include cerulenin, C75 and orlistat.
▪ The mode of action of these compounds is not entirely understood but may involve fatty acid depletion.
▪ Therapeutic use of these compounds has been limited due to their poor pharmacologic or pharmaceutical properties and to their effect on feeding behavior.
▪ Recently developed FASN inhibitors such as C93, FAS31, 3-aryl-4-hydroxyquinolin-2(1H)-one derivatives or bisamide derivatives reported herein may offer new opportunities to access FASN inhibitors with greater efficacy and reduced side effects.
▪ FASN overexpression has a role in drug resistance, which can be exploited therapeutically with FASN inhibitors.
▪ Novel sources of FASN inhibitors, such as green tea and dietary soy, make both dietary manipulation and chemoprevention potential alternative modes of therapy in the future.
▪ Obese patients whose tumors overexpress FASN may have the most to gain from FASN inhibition and could be ideal candidates for FASN trials as more tolerable compounds are made available.
Acknowledgments
Support to Massimo Loda was obtained from the Prostate Cancer Foundation, the National Cancer Institute (RO1CA131945, PO1CA89021 and P50 CA90381), the Linda and Arthur Gelb Center for Translational Research, a gift from Nuclea Biomarkers to the Jimmy Fund and the Loda laboratory. Massimo Loda is the recipient of a grant from the Dana Farber Cancer Institute–Novartis Drug Development Program.
Footnotes
Financial & competing interests disclosure The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Richard Flavin, Center for Molecular Oncologic Pathology, Dana Farber Cancer Institute , Harvard Medical School, Boston, MA, USA and Brigham & Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Stephane Peluso, Infinity Pharmaceuticals, Cambridge, MA, USA.
Paul L Nguyen, Department of Radiation Oncology, Dana Farber Cancer Institute, Harvard Medical School, Boston, MA, USA and Brigham & Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Massimo Loda, Department of Medical Oncology, Dana Farber Cancer Institute, Harvard Medical School, Boston, MA, USA and Department of Pathology, Brigham & Women’s Hospital, Harvard Medical School, Boston, MA, USA and The Broad Institute of Harvard & MIT, Cambridge, MA, USA.
Bibliography
▪ of interest
▪▪ of considerable interest
- 1.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer. 2007;7(10):763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 2.Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16(3):202–208. doi: 10.1016/s0899-9007(99)00266-x. [DOI] [PubMed] [Google Scholar]
- 3.Kuhajda FP. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res. 2006;66(12):5977–5980. doi: 10.1158/0008-5472.CAN-05-4673. [DOI] [PubMed] [Google Scholar]
- 4.Menendez JA, Lupu R. Fatty acid synthase-catalyzed de novo fatty acid biosynthesis: from anabolic-energy-storage pathway in normal tissues to jack-of-all-trades in cancer cells. Arch. Immunol. Ther. Exp. (Warsz.) 2004;52(6):414–426. [PubMed] [Google Scholar]
- 5.Menendez JA, Lupu R. Oncogenic properties of the endogenous fatty acid metabolism: molecular pathology of fatty acid synthase in cancer cells. Curr. Opin. Clin. Nutr. Metab. Care. 2006;9(4):346–357. doi: 10.1097/01.mco.0000232893.21050.15. [DOI] [PubMed] [Google Scholar]
- 6.Menendez JA, Lupu R, Colomer R. Targeting fatty acid synthase: potential for therapeutic intervention in HER2/neu-overexpressing breast cancer. Drug News Perspect. 2005;18(6):375–385. doi: 10.1358/dnp.2005.18.6.927929. [DOI] [PubMed] [Google Scholar]
- 7.Swinnen JV, Brusselmans K, Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Curr. Opin. Clin. Nutr. Metab. Care. 2006;9(4):358–365. doi: 10.1097/01.mco.0000232894.28674.30. [DOI] [PubMed] [Google Scholar]
- 8.Rossi S, Ou W, Tang D, et al. Gastrointestinal stromal tumours overexpress fatty acid synthase. J. Pathol. 2006;209(3):369–375. doi: 10.1002/path.1983. [DOI] [PubMed] [Google Scholar]
