Abstract
Hepatocyte growth factor-regulated tyrosine kinase substrate that is encoded by Hgs promotes degradation of ubiquitinated signaling molecule in the early endosome. We previously reported that a targeted mutation in Hgs results in embryonic lethality soon after gastrulation in the mouse. Here, we report that downstream target genes for BMP signaling were highly down regulated in the Hgs mutant embryos. We also showed that Hgs is required for phosphorylation of SMAD1/5/8 and TAK1/p38 to transduce BMP signaling. Furthermore, we found that HGS functions to localize TAK1 in early endosome for its activation. These results suggest that HGS is critical to localize TAK1 to early endosome for transducing BMP signaling for proper development. Our data revealed a new mechanism to modify BMP signaling by Hgs during early mouse development.
Keywords: gastrulation, signal transduction, endosome
INTRODUCTION
Members of the Transforming growth factor-beta (TGF-β) superfamily, such as Activin, Nodal, and Bone morphogenetic proteins (BMPs) bind to their specific cell surface receptors, which are composed of two distinct subfamilies retaining serine/threonine kinases and are known as the type I and type II receptors (Kishigami and Mishina, 2005). Upon ligand binding, the type II receptor kinase phosphorylates and activates the type I receptor kinase, which induces phosphorylation and activation of signal-transducing effector molecules known as SMAD1, 2, 3, 5, and 8; subsequently, these receptor-regulated SMAD proteins form complexes with SMAD4, a collaborating Smad protein. The TGF/Activin and BMP receptor kinases activate SMAD2 or SMAD3 and SMAD1 or SMAD5, respectively, followed by formation of a complex of each SMAD with SMAD4, which transmits signals from the receptor complex in the plasma membrane to the nucleus.
TGF-β-activating kinase 1 (TAK1) is a member of the mitogen-activated protein kinasekinase kinase (MAPKKK) family and is activated by cytokinessuch as interleukin-1 (Ninomiya-Tsuji et al., 1999). TAK1 also playsimportant roles in signaling downstream of a variety of othermolecules, including BMPs. TAK1 functions to transduce BMP signaling in dorsoventral patterning and neural induction of Xenopus embryo (Shibuya et al., 1998; Yamaguchi et al., 1999). TAK1 is an ubiquitin-dependentkinase. Its activation requires ubiquitin ligase, deubiquitinating enzymes inhibit TAK1 activation and polyubiquitin plays an important role as a scaffold protein that facilitate autophosphorylation of TAK1 (Adhikari et al., 2007). Despite these reports, little is known about involvement of TAK1 in BMP signaling during mouse embryogenesis as well as other mechanism(s) of TAK1 phosphorylation occurring in vivo.
Hepatocyte Growth factor-regulated tyrosine kinase Substrate (HGS) is an evolutionarily conserved vesicular sortingprotein belonging to the endosomal-sorting proteins requiredfor transport (ESCRT) (Komada et al., 1997). HGS possesses a FYVE domain, whichenables the molecule to anchor to the internal surface of thelipid bilayer (Raiborg et al., 2001), and a UIM domain, through which HGSbinds monoubiquitinated receptors (Komada et al., 1997). By associating with ubiquitinated receptors such as MET or EGFRs,HGS promotes their degradation through the endosomal/lysosomalpathway and thus regulates Hepatocyte Growth Factor or Epidermal Growth Factor signaling (Lloyd et al., 2002; Toyoshima et al., 2007).
Targeted mutations in Hgs result in early embryonic lethality soon after gastrulation (Komada and Soriano, 1999; Miura et al., 2000). We also reported that HGS enhance the association of ACTR1 and SMAD2 for ACTIVIN signaling (Miura et al., 2000). Here we report that the phosphorylation of SMAD1 is decreased, and that of TAK1/p38 is abrogated in Hgs−/− embryos at E7.5. As a consequence, the expression of the target genes of BMP signaling is lost or strongly down regulated. Results from selective inhibitions of TAK1 indicate that TAK1 is responsible for Flk1 expression. Subcellular localization of TAK1 in the early endosome is largely displaced in the Hgs−/−embryos, which might be a cause of loss of phosphorylation of TAK1. Together, these results suggest that HGS is a critical component to localize TAK1 to the early endosome for transducing BMP signaling for proper development.
RESULTS
Hgs−/− represents null allele of Hgs gene
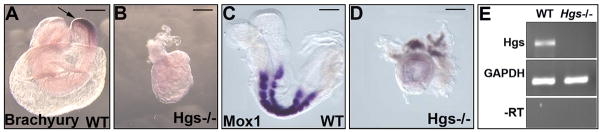
We previously reported that homozygous embryos for the targeted Hgs allele (Hgs−/−) show severe gastrulation defect and die around E9.5 (Miura et al., 2000). Primitive steak formation is not observed or strongly deteriorated as judged by downregulated expression of Brachyury (Fig. 1A, B). Also, Hgs−/− embryos develop no mesoderm, as evidenced by the lack of Mox1 expression (Fig.1C, D). The Hgs mutant allele is not transcribed as shown by RT-PCR (Fig. 1E), indicating that the targeted allele for Hgs is likely functionally null.
Fig. 1. Hgs−/− is a null mutation of Hgs gene.
