Abstract
Background & Aims
The Atonal Homolog 1 (Atoh1 or Math1) transcription factor is required for intestinal secretory (goblet, Paneth, enteroendocrine) cell differentiation. Notch/γ-secretase inhibitors (GSIs) block proliferation and induce secretory cell differentiation in the intestine. We used genetic analyses of mice to determine whether Atoh1 mediates the effects of GSIs in normal and cancerous intestinal epithelia.
Methods
We studied mice with intestine-specific disruption of Atoh1 (Atoh1Δintestine), the APCmin mutation, both mutations [Atoh1Δintestine; APCmin], or littermate controls; mice were given GSI or vehicle. Colorectal cancer (CRC) cell lines were treated with GSI or vehicle and with small hairpin (sh) RNAs to reduce ATOH1. Differentiation and homeostasis was assessed by protein, RNA, and histologic analyses.
Results
GSIs failed to induce secretory cell differentiation or apoptosis or decrease proliferation of Atoh1-null progenitor cells, compared with wild-type cells. Exposure of APCmin adenomas to GSIs decreased proliferation and increased secretory cell numbers in an Atoh1-dependent manner. In CRC cells treated with GSI, ATOH1 levels were inversely correlated with proliferation. ATOH1 was required for secretory cell gene expression in cell lines and in mice.
Conclusions
ATOH1 is required for all effects of GSIs in intestinal crypts and adenomas; Notch has no unique function in intestinal progenitors and cancer cells other than to regulate ATOH1 expression. Reducing ATOH1 activity might mitigate intestinal toxicity from systemic GSI therapy for non-intestinal diseases. Among gastrointestinal malignancies, ATOH1 mediates the effects of GSIs, so ATOH1 expression levels might predict responses to these inhibitors. We propose that only the subset of CRCs that retain ATOH1 expression will respond to GSIs.
Keywords: biomarkers, cell fate specification, basic helix-loop-helix transcription factor, notch intracellular domain
Molecular pathways that regulate normal development and homeostasis are frequently misregulated in cancers and represent potential targets for clinical intervention. The Notch signaling pathway is key to determining self-renewal versus differentiation of intestinal stem cells. The importance of Notch signaling in colorectal cancer (CRC) tumorigenesis has only recently been recognized, suggesting that this pathway is a target for new CRC therapeutics 1.
The intestinal epithelium is composed of three main cell types: “absorptive” enterocytes, and three “secretory” cell types: Paneth, enteroendocrine, and goblet cells. Notch signaling controls the fate of intestinal progenitors by differentially regulating two opposing basic helix-loop-helix (bHLH) transcription factors, HAIRY/ENHANCER OF SPLIT 1 (HES1, also called HRY)2 and ATONAL HOMOLOG 1 (ATOH1, also called Math1 or HATH1)3. Progenitors with low levels of Notch express high levels of ATOH1 and commit to a secretory cell fate, whereas progenitors with high levels of active Notch express HES1, which in turn repress ATOH1, and become absorptive enterocytes1, 4–9. This model of alternate fate selection is based upon regulation of Notch, atonal, and enhancer-of-split genes via lateral inhibition in Drosophila melanogaster 10–11. Thus, ATOH1 is thought to be a critical gatekeeper for the program of Notch-directed differentiation of intestinal stem cells.
Recent studies report loss of ATOH1 expression in human CRCs 12. We recently confirmed that ~80% of human CRCs silence ATOH1, and showed that the mechanism includes both genetic and epigenetic silencing 13. Moreover, we showed that Atoh1 mutant mice (Atoh1Δintestine) are highly susceptible to tumor formation using both azoxymethane and APCmin/+ mouse models of CRC 13. Taken together, these results suggest that ATOH1 may be the key target of the Notch pathway regulating differentiation and proliferation within CRCs.
γ-secretase inhibitors (GSIs) are small molecules first developed for their ability to inhibit processing of the Alzheimer’s related β-amyloid peptide (Aβ) from the amyloid precursor protein (APP) 14. Subsequently, these drugs were shown to also inhibit ligand-dependent Notch cleavage and activation 15. More recently, Notch-sparing GSIs have been developed that selectively inhibit APP processing but have less effect on Notch processing, and thus avoid potential side effects on Notch-regulated organ systems such as the intestine 16. In contrast, nonselective GSIs cause a dose-dependent conversion of small intestinal and colonic progenitors to the secretory cell fate, accompanied by activation of Atoh1 and downregulation of Hes1 expression in intestinal crypt progenitors 1, 17–19. Additionally, treatment of APCmin/+ mice with GSIs resulted in reduced proliferation, Atoh1 overexpression, and differentiation of some adenoma cells to nonproliferating goblet cells 1. More recently, GSI treatment was shown to quantitatively shrink adenomas in APCmin/+ mice 20. Experiments in CRC cell lines showed a minimal effect of GSI alone, but a pro-apoptotic effect when given in combination with cytotoxic drugs such as taxanes or platinum compounds 21–23. In addition to these effects, GSIs have been proposed as chemotherapeutic agents in Barrett’s esophagus, gastric cancer, and several non-GI cancers 23–25. Thus, Notch-targeted GSIs are a promising class of small molecules for treatment of gastrointestinal neoplasias. However, in these studies the role of ATOH1 in mediating these effects of Notch inhibitors was not examined. Here we test the hypothesis that GSI mediated Notch inhibition critically requires ATOH1, in normal and cancer cells, for growth arrest and differentiation into secretory cells.
Materials and Methods
Complete materials and methods are available in the online supplementary methods.
Mice and treatments
Atoh1WT, Atoh1Δintestine, APCmin and APCmin; Atoh1Δintestine mice were treated either with vehicle or Gamma secretase inhibitor-20 (GSI-20; EMD) at 10μM/kg once (1×GSI) or twice (2×GSI) a day for five days.
Immunohistochemistry
Sections were stained for BrdU (Developmental Studies Hybridoma Bank), cleaved caspase-3 (Cell Signaling Technology), lysozyme (Invitrogen) and chromogranin A (ImmunoStar incorporated). Two-way ANOVA and Bonferroni posthoc analysis was used to measure significance (for BrdU and C-caspase-3 analysis).
Cell culture and treatments
HCT116, HT29, LOVO, LS174T, RKO, and SW480 were treated with DMSO or 5μM DAPT (SIGMA) for four days. Non-targeting and ATOH1-shRNA constructs (SIGMA) packaged into lentivirus were used to infect cells before treatment. Cell Counting-8 Kit (Dojindo) and BrdU cell proliferation kit (Millipore) were used to measure proliferation.
Immunobloting
CRC cell and intestinal protein lysates were used for immunoblotting. The following antibodies were used mouse IgM anti- actin (Developmental Studies Hybridoma Bank), rabbit polyclonal anti- HES1 (Dr. Nadean Brown, Cincinnati Children’s Hospital), rabbit polyclonal anti-cleaved Notch 1 (Val1744; Cell Signaling Technology), mouse monoclonal anti-Notch1 (mN1A; Santa Cruz Biotechnology), mouse monoclonal anti-p27[Kip1] (BD Transduction Laboratories) rabbit polyclonal anti-TFF3 (Dr. Daniel Podolsky, University of Texas), and rabbit polyclonal anti-poly-ADP ribose polymerase (PARP) (Cell Signaling Technology).