- 9.Alo PL, Galati GM, Sebastiani V, et al. Fatty acid synthase expression in Paget’s disease of the vulva. Int. J. Gynecol. Pathol. 2005;24(4):404–408. doi: 10.1097/01.pgp.0000170065.53813.81. [DOI] [PubMed] [Google Scholar]
- 10.Wang WQ, Zhao XY, Wang HY, Liang Y. Increased fatty acid synthase as a potential therapeutic target in multiple myeloma. J. Zhejiang Univ. Sci. B. 2008;9(6):441–447. doi: 10.1631/jzus.B0740640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Innocenzi D, Alo PL, Balzani A, et al. Fatty acid synthase expression in melanoma. J. Cutan. Pathol. 2003;30(1):23–28. doi: 10.1034/j.1600-0560.2003.300104.x. [DOI] [PubMed] [Google Scholar]
- 12.Kusakabe T, Nashimoto A, Honma K, Suzuki T. Fatty acid synthase is highly expressed in carcinoma, adenoma and in regenerative epithelium and intestinal metaplasia of the stomach. Histopathology. 2002;40(1):71–79. doi: 10.1046/j.1365-2559.2002.01289.x. [DOI] [PubMed] [Google Scholar]
- 13.Milgraum LZ, Witters LA, Pasternack GR, Kuhajda FP. Enzymes of the fatty acid synthesis pathway are highly expressed in in-situ breast carcinoma. Clin. Cancer Res. 1997;3(11):2115–2120. [PubMed] [Google Scholar]
- 14.Piyathilake CJ, Frost AR, Manne U, et al. The expression of fatty acid synthase (FASE) is an early event in the development and progression of squamous cell carcinoma of the lung. Hum. Pathol. 2000;31(9):1068–1073. doi: 10.1053/hupa.2000.9842. [DOI] [PubMed] [Google Scholar]
- 15.Ogino S, Nosho K, Meyerhardt JA, et al. Cohort study of fatty acid synthase expression and patient survival in colon cancer. J. Clin. Oncol. 2008;26(35):5713–5720. doi: 10.1200/JCO.2008.18.2675. ▪ Epidemiological study suggesting an interaction between obesity and fatty acid synthase (FASN).
- 16.Rossi S, Graner E, Febbo P, et al. Fatty acid synthase expression defines distinct molecular signatures in prostate cancer. Mol. Cancer Res. 2003;1(10):707–715. [PubMed] [Google Scholar]
- 17.Shurbaji MS, Kalbfleisch JH, Thurmond TS. Immunohistochemical detection of a fatty acid synthase (OA-519) as a predictor of progression of prostate cancer. Hum. Pathol. 1996;27(9):917–921. doi: 10.1016/s0046-8177(96)90218-x. [DOI] [PubMed] [Google Scholar]
- 18.Takahiro T, Shinichi K, Toshimitsu S. Expression of fatty acid synthase as a prognostic indicator in soft tissue sarcomas. Clin. Cancer Res. 2003;9(6):2204–2212. [PubMed] [Google Scholar]
- 19.Visca P, Sebastiani V, Botti C, et al. Fatty acid synthase (FAS) is a marker of increased risk of recurrence in lung carcinoma. Anticancer Res. 2004;24(6):4169–4173. [PubMed] [Google Scholar]
- 20.De Schrijver E, Brusselmans K, Heyns W, Verhoeven G, Swinnen JY. RNA interference-mediated silencing of the fatty acid synthase gene attenuates growth and induces morphological changes and apoptosis of LNCaP prostate cancer cells. Cancer Res. 2003;63(13):3799–3804. [PubMed] [Google Scholar]
- 21.Graner E, Tang D, Rossi S, et al. The isopeptidase USP2a regulates the stability of fatty acid synthase in prostate cancer. Cancer Cell. 2004;5(3):253–261. doi: 10.1016/s1535-6108(04)00055-8. ▪ Illustrates the role of post-translational control of FASN expression.
- 22.Kridel SJ, Axelrod F, Rozenkrantz N, Smith JW. Orlistat is a novel inhibitor of fatty acid synthase with antitumor activity. Cancer Res. 2004;64(6):2070–2075. doi: 10.1158/0008-5472.can-03-3645. ▪ Highlights inhibition of the thioesterase domain of FASN with induction of tumor cell apoptosis.
- 23.Pizer ES, Thupari J, Han WF, et al. Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced by fatty-acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res. 2000;60(2):213–218. [PubMed] [Google Scholar]
- 24.Migita T, Ruiz S, Fornari A, et al. Fatty acid synthase: a metabolic enzyme and candidate oncogene in prostate cancer. J. Natl Cancer Inst. 2009;101(7):519–532. doi: 10.1093/jnci/djp030. ▪▪ Establishes FASN as a bona fide oncogene in prostate cancer.