A–D: Whole mount in situ analysis of E8.5 embryos. Brachyury was expressed in the regressing primitive streak in WT (A, arrow). In Hgs−/− embryos, the expression was strongly decreased (B). Mox1, a somite marker (C), was not expressed in Hgs−/− embryos (D). Bar = 300 μm. E: Semi-quantitative RT-PCR analyses. The expressions of Hgs or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a loading control of mRNA in WT or Hgs−/− embryos are shown. −RT is a negative control sample without reverse transcriptase.
Target genes of BMP signaling are down regulated in Hgs−/− embryos
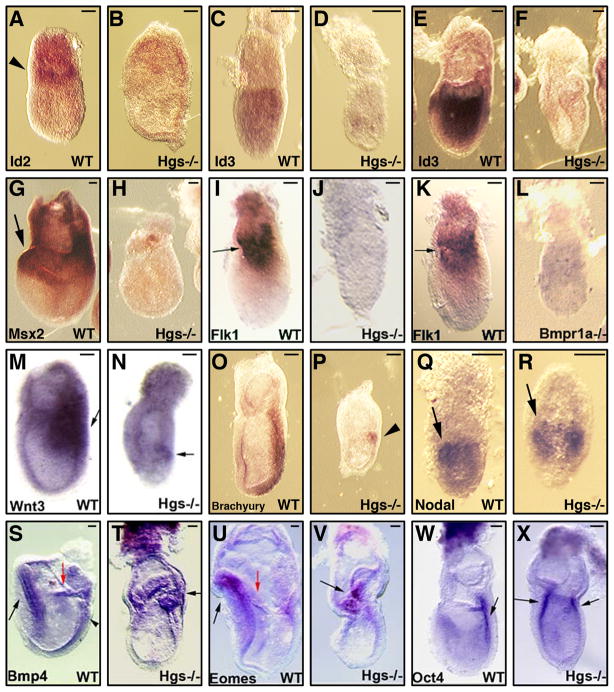
During the examination of the expression of markers of embryonic development at the time of gastrulation, we noticed that target genes of BMP signaling were strongly down regulated in Hgs−/− embryos (Fig. 2). Id2 and Id3 are target genes of BMP signaling during mouse development (Miyazono and Miyazawa, 2002; Ying et al., 2003). In Hgs−/−embryos, the expressions of both genes were highly reduced (Fig. 2A–F). Msx2 expression was also significantly decreased in Hgs−/− embryos (Fig. 2G, H). along with Flk1 (Fig. 2I, J). We observed the same results in Bmpr1a−/− embryos, homozygous null embryos for BMP type IA receptor (Mishina et al., 1995) (Fig. 2K, L). Wnt3 is also positively regulated by BMP signaling in early mouse embryos (Ben-Haim et al., 2006). The expression of Wnt3 (Fig. 2M, N) and its target gene Brachyury was largely down regulated in Hgs−/− embryos (Fig. 1A, B for E8.5, Fig. 2O, P for E7.5). Nodal was apparently expressed in Hgs−/− embryos (Fig. 2Q, P). Expression of Bmp4 (Winnier et al., 1995) and Eomes (Russ et al., 2000) in the extraembryonic ectoderm or Oct4 (Nichols et al., 1998) in epiblast were detected in Hgs−/− embryos (Fig. 2T, V, X), suggesting that the extraembryonic ectoderm and epiblast were formed in the mutant embryos. These expression data indicate that down-regulation of Id2, Id3, Msx2, Flk1 or Wnt3 in Hgs−/−embryos was not due to the loss of these tissues. Despite the comparable levels of Bmp4 expression (Fig. 2T), the reductions of these target genes for BMPs in the mutant embryos suggests that Hgs is involved in BMP signaling in early mouse embryo.
Fig. 2. BMP signaling is downregulated in Hgs−/− embryos.
A–V: Whole mount in situ analysis of E6.5 (C–D, Q–R) or E7.5 WT and Hgs−/− embryos A–B, E–P, S–X). Id2 is expressed in the extraembryonic tissues of WT embryos (A, arrowhead), but not in Hgs−/− embryos (B). Id3 expression in the extraembryonic and/or epiblast of WT embryos (C, E) was highly reduced in Hgs−/− embryos (D, F). Msx2 that is expressed in the extraembryonic tissues and proximal epiblast (G, arrow) was largely down regulated in Hgs−/− embryos (H). Flk1 that is expressed in the extraembryonic and proximal-lateral embryonic mesoderm (I, K, arrow) was highly down regulated in Hgs−/−embryos (J) and Bmpr1a −/− embryos (L). The expression of Wnt3 in the primitive streak and overlying visceral endoderm (M, arrow) was also decreased in Hgs−/− embryos (N, arrow). The expression of Brachyury in the primitive streak (O) was also decreased in Hgs−/− embryos (P, arrowhead). Nodal expression was observed normally in the Hgs−/−embryos (Q, R, arrows). Bmp4 expression is observed in the extraembryonic membrane (S, red arrow), neurectoderm (S, arrow) and proximal primitive streak (S, arrowhead) of WT embryos. In Hgs−/− embryos, the expression was observed in the extraembryonic ectoderm (T, arrow). In WT embryos, Eomes is expressed in the extraembryonic membrane (U, red arrow) and neurectoderm (U, arrow). In Hgs−/− embryos, expression is observed in the extraembryonic ectoderm (V, arrow). The expression of Oct4 is observed in the posterior epiblast of WT embryos (W, arrow). In Hgs−/− embryos, the expression was also observed in the epiblast (X, arrows). At least 3 embryos are used for each probe. Bar = 100 μm.
