Abstract
The Notch signaling pathway has been recently shown to contribute to T cell differentiation in vitro. However, the in vivo function of Notch signaling in transplantation remains unknown. In this study, we investigated the importance of Delta1 in regulating the alloimmune response in vivo. Delta1 expression was upregulated on DCs and monocytes/Mφ upon transplantation in a BALB/c into B6 vascularized cardiac transplant model. While administration of anti-Delta1 mAb only slightly delayed survival of cardiac allografts in this fully MHC mismatched model, it significantly prolonged graft survival in combination with sCTLA4-Ig or in CD28KO recipients. The prolongation of allograft survival was associated with Th2 polarization and a decrease in Th1 and granzyme B-producing cytotoxic T cells. The survival benefit of Delta1 blockade was abrogated after IL-4 neutralization and in STAT6KO recipients, but was maintained in STAT4KO recipients, reinforcing the key role of Th2 cell development in its graft-prolonging effects. These data demonstrate for the first time an important role of Delta1 in alloimmunity, identifying Delta1 ligand as a potential novel target for immunomodulation in transplantation.
Keywords: mice, cell differentiation, Th1/Th2 cells, transplantation, cytokines, Notch signaling
Introduction
The adaptive immune system has permitted the development of specific defense mechanisms against a large variety of pathogens and abnormal cells. In transplantation, both CD4+ and CD8+ T cells play a dominant role in cell-mediated immunity against alloantigens and have been shown to be of key importance in allograft rejection (1-3). After antigen recognition, naïve CD4+ T cells can differentiate into Th1, Th2, Th17 or regulatory T cells (Tregs), while naïve CD8+ T cells can become cytotoxic T lymphocytes (CTLs). The type of differentiation is determined by signals delivered by antigen-presenting cells (APC) and the cytokine microenvironment (4). Accumulating evidence indicates that Notch and its ligands on APCs might be important mediators of T cell differentiation (5-11).
The Notch pathway is an important intercellular signaling pathway that plays a major role in controlling cell fate (5, 7). The Notch family consists of 4 highly conserved receptors (Notch1, 2, 3 and 4), which can interact with at least five Notch ligands (Jagged1 and 2; Delta1, 3 and 4). Within the immune system, the ligands are mainly expressed on APCs whereas the Notch receptors are expressed on T cells (6, 7, 12). At the molecular level, Notch is a heterodimeric surface receptor consisting of an extracellular ligand-binding region noncovalently associated with a transmembrane polypeptide with a long intracellular tail, which is cleaved during activation. This intracellular domain (ICD) then translocates into the nucleus where it forms a complex with CSL/RBP-Jκ/MAML/p300 that activates the expression of target genes (7, 13).
Depending on the predominant Notch ligand expressed on APCs, different target genes are activated by Notch signaling. As an example, Delta1-expressing dendritic cells have increased ability to activate naïve CD4+ T cells and promote Th1 cell development in vitro via upregulation of T-bet and IFN-γ, and inhibition of IL4 receptor signaling (6, 11). In contrast, Jagged-expressing APCs lead to Th2-cell differentiation via enhancement of GATA3 transcription (6). Finally, transfected Delta1 on DCs are also able to direct differentiation of naïve CD8+ T cells into cytotoxic T lymphocytes by promoting granzyme B and Eomes transcription (10, 14).
In transplantation, a Th1 response is the dominant phenotype in allograft rejection, while a Th2 response favors long-term survival in some transplant models (15-17). Therefore, we decided to study if blockade of Delta1 could potentially tip the balance towards a favorable T cell subtype and improve allograft survival. In this report, we demonstrate that an anti-Delta1 blocking mAb is able to prolong allograft survival in a fully MHC mismatch model of cardiac transplantation, in particular with blockade of the B7: CD28 costimulation. This effect was characterized by an increase in Th2 polarization and a decrease in Th1 and cytotoxic T cells. Finally, the prolongation in allograft survival was shown to require the presence of Th2 cytokines, in particular IL-4. These data lead us to conclude that Delta1 signaling is important in alloimmunity in vivo and blockade of this pathway could be a potential additional target to improve graft outcome.
Material and Methods
Mice
C57BL/6 (H-2b, B6), BALB/c (H-2d), CD28-deficient mice on B6 background (B6.129S2-Cd28tm1Mak/J), STAT4-deficient and STAT6-deficient mice on BALB/c background (C.129S2-Stat4tm1Gru/J and C.129S2-Stat6tm1Gru/J, respectively) were purchased from The Jackson Laboratory. All mice were 8–12 weeks of age and housed in accordance with institutional and NIH guidelines.
Heterotopic heart transplantation
Vascularized heart grafts were placed in an intra-abdominal location using microsurgical techniques as described by Corry et al. (18). Graft function was assessed by palpation of the heartbeat. Rejection was determined by complete cessation of palpable heartbeat and was confirmed by direct visualization after laparotomy. Graft survival is shown as the median survival time (MST) in days.