- 25.Vazquez-Martin A, Colomer R, Brunet J, Lupu R, Menendez JA. Overexpression of fatty acid synthase gene activates HER1/HER2 tyrosine kinase receptors in human breast epithelial cells. Cell Prolif. 2008;41(1):59–85. doi: 10.1111/j.1365-2184.2007.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiorentino M, Zadra G, Palescandolo E, et al. Overexpression of fatty acid synthase is associated with palmitoylation of WNT1 and cytoplasmic stabilization of β-catenin in prostate cancer. Lab. Invest. 2008;88(12):1340–1348. doi: 10.1038/labinvest.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mashima T, Seimiya H, Tsuruo T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br. J. Cancer. 2009;100(9):1369–1372. doi: 10.1038/sj.bjc.6605007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bandyopadhyay S, Pai SK, Watabe M, et al. FAS expression inversely correlates with PTEN level in prostate cancer and a PI3-kinase inhibitor synergizes with FAS siRNA to induce apoptosis. Oncogene. 2005;24(34):5389–5395. doi: 10.1038/sj.onc.1208555. [DOI] [PubMed] [Google Scholar]
- 29.Choi WI, Jeon BN, Park H, et al. Proto-oncogene FBI-1 (Pokemon) and SREBP-1 synergistically activate transcription of fatty-acid synthase gene (FASN) J. Biol. Chem. 2008;283(43):29341–29354. doi: 10.1074/jbc.M802477200. ▪ New role for FBI-1 (Pokemon) in regulating FASN expression through synergistic activity with sterol regulatory element-binding protein 1c.
- 30.Martel PM, Bingham CM, McGraw CJ, et al. S14 protein in breast cancer cells: Direct evidence of regulation by SREBP-1c, superinduction with progestin, and effects on cell growth. Exp. Cell Res. 2006;312(3):278–288. doi: 10.1016/j.yexcr.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 31.D’Erchia AM, Tullo A, Lefkimmiatis K, Saccone C, Sbisa E. The fatty acid synthase gene is a conserved p53 family target from worm to human. Cell Cycle. 2006;5(7):750–758. doi: 10.4161/cc.5.7.2622. [DOI] [PubMed] [Google Scholar]
- 32.Shah US, Dhir R, Gollin SM, et al. Fatty acid synthase gene overexpression and copy number gain in prostate adenocarcinoma. Hum. Pathol. 2006;37(4):401–409. doi: 10.1016/j.humpath.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 33.Yoon S, Lee My, Park SW, et al. Up-regulation of acetyl-CoA carboxylase α and fatty acid synthase by human epidermal growth factor receptor 2 at the translational level in breast cancer cells. J. Biol. Chem. 2007;282(36):26122–26131. doi: 10.1074/jbc.M702854200. [DOI] [PubMed] [Google Scholar]
- 34.Oliveras-Ferraros C, Vazquez-Martin A, Fernandez-Real JM, Menendez JA. AMPK-sensed cellular energy state regulates the release of extracellular fatty acid synthase. Biochem. Biophys. Res. Commun. 2009;378(3):488–493. doi: 10.1016/j.bbrc.2008.11.067. [DOI] [PubMed] [Google Scholar]
- 35.Furuta E, Pai SK, Zhan R, et al. Fatty acid synthase gene is up-regulated by hypoxia via activation of AKT and sterol regulatory element binding protein-1. Cancer Res. 2008;68(4):1003–1011. doi: 10.1158/0008-5472.CAN-07-2489. [DOI] [PubMed] [Google Scholar]
- 36.Menendez JA, Decker JP, Lupu R. In support of fatty acid synthase (FAS) as a metabolic oncogene: extracellular acidosis acts in an epigenetic fashion activating FAS gene expression in cancer cells. J. Cell. Biochem. 2005;94(1):1–4. doi: 10.1002/jcb.20310. ▪ Highlights the epigenetic role of modifying FASN expression.