Hgs is required for phosphorylation of SMAD1/5/8 and TAK1
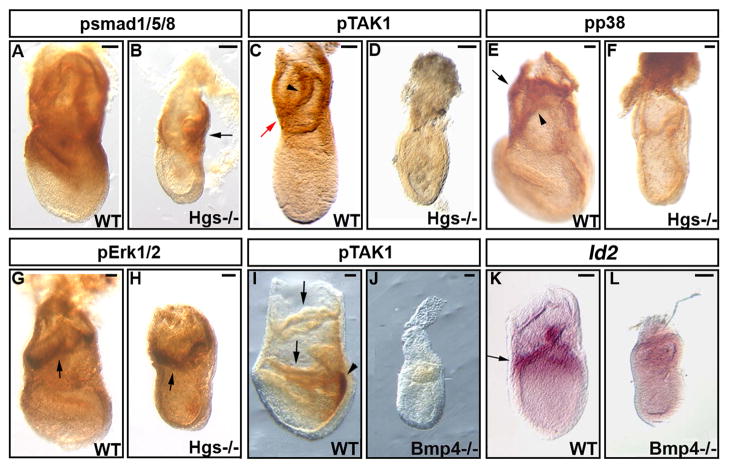
The above data prompted us to examine the status of phosphorylation of SMAD1/5/8 and TAK1/p38 as a measurement for levels of BMP signaling pathway. In control embryos, pSMAD1/5/8 was observed in proximal epiblast/extraembryonic tissues such as extraembryonic ectoderm and extraembryonic visceral endoderm (Fig. 3A) in consistent with the previous report (Yang and Klingensmith, 2006). In mutants, an apparent decrease of pSMAD1/5/8 staining was observed in these tissues (Fig. 3B). HGS binds to TAK1 and overexpression of Hgs increases TAK1 activity (Sasaki and Sugamura, 2001). Thus, we speculated that Hgs was also involved in BMP signaling through TAK1 in early embryogenesis. The total level of TAK1 showed no apparent difference between WT and Hgs −/− embryos (data not shown). However, levels of pTAK1 were markedly decreased in Hgs−/− embryos (Fig. 3D). Moreover, phosphorylation of p38, a MAPK downstream of TAK1 (Ninomiya-Tsuji et al., 1999), was strongly down regulated in Hgs−/− embryos (Fig. 3F). Phosphorylation of ERK1/2, another MAPK, was apparently normal in Hgs−/− embryos (Fig. 3G, H). These results showed that Hgs is involved in phosphorylation of SMAD1/5/8 and TAK1/p38.
Fig. 3. Phosphorylation of signaling molecules for BMPs is highly downregulated in Hgs−/− embryos.
A–J: Whole mount Immunohistochemistry at E7.5. Presence of pSmad1/5/8 is detected mainly in the extraembryonic tissues of WT (A) and Hgs−/− embryos (B, arrow). Presence of pTAK1 is detected in the extraembryonic visceral endoderm (C, red arrow) and chorion (C, arrowhead) at the bud stage. In Hgs−/− embryos, pTAK1 expression is not detected (D). Presence of pp38 is detected in the extraembryonic visceral endoderm (E, arrow) and chorion (E, arrowhead). In Hgs−/− embryos, pp38 expression is not detected (F). pErk1/2 is mainly detected in the chorion of E7.5 of WT (G, arrow) and Hgs−/− embryos (arrow, H). pTAK1 detected in the extraembryonic tissues (I, arrow) and proximal part of the primitive streak (I, arrowhead) at the head-fold stage was highly reduced in Bmp4−/−embryos (J). K,L: Whole mount in situ analyses at E7.5. Expression of Id2 was strongly decreased in Bmp4−/− embryos (L) compared to WT embryos (K, arrow). At least 3 embryos are used for each antibody. Bar = 100 μm.
Bmp4 is critical for phosphorylation of TAK1
TAK1 is a MAPKKK that transduces variety of signals (Adhikari et al., 2007). Importantly, TAK1 is a potential transducer of BMP signaling in vertebrate embryogenesis (Shibuya et al., 1998; Yamaguchi et al., 1999). However, the status of TAK1 was not reported in mutant mouse embryos for BMPs. Therefore, we examined the total or phosphorylation level of TAK1 in mouse mutant embryos for Bmp4 (Winnier et al., 1995). Total level of TAK1 observed by whole mount immunohistochemistry was seemingly normal in Bmp4−/− embryos (data not shown). However, phosphorylation of TAK1 (pTAK1) is strongly down regulated (Fig. 3J). The expression of Id2, Id3 and Msx2 were also decreased in Bmp4−/− embryos (Fig. 3L and data not shown). These results also support the idea that TAK1 may play a role in BMP signaling in early mouse embryo.
TAK1 is involved in regulation of Flk1 (along with Smad signaling)
The aforementioned observations suggest that HGS is involved in BMP signaling by modulating both SMAD signaling and TAK1-p38 signaling pathways. To further investigate this possibility, we employed chemical inhibitors specific for individual pathways. We chose Flk1, Id2 and Id3 for readout of BMP signaling because they are transcriptonally activated by SMAD1 when tested using cell line (Park et al., 2004; Chen et al., 2006; Du and Yip, 2010).