Antibodies and in vivo treatment protocol
The anti-Delta1 (HMD1-5) monoclonal antibody (mAb) was generated as previously described (19). This mAb was manufactured and purified from the original hybridoma by a commercial source, BioXCell (West Lebanon, NH). Control hamster IgG was also obtained from the same source. Cardiac allograft recipients were treated with mAbs i.p. at 500 μg at day 0, and 250 μg at days 2, 4, 6, 8, and 10 after transplantation. CTLA4-Ig is a fusion protein composed of a human IgG1 Fc fused to the extracellular domain of CTLA4, which was purchased from Bristol-Myers-Squibb. CTLA4-Ig was given i.p. at day 2 after transplantation (250 μg). For IL-4 neutralization, 1 mg of anti-IL4 mAb (11B11) was administered on days 0, 1, 3, 5 and 8 after transplantation (BioXCell).
Measurement of cytokines by ELISPOT and Luminex assay
Splenocytes harvested at 10-14 days after transplantation from B6 recipients of BALB/c heart allografts were restimulated by irradiated donor-type splenocytes. The ELISPOT assay (R&D Systems, Minneapolis, MN) was adapted to measure the frequency of alloreactive T cells producing granzyme B, as described previously (20). The frequencies of cytokine-secreting alloreactive cells were expressed as the number of cytokine-producing cells per 0.5 ×106 responder cells. For Luminex assay, cell-free supernatants of individual wells were removed after 48 h of incubation and analyzed by a multiplexed cytokine bead–based immunoassay using a preconfigured 21-plex mouse cytokine detection kit (Millipore) as described previously (21). All samples were tested in triplicate wells.
Flow Cytometry
Splenocytes from recipients at 10-14 days after transplantation were stained with fluorochrome-labeled monoclonal antibodies (mAbs) against CD4, CD8, CD11b, CD11c, B220, CD62 ligand (CD62L), CD44, CD25, CD80, CD86, PDL-1, CD1d, CD5, CD21 and FoxP3 (BD Biosciences, San Jose, CA). Intracellular FoxP3 staining was performed using the Cytofix/Cytoperm intracellular staining kit. Flow cytometry was performed with a FACSCalibur system (BD Biosciences) and analyzed using FlowJo software. In order to characterize Delta1 expression, we first incubated splenocytes from naïve and transplanted animals 7 days after transplantation with anti-mouse CD16/CD32 purified (BD Bioscience) to block non-specific binding to FcγR. Then cells were incubated with biotinylated anti-mouse Delta1 (HMD1-5) and Delta4 (HMD4-2) Abs, generated as previously described (19), and biotinylated control hamster IgG (eBioscience) for 15 min on ice. After washing the cells, APC-Streptavidin was added. Washed cells were also stained for 7-AAD (BD Pharmingen) to exclude nonviable cells from flow cytometric analysis. For Notch receptors’ characterization on T cells, the following flurochrome-conjugated antibodies were used: Notch1 PE (HMN1-12), Notch2 APC (HMN2-35), Notch3 PE (HMN3-133) and Notch4 APC (HMN4-14) with respective isotype Hamster IgG controls (Biolegend)(19).
Intracellular cytokine staining
In order to isolate cells from the allografts, hearts were excised, minced and digested with ~500 U/ml collagenase (Worthington Biochemical) for 30 min at 37°C. Cells were then mashed through 70 μm filters and red blood cells were lysed. Cells (0.5 × 106) were resuspended in HL-1 medium (BioWhittaker); supplemented with 1% l-glutamine (BioWhittaker), 1% penicillin (BioWhittaker), and 10% FCS (BioWhittaker); and restimulated with PMA (5 ng/ml) (Sigma-Aldrich) plus ionomycin (500 ng/ml) (Sigma-Aldrich); and brefeldin A (10 μg/ml) (Sigma-Aldrich) was added. Cells were incubated for 4 hours at 37° C. After staining for the surface markers (CD4 and CD8), cells were fixed and permeabilized with Cytofix/Cytoperm solution (BD Biosciences), according to the manufacturer’s instructions, and incubated with PE-conjugated anti-IFN-γ (XMG1.2) and APC-conjugated anti-IL-17 (eBio17B7) for 30 minutes at 4°C. A gate was set on CD4+ and CD8+ cells, and the percentage of IFN-γ and IL-17 cells was determined by flow cytometric analysis.
Ex vivo cytotoxicity assay
Splenocytes from control and anti-Delta1 treated recipients were harvested after transplantation and CD8+ T cells were isolated by magnetic active cell sorting (CD8a; Ly-2 microbeads from Miltenyi Biotec). B cells from allogeneic BALB/c mice were used as target cells after cell sorting with CD19 microbeads (Miltenyi Biotec) with greater than 95% purity. For the cytotoxicity assay, CD8+ T cells and allogeneic target cells were incubated for 6 hours at 1:1 and 5:1 effector:target cell ratio at 37° C, followed by staining of target cells by fluorochrome-B220 Ab (APC; eBioscience) and ethidium/calcein AM according to protocol from LIVE/DEAD® viability/cytotoxicity kit (Invitrogen) (22). The percentage of Calcein AM negative (apoptotic cells) and ethidium positive (dead cells) was determined by flow cytometry to differentiate apoptotic/dead target cells from viable ones. The specific cytotoxicity of CD8+ cells was calculated as follows: % specific cytotoxicity = (% of dead/apoptotic target cells in the presence of effector cells) − (% of dead/apoptotic target cells alone) (23).