- 37.Maier T, Leibundgut M, Ban N. The crystal structure of a mammalian fatty acid synthase. Science. 2008;321(5894):1315–1322. doi: 10.1126/science.1161269. [DOI] [PubMed] [Google Scholar]
- 38.Maier T, Jenni S, Ban N. Architecture of mammalian fatty acid synthase at 4.5 Å resolution. Science. 2006;311(5765):1258–1262. doi: 10.1126/science.1123248. [DOI] [PubMed] [Google Scholar]
- 39.Asturias FJ, Chadick JZ, Cheung IK, et al. Structure and molecular organization of mammalian fatty acid synthase. Nat. Struct. Mol. Biol. 2005;12(3):225–232. doi: 10.1038/nsmb899. [DOI] [PubMed] [Google Scholar]
- 40.Brink J, Ludtke SJ, Kong Y, Wakil SJ, Ma J, Chiu W. Experimental verification of conformational variation of human fatty acid synthase as predicted by normal mode ana lysis. Structure. 2004;12(2):185–191. doi: 10.1016/j.str.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 41.Chirala SS, Wakil SJ. Structure and function of animal fatty acid synthase. Lipids. 2004;39(11):1045–1053. doi: 10.1007/s11745-004-1329-9. [DOI] [PubMed] [Google Scholar]
- 42.Smith S, Witkowski A, Joshi AK. Structural and functional organization of the animal fatty acid synthase. Prog. Lipid Res. 2003;42(4):289–317. doi: 10.1016/s0163-7827(02)00067-x. [DOI] [PubMed] [Google Scholar]
- 43.Pemble CWT, Johnson LC, Kridel SJ, Lowther WT. Crystal structure of the thioesterase domain of human fatty acid synthase inhibited by orlistat. Nat. Struct. Mol. Biol. 2007;14(8):704–709. doi: 10.1038/nsmb1265. ▪▪ Landmark paper where the crystal structure of the thioesterase domain of human FASN is outlined.
- 44.Funabashi H, Kawaguchi A, Tomoda H, Omura S, Okuda S, Iwasaki S. Binding site of cerulenin in fatty acid synthetase. J. Biochem. 1989;105(5):751–755. doi: 10.1093/oxfordjournals.jbchem.a122739. [DOI] [PubMed] [Google Scholar]
- 45.Pizer ES, Jackisch C, Wood FD, Pasternack GR, Davidson NE, Kuhajda FP. Inhibition of fatty acid synthesis induces programmed cell death in human breast cancer cells. Cancer Res. 1996;56(12):2745–2747. [PubMed] [Google Scholar]
- 46.Pizer ES, Wood FD, Heine HS, Romantsev FE, Pasternack GR, Kuhajda FP. Inhibition of fatty acid synthesis delays disease progression in a xenograft model of ovarian cancer. Cancer Res. 1996;56(6):1189–1193. [PubMed] [Google Scholar]
- 47.Kuhajda FP, Pizer ES, Li JN, Mani NS, Frehywot GL, Townsend CA. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc. Natl Acad. Sci. USA. 2000;97(7):3450–3454. doi: 10.1073/pnas.050582897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rendina AR, Cheng D. Characterization of the inactivation of rat fatty acid synthase by C75: inhibition of partial reactions and protection by substrates. Biochem. J. 2005;388(Pt 3):895–903. doi: 10.1042/BJ20041963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alli PM, Pinn ML, Jaffee EM, Mcfadden JM, Kuhajda FP. Fatty acid synthase inhibitors are chemopreventive for mammary cancer in neu-N transgenic mice. Oncogene. 2005;24(1):39–46. doi: 10.1038/sj.onc.1208174. [DOI] [PubMed] [Google Scholar]
- 50.Wang X, Lin J, Chen Y, et al. Novel fatty acid synthase (FAS) inhibitors: design, synthesis, biological evaluation, and molecular docking studies. Bioorg. Med. Chem. 2009;17(5):1898–1904. doi: 10.1016/j.bmc.2009.01.050. ▪ Highlights novel FASN inhibitors under current development.