E6.5 embryos were incubated with different concentrations of Dorsomorphin (Yu et al., 2008), an inhibitor for phosphorylation of BMP SMADs (SMAD1/5/8), for 20 hrs and examined for the expression levels of Flk1, Id2 and Id3. Flk1 showed about an 80% reduction and Id2 and Id3 showed about a 90% reduction, indicating these genes are dependent on SMAD mediated BMP signaling in vivo as well as in vitro (Fig. 4A). Treatment with 11,12-dihydro-5Z7-oxo-zeaenol, an inhibitor for TAK1 kinase activity (Yao et al., 2007), resulted in more than a 99% reduction of Flk1 expression, in contrast to the 2–3 fold increase in Id2 and Id3 expressions (Fig. 4B). These results suggest that Id genes are regulated mostly by Smad signaling, whereas, Flk1 expression is regulated by both SMAD and MAPK signaling pathways.
Fig. 4. Expression levels of BMP target genes are differentially regulated by SMAD and TAK1 pathways.
A,B: Quantitative RT-PCR of downstream target genes for BMP signaling. WT embryos harvested at E7.5 are cultured with Dorsomorphin (A) or dihydro-5Z7-oxo-zeaenol (B) with indicated concentration for 20 hrs and levels of gene expression are measured. At least 6 embryos are analyzed for each concentration.
Subcellular localization of TAK1 is abnormal in Hgs−/− embryos
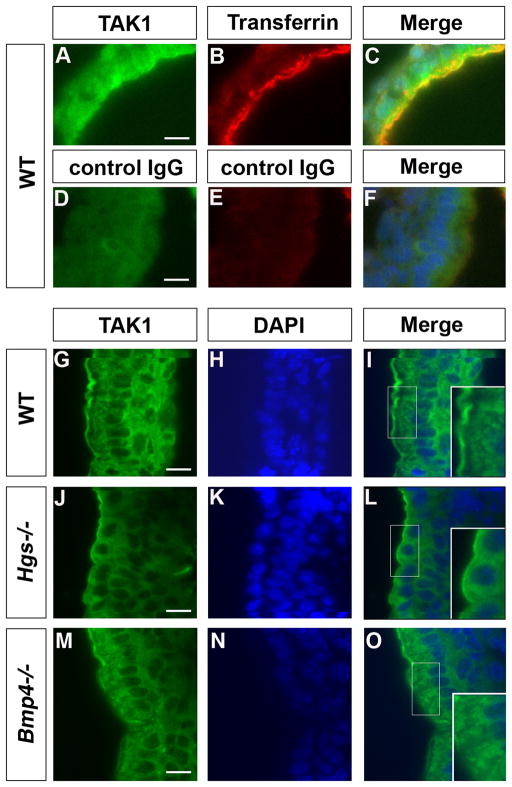
As HGS is normally localized in the early endosome (Komada et al., 1997), we hypothesized that a large part of TAK1 is also localized in the early endosome, and such subcellular localization might not be maintained in Hgs−/− embryos. We observed that TAK1 and transferrin, an early endosomal marker (Gorvel et al., 1991), partially co-localize in WT E7.5 embryos (Fig. 5A–C, G–I). However, in Hgs−/− embryos, such distinct pattern was not observed (Fig. 5J–L). These data suggest that Hgs is required to maintain normal TAK1 subcellular localization in early endosome and this may be critical for TAK1 phosphorylation and subsequent signal transduction.
Fig. 5. Localization of TAK1 in the early endosome is not maintained in Hgs−/− embryos.
A–F: Double immunostaining for TAK1 and Transferrin, an early endosome marker, in the E7.5 WT embryos. G–O: Immunostaining of TAK1 of E7.5 WT (G–I), Hgs−/− (J–L), and Bmp4−/− embryos (M–O). Insets in I, L, O are enlarged images of boxed region in each panel. In Hgs−/− embryos, TAK1 is homogenously expressed in the cytoplasm (L). In Bmp4−/− embryos, TAK1 was expressed in dot-like structures (O, inset), as observed in WT embryos (I, inset). Bar = 10 μm.
In Bmp4−/− embryos, subcellular localization of TAK1 appears to be the same with WT embryos (Fig. 5M–O). This indicated that BMP signaling is not required for localization of TAK1 into the early endosome, suggesting that HGS is critical to anchor TAK1 to early endosome.
DISCUSSION
In this study, we demonstrate that HGS is required for phosphorylation of both TAK1 and SMAD1/5/8. We also show that levels of faithful downstream target genes of BMP signaling are highly down regulated in the Hgs mutant embryos. These results are similar to those found in the mutant embryos for BMP4 and BMP type 1A receptor, indicating that HGS contributes to BMP signaling. We also demonstrate that subcellular localization of TAK1, which is found in early endosome in WT embryos, is altered in Hgs mutant embryos, but not in Bmp4 mutant embryos. Based on these notions, we propose a model of Hgs function in BMP signaling (Fig. 6). In normal development, SMAD1/5/8 and TAK1 interact to HGS, and at least in part, these molecules are co-localizing in the early endosome. BMP signaling and HGS function for phosphorylation of SMAD1/5/8 and of TAK1. pSAMD1/5/8 are required for the transcription of Id2, Id3 and Flk1 whereas TAK1/p38 are also required for the transcription of Flk1 (Fig. 6A). In Hgs−/− embryos, phosphorylation of SMAD1/5/8 decreased significantly but partly, probably because other molecules such as SARA (Itoh et al., 2002) compensate the loss of HGS. Id1 and Id2 expression are also significantly deceased. Phosphorylation of TAK1/p38 is abrogated and accumulation of TAK1 in the early endosome is apparently less significant. In sum, Flk1 expression is highly downregulated in Hgs−/− embryos (Fig. 6B). It is noteworthy that the cells in Hgs−/− embryos seem to have an altered apical-basal polarity (Fig. 5, J–L). Localization of TAK1 in the early endosome may be disrupted as a consequence of this phenomenon. Further studies are needed to clarify this issue.
