Abstract
Inhalation of ambient ozone alters populations of lung macrophages. However, the impact of altered lung macrophage populations on the pathobiology of ozone is poorly understood. We hypothesized that sub-populations of macrophages modulate the response to ozone. We exposed C57BL/6 mice to ozone (2 ppm × 3h) or filtered air. 24 h after the exposure, the lungs were harvested and digested and the cells underwent flow cytometry. Analysis revealed a novel macrophage subset present in ozone exposed mice, which were distinct from resident alveolar macrophages (AM) and identified by enhanced Gr-1+ expression (Gr-1 Macs). Further analysis identified that Gr-1+ Macs exhibited high expression of MARCO, CX3CR1, and NQO1. Gr-1+ Macs were present in the absence of CCR2, suggesting that they were not derived from a CCR2-dependent circulating intermediate. Using PKH26-PCL to label resident phagocytic cells, we demonstrated that Gr-1 Macs were derived from resident lung cells. This new subset was diminished in the absence of CX3CR1. Interestingly, CX3CR1-null mice exhibited enhanced responses to ozone, including increased airway hyperresponsiveness (AHR), exacerbated neutrophil influx, accumulation of 8-isoprostanes and protein carbonyls, and increased expression of cytokines (CXCL2, IL-1β, IL-6, CCL2, and TNF-α). Our results identify a novel subset of lung macrophages, which are derived from a resident intermediate, dependent upon CX3CR1, and appear to protect the host from the biological response to ozone.
Introduction
Ozone, a gas formed via a chemical reaction between oxides of nitrogen and volatile organic compounds in the presence of sunlight, is a source and cause of environmental lung injury. Inhaled oxidants, such as ozone, are important contributors to a variety of respiratory diseases including chronic obstructive pulmonary disease, asthma, cystic fibrosis, and adult respiratory distress syndrome (1). The mechanism of action for the response to ozone appears to be through a combination of several mechanisms including the alteration of barrier functions in the epithelium (2, 3), the generation of oxidized species such as 5β, 6β-epoxycholesterol found in the epithelial lining fluid (4), and the fragmentation of matrix proteins such as hyaluronan to low molecular weight fragments (5). Inhalation of ozone results in the activation and recruitment of many cell types within the lung (6).
Macrophages are increased in the airspace after inhalation of ambient ozone and appear central to the host response to ozone (7, 8). Macrophages can produce pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6 and IL-8, which contribute to the biological response to ozone (7, 9). Despite the evidence suggesting a pro-inflammatory role of macrophages, additional evidence suggests that macrophages can be protective during ozone-induced lung injury (10). Macrophages are important for clearance of both apoptotic cells and of oxidized species generated by ozone, which if not cleared can serve to exacerbate inflammation (10, 11). The understanding of how macrophages can cause both pro- and anti-inflammatory outcomes remains poorly understood. We hypothesized that individual macrophage sub-populations within the lung account for the divergent reported functions of macrophages in the setting of ozone inhalation.
There is increased interest in identifying macrophage subpopulations in the lung and in other organ systems as a means to understand their diverse function during injury and repair. Given the diverse functional roles of macrophages, it is not surprising that sub-populations of macrophages exist that are either recruited or mature from resident cells in response to environmental challenge. Recent studies have identified distinct macrophage sub-populations with unique functions in multiple tissues (12). These data also support plasticity of macrophages in a manner dependent on microenvironment. In the lung, distinct macrophage sub-populations have been characterized in murine models of infectious disease and asthma (13–16). Work from our lab identified a lung macrophage sub-population, exudative macrophage (ExMac), that are recruited following non-infectious lung injury (17). Separable macrophage lineages are a result of the heterogeneity in macrophage precursor cells (i.e. monocytes) and the specific developmental pathways that are initiated by stimulatory local conditions at the time of monocyte differentiation (12). The focus of this work has largely been upon factors of macrophage recruitment and development from circulatory monocytes during pulmonary injury. However, it is unclear whether resident lung macrophages can extend beyond their primary alveolar membrane defense phenotype, adapt and undergo development into unique populations in the setting of acute lung injury.
Macrophages are derived either from monocytes or by proliferation of local macrophages. Two unique monocyte populations are present in the circulation (18). Constitutive (Gr-1-) monocytes enter tissues under steady state conditions in the lung and develop into resident tissue interstitial macrophages and alveolar macrophages (18–20). This homeostatic mechanism appears to be dependent on the chemokine receptor CX3CR1 (or the fractalkine receptor). In contrast, inflammatory (Gr-1+) monocytes, are recruited to the lung during inflammation in a CCR2-dependent manner and develop into either an activated macrophage population known as exudative macrophages or into monocyte-derived dendritic cells (18, 21). The morphology, phenotype, and effector functions of constitutive monocyte-derived macrophages differ markedly from inflammatory monocyte-derived macrophages (16). We and others identified that recruited ExMacs, but not resident lung macrophages, express high levels of MHCII and costimulatory molecules, stimulate T cell activation, and are a major source of inflammatory cytokines and chemokines such as TNF-α, CXCL2, CXCL10 and NOS2 (16, 17). Despite this characterization of recruited macrophages, little is known about the unique functional roles of resident lung macrophages in the context of non-infectious lung injury.
In the present study, we identify a novel subset of macrophages present in the lung after inhalation of ozone. This subset is separable from resident macrophages and characterized by expression of unique cell surface markers and gene expression. Using cell labeling of resident phagocytic cells, we identify that these macrophages are derived from a resident lung intermediate and not a circulating precursor. Finally, we identify that the development of this macrophage subset is diminished in mice deficient in CX3CR1. In the setting of CX3CR1-deficiency and reduced numbers of Gr1+ Macs, mice have enhanced response to ozone. In conclusion, our data support a novel macrophage sub-population that is derived from resident macrophages and can protect the lungs from oxidant-related lung injury.
Methods and Materials
Mice
C57BL/6 and CX3CR1GFP/GFP mice were purchased from Jackson Laboratory (Bar Harbor, ME). CX3CR1GFP/GFP mice were crossed with C57BL/6 mice to produce CX3CR1+/GFP mice. CCR2 −/−mice were backcrossed on a C57BL/6 background for > 10 generations. Experimental groups consisted of 6–10 week old female mice for all experiments. Animal experiments were conducted in accordance with National Institute of Health guidelines and protocols approved by the Animal Care and Use Committee at Duke University.
Exposure Protocol
C57BL/6, CCR2 −/−, CX3CR1+/GFP, and CX3CR1GFP/GFP were exposed to either filtered air or ozone. Ozone exposure occurred for 3 h at a dose of 2 ppm. The ozone was generated by directing 100% oxygen through a UV light generator. This was then mixed with the air supply to the chamber. Temperature and humidity of chamber air were monitored continuously as was the ozone concentration with a UV light photometer. The mice receiving filtered air were also placed in chambers for 3 h without ozone instillation. The mice were then removed from the chamber and allowed to recover for 24 h.
Lung Digestion and Flow Cytometry
Mice were euthanized 24 h following ozone or filtered air exposure. The lungs were perfused with Hanks Buffered Saline Solution (HBSS) to remove residual red blood cells from the pulmonary circulation. They were minced and digested for 40 minutes at 37°C in HBSS with 1 mg/mL collagenase A (Roche, Indianapolis, IN, USA) and 0.2 mg/mL of DNase1 (Sigma, St. Louis, MO, USA). The digestion solution was passed through a 70-μm mesh strainer and centrifuged at 535 × g at room temperature over an 18% nycodenz (Accurate Chemical Co) cushion. Low density cells were collected, underwent red blood cell lysis and then washed × 2. The following antibodies were used: anti-I-A/I-E FITC, anti-I-A/I-E PE (used in experiments with CX3CR1GFP/WT mice), anti-Ly6-G PE, anti-Mac-3 PE, anti-Gr-1 APC, and anti-CD11b APC Cy7 (all from BD Pharmagen, Franklin Lakes, NJ, USA), anti-CD11c PE Cy5.5, and anti-F480 APC (eBioscience, San Diego, CA, USA) and anti-MARCO PE (AbD Serotec, Raleigh, NC, USA). Isotype controls were generated for MARCO using a rat IgG1 negative control (catalogue #MCA1211, AbD Serotec) which was conjugated to PE fluorochrome using an antibody conjugation kit (catalogue #LNK021RPE, AbD Serotec). All staining was performed in PBS with 3% FBS, 10mM EDTA, 5% normal mouse serum, 5% normal rat serum and 1% FcBlock (BD Pharmagen). To reduce non-specific binding, the cells were incubated in staining buffer at 4°C for 10 minutes prior to the addition of antibodies. Following staining, the cells were washed × 3 and analyzed using a BD Canto II flow cytometer. Data analysis was performed using FloJo (version 8.8.6, Ashland, OR, USA) software. Cell sorting occurred using a BD FACSAria cell sorter.