- 51.Loftus TM, Jaworsky DE, Frehywot GL, et al. Reduced food intake and bodyweight in mice treated with fatty acid synthase inhibitors. Science. 2000;288(5475):2379–2381. doi: 10.1126/science.288.5475.2379. [DOI] [PubMed] [Google Scholar]
- 52.Thupari JN, Landree LE, Ronnett GY, Kuhajda FP. C75 increases peripheral energy utilization and fatty acid oxidation in diet-induced obesity. Proc. Natl Acad. Sci. USA. 2002;99(14):9498–9502. doi: 10.1073/pnas.132128899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chakravarthy MY, Zhu Y, Lopez M, et al. Brain fatty acid synthase activates PPARα to maintain energy homeostasis. J. Clin. Invest. 2007;117(9):2539–2552. doi: 10.1172/JCI31183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buettner C. Does FASing out new fat in the hypothalamus make you slim? Cell Metab. 2007;6(4):249–251. doi: 10.1016/j.cmet.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 55.Tian WX. Inhibition of fatty acid synthase by polyphenols. Curr. Med. Chem. 2006;13(8):967–977. doi: 10.2174/092986706776361012. [DOI] [PubMed] [Google Scholar]
- 56.Brusselmans K, Vrolix R, Verhoeven G, Swinnen JY. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J. Biol. Chem. 2005;280(7):5636–5645. doi: 10.1074/jbc.M408177200. [DOI] [PubMed] [Google Scholar]
- 57.Li BH, Tian WX. Inhibitory effects of flavonoids on animal fatty acid synthase. J. Biochem. 2004;135(1):85–91. doi: 10.1093/jb/mvh010. [DOI] [PubMed] [Google Scholar]
- 58.Wang X, Tian W. Green tea epigallocatechin gallate: a natural inhibitor of fatty-acid synthase. Biochem. Biophys. Res. Commun. 2001;288(5):1200–1206. doi: 10.1006/bbrc.2001.5923. [DOI] [PubMed] [Google Scholar]
- 59.Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (-)-epigallocatechin-3-gallate. Cancer Res. 2006;66(5):2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 60.Brusselmans K, De Schrijver E, Heyns W, Verhoeven G, Swinnen JV. Epigallocatechin-3-gallate is a potent natural inhibitor of fatty acid synthase in intact cells and selectively induces apoptosis in prostate cancer cells. Int. J. Cancer. 2003;106(6):856–862. doi: 10.1002/ijc.11317. [DOI] [PubMed] [Google Scholar]
- 61.Puig T, Turrado C, Benhamu B, et al. Novel inhibitors of fatty acid synthase with anticancer activity. Clin. Cancer Res. 2009;15(24):7608–7615. doi: 10.1158/1078-0432.CCR-09-0856. [DOI] [PubMed] [Google Scholar]
- 62.Menendez JA, Vellon L, Lupu R. Antitumoral actions of the anti-obesity drug orlistat (Xenical™) in breast cancer cells: blockade of cell cycle progression, promotion of apoptotic cell death and PEA3-mediated transcriptional repression of HER2/neu (ErbB-2) oncogene. Ann Oncol. 2005;16(8):1253–1267. doi: 10.1093/annonc/mdi239. [DOI] [PubMed] [Google Scholar]
- 63.Carvalho MA, Zecchin KG, Seguin F, et al. Fatty acid synthase inhibition with orlistat promotes apoptosis and reduces cell growth and lymph node metastasis in a mouse melanoma model. Int. J. Cancer. 2008;123(11):2557–2565. doi: 10.1002/ijc.23835. [DOI] [PubMed] [Google Scholar]
- 64.Dowling S, Cox J, Cenedella RJ. Inhibition of fatty acid synthase by orlistat accelerates gastric tumor cell apoptosis in culture and increases survival rates in gastric tumor bearing mice in vivo. Lipids. 2009;44(6):489–498. doi: 10.1007/s11745-009-3298-2. [DOI] [PubMed] [Google Scholar]
- 65.Hoover HS, Blankman JL, Niessen S, Cravatt BF. Selectivity of inhibitors of endocannabinoid biosynthesis evaluated by activity-based protein profiling. Bioorg. Med. Chem. Lett. 2008;18(22):5838–5841. doi: 10.1016/j.bmcl.2008.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhi J, Melia AT, Funk C, et al. Metabolic profiles of minimally absorbed orlistat in obese/overweight volunteers. J. Clin. Pharmacol. 1996;36(11):1006–1011. doi: 10.1177/009127009603601104. [DOI] [PubMed] [Google Scholar]