Morphology
Cardiac graft samples from transplanted mice were harvested from rejecting (cessation of heartbeat by palpation) mice and at 7-14 days after transplantation when some of the grafts started to reject. Grafts were then fixed in 10% formalin, embedded in paraffin, transversely sectioned, and stained with hematoxylin and eosin stain (H&E) and Elastin Van Gieson stain. Immunoperoxidase staining using CD4 and CD8 antibodies was also performed on smaller pieces of frozen sections following air drying and fixation with acetone. For each immunoperoxidase Ab, the number of stained leukocytes per high power field (hpf) was recorded by averaging the number of positively stained leukocytes in the most affected five high power fields. Acute cellular rejection was semi-quantitatively graded (0R-3R) using the revised International Society of Heart and Lung Transplantation (ISHLT) classification (24). Since the revised ISHLT histologic schema was proposed to grade small endomyocardial biopsies rather than resection specimens, additional parameters were utilized to further assess the severity and extent of the histologic changes. The extent of the cellular infiltration (% inflammation) and myocyte loss was assessed and expressed as the percentage of surface areas involved by the aforementioned processes (25, 26). A transplant pathologist blinded to the groups read all the samples (I.B.).
Statistics
Graft survival was expressed graphically using the Kaplan-Meier method, and statistical differences in survival between the groups were assessed by the log-rank test. Student’s t-test was used for comparison of means. A p<0.05 was considered statistically significant.
Results
Delta1 expression is upregulated on APCs in an acute rejection model
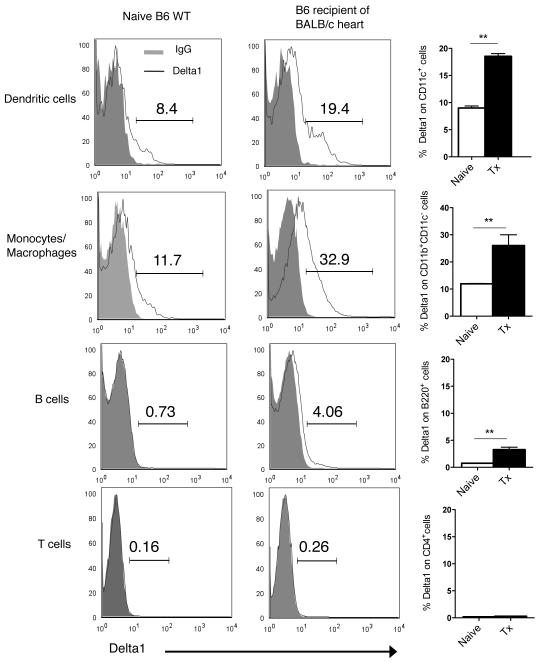
First, we decided to characterize Delta1 and Delta4 ligand expression in the naive and transplant setting. We analyzed their expression on hematopoietic cells from lymph nodes and spleens of naïve B6 WT animals by flow cytometry. Interestingly, Delta1 was predominantly expressed on dendritic cells (CD11c+) and monocytes/macrophages (CD11b+ CD11c−), while it was minimally present on B cells and T cells (Fig. 1). In contrary, Delta4 expression was low in all hematopoietic cells (e.g. CD11c+, 0.41 ±0.08, Supplemental Fig.1). Next, we performed cardiac transplants in a fully allogeneic model of cardiac transplantation and noted that Delta1 expression was significantly upregulated upon transplantation, especially close to the time of allograft rejection (7 days) (Fig. 1), while Delta4 did not significantly varied (Supplemental Fig.1). This observation suggests a potential role of Delta1 in allograft rejection, however, it doesn’t prove a direct contribution of this Notch ligand to the alloimmune response. Since Delta1 ligand signals through Notch receptors, we also evaluated the expression of these receptors on naïve and transplanted T cells. Notch2 was the predominant receptor on CD4+ cells (18.96% ±0.33), while CD8+ T cells expressed similar amounts of both Notch2 and Notch3 receptors (~19%) (Supplemental Fig. 2). Both Notch2 and Notch3 receptors were upregulated upon transplantation on T cells, whereas Notch1 and Notch4 remained low (<1%) in both naïve and transplant settings (Supplemental Fig. 2).
Figure 1. Expression of Delta1 on APCs is significantly upregulated upon transplantation.
By using a biotinylated anti-Delta1 Ab, we were able to demonstrate a predominant expression of Delta1 on antigen-presenting cells, mainly dendritic cells (CD11c+) and monocyte/macrophages (CD11b+ CD11c−). On the left and middle columns, representative histograms of Delta1 expression on different cell subtypes from splenocytes of naïve B6 mice or B6 recipients of BALB/c allografts 7 days after transplantation (Tx). Isotype control IgG is shown with solid grey line. Right column demonstrates the significant upregulation of Delta1 in the setting of transplantation on APCs (n = 6 each group) (** p<0.001).