Resident Lung Phagocytic Labeling
Phagocytic labeling was based on a protocol from Maus, U. et al (22). In brief, mice were anesthetized using 1–3% isoflurane. PKH26-PCL (Sigma), a red fluorescent dye that labels phagocytic cells was diluted by a factor of 20 using the diluent B solution (Sigma). Under sterile conditions, 100 μl of the diluted PKH26-PCL was administered by retro-orbital injection. The mice were allowed to recover for 24 h, then underwent filtered air or ozone exposure and were euthanized and harvested for flow cytometric analysis as described in the above protocols. In addition to lung harvest, blood was drawn into a 23-gauge needle with a 1-mL syringe containing EDTA. The blood underwent red blood cell lysis using ACK Lysis Buffer to separate the RBCs from peripheral blood leukocytes. The leukocytes were washed in sterile PBS and then centrifuged at 1600 rpm for 5 min at 4°C. The whole blood leukocytes underwent flow cytometric analysis for PKH26-PCL. PKH26-PCL was visualized in the PE channel.
Airway Physiology
Direct measurements of respiratory mechanics in response to methacholine were made using the flexiVent system (SCIREQ, Montreal, Canada) and reported as total resistance (RT) cmH2O/mL/s. Anesthesia was achieved with 60 mg/kg pentobarbital sodium injected i.p. Mice were then given a neuromuscular blockade (0.8 mg/kg pancuronium bromide). Mice were ventilated with a computer-controlled ventilator (flexiVent, SCIREQ) with a tidal volume of 7.5 mL/kg and a positive end-expiratory pressure of 3 cmH2O. Measurements of respiratory mechanics were made by the forced oscillation technique. Response to aerosolized methacholine (0 mg/mL, 10 mg/mL 25 mg/mL, and 100 mg/mL) was determined by resistance measurements every 30 s for 5 min, ensuring the parameters calculated had peaked. Total lung capacity breaths were administered after each dose of methacholine to maintain patent airways and return the measurements back to baseline. The resistance measurements were then averaged at each dose and graphed (RT cmH2O/mL/s) along with the initial baseline measurement.
Bronchoalveolar lavage fluid (BALF)
Immediately after pulmonary function measurements, mice were overdosed with Nembutal (100mg/kg) to euthanize. The chest was opened, the trachea was exposed, and bronchoalveolar lavage (BAL) was performed by inserting a catheter into the mouse trachea with PE-90 tubing and instilling saline until the lung reached total lung capacity. This was repeated three times. The total volume returned was the lavage return volume. Cells from the BALF were isolated using centrifugation (1500 rpm, 15 min) and the supernatant was stored at −80°C for assessment of 8-isoprostanes, protein carbonyls and cytokines. The cells were used for cell counts and differentials.
Cytokine Measurements
Cytokines and chemokines were analyzed using a MILLIPLEX™ 5-MAP assay kit (Millipore, Billerica, MA, USA) per the manufacturer’s protocol. This is a bead-based suspension array using the Luminex technology (Bio-Rad, Hercules, CA, USA) in which fluorescent-coded beads, known as microspheres, have cytokine capture antibodies on the bead surface to bind the proteins. CX3CL1 was measured from BAL using commercially available ELISA kit (R&D Systems Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions. The plate was read on a plate reader at a wavelength of 450 nm.
8-Isoprostane Measurements
8-isoprostanes were measured in both the BALF supernatant and the cell supernatant using purification columns and an EIA assay kit from Cayman Chemical Company (Ann Harbor, MI, USA). Samples were briefly diluted 1:2 with column buffer and applied to the purification columns. The sample passed entirely through the column. The column was then washed with column buffer and ultrapure water and the washes were discarded. 5 mL of elution solution was added to the column and allowed to pass through in order to elute the 8-isoprostane. The solution that passed through the column was then collected in a 5 mL tube and the elution solution was evaporated to dryness using a stream of dry nitrogen gas in order to remove all quantities of organic solvent. The purified samples were then reconstituted with saline and used for the EIA kit (Cayman Chemical) following the manufacturer’s protocol. Samples, standards, buffer, bound 8-isoprostane AChE Tracer, and antiserum were added the plate and incubated at 40oC for 18 h. The plate was then washed 5 times with wash buffer and the Ellman’s reagent (substrate for AChE tracer) was added. After 90 minutes, the plate was read on a plate reader at a wavelength of 405 nm. The 8-isoprostane concentrations was calculated by plotting the percent ratio of standard bound/maximum bound for each of the standards using linear and log axes and performing a 4-paramter logistic fit.
Protein Carbonyl
Protein carbonyls were measured in both the BALF supernatant and the cell supernatant using an OxiSelect Protein Carbonyl ELISA kit following the manufacturer’s protocol (Cell Biolabs, Inc., San Diego, CA, USA). Briefly, BSA standards or samples were absorbed onto a 96-well plate for 2 h at 37°C. The protein carbonyls present in the sample or standard were derivatized to DNA hydrazone and probed with an anti-DNP antibody, followed by an HRP conjugated secondary antibody. The plate was then read at 405 nm by a plate reader. The protein carbonyl content in the sample was determined by comparison with a standard curve that was prepared from predetermined reduced and oxidized BSA standards.
Real Time PCR
Total RNA was extracted from flow sorted macrophages using the RNeasy mini kit (Qiagen Inc., Valencia, CA, USA), according to the manufacturer’s protocol. Samples are treated with DNase treatment and removal reagents to remove DNA contamination (Catalogue #AM1906, Ambion Inc., Austin, TX, USA). RNA samples were then reverse-transcribed into cDNA using SuperScript II RT (Invitrogen Corp., Carlsbad, CA, USA) following the manufacturer’s instruction. All real-time quantitative PCRs were performed using ABI SDS 7500 and SYBR Green reagent (Applied Biosystems, Foster City, CA, USA). Primers were designed using ABI software and were produced by IDT, Inc. (Coralville, IA, USA). The sequences are as follows: NQO1 forward, GTGGTTTGGGGTGCCAGCCAT; NQO1 reverse, AGGATGCCACTCTGAATCGGCC; CYP1β1 forward, CCAGGCGTCGCACTTGTAC; CYP1β1 reverse, TGGAAAACGTCGCCATAGC; SOD1 forward, GCCCGGCGGATGAAG; SOD1 reverse, CCTTTCCAGCAGTCACATTGC; HO-1 forward, CAGCCCCACCAAGTTCAAA; and HO-1 reverse, TCAGGTGTCATCTCCAGAGTGTTC. PCR amplification was performed by the following program: 50 °C, 2 min; 95 °C, 10 min; 95 °C 15s, 60°C 1 min, for 40 cycles. Gene expression values were normalized to the housekeeping gene 18s. The data were presented as a fold change compared to 18s.
Statistics
Data are expressed as mean ± standard error. The statistical difference between groups was assessed by Student’s t test. A two-tailed p value of < 0.05 was considered statistically significant.