Quantitative (q)RT-PCR
CRC cells treated with DMSO or DAPT (and shRNA) were harvested and used for RNA purification (Qiagen), DNase digestion, cDNA synthesis (Invitrogen) and SYBR Green-based qRT-PCR (Stratagene). A similar procedure was used for qRT-PCR from intestinal tissues treated with vehicle or GSI (GSI-20, 10μM/kg once a day for five days). The primers for the qRT-PCR are listed in the supplementary Tables 1 and 2. Two tailed Student’s T-test and two-way ANOVA with Bonferroni posthoc test was used for bivariate analysis.
Results
Atoh1 is required for the effects of Notch inhibition on differentiation in the intestine
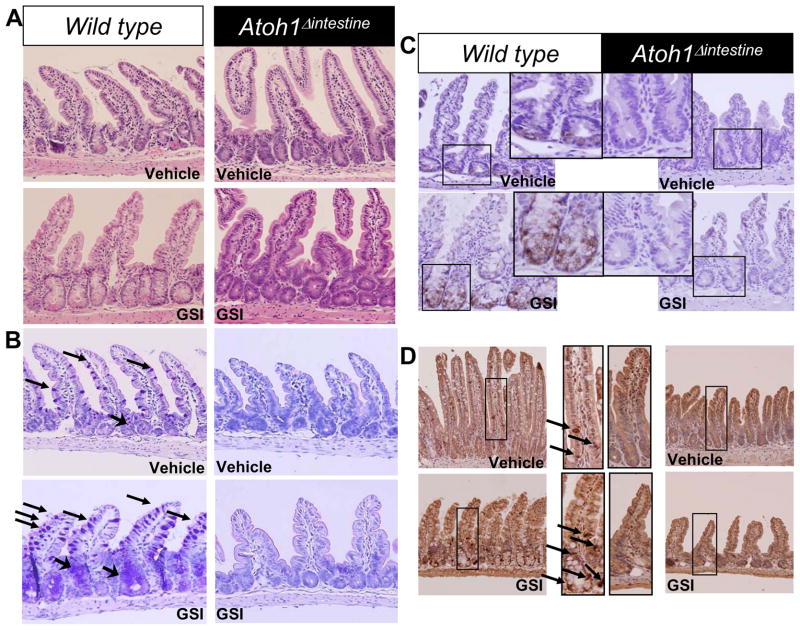
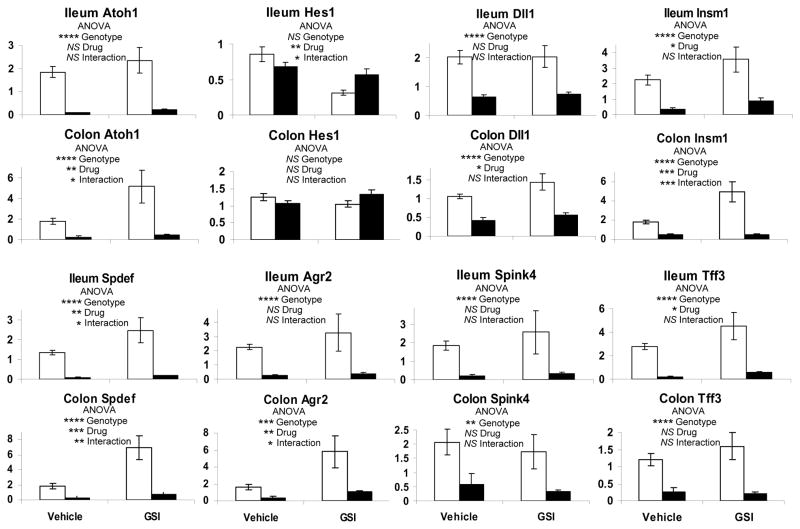
To genetically determine the role of Atoh1 in mediating the effects of Notch inhibition in the intestine, we treated Wild type (WT) and Atoh1Δintestine mice with vehicle or GSI. GSI treatment effectively inhibited Notch activation, as determined by a significant decrease in NICD (supplementary Figure 2). Atoh1Δintestine mice are mosaic, such that in the distal ileum and colon they harbor ~75% Atoh1-null crypts that lack all secretory cells, with ~25% of adjacent crypts retaining a functional copy of Atoh1 (Atoh1-WT crypts) 26. Histological analysis of intestines from WT and Atoh1Δintestine mice treated with vehicle or GSI showed no overt architectural changes to the mucosa in any of the mice (Figure 1A). Next, we stained the carbohydrate-rich goblet cell mucins with PAS/Alcian blue. As expected 1, 17–19, GSI treatment of WT mice results in a dramatic increase in the number of goblet cells in the distal ileum (Figure 1B). However, neither vehicle-nor GSI-treated Atoh1-null crypts produced goblet cells, confirming the requirement of Atoh1 for goblet cell differentiation and indicating that GSI treatment cannot overcome this requirement. Similarly, GSI treatment of WT animals results in increased production of Paneth cells (stained for Lysozyme; Figure 1C) and enteroendocrine cells (stained for Chromogranin A; Figure 1D). GSI treatment of Atoh1-null crypts shows no such increase in Paneth nor enteroendocrine cell differentiation (Figure 1C and D). Similar results for goblet and enteroendocrine cells were observed in the colon (data not shown). Of note, the remaining Atoh1-WT crypts in GSI-treated Atoh1Δintestine mice showed similar increases in secretory lineage cells as shown for WT mice, indicating a cell-autonomous role for Atoh1 in mediating these effects (data not shown). Gene expression analyses of ileum and colon from GSI treated WT and Atoh1Δintestine mice confirmed these observations: Atoh1 and secretory cell markers (Agr2, Spink4, Tff3) and differentiation factors (Insm1, Spdef) were upregulated by GSI treatment of WT mice, but were dramatically reduced in Atoh1Δintestine mice (Figure 2). We also observed reduced expression of the Notch target Hes1 in the ileum of GSI treated WT mice, but no effect in GSI treated Atoh1Δintestine mice. Interestingly, we observed increased colonic expression of the Notch ligand Dll1 in GSI-treated WT mice, which was also dependent upon Atoh1 for its expression. Together, these results demonstrate that Atoh1 is critically required for the effects of Notch/γ-secretase inhibitors on intestinal epithelial differentiation.
Figure 1. Atoh1 mediates GSI differentiation into secretory cells.
A) H&E staining of distal ileum. Vehicle and GSI treated (GSI-20, 10μM/kg twice a day for 5 days) Wild type and Atoh1Δintestine mice. B) Alcian blue/PAS staining of ileal sections. Arrows point to goblet cells in the villi and crypts. C) lysozyme staining of paneth cells. Middle panels represent magnification of ileal crypts of the smaller highlighted boxes. D) Chromogranin staining of the ileal tissues. Middle panels represent magnification of the smaller highlighted boxes; arrow point to chromogranin positive cells.
Figure 2. Atoh1 mediates GSI-induced secretory cell gene expression in the intestine.
Quantitative RT-PCR for gene expression in ileal and colonic tissues treated with vehicle or GSI (10μM/kg once a day for five days), normalized to Gapdh expression. White bars represent WT tissues and black bars Atoh1Δintestine tissues. P values shown were calculated using 2-way ANOVA for Drug (GSI) and Genotype (WT vs Atoh1Δintestine) effects. * p< 0.05, ** p< 0.001, *** p< 0.0001, **** p< 0.00001, NS (not significant). The error bars represent standard error of the mean.