Fig. 6. Proposed Model.
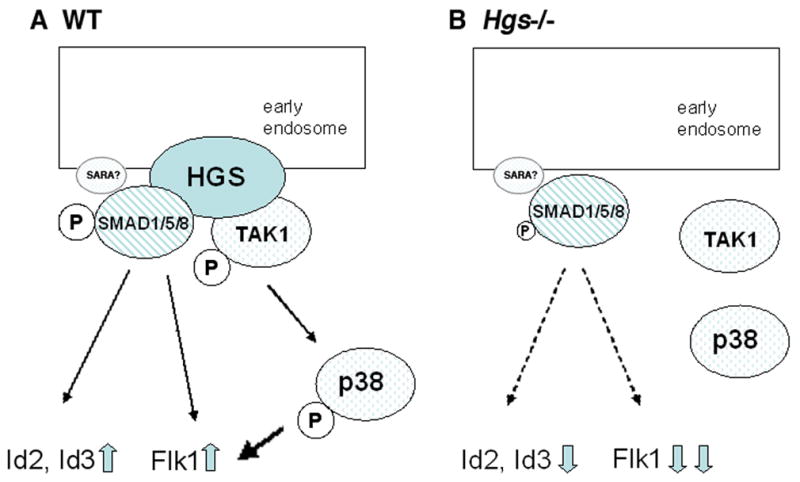
A: In normal development, TAK1 binds to HGS, ensuring its localization in the early endosome. HGS probably acts as a scaffold to facilitate both phosphorylation of SMADs and autophosphorylation of TAK1 to transduce non-SMAD BMP signaling. B: In Hgs−/−embryos, TAK1 is not localized in the early endosome, thus BMP signaling mediated by TAK1 and subsequent MAK kinase cascades are largely compromised. The canonical BMP signaling mediated by phosphorylation of SMADs is less affected probably due to compensation by other molecules.
Generation of a different mutant allele of Hgs gene, Hrs allele, is reported previously and show that Hrs−/− embryos develop mesoderm (Komada and Soriano, 1999), which is a milder phenotype than that of Hgs−/− described here. Trans-heterozygote embryos for both alleles (Hgs−/Hrsminus;) showed a phenotype between that of Hrs−/− and Hgs−/− embryos (our unpublished data). Further, transcription from the Hgs mutant allele is not detected by RT-PCR (Fig. 1E). Taken together these data suggest that the Hgs mutant allele we used here probably represents a null allele of Hgs gene and the Hrs allele is a hypomorphic allele.
BMP signaling is transduced by SMAD1/5/8 as receptor-regulated SMADs and SMAD4. A complex of each receptor-regulated SMAD and SMAD4 translocates to the nucleus and transcribes a number of genes (Mishina, 2003). However, although Smad4 mutant embryos in the epiblast still forms rudimentary heart (Chu et al., 2004), no heart mesoderm remains in E8.5 embryos with Bmpr1a deleted in the epiblast (Miura et al., 2006). This suggests that not all BMP signaling are transduced by the SMAD pathway, but there are non-SMAD mediated pathways existing to transduce BMP signaling. In Xenopus embryos, a dominant-negative form of TAK1 reverses phenotypes caused by ectopic expression of SMAD1 and SMAD5, such as ventralization or loss of neutralization (Shibuya et al., 1998), suggesting that TAK1 and SMAD1/5 cooperate to transduce BMP signaling. Our data also indicate that TAK1 functions as a component of BMP signaling in the mouse embryo. As phosphorylation of pTAK1-p38 as well as that of SMAD1/5/8 is affected in Hgs−/− embryos, HGS contributes to both SMAD and non-SMAD mediated pathway of BMP signaling. It is interesting that the reduction of phosphorylation of TAK1 and SMAD1/5/8 are largely found in the extraembryonic regions (Fig. 3). It is reported that BMP signaling in the extraembryonic tissues is critical for appropriate development of the epiblast (Davis et al., 2004; Kishigami et al., 2004; Miura et al., 2006; Di-Gregorio et al., 2007; Yamamoto et al., 2009; Miura et al., 2010). These results suggest that HGS in the extraembryonic tissues is involved in non-cell autonomous function of BMP signaling towards the epiblast.
We previously reported that Hgs−/− embryos show a significant reduction in responding to ACTIVIN stimulation at E8.5 (Miura et al., 2000), suggesting that the ACTIVIN signaling pathway is also disrupted in Hgs−/− embryos. However, Nodal expression, which is regulated by NODAL signaling (Gu et al., 1998), is apparently expressed at E6.5 in Hgs−/− embryos. Because both ACTIVIN and NODAL share the same receptor system to signal, such as ACVR1B (ALK4), these results suggest that machinery required for ACTIVIN signaling is mostly not affected at E6.5 in Hgs−/− embryos. As BMP signaling is required for the maintenance of Nodal expression and ACTIVIN signaling (Di-Gregorio et al., 2007), we believe that the observed defect in ACTIVIN signaling at E8.5 is an indirect outcome caused by the downregulation of BMP signaling during gastrulation in Hgs−/− embryos.