Results
Ozone exposure results in development of a Gr-1 positive subset of macrophages
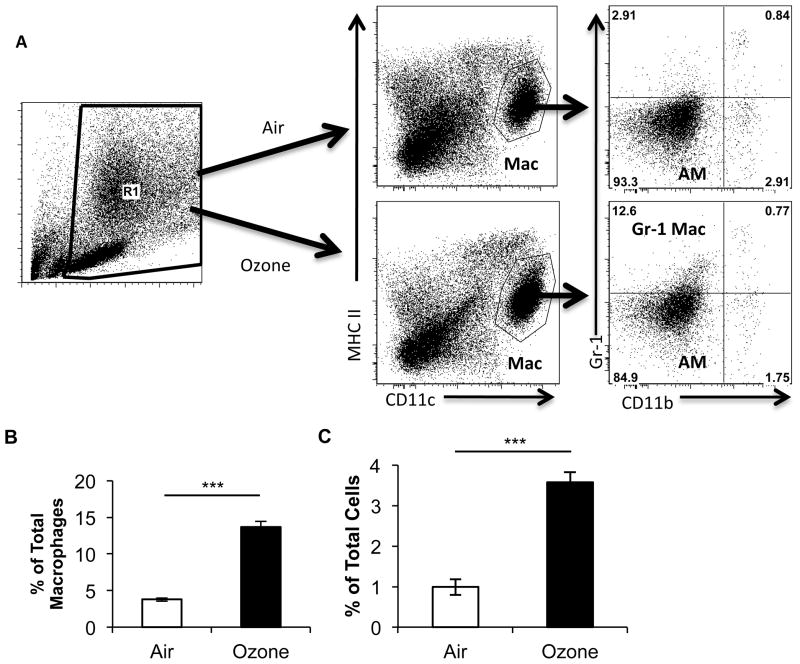
To further characterize the influx of macrophages after ozone exposure, we performed multicolor flow cytometric analysis on whole lung macrophages using a previously described technique (16, 17). Lung macrophages are identified as autofluorescent+, MHCIIint, CD11chi by flow cytometry. In previous work, we identified two types of macrophages: resident alveolar macrophages (AM) and exudative macrophages (ExMacs) (17). ExMacs were separated from AMs by enhanced cell surface expression of both CD11b and CX3CR1. To our surprise, flow cytometric analysis of whole lung macrophages at 24-h post-ozone exposure did not reveal a population of CD11b+ macrophages consistent with previously reported ExMacs (Figure 1A). Rather, we observed a population of autofluorescent+ MHCIIint CD11chi macrophages, which were Gr-1hi CD11bint. These were separate from other alveolar macrophages, which were Gr-1low CD11bneg. The Gr-1 positive subset was not present in air-exposed mice. AMs and the Gr-1 positive subset both stained positive for the macrophage markers F4/80 and Mac-3 (Figure S1) confirming that they were in fact macrophages. Graphic representation of the percentage of Gr-1 positive macrophages as a percentage of total macrophages (Figure 1B) and of total cells (Figure 1C) confirmed this identification. This observation suggested that ozone exposure resulted in development of a novel macrophage subset, which was defined by increased Gr-1 expression.
Figure 1. Flow cytometry of macrophages after ozone challenge.
A, Flow profile of total lung cells isolated 24 h after exposure to filtered air or 2ppm of ozone. Lungs are digested and then enriched for mononuclear cells over an 18% Nycodenz gradient. Flow cytometry was then performed. Live cells are gated on the basis of FSC and SSC (R1 Gate). The cells from R1 are analyzed by CD11c vs. MHCII expression to identify individual cell populations. Macrophages are identified as MHCIIint and CD11c+ (Mac). Subgate analysis from the Mac Gate by CD11b and Gr-1 after air exposure identifies macrophages as CD11blow Gr-1low (AM). After ozone exposure, in addition to CD11blow Gr-1low macrophages a population of CD11blow Gr-1high macrophages (Gr-1 Macs) develops. B, A graphic representation of the number of CD11blow Gr-1high macrophages as a percentage of total macrophages (defined as MHCIIint and CD11c+) after either filtered air or ozone. C, A graphic representation of the number of CD11blow Gr-1high macrophages as a percentage of total cells. The flow plots are from a representative sample of C57BL/6 mice given filtered air or ozone. Data were derived from 3–4 mice with both exposures and are representative of two separate experiments (***P<0.0005 between groups of Gr-1high macrophages in either air or ozone exposure groups).
Gr-1 positive macrophages are distinct from resident alveolar macrophages
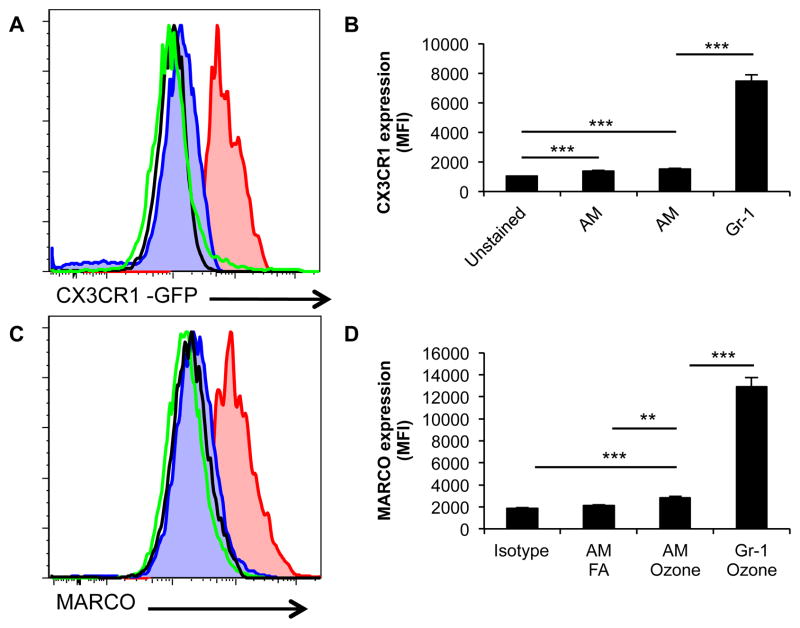
We were interested to determine whether unique differences could be defined between Gr-1 positive macrophages after ozone and resident macrophages (AM). In addition, we hoped to gain insight into a potential role of this subset in the host response to ozone lung injury. Flow cytometry was performed to identify additional cell surface markers, which could delineate Gr-1 positive macrophages from AMs. By utilizing mice in which an allele for CX3CR1 is replaced with GFP (CX3CR1GFP/WT) to track CX3CR1 expression, we identified that Gr-1 macrophages had enhanced CX3CR1 expression over AMs after ozone exposure. This was confirmed both by histogram (Figure 2A) and by a significant difference in mean fluorescent intensity (Figure 2B). We also demonstrated that Gr-1 macrophages had enhanced expression of MARCO by histogram analysis (Figure 2C). This was confirmed by a statistically higher MFI in the Gr-1 macrophage population (Figure 2D). MARCO previously has been suggested to have a role in protection of the host from ozone induced lung injury (10).
Figure 2. Cell surface expression of Gr-1high macrophages.
A–B, CD11blow Gr-1low and CD11blow Gr-1high macrophages are analyzed for cell surface expression of CX3CR1 using CX3CR1GFP/WT mice after ozone exposure. Gr-1 macrophages (Red) have enhanced surface expression of CX3CR1 as compared to AM (Blue) by histogram analysis (Fig. A) and mean fluorescent intensity (MFI) (Fig. B). Black open histogram represents unstained FITC channel autofluorescence and Green open histogram represents air exposed macrophages. C–D, Gr-1 Macs and AMs are analyzed for cell surface expression of MARCO. Gr-1 Macs (Red) have enhanced cell surface expression for MARCO by histograms (Fig. C) and MFI (Fig. D) as compared to ozone exposed AMs (Blue), isotype control (Black) and air exposed resident macrophages (Green). Histograms are a representative sample of C57BL/6 and CX3CR1GFP/WT mice. MFI data is generated from 4 mice with both FA and ozone exposures with two repeats (***P<0.0005).