Delta1 blockade prolongs cardiac graft survival
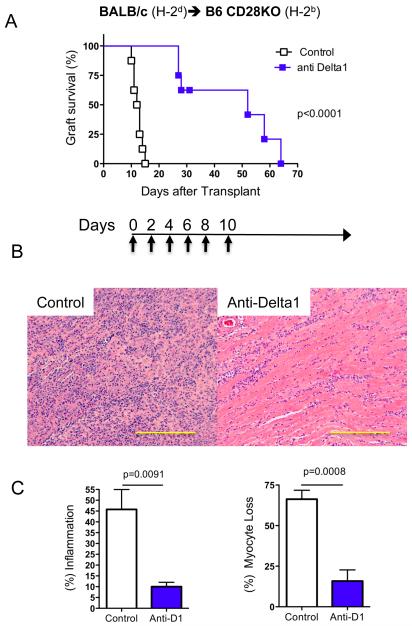
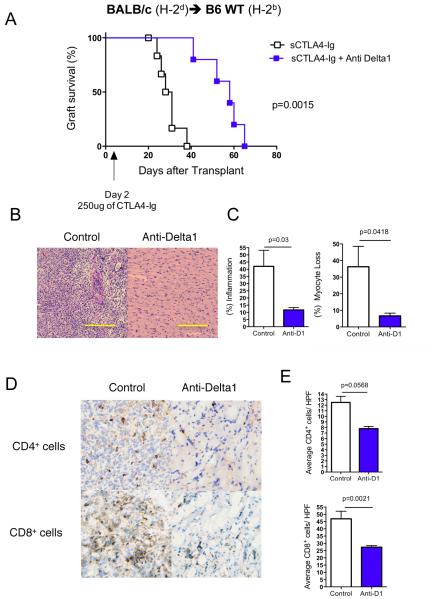
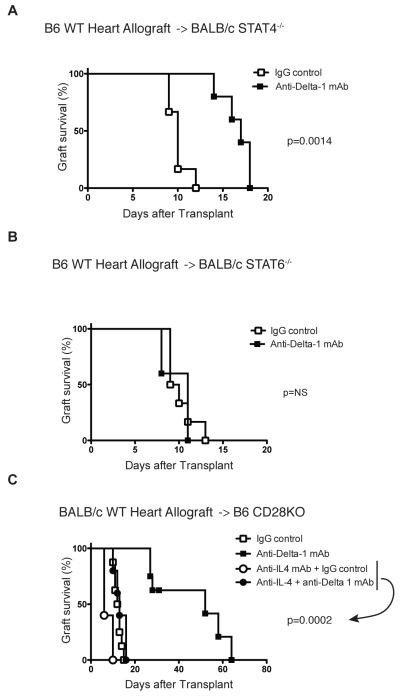
In order to evaluate if Delta1 plays a role in allograft rejection, we decided to use a Delta1 blocking mAb (HMD1-5) in vivo. We transplanted BALB/c hearts into B6 recipients and administered either hamster IgG control or anti-Delta1 mAb for 10 days (500 μg on day 0, 250 μg on days 2, 4, 6, 8,10). While control animals had a median survival time (MST) of 7 days, anti-Delta1-treated group had a slight delayed graft survival (MST=9 days, n=6, p=0.0005). However, when CD28KO mice were used as recipients, Delta1 blockade significantly delayed allograft rejection (MST=52 days vs. 12 days in controls, n=8, p<0.0001) (Fig. 2A). Similar finding was also observed when B6 wild-type recipients were treated with anti-Delta1 in combination with a single dose of CTLA4-Ig (250 μg on day 2) (MST=58 vs. 29 days in sCTLA4-Ig alone, n=7, p<0.0001) (Fig. 3A). These data suggest that brief Delta1 blockade is able to significantly delay allograft rejection, especially in the absence of strong B7: CD28 costimulation.
Figure 2. Anti-Delta1 mAb delays rejection of BALB/c cardiac allografts in CD28KO recipients.
The hearts from BALB/c mice were transplanted into CD28KO recipients on B6 background, which were treated with anti-Delta1 mAb or control hamster IgG. (A) While control-group cardiac grafts had a median survival time (MST) of 12 days (n=8), anti-Delta1-treated group had a delayed rejection with MST of 52 days (n=8, p<0.0001). Days of administration of mAb is depicted on arrow below survival curves. (B) Representative photomicrographs of H&E staining demonstrate more prominent cellular infiltrate on control group compared to anti-Delta1 at 2 weeks after transplantation. (C) At similar time point, scoring of pathology samples from both groups showed significant higher extent of interstitial inflammation and myocyte loss on the control group (n=5 each group). Bars, 200 μm.
Figure 3. Anti-Delta1 mAb prolongs BALB/c cardiac graft survivals in synergy with sCTLA4-Ig in B6 WT recipients.
The hearts from BALB/c mice were transplanted into B6 WT recipients, which were treated with single dose of CTLA4-Ig (sCTLA4-Ig) in combination with either anti-Delta1 mAb or control hamster IgG. (A) While control cardiac grafts had a MST of 29 days (n=7), anti-Delta1-cotreated group had a delayed rejection with MST of 58 days (n=7, p=0.0015). (B) Representative photomicrographs of H&E staining demonstrate more prominent cellular infiltrate on control group compared to anti-Delta1-treated group at 2 weeks after transplantation. (C) At similar time point, scoring of pathology samples from both groups showed significant higher extent of interstitial inflammation and myocyte loss on the control group (n=5 each group). (D) Representative photomicrographs of CD4+ and CD8+ stained sections showed less CD8+ and to a lesser extent CD4+ infiltration on the combined treated group with significant less T cells/high power field (HPF) as depicted on (E). Bars, 200 μm.