In this study, we have revealed that HGS is required for phosphorylation of TAK1 as well as SMAD1/5/8, thus showing specific contribution of HGS to BMP signaling. Tak1−/− embryos die around E12.5 by defects in vasculogenesis (Jadrich et al., 2006). Thus, phenotypes of Hgs−/− embryos cannot be only attributed to failure of activation of TAK1-p38 MAPK pathway. A PI3K specific inhibitor wortmannin induces expansion of early endosome in cultured cell lines (Spiro et al., 1996). Because the expansion of early endosome is a well-recognized phenotype found in the Hgs−/− embryos (Komada and Soriano, 1999), it is an interesting possibility that PI3K pathway is abrogated by mutation of Hgs gene. Recent reports suggest that BMP signaling is, in part, transduced via PI3K pathway (Mandal et al., 2009). Thus, it is reasonable to speculate that HGS may play a critical role in BMP signaling pathways mediated by SMAD, TAK1 and PI3K.
EXPERIMENTAL PROCEDURES
Mouse
Mouse strains and genotyping were described elsewhere (Winnier et al., 1995; Komada and Soriano, 1999; Miura et al., 2000). All mouse experiments were performed in accordance with NIEHS/NIH guidelines covering the humane care and use of animals in research.
Whole mount in situ hybridization
Whole mount in situ hybridization was performed as described previously (Belo et al., 1997). Probes used were Mox1 (Candia et al., 1992) and Nodal (Conlon et al., 1994), Wnt3 (Liu et al., 1999), Foxf1 (Mahlapuu et al., 2001), Id2 (Jen et al., 1996), Id3 (Ellmeier and Weith, 1995), Msx2 (Catron et al., 1996), Bmp4 (Winnier et al., 1995), Eomes (Russ et al., 2000) and Oct4 (Yang and Klingensmith, 2006). Three or more embryos were used for each probe.
Immunohistochemistry
E7.5 embryos were fixed in 4% paraformaldehyde, dehydrated through graded alcohol embedded in paraffin. Sections (7μm) were deparaffinized, and stained with anti-TAK1 rabbit antibody (2.5μg/ml) (Abgent) and Alexa anti-rabbit IG according to manufacturer’s protocols. Whole mount Immunohistochemistry was performed as described (Miura et al., 2006). Briefly, primary antibodies were used at a dilution of 1:100 for anti-phospho-TAK1 antibody (Cell Signaling), 1:200 for anti-phospho-p38 antibody (Sigma), 1:200 for anti-phospho-Smad1/5/8 antibody (Cell Signaling) and 1:100 for anti-phospho-Erk1/2 antibody (Cell Signaling) in blocking solution. After primary antibody incubation, embryos were washed and then incubated with biotinylated goat anti-rabbit antibody (Vector Lab), diluted 1:200 in blocking solution. After several washes in PBS with 0.1% TritonX-100 (Fisher), embryos were incubated with HRP-conjugated biotin-streptavidin system (Vector Lab). Staining was then developed with the ImmunoPure Metal-enhanced DAB substrate kit (Pierce). Stained embryos were embedded in paraffin and sectioned and counter stained with eosin. Three or more embryos were used for each antibody.
RT-PCR analysis
Total RNA was extracted from each embryo using PicoPure RNA isolation kit (Arkus). Total RNA harvested was measured with Nanodrop 1000 (Thermo Scientific) and same amount of total RNA was used for each RT-PCR reaction. RT-PCR was performed using One step RT-PCR kit (Qiagen) according to manufacture’s protocol. Hrs primes are Hrs-F (5’-ctacagatctgcgacctgat ccgt-3’) in exon 1 and Hrs-R (5’-tcctcagcatccacccagtcagg-3’) locating in exon 6, yielding a 410-bp product.
Acknowledgments
We thank Drs. M. Komada for Hrs mice, B. Hogan for Bmp4 mice; B.G. Herrmann, J. Klingensmith, M. Kuehn, R. Benezra, A. Russ, B. Hogan for in situ probes. We also thank Comparative Medicine Branch and the Knockout Core at NIEHS for mouse activity, Drs. Manas Ray, Greg Scott, Kelly McCann and Catherine Krull for comments on the manuscript. This research was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences (ES071003-11) and a conditional gift from RIKEN Brain Science Institute to Y.M.