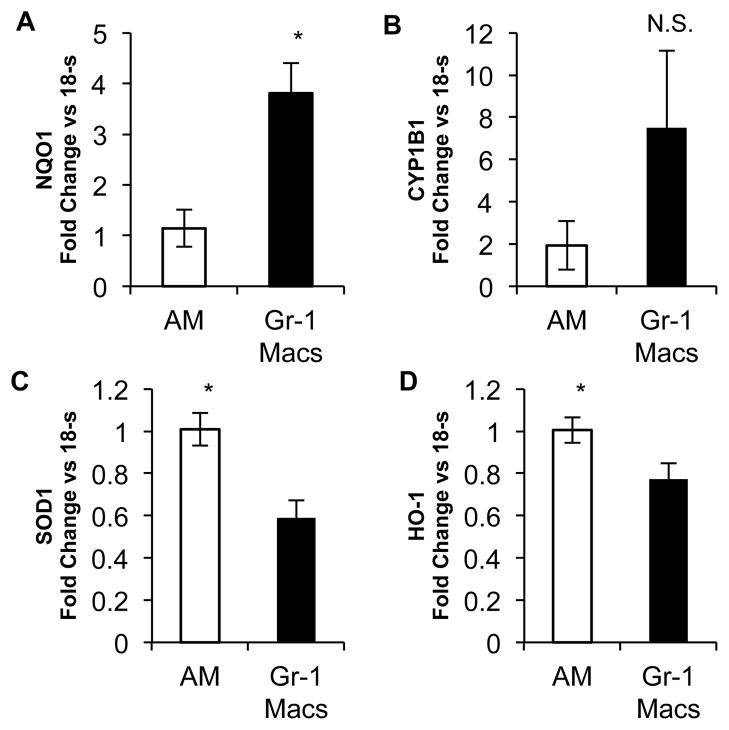
To further understand the difference between Gr-1 macrophages and AMs, we performed flow sorting experiments. Mice were exposed to ozone and then whole lungs were processed for flow cytometry. Gr-1 macrophages were sorted from AMs. The cells then underwent processing for mRNA analysis. Given the findings of MARCO expression, we utilized real time PCR to identify anti-oxidant gene expression. We did not identify significant difference in expression between Gr-1 and AMs for the following genes: thioredoxin reductase, glutathione s-transferase A1, CCAAT/enhancer binding protein-beta, colony stimulating factor 1 receptor, glutathione peroxidase 1, runt-related transcription factor 3, glutathione s-transferase theta 1, glutathione s-transferase M1, glutathione s-transferase P1 (Figure S2). However, Gr-1 macrophages demonstrated significantly enhanced expression of NADP (H): quinoneoxidoreductase 1 (NQO1) (Figure 3A) and exhibited a trend towards enhanced expression of cytochrome P450, family 1, subfamily B, polypeptide 1 (CYP1B1) (Figure 3B). Gr-1 macrophages had decreased expression of heme oxygenase-1 (HO-1) and superoxide dismutase 1 (SOD1) (Figure 3C, D). Differential cell surface markers and differential mRNA expression supports that Gr-1 macrophages are, in fact, a unique subset that develops after ozone exposure. In addition, the profile of surface markers and gene expression suggested that this novel subset of macrophages may serve a protective role after inhalation of ozone.
Figure 3. mRNA expression of Gr-1 Macs and Resident Macs after ozone exposure.
C57BL/6 mice were exposed to 2ppm of ozone for three h and then the lungs were harvested at 24 h. After digestion and centrifugation over an 18% Nicodenz cushion, the cells are sorted by flow cytometry. Both AMs and Gr-1 Macs are sorted and then processed for mRNA analysis. A–B, Enhanced expression of Gr-1 Macs over AMs after ozone demonstrates enhanced NQ01 expression (Fig. A) and CYP1B1 expression (Fig. B). C–D, Reduced expression in Gr-1 Macs over AMs after ozone demonstrates reduced SOD1 (Fig. C) and HO-1 (Fig. D). Data is from pooled samples of 15 mice which were performed 4 times. mRNA was analyzed for each sample as fold change vs. 18s. (*P<0.05)
Gr-1 positive macrophages are derived from a resident lung source
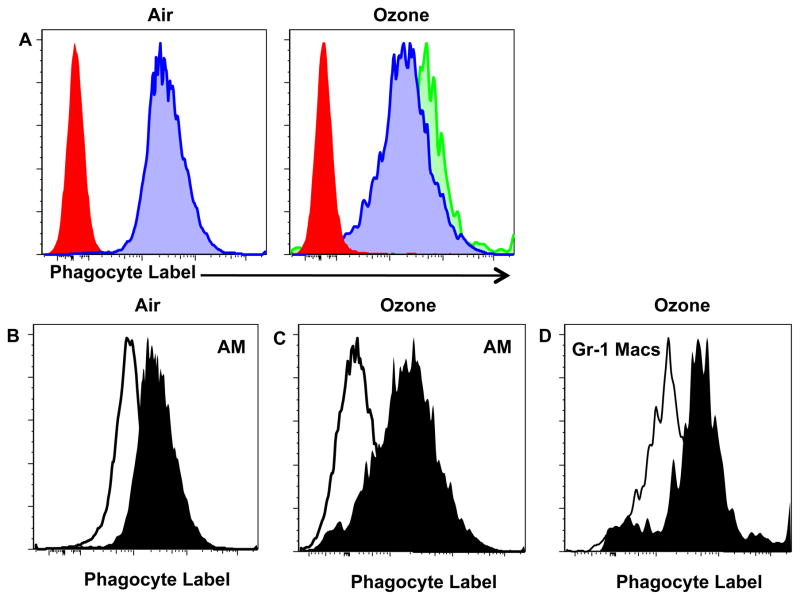
We and others have previously identified that the recruitment of ExMacs are dependent on recruitment of a CCR2-dependent circulating intermediate. We were therefore interested to determine if the source of Gr-1 positive macrophages after ozone was also due to recruitment into the lung. Recruitment of inflammatory monocytes, which mature into exudative macrophages and monocyte-derived dendritic cells, is dependent on the chemokine receptor CCR2 and its ligand CCL2. Using C57BL/6 and CCR2-null mice, we identified that there was no difference in number of Gr-1 macrophages after ozone exposure in a manner dependent on CCR2 (Figure S3). This observation suggested that our novel macrophage subset was not derived from a circulatory intermediate. Based on this result, we considered that Gr-1 positive macrophages could be derived from a resident lung intermediate. Therefore, we utilized a technique described by Maus et al (22) and utilized by others (23–25), which uses the fluorescent dye PKH26-PCL (Sigma) to label resident phagocytic cells in the lung. Mice were given PKH26-PCL via retro-orbital injection. 24 h after injection, the mice were exposed to either ozone or filtered air and processed for flow cytometry. As previously demonstrated by Maus et al., this dye does not label circulating blood cells as identified by low cell surface expression of the phagocytic label (Figure 4A, solid red histogram). Resident alveolar macrophages (defined as MHCIIint CD11chi Gr-1low CD11blow) successfully stain with the phagocyte label after either filtered air or ozone exposure (Figure 4A, blue shaded histogram). Interestingly, Gr-1 positive macrophages (defined as MHCIIint CD11chi Gr-1hi CD11bint) also stained for the phagocyte label. To make sure that this staining of the phagocytic label was not due to the autofluorescence of macrophages we compared both non-phagocyte labeled (open histogram) and phagocyte labeled (closed histogram) groups for both air exposed AMs (MHCIIint CD11chi) (Figure 4B), AMs after ozone (MHCIIint CD11chi Gr-1low CD11blow) (Figure 4C), and Gr-1 positive macrophages (MHCIIint CD11chi Gr-1hi CD11bint) (Figure 4D). These data confirmed that the phagocyte labeling was not due to autofluorescence. These data further support that the Gr-1 positive macrophages present after ozone exposure are derived from a resident lung source and not recruited/derived from a circulating intermediate.
Figure 4. Analysis of macrophages after cell labeling with the dye PKH26-PCL.
C57BL/6 mice are given the dye PKH26-PCL or control dilutant via retro-orbital objection 24 h prior to exposure to 3 h of FA or 2ppm of ozone. The mice were then harvested and analyzed by flow cytometry. At the same time whole blood was obtained from the FA and ozone exposed mice. A, Histogram analysis of dye PKH26-PCL expression from whole blood (red histogram), AMs (blue histogram) and Gr-1 Macs (green histograms) after filtered air or ozone. B–D Histogram analysis of phagocytic label with reagent dilutent (control) vs. PKH26-PCL for AMs after air exposure (Fig. B), for AMs after ozone exposure (Fig. C) and for Gr-1 Macs after ozone exposure (Fig. D). Data is representative of 4 mice per exposure with 1 repeat.
CX3CR1 deficiency results in a reduction in Gr-1+ macrophages and in enhanced response to ozone
The previously-described labeling of resident lung cells supports that our macrophage subset was derived from a resident source. Our present understanding of macrophages during lung injury has focused on macrophage differentiation from inflammatory monocytes recruited in a CCR2 dependent fashion. However, maturation of resident macrophages in context of injury remains poorly understood. CX3CR1 has previously been implicated in the maintenance of resident cell populations. As CX3CR1 expression was enhanced in Gr-1+ macrophages, we were interested to determine the effect of CX3CR1 deficiency on the maturation of Gr-1 macrophages. Flow cytometry was performed on CX3CR1GFP/WT (WT) and CX3CR1GFP/GFP (null) after exposure to either air or ozone. Gr-1+ macrophages were then identified. There was no difference in the air-exposed groups (Figure 5A). However, we observed a significant reduction in the Gr-1 sub-population after ozone exposure in the CX3CR1-null mice when compared to the WT mice. These data suggest that CX3CR1 was important for the maturation of Gr-1+ macrophages.