Anti-Delta1 decreases cellular infiltration and myocyte loss of cardiac allografts
To elucidate the delayed rejection in anti-Delta1-treated group, we started by analyzing cardiac grafts at 2 weeks after transplantation, when control grafts started to be rejected. While control grafts had intense inflammatory infiltrates and myocyte losses, Delta1 blockade on CD28KO recipients led to significant less myocyte loss and inflammatory infiltrates (Fig. 2B, C), with lower rejections scores by ISHLT classification (1.8±0.1 vs. 2.4±0.24, p<0.05). Similar picture was also observed in B6 WT recipients treated with sCTLA4-Ig and anti-Delta1 (Fig. 3B, C). Furthermore, the latter grafts had less CD4+ and CD8+ infiltrating cells when analyzed by immunohistochemistry (Fig. 3D, E). These findings suggest that Delta1 blockade leads to a decrease in the intensity of the alloimmune response.
Anti-Delta1 reduced the frequency of CD4 and CD8 effector/ memory cells
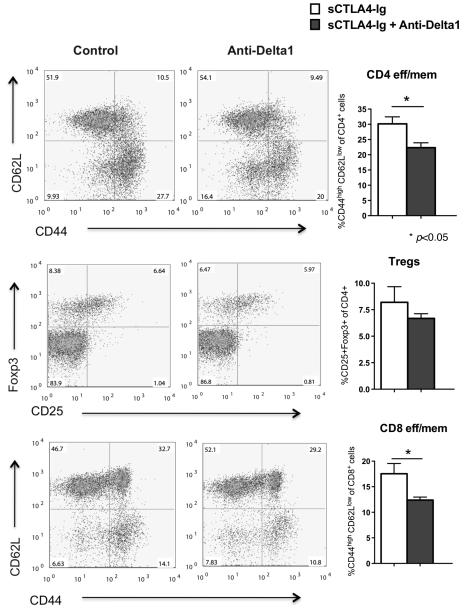
In order to investigate the mechanisms of reduced alloimmunity in response to Delta1 blockade, we first measured the frequency of lymphocyte subtypes in transplanted groups 14 days after transplantation. Anti-Delta1 administration resulted in a significant decrease in both CD4 and CD8 effector/ memory T cells (CD44hiCD62Llo) in CD28KO recipients (p=0.001) (Supplemental Fig. 3) as well as in B6 WT recipients co-treated with sCTLA4-Ig (p<0.05) when compared to controls (Fig. 4). Tregs were not significantly different between groups (Fig. 4). Furthermore, the percentage of APCs subtypes was similar between groups as well as the activation status of DCs as assessed by CD80, CD86 and PDL1 expression (Supplemental Fig. 4A). Since Delta1 has been shown to be important in the development of marginal zone B cells, we also checked the frequency of this subpopulation in the spleens of treated and control animals. Despite a lower trend in percentage of total B cells as well as subtypes (Bregs and MZ B cells), there was no statistical significant difference between anti-Delta1 group and controls (Supplemental Fig. 4B). In summary, anti-Delta1 lowered the frequency of effector/ memory CD4 and CD8 cells, while it did not affect the activation status of DCs or other APC subtypes.
Figure 4. Lower frequency of CD4 eff/ mem and CD8 eff/ mem in the combined sCTLA4-Ig/anti-Delta1-treated group.
Flow cytometry analysis of splenocytes from B6 WT recipients of BALB/c hearts 14 days after transplantation showed that anti-Delta1 Ab significantly decreased the percentage of CD4+CD44highCD62Llow and CD8+CD44highCD62Llow cells (CD4 and CD8 eff/ mem, respectively) when compared to sCTLA4-Ig alone. CD4+CD25+Foxp3+ regulatory T cells were no different between groups (p=0.3820). Panels on the left are representative examples of dot plots. Data are representative of three independent experiments.
Anti-Delta1 mAb tipped the balance towards Th2 cells
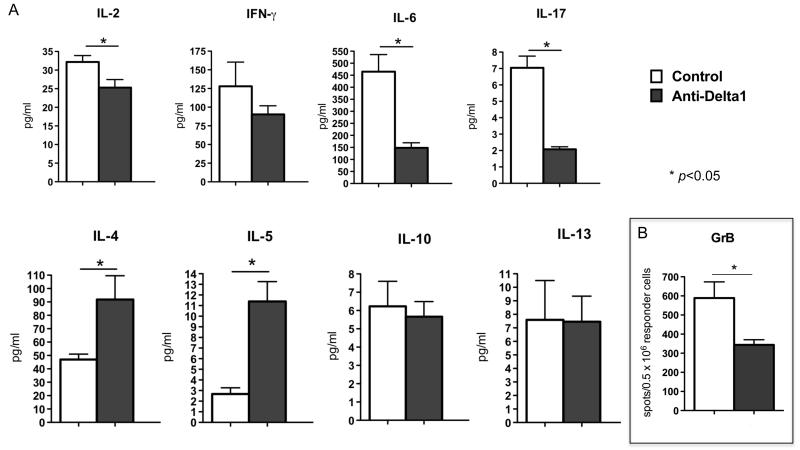
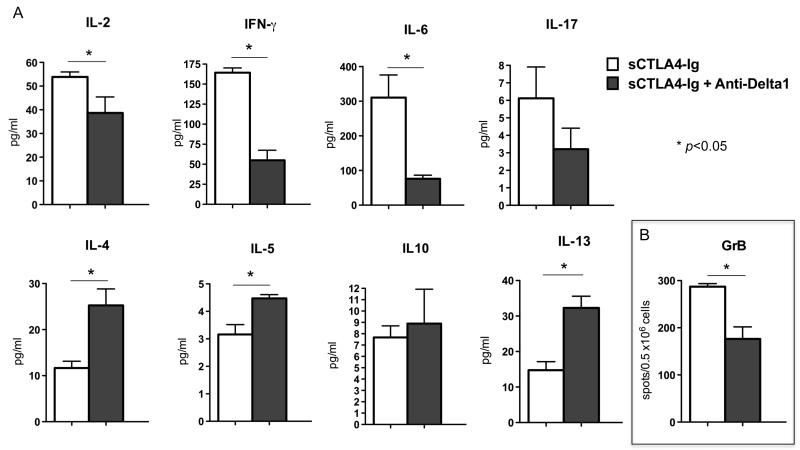
Next, we sought to characterize the influence of Delta1 blockade on T helper cell phenotype in lymph nodes and spleens of CD28KO recipients two weeks after transplantation. The anti-Delta1-treated group showed increased production of IL-4 and IL-5, while IFN-γ and IL-17 levels were decreased as measured by Luminex bead-based assay (Fig. 5A). Similar observation was also noted in B6 WT recipients co-treated with sCTLA4-Ig and anti-Delta1 (Fig. 6A), however the decrease in IFN-γ was more dramatic in this model. Moreover, Delta1 blockade also led to lower frequency of granzyme B-producing cells as demonstrated by ELISPOT (Fig. 5B, 6B). This was confirmed by an in vitro cytotoxicity assay, in which CD8+ T cells from anti-Delta1 treated group demonstrated lower cytotoxicity activity against allogeneic target cells when compared to controls (8% apoptotic/dead cells vs. 16% on controls, p=0.0001)(Fig. 7).