- 67.Ma G, Zancanella M, Oyola Y, Richardson RD, Smith JW, Romo D. Total synthesis and comparative ana lysis of orlistat, valilactone, and a transposed orlistat derivative: inhibitors of fatty acid synthase. Org. Lett. 2006;8(20):4497–4500. doi: 10.1021/ol061651o. [DOI] [PubMed] [Google Scholar]
- 68.Purohit VC, Richardson RD, Smith JW, Romo D. Practical, catalytic, asymmetric synthesis of β-lactones via a sequential ketene dimerization/hydrogenation process: inhibitors of the thioesterase domain of fatty acid synthase. J. Org. Chem. 2006;71(12):4549–4558. doi: 10.1021/jo060392d. [DOI] [PubMed] [Google Scholar]
- 69.Richardson RD, Ma G, Oyola Y, et al. Synthesis of novel β-lactone inhibitors of fatty acid synthase. J. Med. Chem. 2008;51(17):5285–5296. doi: 10.1021/jm800321h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang W, Richardson RD, Chamni S, Smith JW, Romo D. β-lactam congeners of orlistat as inhibitors of fatty acid synthase. Bioorg. Med. Chem. Lett. 2008;18(7):2491–2494. doi: 10.1016/j.bmcl.2008.02.043. [DOI] [PubMed] [Google Scholar]
- 71.McFadden JM, Medghalchi SM, Thupari JN, et al. Application of a flexible synthesis of (5R)-thiolactomycin to develop new inhibitors of type I fatty acid synthase. J. Med. Chem. 2005;48(4):946–961. doi: 10.1021/jm049389h. [DOI] [PubMed] [Google Scholar]
- 72.Orita H, Coulter J, Lemmon C, et al. Selective inhibition of fatty acid synthase for lung cancer treatment. Clin. Cancer Res. 2007;13(23):7139–7145. doi: 10.1158/1078-0432.CCR-07-1186. [DOI] [PubMed] [Google Scholar]
- 73.Orita H, Coulter J, Tully E, Kuhajda FP, Gabrielson E. Inhibiting fatty acid synthase for chemoprevention of chemically induced lung tumors. Clin. Cancer Res. 2008;14(8):2458–2464. doi: 10.1158/1078-0432.CCR-07-4177. ▪ Outlines the effect of FASN inhibition with C93 for chemoprevention in chemically induced lung tumors.
- 74.Zhou W, Han WF, Landree LE, et al. Fatty acid synthase inhibition activates AMP-activated protein kinase in SKOV3 human ovarian cancer cells. Cancer Res. 2007;67(7):2964–2971. doi: 10.1158/0008-5472.CAN-06-3439. [DOI] [PubMed] [Google Scholar]
- 75.McFadden JM, Frehywot GL, Townsend CA. A flexible route to (5R)-thiolactomycin, a naturally occurring inhibitor of fatty acid synthesis. Org. Lett. 2002;4(22):3859–3862. doi: 10.1021/ol026685k. [DOI] [PubMed] [Google Scholar]
- 76.El Meskini R, Medghalchi SM, Vadlamudi A, et al. Fatty acid synthase inhibition for ovarian cancer. Presented at: AACR Annual Meeting; San Diego, CA, USA. Apr, 2008. pp. 12–16. (Poster 5667) [Google Scholar]
- 77.Rivkin A, Kim YR, Goulet MT, et al. 3-aryl-4-hydroxyquinolin-2(1H)-one derivatives as type I fatty acid synthase inhibitors. Bioorg. Med. Chem. Lett. 2006;16(17):4620–4623. doi: 10.1016/j.bmcl.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 78.Vazquez MJ, Leavens W, Liu R, et al. Discovery of GSK837149A, an inhibitor of human fatty acid synthase targeting the β-ketoacyl reductase reaction. FEBS J. 2008;275(7):1556–1567. doi: 10.1111/j.1742-4658.2008.06314.x. [DOI] [PubMed] [Google Scholar]
- 79.Wang J, Soisson SM, Young K, et al. Platensimycin is a selective FABF inhibitor with potent antibiotic properties. Nature. 2006;441(7091):358–361. doi: 10.1038/nature04784. [DOI] [PubMed] [Google Scholar]
- 80.Thupari JN, Pinn ML, Kuhajda FP. Fatty acid synthase inhibition in human breast cancer cells leads to malonyl-CoA-induced inhibition of fatty acid oxidation and cytotoxicity. Biochem. Biophys. Res. Commun. 2001;285(2):217–223. doi: 10.1006/bbrc.2001.5146. [DOI] [PubMed] [Google Scholar]
- 81.Zhou W, Tu Y, Simpson PJ, Kuhajda FP. Malonyl-CoA decarboxylase inhibition is selectively cytotoxic to human breast cancer cells. Oncogene. 2009;28(33):2979–2987. doi: 10.1038/onc.2009.160. [DOI] [PubMed] [Google Scholar]
- 82.Little JL, Wheeler FB, Fels DR, Koumenis C, Kridel SJ. Inhibition of fatty acid synthase induces endoplasmic reticulum stress in tumor cells. Cancer Res. 2007;67(3):1262–1269. doi: 10.1158/0008-5472.CAN-06-1794. [DOI] [PubMed] [Google Scholar]