Figure 5. Ozone exposure in CX3CR1 null mice.
C57BL/6 and CX3CR1GFP/GFP (CX3CR1 null) mice were exposed to FA or 2ppm of ozone. A, Flow cytometric analysis of Gr-1 Macs in WT (open box) and CX3CR1 null mice (closed box) after FA and ozone as a percentage of total cells. B, CX3CL1 protein expression was analyzed by ELISA in bronchoalveolar lavage (BAL) from WT and CX3CR1 null mice after FA (open box) and ozone (closed box) exposure. C, Airway hyperresponsiveness after increasing doses of methacholine in WT and CX3CR1 null mice after either FA or ozone exposure for 3 h. D, Total Cells and neutrophils (PMN) from BAL cell count differentials in WT (open box) and CX3CR1 null mice (closed box) 24 hours after FA and ozone exposure. E–G, Analysis of total protein (Fig. E), 8-isoprostanes and protein carbonyls (Fig. F) and cytokines (CXCL2, IL-1β, IL-6, CCL2 and TNF-α) (Fig. G) from BAL in WT (open box) and CX3CR1 null mice (closed box) after FA and ozone. Data is from 4–6 mice per group (WT-FA, WT-ozone, CX3CR1 null-FA and CX3CR1 null-ozone) with one repeat (*P<0.05, **P<0.005)
Next, we determined the biological effect of CX3CR1 deficiency and the absence of Gr-1+ macrophages in the context of ozone exposure. The role of CX3CR1 in response to ozone was unknown. CX3CL1 is the only known ligand for CX3CR1. We assessed the expression of CX3CL1 in the bronchoalveolar lavage of both WT and CX3CR1 null mice. CX3CL1 was increased in the lavage of WT ozone exposed mice as compared to WT air-exposed mice (Figure 5B). CX3CR1-null mice had elevated levels of CX3CL1 in the BAL in both ozone-exposed and air-exposed mice. This observation is consistent with prior reports demonstrating the CX3CL1 is increased in CX3CR1 null mice at baseline due to failed clearance of the ligand by the receptor (26). Based on the increased expression of CX3CL1 after ozone, we were interested to determine whether CX3CR1-null mice had enhanced airway hyperresponsiveness (AHR) to methacholine challenge. There was no difference in the AHR between air-exposed WT and CX3CR1-null mice (Figure 5C). WT mice exposed to ozone were noted to have enhanced AHR as compared to air controls. CX3CR1-null mice had even further enhanced AHR after ozone exposure when compared to similarly exposed WT mice. This observation demonstrated that ozone exposed CX3CR1 null mice develop enhanced AHR to methacholine challenge when compared to ozone-exposed WT mice. Based on this observation, we were interested to determine potential mechanisms that could account for this enhanced AHR. There was evidence of enhanced total cells and neutrophils in the BAL of CX3CR1 null mice after ozone as compared to WT mice consistent with enhanced inflammation (Figure 5D). There was no significant difference in BAL total protein between ozone-exposed WT and CX3CR1 null mice (Figure 5E). However, there was evidence of enhanced oxidative stress as measured by both 8-isoprostanes and protein carbonyl levels (Figure 5F). BAL cytokines, recognized to be elevated in the setting of ozone exposure, were further enhanced in ozone-exposed CX3CR1 null mice (Figure 5G). CX3CR1-deficiency was associated with enhanced AHR, oxidative stress, and pro-inflammatory cytokines. Together these findings suggest that CX3CR1-dependent Gr-1+ macrophages are protective to the lung after ozone exposure.
Discussion
Our results demonstrate that inhalation of ozone results in the development of a novel macrophage subset characterized by enhanced expression of the cell surface marker Gr-1. These macrophages are not recruited from a circulating intermediate in a manner dependent on CCR2, but rather appear to be derived from a resident lung source. We demonstrate that this novel macrophage subset has enhanced cell surface expression of CX3CR1 and MARCO and a unique mRNA expression profile when compared to other resident macrophages. The development of this novel subset of macrophages is dependent on the chemokine receptor CX3CR1. Furthermore, CX3CR1-null mice have enhanced biological response to inhaled ozone. Our observations, in total, support a novel macrophage subset, which appears to protect the lung from the biological response to ambient ozone.
Ambient ozone remains a significant health burden, which can exacerbate chronic respiratory conditions such as asthma. Macrophages appear to be critical in the response to inhaled ozone. Pendino et al. demonstrated that when the macrophage inhibitor gadolinium chloride was provided to rats prior to ozone exposure, these rats were protected from lung injury as measured by decreased lavage fluid protein levels, inflammatory cells, cytokines and nitric oxide/inducible nitric oxide synthase (7). We and others have identified macrophage-derived mediators present in the lung after ozone exposure (27, 28). However, this observation remains somewhat inconsistent and may be dependent on the intensity and duration of exposure, as other groups have demonstrated that ozone exposure results in a decrease in macrophage-derived cytokines (29–31). Macrophages can also serve a protective role in the setting of ozone-induced lung injury. Work by Dahl et al demonstrated that macrophage expression of both MARCO and scavenger receptor AI and AII was critical to protection against ozone injury in the lung via scavenging of oxidized lipid moieties (10). These ostensibly conflicting observations suggest that individual macrophage sub-populations may be present after ozone exposure and have divergent roles in the development of the host response.
Heightened interest in macrophage heterogeneity has evolved as a result of our understanding that macrophages have a variety of functions that are dependent on the local cytokine micro-environment with which they are exposed (12, 32). We previously demonstrated that after both infectious and non-infectious lung injury there is recruitment of exudative macrophages from a blood borne intermediate (16, 17). We anticipated recruitment of ExMacs into the lung after ozone exposure. To our surprise, there was no significant accumulation of CD11b+ exudative macrophages at 24 h after inhalation of ozone. There was also no CCR2 dependent recruitment of macrophages. This suggests that exudative macrophages are not recruited to the lung in the setting of ozone exposure. This could be an issue of the timing after injury, or reflect the difference in the severity of the injury models between ozone and bleomycin or influenza.
In this study, we identified a macrophage subset that was not dependent on CCR2 and was delineated from other macrophages by expression of Gr-1 and not CD11b. The Gr-1 epitope is expressed on the two molecules Ly-6G and Ly-6C. The expression of Ly-6G is restricted to granulocytes therefore Gr-1+/Ly-6G+ cells are defined as neutrophils (33). Excluding neutrophils, Gr-1+ expression within the lung has been identified with monocytes, monocyte derived dendritic cells and under certain injury conditions with exudative macrophages (16, 17). It is generally accepted that macrophages and dendritic cells derived from monocytes in the setting of inflammation have increased expression of Gr-1 which reflects this recent differentiation from monocytes. In this setting, all of these cells are also characterized by being CD11b+ and dependent on CCR2 for their migration. There have been several other descriptions of pulmonary macrophages with low levels of Gr-1 expression. Gr-1dull macrophages which also expression CD11c, and low levels of both CD11b and F4/80 were identified after pulmonary infection with Streptococcus pneumonia and were responsible for early production of TNF-α (34). Recent work described Gr-1dim/Ly-6Gdim cells which were also low expressing for F4/80 (35). The presence of these cells was associated with the progression of Mycobacterium tuberculosis pulmonary infection. Though there are several descriptions of Gr-1 macrophages and their function, it remains unclear if there is a common cell among the various descriptions. Gr-1 macrophages are associated with a pro-inflammatory and not protective function in these experimental models. None of the prior studies that we are aware of have identified a Gr-1 positive macrophage population from resident cells. This suggests that the Gr-1 macrophages we describe are unique from prior descriptions. It remains unknown whether Gr-1 have functional implications or represent a useful marker for identification of sub-populations of myeloid cells.