Figure 5. Anti-Delta1 mAb upregulates Th2 cytokines.
(A) Donor specific Th1, Th2 and proinflammatory cytokine production of splenocytes from CD28KO recipients of BALB/c allografts were assessed 14 days after transplantation by Luminex assay. Anti-Delta1 treatment led to a significant increase in the production of Th2 cytokines (IL-4, IL-5), while it decreased the production of IL-2, IL-6 and IL-17. (B) The frequency of GrB-producing cells was also decreased on the Delta1 blockade group by ELISPOT. There was a trend towards lower IFN-γ but it did not reach statistical significance. Data are representative of 3 independent experiments and indicate the mean of triplicate results in each experiment (* p<0.05).
Figure 6. Combination of sCTLA4-Ig and anti-Delta1 lowered GrB and IFN-γ production, while it increased Th2 cytokines.
(A) Donor specific cytokine production of splenocytes from B6 WT recipients of BALB/c allografts were assessed 14 days after transplantation by ELISPOT and Luminex assay. GrB (B), IL-2, IFN-γ and IL-6 was significantly decreased in the combined sCTLA4-Ig /anti-Delta1 group, while Th2 cytokines (IL-4, IL-5 and IL-13) were increased when compared to sCTLA4-Ig alone group. Data are representative of 3 independent experiments and indicate the mean of triplicate results in each experiment. (* p<0.05).
Figure 7.
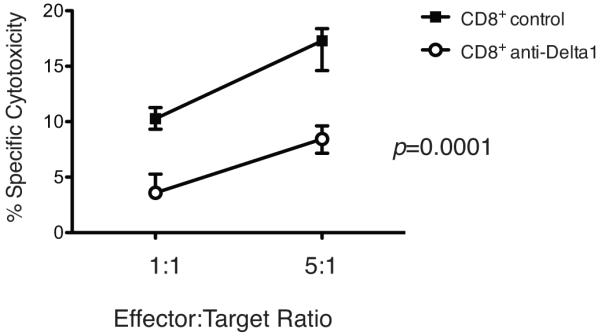
Cytotoxicity assay. CD8+ T cells from control and anti-Delta1 treated recipients were incubated with allogeneic target cells for 6 hours at effector:target ratios of 1:1 and 5:1 and cytotoxicity activity of these T cells was measured using a live/dead® Viability/Cytotoxicity Kit (Invitrogen). Percentage of specific cytotoxicity with either control or anti-Delta1 CD8+ T cells was calculated as described in Materials and Methods (n=3-4, p<0.0001). Data are representative of three independent experiments.
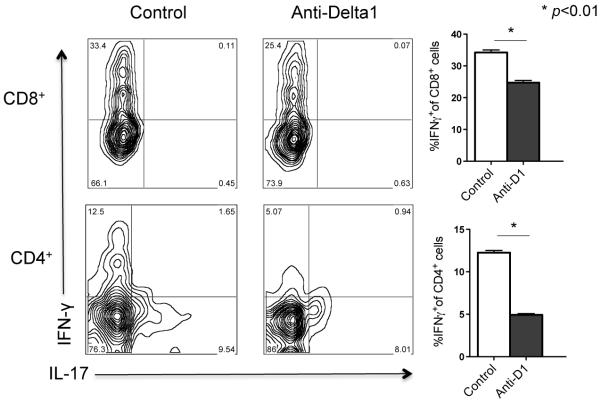
Finally, when lymphocytes infiltrating the allografts were isolated and cultured with irradiated donor cells, the frequency of both CD4+ and CD8+ IFN-γ+ T cells was significantly lower in the anti-Delta1 group (Fig. 8). These results indicate that Delta1 blockade leads to polarization into Th2 cells and decreases Th1 and cytotoxic T cells, potentially explaining the delayed in allograft rejection.
Figure 8. Lower frequency of IFN-γ-producing CD4+ and CD8+ T cells isolated from heart allografts cotreated with anti-Delta1.