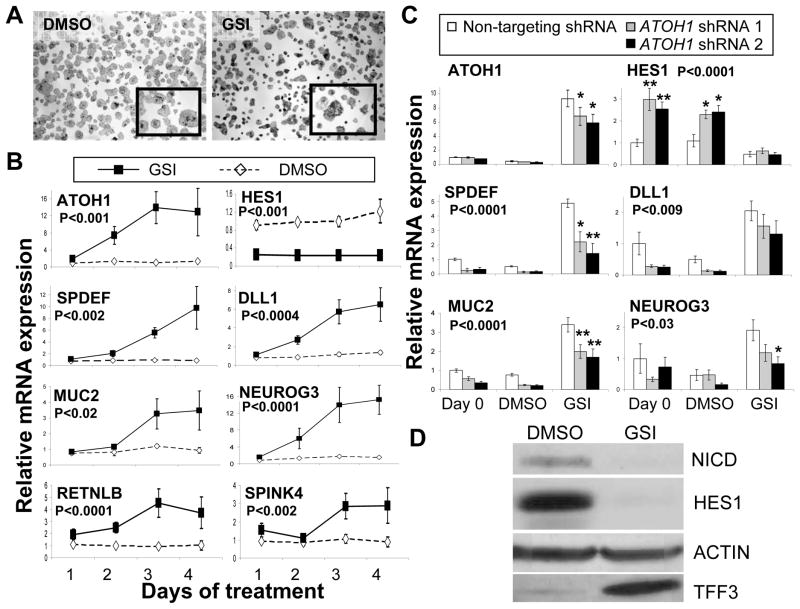
We next tested if Notch inhibition resulted in upregulation of ATOH1 and markers of secretory cell differentiation in human CRC cells, in a manner similar to the effect in mouse adenomas 1. We chose the mucinous adenocarcinoma cell line LS174T, which was reported to express low but detectable levels of ATOH1 mRNA 12, to characterize the effect of Notch inhibition and the role of ATOH1 in regulating secretory cell marker expression. Figure 3A shows an increase in mucin production after GSI treatment compared to DMSO vehicle, evident by increased Alcian blue staining of the cells. A time course study of GSI treatment showed significant accumulation of ATOH1 mRNA levels over 4 days (Figure 3B). Notch activation was inhibited as indicated by loss of the activated form of Notch (NICD; Figure 3D) and down regulation of its transcriptional target HES1 (Figure 3B and 3D). Several secretory cell mRNAs were upregulated, including the goblet cell markers SPDEF, MUC2, RETNLB and SPINK4; and the enteroendocrine progenitor cell marker NEUROG3 (Figure 3B). The secreted goblet cell protein TFF3 was similarly upregulated upon GSI treatment of LS174T cells (Figure 3D).The accumulation of secretory cell mRNAs correlated with the accumulation of ATOH1 mRNA. Interestingly, ATOH1 mRNA accumulation also correlated with increased expression of DLL1 (Figure 3B). Thus Notch/γ-secretase inhibition in CRC cells activates expression of ATOH1 and secretory cell markers.
Figure 3. ATOH1 mediates GSI-induced secretory cell gene expression in colon cancer cells.
A) Alcian blue/PAS staining of LS174T cells treated either with DMSO or GSI (5μM DAPT) for 4 days. The insets are higher magnification of stained cells. B) Quantitative RT-PCR for gene expression in LS174T cells treated with DMSO or GSI (5μM DAPT), normalized to expression at day 0. P values represent GSI effect using 2 way ANOVA analysis. C) Quantitative RT-PCR for gene expression in LS174T cells transduced with lentivirus encoding either non-targeting (white bars), or ATOH1-targeting (grey and black bars) shRNA, normalized to expression of cells transduced with non-targeting shRNA at day 0. 48 hours post transduction (Day 0) cells were treated with DMSO or GSI (5μM DAPT) for four days. P values shown were calculated using 2-way ANOVA for shRNA effect, Bonferroni posttests calculated individual group differences, * p< 0.05, ** p< 0.001. The error bars represent standard error of the mean. D) A representative immunoblot of LS174T after 4 days of treatment with DMSO or GSI (5μM DAPT). Antibodies recognized the Notch intracellular domain (NICD), HES1, TFF3, and ACTIN (as a loading control).
Next, we tested whether upregulation of ATOH1 upon Notch inhibition is required for activation of secretory cell markers. We measured the effect on GSI-induced gene expression of two different shRNAs that specifically reduce ATOH1 expression. Figure 3C shows 30–40% reduction in GSI-induced ATOH1 mRNA levels following shRNA treatment. Knockdown of endogenous ATOH1 expression in these cells results in upregulation of HES1 mRNA levels as shown for day 0 and day 4 for the vehicle treatment (Figure 3C). HES1 levels are reduced following GSI treatment in both non-targeting (control) and ATOH1-targeting shRNA treatments. The secretory cell markers SPDEF, MUC2 and NEUROG3 are significantly reduced with the ATOH1 shRNA knockdown but not in the non-targeting shRNA treatments (Figure 3C). Similarly, DLL1 levels are also reduced with ATOH1 shRNA knockdown (Figure 3C). These data indicate 1) that ATOH1 and HES1 are reciprocally regulated, and 2) that ATOH1 is required for secretory cell gene activation by Notch/γ-secretase inhibitors in LS174T colon cancer cells.
Atoh1 is required for the effects of Notch inhibition on intestinal proliferation and apoptosis
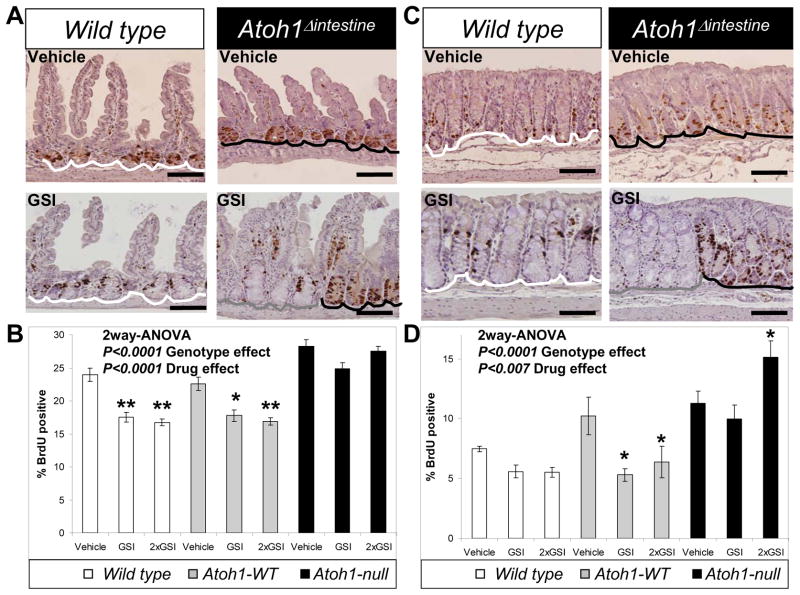
Notch/γ-secretase inhibitors have been reported to reduce proliferation and enhance apoptosis of intestinal epithelial cells in vivo 1, 17, 20. To test whether these effects of GSI are dependent upon Atoh1, WT and Atoh1Δintestine mice were treated with vehicle or GSI and the effect on proliferation and apoptosis was measured in both the distal ileum and colon. Consistent with previous reports, GSI treatment showed a 20–50% reduction in proliferating crypt cells both in WT mice and in Atoh1-WT crypts from Atoh1Δintestine animals, with no difference between these two control groups (Figure 4; white and grey bars, respectively). By contrast, Atoh1-null crypts treated with GSI did not show reduced proliferation and in fact we observed higher proliferation in 2×GSI treated Atoh1-null colon crypts (Figure 4; black bars). A similar pattern of GSI-induced, Atoh1-dependent reduction in proliferation was observed in both ileum and colon (Figure 4B and D). This striking difference in proliferative response to GSI was particularly apparent in Atoh1Δintestine mice where Atoh1-WT and Atoh1-null crypts appeared adjacent to each other (Figure 4A and C). We also noted higher levels of proliferation in the ileum of vehicle-treated Atoh1-null crypts compared to WT and Atoh1-WT, consistent with our previous reports 13, 26. We next assessed GSI-induced apoptosis in ileum and colon sections by quantifying crypt cells stained by cleaved caspase 3 (CC3) in WT and Atoh1Δintestine mice. Ileal crypts showed a significant increase in CC3-positive cells in WT and Atoh1-WT crypts (Supplemental Figure 3A; white and grey bars), whereas Atoh1-null crypts showed no increase in apoptosis (Supplemental Figure 3A; black bars). A similar pattern of Atoh1-dependent, GSI-induced apoptosis was observed in the colon (Supplemental Figure 3B). Together, these results demonstrate that Atoh1 mediates the effects of Notch/γ-secretase inhibitors on intestinal crypt progenitor proliferation and apoptosis.