The appearance of this novel sub-population after ozone suggested that it may have unique functions when compared to previously described macrophages. We demonstrated that Gr-1 Macs had enhanced cell surface expression of both CX3CR1 and MARCO. The high level of expression of MARCO suggested that these macrophages may be protective in the response to ozone based on prior observations that MARCO served to scavenge oxidized lipid species produced after ozone exposure to the epithelial lining fluid (10). Drawing on this finding, we hypothesized that the expression profile would be unique between Gr-1 Macs and the Gr-1 negative lung macrophages. After sorting, real time PCR analysis revealed that these macrophages had enhanced expression of NAD(P)H:quinine oxioreductase 1 (NQO1) and reduced expression of both SOD-1 and HO-1. NQO1 is a detoxifying 2-electron reductase that can act as an anti-oxidant through regeneration of anti-oxidant forms of ubiquinone and vitamin E quinine. Toxicology studies identify a cyto-protective role for NQO1. For example, NQO1 detoxifies quinones derived from the oxidation of phenolic metabolites of benzene (36) and absence of NQO1 results in increased toxicity to menadione (37). These results established a role for NQO1 in protection against quinone toxicity. Myeloid expression of NQ01 is both inducible by the anti-oxidant response element nuclear factor E2-related factor 2 (Nrf2) and is associated with resistance to oxidant-induced cell injury (38, 39). However, NQO1 has previously been reported to function as either an anti-oxidant or pro-oxidant depending on the quinine substrate and cell-type. The high level of expression of both MARCO and NQO1 is suggestive of a protective phenotype for this sub-population of macrophages.
Much of the work on macrophage sub-populations in the lung has focused on recruited cells from the bone marrow. Inflammatory monocytes are recruited into the lung in a manner dependent on CCR2 and then appear to differentiate into exudate macrophages (16, 22). In the present work we did not identify macrophages recruited to the lung dependent on CCR2, which suggested that our macrophage subset may be derived from a resident lung population. Using a labeling technique described by Maus et al. (22) and utilized by others (23–25), we determined that Gr-1 positive macrophages were in fact derived from a resident lung intermediate. To our knowledge, this is the first report of a resident lung subset identified by flow cytometry after inhalation of ozone. Prior reports have identified that resident lung monocytes and macrophages have altered gene expression profiles from recruited cells (40). Other work supports that resident lung macrophages contribute to alveolar cytokine release and recruitment of neutrophils and monocyte recruitment into the alveolar space after exposure to LPS (41–43). These findings suggest that resident lung macrophages are critical for the host response to inflammation, but that they remain poorly characterized in the setting of inflammation. It is also clear that some macrophages function to limit pulmonary inflammation by clearance of inflammatory mediators such as oxidized lipid species and apoptotic cells (10, 44, 45). To better understand the complex functional role of lung macrophages after environmental challenges, clear appreciation of macrophage sub-populations in the lung will be necessary.
We made the interesting discovery that Gr-1 macrophages had enhanced CX3CR1 expression. CX3CR1 expression on blood monocytes defines them as constitutive monocytes important for the development and replenishment of resident niches of monocytes/macrophages (18). CX3CR1 is expressed on monocytes, macrophages, mucosal DCs, natural killer cells, CD8 T cells and CD4 T cells (18, 46, 47). CX3CR1 has only one known ligand, CX3CL1 (also known as fractalkine). Despite the characterization that blood monocytes express CX3CR1 and data from other organ systems which suggest a critical role for the chemokine axis in inflammation (48–50), until recently there has been a dearth of information on the effect(s) this receptor/ligand axis exercises on the recruitment of cells into the lung during lung injury. CX3CL1 is constitutively expressed on lung macrophages (51), bronchial epithelium (52) and pulmonary endothelium (53). Multiple stimuli including IFN-γ (52), chronic cigarette smoke (54), and allergen challenge (55) will cause increased pulmonary CX3CL1 expression. We identified that levels of CX3CL1 were increased in the lavage fluid of ozone-challenged C57BL/6 mice when compared to filtered air controls. The precise effect of CX3CL1 after inhalation of ozone remains unknown. Our findings suggest that CX3CL1 may contribute to the maturation of Gr-1 Macs. Prior work demonstrated that increased CX3CL1 appeared to be associated with the recruitment of CX3CR1+ mononuclear phagocytes during chronic smoke exposure (54). A follow up study identified that CX3CL1, in fact, failed to recruit significant numbers of CX3CR1+ cells into the airspaces, but rather was required for amplifying a subset of CD11b expressing macrophages, which were spatially confined to the interstitium and were a source of IL-6 and TNF-α (51). This description of cells most consistently fits with prior descriptions of exudative macrophages (16, 17). We demonstrated a CX3CR1+ macrophage population from the whole lung that did not have enhanced CD11b expression but instead enhanced Gr-1. Gr-1+ macrophages also appear to be dependent on CX3CR1 for their development after ozone. Gr-1+ macrophages appear to be a unique macrophage subset when compared to circulatory derived phagocytes present in the lung following exposure to cigarette smoke (54).
We identify that CX3CR1-deficiency was associated with enhanced ozone induced lung inflammation. This observation is in apparent conflict with prior reports demonstrating a protection from the development of emphysema in a model of chronic smoke exposure (51) and of protection from allergic airways disease (56). We suspect that the discrepancy is related to the different models. Allergen challenge is likely to affect the adaptive immune system, while the acute response to ozone is predominantly dependent on the innate immune system (6). Chronic smoke exposure appears to cause chronic inflammation, which may affect different cell types and mechanisms. Cigarette smoke appears to stimulate/induce a CX3CR1-dependent recruitment of macrophages from circulating intermediates, and a similar process does not appear to evolve in our acute ozone exposure model. Our results provide strong support that following exposure to environmental toxicants such as ozone, CX3CR1 is protective and additionally contributes to maturation of resident macrophages.
In summary, we identify a novel macrophage subset in the lungs of mice after inhalation of ozone. This unique macrophage subset is characterized by enhanced Gr-1 expression and enhanced cell surface expression of both MARCO and CX3CR1. These macrophages have a unique mRNA expression profile when compared to residual resident macrophages. The mRNA profile is characterized by enhanced NQO1 expression, which is suggestive of a detoxification phenotype. This macrophage sub-population appears to be derived from a resident lung phagocyte cells, and not from a circulating intermediate. Finally, we establish that the presence of this macrophage sub-population is dependent on CX3CR1. Furthermore following ozone CX3CR1 adds a protective element to lung defenses, since in the absence of CX3CR1 both biological (neutrophilic inflammation, oxidant stress, and pro-inflammatory cytokine production) and physiological (AHR) phenotypes associated with ozone, are enhanced. Together these observations support a novel sub-population of protective detoxifying lung macrophages which we now term ToxMacs. We speculate that ToxMacs limit the biological and physiological responses to ozone due to their ability to scavenge oxidized species and/or manage oxidant balance. Improved understanding of macrophage sub-populations may provide insights into potential treatments to protect against oxidant-induced lung pathology.
Supplementary Material
Footnotes
This work was supported by National Institutes of Health Grants ES016126 (to J.W.H.), ES016347 (to W.M.F.) and HL077291 (to P.W.N.)