Lymphocytes were isolated from heart allografts two weeks after transplantation and were restimulated in vitro for 4 hours. Subsequent intracellular cytokine staining by flow cytometry revealed a lower frequency of IFN-γ-producing T cells in the group treated with both sCTLA4-Ig and anti-Delta1 compared with control (only sCTLA4-Ig). Contour plots are representative examples. Graphs on the right are representative of three independent experiments. (* p<0.01).
Anti-Delta1 prolongation of allograft survival is dependent on Th2 cytokines
Th1 and Th2 development is dependent on STAT4 and STAT6 signaling, respectively (27). To evaluate if Delta1 signaling may induce Th1 differentiation indirectly via suppression of Th2 cells through IL-4 signaling blockade, we decided to dissect what was the dominant beneficial consequence of Delta1 blockade. Since STAT4KO mice were only commercially available on BALB/c background, we inverted the strains for this experiment. First, we confirmed the effect of anti-Delta1 mAb in this strain combination by demonstrating a slight prolongation of graft survival on BALB/c recipients of B6 hearts treated with anti-Delta1 mAb (MST=11) when compared to controls (MST=9, n=5/group, p=0.0025), similarly to what was observed on the BALB/c into B6 model. By transplanting B6 hearts into STAT4KO or STAT6KO recipients on BALB/c background, we were able to observe that prolongation of allograft survival was only maintained in STAT4KO treated with anti-Delta1 (Fig. 9A), while STAT6KO recipients had similar accelerated rejection as controls (Fig. 9B). Furthermore, neutralization of IL-4 abrogated the prolonging effects of Delta1 blockade in CD28KO recipients of BALB/c hearts (MST=52 vs. 13 in the co-treated group with anti-Delta1 and anti-IL-4 mAb, n=5, p=0.0002) (Fig. 9C). These findings suggest that the improved survival by Delta1 blockade is mainly dependent on Th2 polarization.
Figure 9. Prolongation of allograft survival on anti-Delta1-treated group is dependent on Th2 cytokines.
The hearts from B6 mice were transplanted into STAT4KO (A) or STAT6KO (B) recipients on BALB/c background, which were treated with anti-Delta1 mAb or control hamster IgG. While anti-Delta1 delayed allograft rejection in STAT4KO recipients (n=6, p=0.0014), it did not affect graft survival in STAT6KO recipients (n=6, p=0.87). (C) The hearts from BALB/c mice were transplanted into CD28KO recipients on B6 background, which were treated with anti-IL4 mAb in combination with either anti-Delta1 mAb or control hamster IgG. IL-4 neutralization abrogated the prolonging effect of Delta1 blockade (MST=13 vs. 52 in controls, n=5/group, p=0.0002).
Discussion
The role of Notch pathway in T cell differentiation in vitro has been increasingly explored lately. In particular, Delta1 ligands expressed on DCs have been showed to promote Th1 cell development and cytotoxic T cell (CTLs) differentiation (6, 10, 11, 14), while Delta4 promotes IL17 production (28). Nevertheless, little is known about the contribution of Notch ligands in the transplant setting in vivo, especially of Delta1 since both Th1 and CTLs are known to play a major role in allograft rejection (15-17, 29). We herein demonstrated that Delta1 is strongly upregulated upon transplantation in APCs in an acute cardiac rejection model (Fig. 1), while Delta4 remains mainly unchanged. The expression of Delta1 on DCs is induced by several stimuli, including infection with virus and bacteria, exposure to TLR ligands and CpG-containing DNA (6, 11, 30). In alloimmunity, multiple stimuli might act in synergy in the upregulation of Delta1, especially TLR ligands released after surgical trauma and ischemic injury (31, 32). Based on observations of Delta1 expression after syngeneic cardiac transplants where Delta1 was not as significantly upregulated as in allotransplants (data not shown), other unknown stimuli must be present to contribute to the dominant Delta1 expression upon transplantation.
Delta1 deficiency in mice is an embryonically lethal condition since Delta1 plays a major role in the compartmentalization of somites during embriogenesis (33). Cre-transgenic mice with selective deletion of Delta1 after birth demonstrated that the proportion of T and B cells was normal in both spleens and lymph nodes when compared to age-matched controls (34), suggesting that Delta1 is dispensable for postnatal T cell development in the thymus. However, a subpopulation of B cells, splenic marginal zone B (MZB) cells, was significantly decreased in these Delta1-null mice (34). Later, it was shown that despite the essential role of Delta1 for the maintenance of MZB cells in normal mice, anti-Delta1 blocking antibody did not significantly affect MZB cells in lupus-prone (NZBxNZW) F1 mice (19), indicating that other signals might contribute to MZB cell maintenance. Similarly to these findings, we noticed that MZB cell subpopulation was not significantly decreased in the transplant setting at both 7 and 14 days after transplantation (Supplemental Fig. 4B), while it was decreased in the naïve B6 mice that received anti-Delta1 without a transplant (data not shown). These findings suggest that Delta1 blockade does not reduce the alloimmune response through MZB cell depletion or by affecting T cell development, however it could be affecting either T cell activation and/or differentiation.