Figure 4. Atoh1 mediates GSI-induced growth inhibition in normal intestinal epithelia.
A) S-phase cells (marked by BrdU) were stained brown in ileal sections from Wild type and Atoh1Δintestine mice treated with vehicle or GSI (GSI-20, 10μM/kg twice a day for 5 days). White lines underline Wild type crypts form Wild type animals; grey line underlines Atoh1-WT crypts in Atoh1Δintestine mice and black lines underline Atoh1-null crypts in Atoh1Δintestine mice. B) Quantification of BrdU positive ileal cells from vehicle or GSI treated Wild type and Atoh1Δintestine mice. Animals were treated for 5 days with 10μM/kg once a day (GSI) or twice a day (2×GSI). The white, grey and black bars represent counts from different set of crypts as described in A. [Wild type animals: n=5 for vehicle, n=4 for GSI, and n=7 for 2×GSI; Atoh1Δintestine animals: n=4 for vehicle, n=8 for GSI, and n=5 for 2×GSI]. A significant difference in proliferation was measured for vehicle treated Wild type versus Atoh1-null crypts (p<0.01), and Atoh1-WT versus Atoh1-null crypts (p<0.001). C) Colonic sections from mice as shown in A. D) Quantification of BrdU positive colonic cells from mice as shown in B. [Wild type animals: n=3 for vehicle, n=8 for GSI, and n=6 for 2×GSI; Atoh1Δintestine animals: n=4 for vehicle, n=4 for GSI, and n=5 for 2×GSI]. (B and D) Error bars represent standard error of the mean. Significance was calculated using 2-way ANOVA for GSI and genotype effects. Bonferroni posttest analysis was used for calculating significance for individual groups. * p< 0.05, ** p< 0.001.
ATOH1 induction is tightly correlated with the growth inhibitory effects of Notch inhibition in colorectal cancer cells
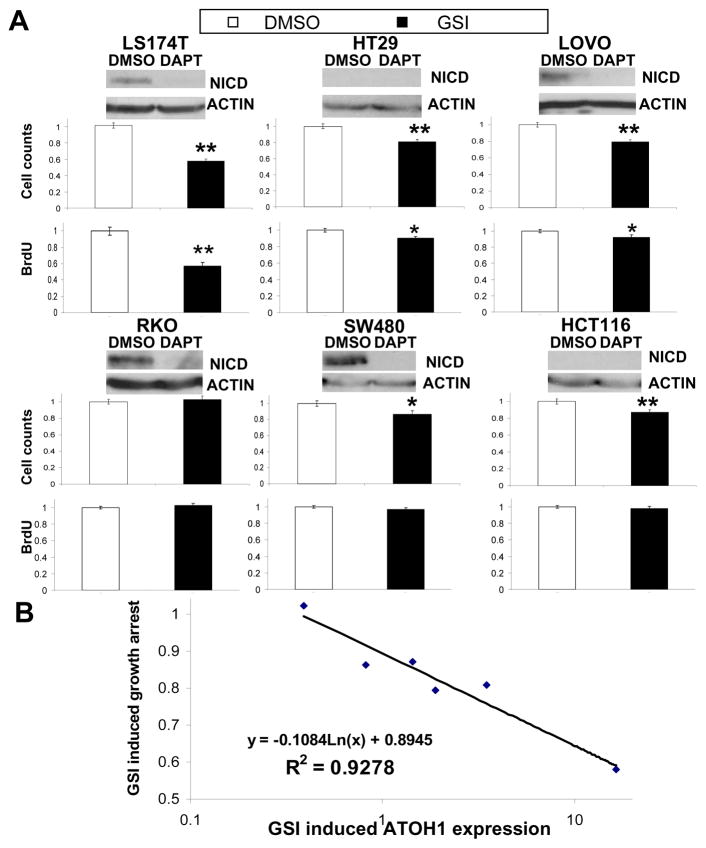
Given the requirement for Atoh1 to mediate the growth inhibitory properties of GSIs on normal intestinal cells (shown above), and recent reports that ATOH1 functions as a tumor suppressor in colorectal cancers 12–13, we next investigated the role of ATOH1 in the growth inhibitory properties of GSIs in human CRC cell lines. We treated six different cell lines (LS174T, HT29, LOVO, RKO, SW480 and HCT116) for four days with vehicle or GSI to assess the effects on cell viability. Active Notch1 (NICD1) was significantly reduced upon GSI treatment in all cells where it was measurable (Figure 5A). LS174T cells showed the greatest reduction in cell viability when treated with GSI (~40%, Figure 5A). Similarly, HT29, LOVO, SW480 and HCT116 each showed small but significant reductions in viability when treated with GSI (10–20%, Figure 5A). In contrast, RKO showed no change in growth with GSI treatment compared to vehicle control (Figure 5A). We confirmed that these growth inhibitory effects of GSI were due to inhibition of proliferation with a BrdU incorporation assay: LS174T, LOVO, and HT29 cells showed a reduction in proliferation corresponding to reduced cell viability; RKO, SW480, and HCT116, which showed little or no effect of GSI on viability, showed no change in BrdU incorporation (Figure 5A). GSI can induce apoptosis in vivo (supplemental figure 3 A and B), therefore, we examined whether a similar response is observed in CRC cells. Immunoblot analysis for cleavage of poly ADP-ribose polymerase (PARP), a marker of apoptosis, showed no increase in the major cleavage product upon GSI treatment, consistent with related reports 21–22 (Supplemental Figure 4). Thus, GSI treatment has variable potency to inhibit growth of CRC cell lines.