Abreviations: AM – Alveolar Macrophages; ExMac – Exudative Macrophages; NQO1 – NAD(P)H: quinine oxioreductase 1; SOD-1 – Superoxide Dismutase 1; HO-1 – Heme Oxygenase 1
References
- 1.Ciencewicki J, Trivedi S, Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol. 2008;122:456–468. doi: 10.1016/j.jaci.2008.08.004. quiz 469–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foster WM, Stetkiewicz PT. Regional clearance of solute from the respiratory epithelia: 18–20 h postexposure to ozone. J Appl Physiol. 1996;81:1143–1149. doi: 10.1152/jappl.1996.81.3.1143. [DOI] [PubMed] [Google Scholar]
- 3.Kehrl HR, Vincent LM, Kowalsky RJ, Horstman DH, O’Neil JJ, McCartney WH, Bromberg PA. Ozone exposure increases respiratory epithelial permeability in humans. Am Rev Respir Dis. 1987;135:1124–1128. doi: 10.1164/arrd.1987.135.5.1124. [DOI] [PubMed] [Google Scholar]
- 4.Pulfer MK, Murphy RC. Formation of biologically active oxysterols during ozonolysis of cholesterol present in lung surfactant. J Biol Chem. 2004;279:26331–26338. doi: 10.1074/jbc.M403581200. [DOI] [PubMed] [Google Scholar]
- 5.Garantziotis S, Li Z, Potts EN, Kimata K, Zhuo L, Morgan DL, Savani RC, Noble PW, Foster WM, Schwartz DA, Hollingsworth JW. Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice. J Biol Chem. 2009;284:11309–11317. doi: 10.1074/jbc.M802400200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 6.Al-Hegelan M, Tighe RM, Castillo C, Hollingsworth JW. Ambient ozone and pulmonary innate immunity. Immunol Res. 2011;49:173–191. doi: 10.1007/s12026-010-8180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pendino KJ, Meidhof TM, Heck DE, Laskin JD, Laskin DL. Inhibition of macrophages with gadolinium chloride abrogates ozone-induced pulmonary injury and inflammatory mediator production. Am J Respir Cell Mol Biol. 1995;13:125–132. doi: 10.1165/ajrcmb.13.2.7542894. [DOI] [PubMed] [Google Scholar]
- 8.Hotchkiss JA, Harkema JR, Kirkpatrick DT, Henderson RF. Response of rat alveolar macrophages to ozone: quantitative assessment of population size, morphology, and proliferation following acute exposure. Exp Lung Res. 1989;15:1–16. doi: 10.3109/01902148909069605. [DOI] [PubMed] [Google Scholar]
- 9.Arsalane K, Gosset P, Vanhee D, Voisin C, Hamid Q, Tonnel AB, Wallaert B. Ozone stimulates synthesis of inflammatory cytokines by alveolar macrophages in vitro. Am J Respir Cell Mol Biol. 1995;13:60–68. doi: 10.1165/ajrcmb.13.1.7598938. [DOI] [PubMed] [Google Scholar]
- 10.Dahl M, Bauer AK, Arredouani M, Soininen R, Tryggvason K, Kleeberger SR, Kobzik L. Protection against inhaled oxidants through scavenging of oxidized lipids by macrophage receptors MARCO and SR-AI/II. J Clin Invest. 2007;117:757–764. doi: 10.1172/JCI29968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishii Y, Hashimoto K, Nomura A, Sakamoto T, Uchida Y, Ohtsuka M, Hasegawa S, Sagai M. Elimination of neutrophils by apoptosis during the resolution of acute pulmonary inflammation in rats. Lung. 1998;176:89–98. doi: 10.1007/pl00007597. [DOI] [PubMed] [Google Scholar]
- 12.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 13.Careau E, Bissonnette EY. Adoptive transfer of alveolar macrophages abrogates bronchial hyperresponsiveness. Am J Respir Cell Mol Biol. 2004;31:22–27. doi: 10.1165/rcmb.2003-0229OC. [DOI] [PubMed] [Google Scholar]
- 14.Maus U, Huwe J, Maus R, Seeger W, Lohmeyer J. Alveolar JE/MCP-1 and endotoxin synergize to provoke lung cytokine upregulation, sequential neutrophil and monocyte influx, and vascular leakage in mice. Am J Respir Crit Care Med. 2001;164:406–411. doi: 10.1164/ajrccm.164.3.2009055. [DOI] [PubMed] [Google Scholar]
- 15.Taut K, Winter C, Briles DE, Paton JC, Christman JW, Maus R, Baumann R, Welte T, Maus UA. Macrophage Turnover Kinetics in the Lungs of Mice Infected with Streptococcus pneumoniae. Am J Respir Cell Mol Biol. 2008;38:105–113. doi: 10.1165/rcmb.2007-0132OC. [DOI] [PubMed] [Google Scholar]
- 16.Lin KL, Suzuki Y, Nakano H, Ramsburg E, Gunn MD. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol. 2008;180:2562–2572. doi: 10.4049/jimmunol.180.4.2562. [DOI] [PubMed] [Google Scholar]
- 17.Tighe RM, Liang J, Liu N, Jung Y, Jiang D, Gunn MD, Noble PW. Recruited Exudative Macrophages Selectively Produce CXCL10 Following Non-Infectious Lung Injury. Am J Respir Cell Mol Biol. 2011 doi: 10.1165/rcmb.2010-0471OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 19.Landsman L, Varol C, Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. J Immunol. 2007;178:2000–2007. doi: 10.4049/jimmunol.178.4.2000. [DOI] [PubMed] [Google Scholar]
- 20.Landsman L, Jung S. Lung macrophages serve as obligatory intermediate between blood monocytes and alveolar macrophages. J Immunol. 2007;179:3488–3494. doi: 10.4049/jimmunol.179.6.3488. [DOI] [PubMed] [Google Scholar]
- 21.Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maus U, Herold S, Muth H, Maus R, Ermert L, Ermert M, Weissmann N, Rosseau S, Seeger W, Grimminger F, Lohmeyer J. Monocytes recruited into the alveolar air space of mice show a monocytic phenotype but upregulate CD14. Am J Physiol Lung Cell Mol Physiol. 2001;280:L58–68. doi: 10.1152/ajplung.2001.280.1.L58. [DOI] [PubMed] [Google Scholar]
- 23.Janssen WJ, Barthel L, Muldrow A, Oberley-Deegan RE, Kearns MT, Jakubzick C, Henson PM. Fas Determines Differential Fates of Resident and Recruited Macrophages During Resolution of Acute Lung Injury. Am J Respir Crit Care Med. 2011 doi: 10.1164/rccm.201011-1891OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson AA, Murphy GJ, Hamakawa H, Kwok LW, Srinivasan S, Hovav AH, Mulligan RC, Amar S, Suki B, Kotton DN. Amelioration of emphysema in mice through lentiviral transduction of long-lived pulmonary alveolar macrophages. J Clin Invest. 2010;120:379–389. doi: 10.1172/JCI36666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jennings JH, Linderman DJ, Hu B, Sonstein J, Curtis JL. Monocytes recruited to the lungs of mice during immune inflammation ingest apoptotic cells poorly. Am J Respir Cell Mol Biol. 2005;32:108–117. doi: 10.1165/rcmb.2004-0108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cardona AE, Sasse ME, Liu L, Cardona SM, Mizutani M, Savarin C, Hu T, Ransohoff RM. Scavenging roles of chemokine receptors: chemokine receptor deficiency is associated with increased levels of ligand in circulation and tissues. Blood. 2008;112:256–263. doi: 10.1182/blood-2007-10-118497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garantziotis S, Li Z, Potts EN, Lindsey JY, Stober VP, Polosukhin VV, Blackwell TS, Schwartz DA, Foster WM, Hollingsworth JW. TLR4 is necessary for hyaluronan-mediated airway hyperresponsiveness after ozone inhalation. Am J Respir Crit Care Med. 2010;181:666–675. doi: 10.1164/rccm.200903-0381OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 28.Pendino KJ, Shuler RL, Laskin JD, Laskin DL. Enhanced production of interleukin-1, tumor necrosis factor-alpha, and fibronectin by rat lung phagocytes following inhalation of a pulmonary irritant. Am J Respir Cell Mol Biol. 1994;11:279–286. doi: 10.1165/ajrcmb.11.3.8086166. [DOI] [PubMed] [Google Scholar]
- 29.Devlin RB, McKinnon KP, Noah T, Becker S, Koren HS. Ozone-induced release of cytokines and fibronectin by alveolar macrophages and airway epithelial cells. Am J Physiol. 1994;266:L612–619. doi: 10.1152/ajplung.1994.266.6.L612. [DOI] [PubMed] [Google Scholar]
- 30.Becker S, Madden MC, Newman SL, Devlin RB, Koren HS. Modulation of human alveolar macrophage properties by ozone exposure in vitro. Toxicol Appl Pharmacol. 1991;110:403–415. doi: 10.1016/0041-008x(91)90042-d. [DOI] [PubMed] [Google Scholar]