References
- Adhikari A, Xu M, Chen ZJ. Ubiquitin-mediated activation of TAK1 and IKK. Oncogene. 2007;26:3214–3226. doi: 10.1038/sj.onc.1210413. [DOI] [PubMed] [Google Scholar]
- Belo JA, Bouwmeester T, Leyns L, Kertesz N, Gallo M, Follettie M, De Robertis EM. Cerberus-like is a secreted factor with neutralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech Dev. 1997;68:45–57. doi: 10.1016/s0925-4773(97)00125-1. [DOI] [PubMed] [Google Scholar]
- Ben-Haim N, Lu C, Guzman-Ayala M, Pescatore L, Mesnard D, Bischofberger M, Naef F, Robertson EJ, Constam DB. The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev Cell. 2006;11:313–323. doi: 10.1016/j.devcel.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Candia AF, Hu J, Crosby J, Lalley PA, Noden D, Nadeau JH, Wright CV. Mox-1 and Mox-2 define a novel homeobox gene subfamily and are differentially expressed during early mesodermal patterning in mouse embryos. Development. 1992;116:1123–1136. doi: 10.1242/dev.116.4.1123. [DOI] [PubMed] [Google Scholar]
- Catron KM, Wang H, Hu G, Shen MM, Abate-Shen C. Comparison of MSX-1 and MSX-2 suggests a molecular basis for functional redundancy. Mech Dev. 1996;55:185–199. doi: 10.1016/0925-4773(96)00503-5. [DOI] [PubMed] [Google Scholar]
- Chen X, Zankl A, Niroomand F, Liu Z, Katus HA, Jahn L, Tiefenbacher C. Upregulation of ID protein by growth and differentiation factor 5 (GDF5) through a smad-dependent and MAPK-independent pathway in HUVSMC. J Mol Cell Cardiol. 2006;41:26–33. doi: 10.1016/j.yjmcc.2006.03.421. [DOI] [PubMed] [Google Scholar]
- Chu GC, Dunn NR, Anderson DC, Oxburgh L, Robertson EJ. Differential requirements for Smad4 in TGFbeta-dependent patterning of the early mouse embryo. Development. 2004;131:3501–3512. doi: 10.1242/dev.01248. [DOI] [PubMed] [Google Scholar]
- Conlon FL, Lyons KM, Takaesu N, Barth KS, Kispert A, Herrmann B, Robertson EJ. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–1928. doi: 10.1242/dev.120.7.1919. [DOI] [PubMed] [Google Scholar]
- Davis S, Miura S, Hill C, Mishina Y, Klingensmith J. BMP receptor IA is required in the mammalian embryo for endodermal morphogenesis and ectodermal patterning. Dev Biol. 2004;270:47–63. doi: 10.1016/j.ydbio.2004.01.048. [DOI] [PubMed] [Google Scholar]
- Di-Gregorio A, Sancho M, Stuckey DW, Crompton LA, Godwin J, Mishina Y, Rodriguez TA. BMP signalling inhibits premature neural differentiation in the mouse embryo. Development. 2007;134:3359–3369. doi: 10.1242/dev.005967. [DOI] [PubMed] [Google Scholar]
- Du Y, Yip H. Effects of bone morphogenetic protein 2 on Id expression and neuroblastoma cell differentiation. Differentiation. 2010;79:84–92. doi: 10.1016/j.diff.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Ellmeier W, Weith A. Expression of the helix-loop-helix gene Id3 during murine embryonic development. Dev Dyn. 1995;203:163–173. doi: 10.1002/aja.1002030205. [DOI] [PubMed] [Google Scholar]
- Gorvel JP, Chavrier P, Zerial M, Gruenberg J. rab5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Gu Z, Nomura M, Simpson BB, Lei H, Feijen A, van den Eijnden-van Raaij J, Donahoe PK, Li E. The type I activin receptor ActRIB is required for egg cylinder organization and gastrulation in the mouse. Genes Dev. 1998;12:844–857. doi: 10.1101/gad.12.6.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh F, Divecha N, Brocks L, Oomen L, Janssen H, Calafat J, Itoh S, Dijke PtP. The FYVE domain in Smad anchor for receptor activation (SARA) is sufficient for localization of SARA in early endosomes and regulates TGF-beta/Smad signalling. Genes Cells. 2002;7:321–331. doi: 10.1046/j.1365-2443.2002.00519.x. [DOI] [PubMed] [Google Scholar]
- Jadrich JL, O'Connor MB, Coucouvanis E. The TGF beta activated kinase TAK1 regulates vascular development in vivo. Development. 2006;133:1529–1541. doi: 10.1242/dev.02333. [DOI] [PubMed] [Google Scholar]
- Jen Y, Manova K, Benezra R. Expression patterns of Id1, Id2, and Id3 are highly related but distinct from that of Id4 during mouse embryogenesis. Dev Dyn. 1996;207:235–252. doi: 10.1002/(SICI)1097-0177(199611)207:3<235::AID-AJA1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Kishigami S, Mishina Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 2005;16:265–278. doi: 10.1016/j.cytogfr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Kishigami S, Yoshikawa S, Castranio T, Okazaki K, Furuta Y, Mishina Y. BMP signaling through ACVRI is required for left-right patterning in the early mouse embryo. Dev Biol. 2004;276:185–193. doi: 10.1016/j.ydbio.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Komada M, Masaki R, Yamamoto A, Kitamura N. Hrs, a tyrosine kinase substrate with a conserved double zinc finger domain, is localized to the cytoplasmic surface of early endosomes. J Biol Chem. 1997;272:20538–20544. doi: 10.1074/jbc.272.33.20538. [DOI] [PubMed] [Google Scholar]
- Komada M, Soriano P. Hrs, a FYVE finger protein localized to early endosomes, is implicated in vesicular traffic and required for ventral folding morphogenesis. Genes Dev. 1999;13:1475–1485. doi: 10.1101/gad.13.11.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Lloyd TE, Atkinson R, Wu MN, Zhou Y, Pennetta G, Bellen HJ. Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell. 2002;108:261–269. doi: 10.1016/s0092-8674(02)00611-6. [DOI] [PubMed] [Google Scholar]