Figure 5. GSI induced Atoh1 expression levels is associated with CRC growth inhibition.
A) CRC cell lines are named above immonoblots and pairs of bar graphs representing cell growth (upper bar graph) and BrdU incorporation (lower bar graph) following 4 days of treatment with DMSO or GSI (5μM DAPT). Antibodies recognized the Notch intracellular domain (NICD), and ACTIN (as a loading control) for the representative immunoblots. Cell growth and proliferation were quantified by cell viability and BrdU incorporation assays and normalized to growth or BrdU incorporation of DMSO treated cells. B) log-linear comparison of the GSI effect on cell growth versus ATOH1 expression (determined by quantitative RT-PCR, Table 1) for CRC cell lines. Linear regression was used to determine the model shown and to determine R2. GSI induced ATOH1 expression represent the ratio of DMSO/DAPT for normalized expression levels of ATOH1. The GSI induced growth arrest represents the ratio of DMSO/DAPT for the normalized average cell count for each treatment. Error bars represent standard error of the mean. Significance was calculated using 2 tailed student T-tests comparing DMSO to GSI treatment. * p< 0.05, ** p< 0.001.
Next, we used GSI- and vehicle-treated CRC cells to quantify Notch pathway related mRNA levels. With few exceptions, NOTCH receptor and JAGGED ligand expression was unchanged by GSI treatment (Table 1). HES1 expression was reduced by GSI treatment of LS174T, LOVO, and RKO cells, but did not correlate closely with NICD1 expression in all cell lines (Table 1 and Figure 5A). ATOH1 induction by GSI was greatest in LS174T cells (~16 fold), whereas HT29 and LOVO cells showed modest induction, and RKO, SW480, and HCT116 had very low basal ATOH1 expression and no induction upon GSI treatment (Table 1). Interestingly, only those cells that induced ATOH1 upon GSI treatment increased expression of MUC2, DLL1, and DLL4. In contrast, HES1 repression was not tightly correlated to this response (see HT29 and RKO cells). Thus, ATOH1 expression was the best predictor of GSI-dependent gene expression changes.
Table 1. Gene expression in Notch/γ-secretase inhibitor treated colorectal cancer cell lines.
2-tailed student T-Test was used to measure differences in gene expression between vehicle (DMSO) and γ-secretase inhibitor (DAPT) treatment (bold number pairs are significantly different, p< 0.05)
| ATOH1 | HES1 | MUC2 | DLL1 | DLL4 | NOTCH1 | NOTCH2 | JAG1 | JAG2 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| LS174T | DMSO | 1.1 +/− 0.1 | 1.6 +/− 0.1 | 2.0 +/− 0.05 | 1.2 +/− 0.02 | 0.8 +/− 0.2 | 0.6 +/− 0.1 | 0.4 +/− 0.07 | 5.5 +/− 0.9 | 0.09 +/− 0.03 |
| DAPT | 17.4 +/− 5.0 | 0.8 +/− 0.05 | 34.4 +/− 5.6 | 11.4 +/− 1.7 | 12.2 +/− 3.0 | 0.9 +/− 0.3 | 0.4 +/− 0.01 | 6.1 +/− 1.2 | 0.2 +/− 0.03 | |
| HT29 | DMSO | 0.4 +/− 0.03 | 1.6 +/− 0.1 | 0.02 +/− 0.002 | 0.07 +/− 0.01 | 0.5 +/− 0.04 | 1.0 +/− 0.2 | 1.0 +/− 0.1 | 1.1 +/− 0.06 | 0.5 +/− 0.1 |
| DAPT | 1.4 +/− 0.1 | 1.5 +/− 0.08 | 0.1 +/− 0.02 | 0.4 +/− 0.06 | 1.7 +/− 0.1 | 1.1 +/− 0.08 | 1.1 +/− 0.07 | 0.8 +/− 0.1 | 0.5 +/− 0.06 | |
| LOVO | DMSO | 1.5 +/− 0.06 | 1.6 +/− 0.06 | 0.06 +/− 0.003 | 0.6 +/− 0.08 | 0.3 +/− 0.03 | 0.7 +/− 0.1 | 2.4 +/− 0.3 | 16.3 +/− 1.1 | 1.4 +/− 0.3 |
| DAPT | 2.8 +/− 0.02 | 0.9 +/− 0.09 | 0.2 +/− 0.02 | 0.8 +/− 0.01 | 0.5 +/− 0.02 | 0.9 +/− 0.03 | 1.6 +/− 0.2 | 9.6 +/− 0.6 | 1.9 +/− 0.2 | |
| RKO | DMSO | 0.0009 +/− 0.0001 | 0.03 +/−0.002 | 0.000006 +/− 0.000004 | 2.0 +/− 0.2 | 0.1 +/− 0.02 | 0.7 +/− 0.04 | 1.4 +/− 0.3 | 0.3 +/− 0.03 | 1.3 +/− 0.3 |
| DAPT | 0.0004 +/− 0.00003 | 0.002 +/− 0.0005 | 0.000006 +/− 0.0000001 | 2.3 +/− 0.1 | 0.2 +/− 0.02 | 1.3 +/− 0.05 | 1.2 +/− 0.09 | 0.4 +/− 0.06 | 2.4 +/− 0.3 | |
| SW480 | DMSO | 0.001 +/− 0.0001 | 0.07 +/− 0.02 | 0.00005 +/− 0.000004 | 0.02 +/− 0.006 | 0.3 +/− 0.06 | 0.7 +/− 0.1 | 2.4 +/− 0.3 | 1.2 +/− 0.3 | 1.3 +/− 0.3 |
| DAPT | 0.001 +/− 0.0001 | 0.04 +/− 0.006 | 0.00004 +/− 0.00001 | 0.03 +/− 0.007 | 0.3 +/− 0.06 | 0.8 +/− 0.08 | 1.6 +/− 0.2 | 1.6 +/− 0.3 | 1.9 +/− 0.3 | |
| HCT116 | DMSO | 0.001 +/− 0.0002 | 0.7 +/− 0.06 | 0.00002 +/− 0.000006 | 0.4 +/− 0.06 | 0.2 +/− 0.03 | 0.5 +/− 0.02 | 1.7 +/− 0.8 | 5.7 +/− 0.4 | 0.9 +/− 0.1 |
| DAPT | 0.002 +/− 0.0002 | 0.5 +/− 0.05 | 0.00005 +/− 0.00002 | 0.3 +/− 0.02 | 0.2 +/− 0.03 | 0.5 +/− 0.1 | 1.2 +/− 0.1 | 4.6 +/− 0.3 | 1.5 +/− 0.1 |
Given the effect of GSI on proliferation and the concomitant upregulation of ATOH1 mRNA levels, we wanted to examine the relationship between the GSI-induced growth inhibition and increased ATOH1 expression. Using a log-linear comparison of the GSI effect on cell growth versus ATOH1 expression for each CRC cell line, regression was used to derive a model (shown in Figure 5B) for which the coefficient of determination, R2 = 0.93. These data suggest a strong inverse relationship between GSI-induced ATOH1 expression and growth inhibition in CRC cells.