- 31.Janic B, Umstead TM, Phelps DS, Floros J. Modulatory effects of ozone on THP-1 cells in response to SP-A stimulation. Am J Physiol Lung Cell Mol Physiol. 2005;288:L317–325. doi: 10.1152/ajplung.00125.2004. [DOI] [PubMed] [Google Scholar]
- 32.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hestdal K, Ruscetti FW, Ihle JN, Jacobsen SE, Dubois CM, Kopp WC, Longo DL, Keller JR. Characterization and regulation of RB6-8C5 antigen expression on murine bone marrow cells. J Immunol. 1991;147:22–28. [PubMed] [Google Scholar]
- 34.Hatta M, Yamamoto N, Miyazato A, Ishii N, Nakamura K, Inden K, Aoyagi T, Kunishima H, Hirakata Y, Suzuki K, Kaku M, Kawakami K. Early production of tumor necrosis factor-alpha by Gr-1 cells and its role in the host defense to pneumococcal infection in lungs. FEMS Immunol Med Microbiol. 2010;58:182–192. doi: 10.1111/j.1574-695X.2009.00616.x. [DOI] [PubMed] [Google Scholar]
- 35.Lyadova IV, Tsiganov EN, Kapina MA, Shepelkova GS, Sosunov VV, Radaeva TV, Majorov KB, Shmitova NS, van den Ham HJ, Ganusov VV, De Boer RJ, Racine R, Winslow GM. In mice, tuberculosis progression is associated with intensive inflammatory response and the accumulation of Gr-1 cells in the lungs. PLoS ONE. 2010;5:e10469. doi: 10.1371/journal.pone.0010469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moran JL, Siegel D, Ross D. A potential mechanism underlying the increased susceptibility of individuals with a polymorphism in NAD(P)H:quinone oxidoreductase 1 (NQO1) to benzene toxicity. Proc Natl Acad Sci U S A. 1999;96:8150–8155. doi: 10.1073/pnas.96.14.8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Radjendirane V, Joseph P, Lee YH, Kimura S, Klein-Szanto AJ, Gonzalez FJ, Jaiswal AK. Disruption of the DT diaphorase (NQO1) gene in mice leads to increased menadione toxicity. J Biol Chem. 1998;273:7382–7389. doi: 10.1074/jbc.273.13.7382. [DOI] [PubMed] [Google Scholar]
- 38.Zhu H, Jia Z, Zhang L, Yamamoto M, Misra HP, Trush MA, Li Y. Antioxidants and phase 2 enzymes in macrophages: regulation by Nrf2 signaling and protection against oxidative and electrophilic stress. Exp Biol Med (Maywood) 2008;233:463–474. doi: 10.3181/0711-RM-304. [DOI] [PubMed] [Google Scholar]
- 39.Ross D, Siegel D, Schattenberg DG, Sun XM, Moran JL. Cell-specific activation and detoxification of benzene metabolites in mouse and human bone marrow: identification of target cells and a potential role for modulation of apoptosis in benzene toxicity. Environ Health Perspect. 1996;1046(Suppl):1177–1182. doi: 10.1289/ehp.961041177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Srivastava M, Jung S, Wilhelm J, Fink L, Buhling F, Welte T, Bohle RM, Seeger W, Lohmeyer J, Maus UA. The inflammatory versus constitutive trafficking of mononuclear phagocytes into the alveolar space of mice is associated with drastic changes in their gene expression profiles. J Immunol. 2005;175:1884–1893. doi: 10.4049/jimmunol.175.3.1884. [DOI] [PubMed] [Google Scholar]
- 41.Maus UA, Janzen S, Wall G, Srivastava M, Blackwell TS, Christman JW, Seeger W, Welte T, Lohmeyer J. Resident alveolar macrophages are replaced by recruited monocytes in response to endotoxin-induced lung inflammation. Am J Respir Cell Mol Biol. 2006;35:227–235. doi: 10.1165/rcmb.2005-0241OC. [DOI] [PubMed] [Google Scholar]
- 42.Blackwell TS, Lancaster LH, Blackwell TR, Venkatakrishnan A, Christman JW. Chemotactic gradients predict neutrophilic alveolitis in endotoxin-treated rats. Am J Respir Crit Care Med. 1999;159:1644–1652. doi: 10.1164/ajrccm.159.5.9806166. [DOI] [PubMed] [Google Scholar]
- 43.Hollingsworth JW, 2nd, Cook DN, Brass DM, Walker JK, Morgan DL, Foster WM, Schwartz DA. The role of Toll-like receptor 4 in environmental airway injury in mice. Am J Respir Crit Care Med. 2004;170:126–132. doi: 10.1164/rccm.200311-1499OC. [DOI] [PubMed] [Google Scholar]
- 44.Borges VM, Vandivier RW, McPhillips KA, Kench JA, Morimoto K, Groshong SD, Richens TR, Graham BB, Muldrow AM, Van Heule L, Henson PM, Janssen WJ. TNFalpha inhibits apoptotic cell clearance in the lung, exacerbating acute inflammation. Am J Physiol Lung Cell Mol Physiol. 2009;297:L586–595. doi: 10.1152/ajplung.90569.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science. 2002;296:155–158. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- 46.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, Yoshie O. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 47.Foussat A, Coulomb-L’Hermine A, Gosling J, Krzysiek R, Durand-Gasselin I, Schall T, Balian A, Richard Y, Galanaud P, Emilie D. Fractalkine receptor expression by T lymphocyte subpopulations and in vivo production of fractalkine in human. Eur J Immunol. 2000;30:87–97. doi: 10.1002/1521-4141(200001)30:1<87::AID-IMMU87>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 48.Suzuki F, Nanki T, Imai T, Kikuchi H, Hirohata S, Kohsaka H, Miyasaka N. Inhibition of CX3CL1 (fractalkine) improves experimental autoimmune myositis in SJL/J mice. J Immunol. 2005;175:6987–6996. doi: 10.4049/jimmunol.175.10.6987. [DOI] [PubMed] [Google Scholar]
- 49.Lesnik P, Haskell CA, Charo IF. Decreased atherosclerosis in CX3CR1−/− mice reveals a role for fractalkine in atherogenesis. J Clin Invest. 2003;111:333–340. doi: 10.1172/JCI15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inoue A, Hasegawa H, Kohno M, Ito MR, Terada M, Imai T, Yoshie O, Nose M, Fujita S. Antagonist of fractalkine (CX3CL1) delays the initiation and ameliorates the progression of lupus nephritis in MRL/lpr mice. Arthritis Rheum. 2005;52:1522–1533. doi: 10.1002/art.21007. [DOI] [PubMed] [Google Scholar]
- 51.Xiong Z, Leme AS, Ray P, Shapiro SD, Lee JS. CX3CR1+ lung mononuclear phagocytes spatially confined to the interstitium produce TNF-alpha and IL-6 and promote cigarette smoke-induced emphysema. J Immunol. 2011;186:3206–3214. doi: 10.4049/jimmunol.1003221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fujimoto K, Imaizumi T, Yoshida H, Takanashi S, Okumura K, Satoh K. Interferon-gamma stimulates fractalkine expression in human bronchial epithelial cells and regulates mononuclear cell adherence. Am J Respir Cell Mol Biol. 2001;25:233–238. doi: 10.1165/ajrcmb.25.2.4275. [DOI] [PubMed] [Google Scholar]
- 53.Balabanian K, Foussat A, Dorfmuller P, Durand-Gasselin I, Capel F, Bouchet-Delbos L, Portier A, Marfaing-Koka A, Krzysiek R, Rimaniol AC, Simonneau G, Emilie D, Humbert M. CX(3)C chemokine fractalkine in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2002;165:1419–1425. doi: 10.1164/rccm.2106007. [DOI] [PubMed] [Google Scholar]
- 54.McComb JG, Ranganathan M, Liu XH, Pilewski JM, Ray P, Watkins SC, Choi AM, Lee JS. CX3CL1 up-regulation is associated with recruitment of CX3CR1+ mononuclear phagocytes and T lymphocytes in the lungs during cigarette smoke-induced emphysema. Am J Pathol. 2008;173:949–961. doi: 10.2353/ajpath.2008.071034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rimaniol AC, Till SJ, Garcia G, Capel F, Godot V, Balabanian K, Durand-Gasselin I, Varga EM, Simonneau G, Emilie D, Durham SR, Humbert M. The CX3C chemokine fractalkine in allergic asthma and rhinitis. J Allergy Clin Immunol. 2003;112:1139–1146. doi: 10.1016/j.jaci.2003.09.041. [DOI] [PubMed] [Google Scholar]
- 56.Mionnet C, Buatois V, Kanda A, Milcent V, Fleury S, Lair D, Langelot M, Lacoeuille Y, Hessel E, Coffman R, Magnan A, Dombrowicz D, Glaichenhaus N, Julia V. CX3CR1 is required for airway inflammation by promoting T helper cell survival and maintenance in inflamed lung. Nat Med. 2010;16:1305–1312. doi: 10.1038/nm.2253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.