- 83.Murakami S, Noguchi T, Takeda K, Ichijo H. Stress signaling in cancer. Cancer Sci. 2007;98(10):1521–1527. doi: 10.1111/j.1349-7006.2007.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bandyopadhyay S, Zhan R, Wang Y, et al. Mechanism of apoptosis induced by the inhibition of fatty acid synthase in breast cancer cells. Cancer Res. 2006;66(11):5934–5940. doi: 10.1158/0008-5472.CAN-05-3197. [DOI] [PubMed] [Google Scholar]
- 85.Heiligtag SJ, Bredehorst R, David KA. Key role of mitochondria in cerulenin-mediated apoptosis. Cell Death Differ. 2002;9(9):1017–1025. doi: 10.1038/sj.cdd.4401055. [DOI] [PubMed] [Google Scholar]
- 86.Li JN, Gorospe M, Chrest FJ, et al. Pharmacological inhibition of fatty acid synthase activity produces both cytostatic and cytotoxic effects modulated by p53. Cancer Res. 2001;61(4):1493–1499. [PubMed] [Google Scholar]
- 87.Pizer ES, Chrest FJ, Digiuseppe JA, Han WF. Pharmacological inhibitors of mammalian fatty acid synthase suppress DNA replication and induce apoptosis in tumor cell lines. Cancer Res. 1998;58(20):4611–4615. [PubMed] [Google Scholar]
- 88.Uddin S, Siraj AK, Al-Rasheed M, et al. Fatty acid synthase and AKT pathway signaling in a subset of papillary thyroid cancers. J. Clin. Endocrinol. Metab. 2008;93(10):4088–4097. doi: 10.1210/jc.2008-0503. [DOI] [PubMed] [Google Scholar]
- 89.Knowles LM, Axelrod F, Browne CD, Smith JW. A fatty acid synthase blockade induces tumor cell-cycle arrest by down-regulating Skp2. J. Biol. Chem. 2004;279(29):30540–30545. doi: 10.1074/jbc.M405061200. [DOI] [PubMed] [Google Scholar]
- 90.Browne CD, Hindmarsh EJ, Smith JW. Inhibition of endothelial cell proliferation and angiogenesis by orlistat, a fatty acid synthase inhibitor. FASEB J. 2006;20(12):2027–2035. doi: 10.1096/fj.05-5404com. [DOI] [PubMed] [Google Scholar]
- 91.Knowles LM, Yang C, Osterman A, Smith JW. Inhibition of fatty-acid synthase induces caspase-8-mediated tumor cell apoptosis by up-regulating DDIT4. J. Biol. Chem. 2008;283(46):31378–31384. doi: 10.1074/jbc.M803384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grunt TW, Wagner R, Grusch M, et al. Interaction between fatty acid synthase- and ErbB-systems in ovarian cancer cells. Biochem. Biophys. Res. Commun. 2009;385(3):454–459. doi: 10.1016/j.bbrc.2009.05.085. [DOI] [PubMed] [Google Scholar]
- 93.Menendez JA, Vellon L, Mehmi I, et al. Inhibition of fatty acid synthase (FAS) suppresses Her2/neu (ErbB-2) oncogene overexpression in cancer cells. Proc. Natl Acad. Sci. USA. 2004;101(29):10715–10720. doi: 10.1073/pnas.0403390101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Van De Sande T, Roskams T, Lerut E, et al. High-level expression of fatty acid synthase in human prostate cancer tissues is linked to activation and nuclear localization of AKT/PKB. J. Pathol. 2005;206(2):214–219. doi: 10.1002/path.1760. [DOI] [PubMed] [Google Scholar]
- 95.Liu X, Shi Y, Giranda Vl, Luo Y. Inhibition of the phosphatidylinositol 3-kinase/AKT pathway sensitizes MDA-MB468 human breast cancer cells to cerulenin-induced apoptosis. Mol. Cancer Ther. 2006;5(3):494–501. doi: 10.1158/1535-7163.MCT-05-0049. [DOI] [PubMed] [Google Scholar]
- 96.Wang HQ, Altomare DA, Skele KL, et al. Positive feedback regulation between AKT activation and fatty acid synthase expression in ovarian carcinoma cells. Oncogene. 2005;24(22):3574–3582. doi: 10.1038/sj.onc.1208463. [DOI] [PubMed] [Google Scholar]
- 97.Swinnen JV, Van Veldhoven PP, Timmermans L, et al. Fatty acid synthase drives the synthesis of phospholipids partitioning into detergent-resistant membrane microdomains. Biochem. Biophys. Res. Commun. 2003;302(4):898–903. doi: 10.1016/s0006-291x(03)00265-1. [DOI] [PubMed] [Google Scholar]
- 98.Liu H, Liu Y, Zhang JT. A new mechanism of drug resistance in breast cancer cells: fatty acid synthase overexpression-mediated palmitate overproduction. Mol. Cancer Ther. 2008;7(2):263–270. doi: 10.1158/1535-7163.MCT-07-0445. ▪ Describes the role of FASN in drug resistance.