Atoh1 is required for the growth inhibitory effects of Notch inhibition on intestinal adenomas
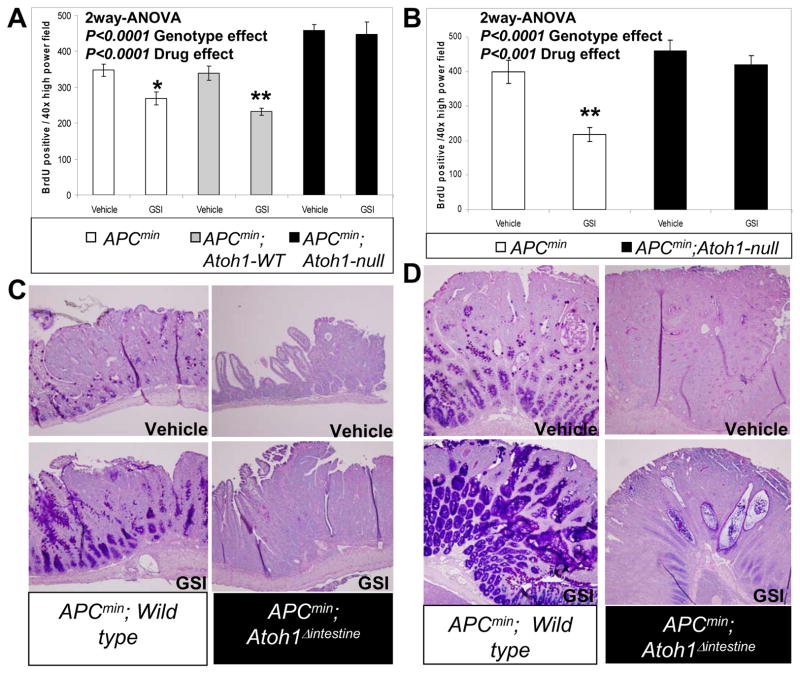
Previous studies showed that GSI treatment of small intestinal adenomas reduced proliferation and enhanced differentiation into goblet cells 1. We examined whether these effects require Atoh1 in GSI treated APCmin and APCmin; Atoh1Δintestine adenomas 13. First, we assessed goblet cell differentiation by Alcian blue/PAS staining of GSI treated APCmin and APCmin; Atoh1Δintestine adenomas (for reference, H&E stained sections of these adenomas are shown in Supplemental Figure 5). Small intestinal adenomas from APCmin mice treated with GSI showed enhanced goblet cell differentiation (Figure 6C). In contrast, goblet cells were absent in Atoh1-null ileal adenomas both in vehicle and GSI treated APCmin; Atoh1Δintestine mice (Figure 6C). Similarly, colonic APCmin adenomas showed an increase in goblet cell differentiation upon GSI treatment, but no such induction was observed in Atoh1-null colonic adenomas (Figure 6D). Given the Atoh1-dependent increased apoptosis in GSI treated ileal and colonic crypts (Supplemental Figure 3A and B), we examined the effect of GSI treatment on APCmin and APCmin; Atoh1Δintestine adenomas. In agreement with our previous finding that ATOH1 regulates apoptosis in cancer cells 13, we find that Atoh1-null adenomas contain less apoptotic cells than control tumors (supplemental Figure 3C and D). However, in contrast to normal crypts we found no induction of apoptosis upon treatment with GSI in ileal or colonic adenomas, regardless of Atoh1 genotype (supplemental Figure 3C and D).
Figure 6. Atoh1 mediates GSI-induced differentiation and growth inhibition in intestinal tumors.
A) Quantification of proliferation in ileal adenomas from mice treated with vehicle or GSI (GSI-20, 10μM/kg twice a day for 5 days). White bars represent adenomas from APCmin animals. Grey bars represent Atoh1-WT adenomas in APCmin;Atoh1Δintestine mice. Black bars represent Atoh1-null adenomas from APCmin;Atoh1Δintestine mice. [APCmin adenomas: n=21 for vehicle, n=33 for GSI; APCmin;Atoh1-WT adenomas: n=27 for vehicle, n=25 for GSI; APCmin; Atoh1-null adenomas: n=15 for vehicle and n=14 for GSI] A significant difference in proliferation was measured for vehicle treated Wild type versus Atoh1-null adenomas (p<0.001), and Atoh1-WT versus Atoh1-null adenomas (p<0.001). B) Quantification of proliferation in colonic adenomas from mice treated with vehicle or GSI (GSI-20, 10μM/kg twice a day for 5 days). The color scheme for bars is the same as in A. [APCmin adenomas: n=9 for vehicle, n=16 for GSI; APCmin;Atoh1-null adenomas: n=10 for vehicle and n=19 for GSI]. Error bars represent standard error of the mean. Significance was calculated using 2-way ANOVA for GSI and genotype effects. Bonferroni posttest analysis was used for calculating significance for individual groups. * p< 0.05, ** p< 0.001. (C and D) Representative Alcian blue/PAS staining of APCmin and APCmin;Atoh1-null ileal and colonic adenomas treated with vehicle and GSI (GSI-20, 10μM/kg twice a day for 5 days).
Finally, we measured proliferation by BrdU incorporation of neoplastic cells in adenomas from the colon and small intestine (Supplemental Figure 6). We observed higher proliferation in Atoh1-null ileal adenomas compared to Atoh1-WT adenomas (Figure 6A). Quantitation of BrdU-positive cells showed a significant 20–25% reduction in proliferation in GSI-treated adenomas from the small intestine of APCmin mice and from Atoh1-WT adenomas in the proximal small intestine of APCmin; Atoh1Δintestine mice (Figure 6A). Similar analysis showed a complete absence of effect of GSI on proliferation in Atoh1-null ileal adenomas from APCmin; Atoh1Δintestine mice (Figure 6A). In the colon, there was a pronounced 45% reduction in proliferation in GSI treated APCmin adenomas compared to vehicle treated colon adenomas (Figure 6B). In contrast, Atoh1-null colonic adenomas from APCmin; Atoh1Δintestine mice treated with GSI showed no such reduction in proliferation (Figure 6B). Thus Atoh1 is essential for differentiation and growth inhibition by Notch/γ-secretase inhibitors in intestinal tumors.
Discussion
In this study we show that ATOH1 is critically required for all effects of Notch/γ-secretase inhibitors on intestinal progenitor cells. Our results suggest a model in which the primary function of Notch in intestinal stem cells is to prevent ATOH1 expression: when Notch is inhibited, ATOH1 is activated and drives cell cycle arrest, apoptosis, and terminal differentiation of progenitors into secretory cells. When both Notch and ATOH1 are inhibited, no change in proliferation or apoptosis is observed, and absorptive enterocyte differentiation proceeds normally. Therefore, Notch has no unique function in intestinal progenitors other than to regulate appropriate ATOH1 expression.
Our findings provide new insights into the mechanism of Notch signaling in the intestine. We found that Delta-like Notch ligands are targets of ATOH1 activation in the intestine (Figures 2 & 3, Table 1). These ligands likely mediate lateral inhibition in the crypts (e.g., restriction of a subset of progenitors to the secretory fate), as shown in the zebrafish intestine 27. Interestingly, we found that Jagged ligands were not regulated by Notch-ATOH1 (Table 1 and unpublished observations), consistent with previous reports that they are targets of the WNT/β-catenin pathway 28. Whether differential responses to distinct Notch ligands regulates the fate of differentiating progenitors or “quiescent” label-retaining stem cells versus “active” crypt-base columnar stem cells is an outstanding question for future investigation. We also found that reduction of ATOH1 induced HES1 expression in a Notch-dependent manner (Figure 3C). This indicates that ATOH1 and Notch reciprocally inhibit one another, suggesting a model of cell fate determination by lateral inhibition in which reciprocal inhibition and positive autoregulation combine to lock in secretory versus absorptive progenitor status.