- Mahlapuu M, Ormestad M, Enerback S, Carlsson P. The forkhead transcription factor Foxf1 is required for differentiation of extra-embryonic and lateral plate mesoderm. Development. 2001;128:155–166. doi: 10.1242/dev.128.2.155. [DOI] [PubMed] [Google Scholar]
- Mandal CC, Ghosh Choudhury G, Ghosh-Choudhury N. Phosphatidylinositol 3 kinase/Akt signal relay cooperates with smad in bone morphogenetic protein-2-induced colony stimulating factor-1 (CSF-1) expression and osteoclast differentiation. Endocrinology. 2009;150:4989–4998. doi: 10.1210/en.2009-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishina Y. Function of bone morphogenetic protein signaling during mouse development. Front Biosci. 2003;8:d855–869. doi: 10.2741/1097. [DOI] [PubMed] [Google Scholar]
- Miura S, Davis S, Klingensmith J, Mishina Y. BMP signaling in the epiblast is required for proper recruitment of the prospective paraxial mesoderm and development of the somites. Development. 2006;133:3767–3775. doi: 10.1242/dev.02552. [DOI] [PubMed] [Google Scholar]
- Miura S, Singh AP, Mishina Y. Bmpr1a is required for proper migration of the AVE through regulation of Dkk1 expression in the pre-streak mouse embryo. Dev Biol. 2010;341:246–254. doi: 10.1016/j.ydbio.2010.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S, Takeshita T, Asao H, Kimura Y, Murata K, Sasaki Y, Hanai JI, Beppu H, Tsukazaki T, Wrana JL, Miyazono K, Sugamura K. Hgs (Hrs), a FYVE domain protein, is involved in Smad signaling through cooperation with SARA. Mol Cell Biol. 2000;20:9346–9355. doi: 10.1128/mcb.20.24.9346-9355.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K, Miyazawa K. Id: a target of BMP signaling. Sci STKE. 2002;2002:pe40. doi: 10.1126/stke.2002.151.pe40. [DOI] [PubMed] [Google Scholar]
- Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe-Nebenius D, Chambers I, Scholer H, Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–391. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- Park C, Afrikanova I, Chung YS, Zhang WJ, Arentson E, Fong GhG, Rosendahl A, Choi K. A hierarchical order of factors in the generation of FLK1- and SCL-expressing hematopoietic and endothelial progenitors from embryonic stem cells. Development. 2004;131:2749–2762. doi: 10.1242/dev.01130. [DOI] [PubMed] [Google Scholar]
- Raiborg C, Bremnes B, Mehlum A, Gillooly DJ, D'Arrigo A, Stang E, Stenmark H. FYVE and coiled-coil domains determine the specific localisation of Hrs to early endosomes. J Cell Sci. 2001;114:2255–2263. doi: 10.1242/jcs.114.12.2255. [DOI] [PubMed] [Google Scholar]
- Russ AP, Wattler S, Colledge WH, Aparicio SA, Carlton MB, Pearce JJ, Barton SC, Surani MA, Ryan K, Nehls MC, Wilson V, Evans MJ. Eomesodermin is required for mouse trophoblast development and mesoderm formation. Nature. 2000;404:95–99. doi: 10.1038/35003601. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Sugamura K. Involvement of Hgs/Hrs in signaling for cytokine-mediated c-fos induction through interaction with TAK1 and Pak1. J Biol Chem. 2001;276:29943–29952. doi: 10.1074/jbc.M104230200. [DOI] [PubMed] [Google Scholar]
- Shibuya H, Iwata H, Masuyama N, Gotoh Y, Yamaguchi K, Irie K, Matsumoto K, Nishida E, Ueno N. Role of TAK1 and TAB1 in BMP signaling in early Xenopus development. EMBO J. 1998;17:1019–1028. doi: 10.1093/emboj/17.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro DJ, Boll W, Kirchhausen T, Wessling-Resnick M. Wortmannin alters the transferrin receptor endocytic pathway in vivo and in vitro. Mol Biol Cell. 1996;7:355–367. doi: 10.1091/mbc.7.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima M, Tanaka N, Aoki J, Tanaka Y, Murata K, Kyuuma M, Kobayashi H, Ishii N, Yaegashi N, Sugamura K. Inhibition of tumor growth and metastasis by depletion of vesicular sorting protein Hrs: its regulatory role on E-cadherin and beta-catenin. Cancer Res. 2007;67:5162–5171. doi: 10.1158/0008-5472.CAN-06-2756. [DOI] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Nagai S, Ninomiya-Tsuji J, Nishita M, Tamai K, Irie K, Ueno N, Nishida E, Shibuya H, Matsumoto K. XIAP, a cellular member of the inhibitor of apoptosis protein family, links the receptors to TAB1-TAK1 in the BMP signaling pathway. EMBO J. 1999;18:179–187. doi: 10.1093/emboj/18.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Beppu H, Takaoka K, Meno C, Li E, Miyazono K, Hamada H. Antagonism between Smad1 and Smad2 signaling determines the site of distal visceral endoderm formation in the mouse embryo. J Cell Biol. 2009;184:323–334. doi: 10.1083/jcb.200808044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YP, Klingensmith J. Roles of organizer factors and BMP antagonism in mammalian forebrain establishment. Dev Biol. 2006;296:458–475. doi: 10.1016/j.ydbio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Yao J, Kim TW, Qin J, Jiang Z, Qian Y, Xiao H, Lu Y, Qian W, Gulen MF, Sizemore N, DiDonato J, Sato S, Akira S, Su B, Li X. Interleukin-1 (IL-1)-induced TAK1-dependent Versus MEKK3-dependent NFkappaB activation pathways bifurcate at IL-1 receptor-associated kinase modification. J Biol Chem. 2007;282:6075–6089. doi: 10.1074/jbc.M609039200. [DOI] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]