- 99.Vazquez-Martin A, Ropero S, Brunet J, Colomer R, Menendez JA. Inhibition of fatty acid synthase (FASN) synergistically enhances the efficacy of 5-fluorouracil in breast carcinoma cells. Oncol. Rep. 2007;18(4):973–980. [PubMed] [Google Scholar]
- 100.Vazquez-Martin A, Colomer R, Brunet J, Menendez JA. Pharmacological blockade of fatty acid synthase (FASN) reverses acquired autoresistance to trastuzumab (herceptin by transcriptionally inhibiting ‘HER2 super-expression’ occurring in high-dose trastuzumab-conditioned SKBR3/Tzb100 breast cancer cells. Int. J. Oncol. 2007;31(4):769–776. [PubMed] [Google Scholar]
- 101.Little JL, Wheeler FB, Koumenis C, Kridel SJ. Disruption of crosstalk between the fatty acid synthesis and proteasome pathways enhances unfolded protein response signaling and cell death. Mol. Cancer Ther. 2008;7(12):3816–3824. doi: 10.1158/1535-7163.MCT-08-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kearney KE, Pretlow TG, Pretlow TP. Increased expression of fatty acid synthase in human aberrant crypt foci: possible target for colorectal cancer prevention. Int. J. Cancer. 2009;125(1):249–252. doi: 10.1002/ijc.24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Puig T, Relat J, Marrero PF, Haro D, Brunet J, Colomer R. Green tea catechin inhibits fatty acid synthase without stimulating carnitine palmitoyltransferase-1 or inducing weight loss in experimental animals. Anticancer Res. 2008;28(6A):3671–3676. [PubMed] [Google Scholar]
- 104.Puig T, Vazquez-Martin A, Relat J, et al. Fatty acid metabolism in breast cancer cells: differential inhibitory effects of epigallocatechin gallate (EGCG) and C75. Breast Cancer Res. Treat. 2008;109(3):471–479. doi: 10.1007/s10549-007-9678-5. [DOI] [PubMed] [Google Scholar]
- 105.Xiao R, Su Y, Simmen RC, Simmen FA. Dietary soy protein inhibits DNA damage and cell survival of colon epithelial cells through attenuated expression of fatty acid synthase. Am J. Physiol. Gastrointest Liver Physiol. 2008;294(4):G868–G876. doi: 10.1152/ajpgi.00515.2007. [DOI] [PubMed] [Google Scholar]
- 106.Menendez JA, Lupu R. Mediterranean dietary traditions for the molecular treatment of human cancer: anti-oncogenic actions of the main olive oil’s monounsaturated fatty acid oleic acid (18:1n-9) Curr. Pharm. Biotechnol. 2006;7(6):495–502. doi: 10.2174/138920106779116900. ▪ Highlights the potential for dietary manipulation in attenuating FASN overexpression.
Patents
- 201.Bostrom J, Brickmann K, Johannesson P, et al. WO2008/059214 Bisamide derivatives and use thereof as fatty acid synthase inhibitors. 2008
- 202.Singh SB, Tota MR, Wang J. WO2008/039327 Method of treatment using fatty acid synthesis inhibitors. 2008