Our results confirm that ATOH1 is critical for normal differentiation and homeostasis of intestinal progenitors 4, 26. Recently, the cell cycle inhibitor p27Kip1 was identified as a target of Notch repression via Hes1, and was found to be de-repressed upon GSI treatment 9. We found reduced p27 expression in Atoh1-mutant colons, which was not restored by Notch inhibition (Supplementary Figure 2 and Bossuyt et al 13). Together, these data suggest a model for regulation of intestinal progenitor proliferation where p27 expression is activated by Atoh1 and repressed by Hes1. De-repression of p27 as a result of Notch/Hes1 inhibition allows increasing Atoh1 to enhance p27 expression and block proliferation. In Atoh1-null cells, reduced p27 is associated with increased proliferation; de-repression of p27 by Notch/Hes1 inhibition is insufficient without coordinate activation of p27 by Atoh1, and thus proliferation is maintained.
Our findings support the hypothesis that ATOH1’s tumor suppressive function 12–13, 29 is mediated by its effects on intestinal progenitors and/or cancer stem cells. Consistent with this tumor suppressive function, we find hyperproliferation and reduced apoptosis of Atoh1-null adenomas (Figure 6 and supplemental Figure 3). Of note, cancer cells respond to GSI not by inducing apoptosis but instead by reducing proliferation in an Atoh1-dependent manner (compare normal progenitors in Figure 4 and Supplemental Figure 3 to cancer cells in Figures 5 and 6 and Supplemental Figure 3 and 4). Atoh1-dependent, GSI-induced cell cycle arrest may enhance tumor cell killing by cytotoxic drugs 21–23; whether ATOH1 mediates the synergistic effect of GSI and chemotherapeutics should be examined in the future.
Our results have several therapeutic implications. GSI therapy for non-intestinal malignancies and other diseases has been limited due to toxicity in the intestine 18, 30. Our results suggest that targeted reduction of ATOH1 may prevent these side effects, and also that the expression level of ATOH1 is predictive of any approach to mitigating intestinal toxicity. As one approach to mitigate intestinal toxicity of GSIs, Real et al. showed that glucocorticoid treatment blocked the effects of GSIs in the intestine while enhancing the anti-leukemic and lymphoid effects 30. The ability of glucocorticoid to block intestinal toxicity of GSIs was suggested to be mediated by downregulation of the transcription factor Klf4; whether Atoh1 is involved in this process remains to be determined. Recently, Notch-sparing GSIs have been developed to selectively inhibit processing of Aβ in Alzheimer’s disease 16. We suggest that ATOH1 expression may be a useful functional marker of intestinal side effects when determining the relative selectivity of the GSIs for Aβ over Notch. Among GI malignancies, we show that ATOH1 is a critical mediator of GSI effects. Therefore, the ability to induce ATOH1 is key for these therapies to be effective. Importantly, we recently showed that ATOH1 is silenced in ~80% of sporadic colorectal cancers 13; therefore GSI therapy of these silenced tumors will be ineffective since they lack expression of the critical mediator of GSI treatment (ATOH1). To utilize GSIs in GI malignancies, we suggest that patients should first be stratified according to ATOH1 silencing status. For example, we predict that ~20% of CRCs that retain ATOH1 expression will respond to GSI therapy. Furthermore, we predict that gastric and esophageal tumors will respond to GSI therapy when they include ATOH1-positive intestinal metaplasia, but will become resistant to GSI therapy if they progress further and silence ATOH1. Together, our results demonstrate that ATOH1 is essential for all effects of Notch/γ-secretase inhibitors in normal and cancerous intestinal cells, and suggest that selective targeting of ATOH1 will allow for tissue specific therapeutic modalities. More broadly, our results support the concept of targeting tissue-specific differentiation factors as an important approach to inhibiting growth of cancers.
Supplementary Material
Acknowledgments
This study was supported by NIH grants T35 DK060444, K01 DK071686, R01 CA142826, and P30 DK078392; an American Cancer Society-Ohio Chapter pilot award; and an American Gastroenterology Association/FDHN Research Scholars Award.
We thank Jefferson Vallance, Tasneem Kaleem, and Allison Price for their technical assistance; Dr. Nadean Brown (Cincinnati Children’s Hospital) for providing the Hes1 polyclonal antibody; Dr. Daniel Podolsky (University of Texas Southwestern Medical School) for anti-TFF3 rabbit polyclonal antibody. This work was supported by a pilot award from the Cincinnati Digestive Health Center supported by P30 DK078392, a Foundation for Digestive Health and Nutrition Research Scholars Award, National Institutes of Health (NIH) K01 DK071686, R01 CA142826, and American Cancer Society (Ohio Affiliate) Pilot Award to NFS. DB was supported by a NIH institutional training award T35 DK060444.
Abbreviations
- ATOH1
Atonal homolog 1
- CRC
colorectal cancer
- BrdU
Bromodeoxyuridine
- RT-PCR
reverse-tanscriptase-PCR
- CC3
Cleaved caspase 3
- APC
adenomatosis polyposis coli
- min
multiple intestinal neoplasia
- HES1
Hairy/Enhancer of Split 1
- GSI
γ-secretase inhibitor
- DAPT
N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester
- PAS
periodic acid Schiff’s
- NICD
Notch IntraCellular Domain
- SPDEF
SAM Pointed Domain containing ETS transcription Factor
- MUC2
MUCIN2
- RETNLB
Resistin Like B
- SPINK4
Serine Peptidase Inhibitor, Kazal type 4
- TFF3
TreFoil Factor 3
- DLL1
Delta Like 1
- DLL4
Delta Like 4
- JAG1
Jagged 1
- JAG2
Jagged 2
- cPARP
cleaved poly-ADP ribose polymerase
- Agr2
Anterior gradient homolog2
- Insm1
Insulinoma-associated 1
Footnotes
The authors have no disclosures to report.
Author contributions: AK, NFS conceived and designed the experiments
AK, TN, DB, JB, NFS performed and interpreted the experiments
AK and NFS wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–63. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 2.Feder JN, Li L, Jan LY, Jan YN. Genomic cloning and chromosomal localization of HRY, the human homolog to the Drosophila segmentation gene, hairy. Genomics. 1994;20:56–61. doi: 10.1006/geno.1994.1126. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Arie N, McCall AE, Berkman S, Eichele G, Bellen HJ, Zoghbi HY. Evolutionary conservation of sequence and expression of the bHLH protein Atonal suggests a conserved role in neurogenesis. Hum Mol Genet. 1996;5:1207–16. doi: 10.1093/hmg/5.9.1207. [DOI] [PubMed] [Google Scholar]
- 4.Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294:2155–8. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 5.Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 6.Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–8. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 7.Stanger BZ, Datar R, Murtaugh LC, Melton DA. Direct regulation of intestinal fate by Notch. Proc Natl Acad Sci U S A. 2005;102:12443–8. doi: 10.1073/pnas.0505690102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vooijs M, Ong CT, Hadland B, Huppert S, Liu Z, Korving J, van den Born M, Stappenbeck T, Wu Y, Clevers H, Kopan R. Mapping the consequence of Notch1 proteolysis in vivo with NIP-CRE. Development. 2007;134:535–44. doi: 10.1242/dev.02733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riccio O, van Gijn ME, Bezdek AC, Pellegrinet L, van Es JH, Zimber-Strobl U, Strobl LJ, Honjo T, Clevers H, Radtke F. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep. 2008;9:377–83. doi: 10.1038/embor.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bjerknes M, Cheng H. Gastrointestinal stem cells. II. Intestinal stem cells. Am J Physiol Gastrointest Liver Physiol. 2005;289:G381–7. doi: 10.1152/ajpgi.00160.2005. [DOI] [PubMed] [Google Scholar]
- 11.Baker NE, Yu S, Han D. Evolution of proneural atonal expression during distinct regulatory phases in the developing Drosophila eye. Curr Biol. 1996;6:1290–301. doi: 10.1016/s0960-9822(02)70715-x. [DOI] [PubMed] [Google Scholar]
- 12.Leow CC, Romero MS, Ross S, Polakis P, Gao WQ. Hath1, down-regulated in colon adenocarcinomas, inhibits proliferation and tumorigenesis of colon cancer cells. Cancer Res. 2004;64:6050–7. doi: 10.1158/0008-5472.CAN-04-0290. [DOI] [PubMed] [Google Scholar]
- 13.Bossuyt W, Kazanjian A, De Geest N, Van Kelst S, De Hertogh G, Geboes K, Boivin GP, Luciani J, Fuks F, Chuah M, VandenDriessche T, Marynen P, Cools J, Shroyer NF, Hassan BA. Atonal homolog 1 is a tumor suppressor gene. PLoS Biol. 2009;7:e39. doi: 10.1371/journal.pbio.1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolfe MS, Citron M, Diehl TS, Xia W, Donkor IO, Selkoe DJ. A substrate-based difluoro ketone selectively inhibits Alzheimer’s gamma-secretase activity. J Med Chem. 1998;41:6–9. doi: 10.1021/jm970621b. [DOI] [PubMed] [Google Scholar]
- 15.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–22. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 16.Augelli-Szafran CE, Wei HX, Lu D, Zhang J, Gu Y, Yang T, Wolfe MS. Discovery of Notch-Sparing gamma-Secretase Inhibitors. Curr Alzheimer Res. 2010 doi: 10.2174/156720510791050920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Milano J, McKay J, Dagenais C, Foster-Brown L, Pognan F, Gadient R, Jacobs RT, Zacco A, Greenberg B, Ciaccio PJ. Modulation of notch processing by gamma-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol Sci. 2004;82:341–58. doi: 10.1093/toxsci/kfh254. [DOI] [PubMed] [Google Scholar]
- 18.Searfoss GH, Jordan WH, Calligaro DO, Galbreath EJ, Schirtzinger LM, Berridge BR, Gao H, Higgins MA, May PC, Ryan TP. Adipsin, a biomarker of gastrointestinal toxicity mediated by a functional gamma-secretase inhibitor. J Biol Chem. 2003;278:46107–16. doi: 10.1074/jbc.M307757200. [DOI] [PubMed] [Google Scholar]
- 19.Wong GT, Manfra D, Poulet FM, Zhang Q, Josien H, Bara T, Engstrom L, Pinzon-Ortiz M, Fine JS, Lee HJ, Zhang L, Higgins GA, Parker EM. Chronic treatment with the gamma-secretase inhibitor LY-411,575 inhibits beta-amyloid peptide production and alters lymphopoiesis and intestinal cell differentiation. J Biol Chem. 2004;279:12876–82. doi: 10.1074/jbc.M311652200. [DOI] [PubMed] [Google Scholar]
- 20.Ghaleb AM, Aggarwal G, Bialkowska AB, Nandan MO, Yang VW. Notch inhibits expression of the Kruppel-like factor 4 tumor suppressor in the intestinal epithelium. Mol Cancer Res. 2008;6:1920–7. doi: 10.1158/1541-7786.MCR-08-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akiyoshi T, Nakamura M, Yanai K, Nagai S, Wada J, Koga K, Nakashima H, Sato N, Tanaka M, Katano M. Gamma-secretase inhibitors enhance taxane-induced mitotic arrest and apoptosis in colon cancer cells. Gastroenterology. 2008;134:131–44. doi: 10.1053/j.gastro.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Aleksic T, Feller SM. Gamma-secretase inhibition combined with platinum compounds enhances cell death in a large subset of colorectal cancer cells. Cell Commun Signal. 2008;6:8. doi: 10.1186/1478-811X-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng RD, Shelton CC, Li Y, Schwartz GK. Effect of targeting the Notch developmental pathway in metastatic gastric cancers on growth suppression, apoptosis, and chemosensitivity. 2009 American Society for Clinical Oncology Gastrointestinal Cancers Symposium; 2009. p. Abstract 25. [Google Scholar]
- 24.Konturek P, Burnat G, Rau T, Hahn EG. Implication of Notch signaling and CDX-2 Expression in Barrett’s Canrinogenesis. Digestive Diseases Week. 2008:Abstract 996. [Google Scholar]
- 25.Aster JC. Deregulated NOTCH signaling in acute T-cell lymphoblastic leukemia/lymphoma: new insights, questions, and opportunities. Int J Hematol. 2005;82:295–301. doi: 10.1532/IJH97.05096. [DOI] [PubMed] [Google Scholar]
- 26.Shroyer NF, Helmrath MA, Wang VY, Antalffy B, Henning SJ, Zoghbi HY. Intestine-Specific Ablation of Mouse atonal homolog 1 (Math1) Reveals a Role in Cellular Homeostasis. Gastroenterology. 2007;132:2478–88. doi: 10.1053/j.gastro.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 27.Crosnier C, Vargesson N, Gschmeissner S, Ariza-McNaughton L, Morrison A, Lewis J. Delta-Notch signalling controls commitment to a secretory fate in the zebrafish intestine. Development. 2005;132:1093–104. doi: 10.1242/dev.01644. [DOI] [PubMed] [Google Scholar]
- 28.Rodilla V, Villanueva A, Obrador-Hevia A, Robert-Moreno A, Fernandez-Majada V, Grilli A, Lopez-Bigas N, Bellora N, Alba MM, Torres F, Dunach M, Sanjuan X, Gonzalez S, Gridley T, Capella G, Bigas A, Espinosa L. Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc Natl Acad Sci U S A. 2009;106:6315–20. doi: 10.1073/pnas.0813221106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsuchiya K, Nakamura T, Okamoto R, Kanai T, Watanabe M. Reciprocal targeting of Hath1 and beta-catenin by Wnt glycogen synthase kinase 3beta in human colon cancer. Gastroenterology. 2007;132:208–20. doi: 10.1053/j.gastro.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 30.Real PJ, Tosello V, Palomero T, Castillo M, Hernando E, de Stanchina E, Sulis ML, Barnes K, Sawai C, Homminga I, Meijerink J, Aifantis I, Basso G, Cordon-Cardo C, Ai W, Ferrando A. Gamma-secretase inhibitors reverse glucocorticoid resistance in T cell acute lymphoblastic leukemia. Nat Med. 2009;15:50–8. doi: 10.1038/nm.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.