T cell activation requires the TCR engagement and co-stimulatory signals. Adding to this complexity, Notch proteins have emerged as potentiators of TCR signaling (5). Notch signaling has been shown to promote the nuclear retention of the NF-κB proteins p50 and p65 (35, 36) and to be involved in a positive-feedback loop that regulates IL-2 production and CD25 expression (37). Furthermore, chemical γ-secretase inhibitors that block Notch signaling decrease T cell proliferation significantly (36). Corroborating with these findings, blockade of Delta1 in our in vivo model led to a decrease in the frequency of both CD4 and CD8 effector/ memory cells suggesting that Delta1 signaling might contribute to T cell proliferation. On the contrary, the transfection of siRNAs into DCs against different Notch ligands led to enhanced IFN-γ production in MLR experiments with CD4+ T cells (38). These discrepancies seem to dependent on the experimental system used and will only be resolved by performing experiments with conditionally gene-targeted mice or perhaps further development of in vivo siRNA technologies.
CD8+ T cells have been reported to be major contributors of chronic rejection (29, 39) and its different requirements for costimulation (3) might pose a challenge to the development of tolerogenic strategies. The differentiation of naïve CD8+ T cells into a functional cytotoxic T lymphocyte (CTL) requires the induction of two key genes: T-bet and eomesodermin (Eomes) (40). CTLs can then mediate direct cell killing via granzyme B, perforin or Fas ligand expression. Delta1-expressing DCs have increased capacity to promote CTL differentiation in vitro via interaction with Notch2 on naïve CD8+ T cells (10). After this interaction, Notch2 intracellular domain migrates to the nucleus in combination with CREB1 to regulate granzyme B expression (10). Furthermore, Cho et al. have shown that Notch1 is also able to directly regulate the expression of Eomes, perforin and granzyme B via binding of the promoters of these crucial effector molecules (14). Reinforcing these findings, Delta1 blockade decreased the frequency of granzyme B-producing cells in our transplant models (Fig. 5B, 6B) and led to lower cytotoxicity activity of CD8+ T cells ex vivo against alloantigen target cell (Fig. 7), suggesting an important role of Delta1-Notch interaction in the regulation of cytolytic effector function of CD8+ T cells. Nonetheless, we realize that our antibody approach is unable to define if Notch signaling is truly required to mount an efficient CTL response in vivo.
In sharp contrast to our findings, Delta1-transfected L cells (carrying both MHC class I and II molecules) injected into recipients 14 days before transplantation were able to prolong cardiac allograft survival in another study (41). This inhibition of rejection was CD8-dependent and presumably due to the emergence of regulatory IL-10-producing CD8+ cells. However, this conclusion was based on in vitro findings using Delta1-Fc, and not Delta1-transfected L cells like the in vivo model, raising concern about their conclusion. Moreover, the experimental approach undertaken with pre-injection of cells in combination with MHC molecules and the properties of Delta1 on L cells could account for the differences observed. Nonetheless, we did not observe any effect of Delta1 blockade on IL-10 production in our experiment in vivo.
Different approaches have been undertaken to prove the role of Delta1 in Th1 cell differentiation, including transfection of Delta1 on APCs (6, 11) and use of Delta1-Fc proteins (30, 42). Furthermore, a neutralization Ab specific for Delta1 ligand (HMD1-5), the same clone used here, was able to decrease the frequency of Th1 cells and reduce the severity of disease in a model of experimental autoimmune encephalomyelitis (42). In agreement with these reports, our anti-Delta mAb was able to decrease Th1 and increase Th2 cells in murine cardiac transplantation, in particular when B7: CD28 pathway was blocked (Fig. 5,6), suggesting an important role of Delta1 signaling in the determination of Th cell polarization in vivo.
It is believed that once Delta1 ligand interacts with the Notch receptor on CD4+ T cells, it activates the transcription of Tbx21 via a RBP-J-dependent mechanism, which encodes the Th1-cell-specifying transcription factor T-bet (43). Moreover, Sun et al. have shown that Delta1 signaling could also induce a preferential Th1 response by inhibiting IL-4-receptor signaling, suggesting that it is actually the suppression of Th2 development that favors Th1 polarization (11). Interestingly, our results with IL-4 neutralization, STAT4- and STAT6-deficient recipients go in support of this hypothesis, where the dominant effect of Delta1 signaling is possibly through its suppressive effect on Th2 cell development. In neutral conditions, Th cells seem to favor Th2 polarization through GATA3 expression (44) and Delta1 affects IL-4 responsiveness to allow Th1 cell development (11). In the transplant setting, Th2 response favors long-term survival in some transplant models (15-17, 45) and tipping the balance from a Th1 to a Th2 response via Delta1 blockade demonstrated to be effective in reducing the alloimmune response. Among the Th2 cytokines, IL-4 was demonstrated in our model to be specifically important for the protective effect of Delta1 blockade, since neutralization of IL-4 abrogated the prolonging graft effect of anti-Delta1. However, the mechanism by which Delta1-initiated signaling blocks Th2 cell development remains to be established.
Taken together, our findings support a key role of Delta1 in the regulation of Th cell polarization and CTL differentiation in transplantation (Fig. 10). We were able to show that Delta1 is significantly upregulated on APCs upon transplantation and using an antibody approach, a brief blockade of Delta1 was able to decrease CTLs and tip the balance towards a Th2 response, in synergy with B7: CD28 blockade. This effect was shown to dependent on Th2 cytokines, in particular IL-4, suggesting a protective role of Th2 polarization in delaying rejection. In face of the poor long-term outcomes of current immunosuppressive drugs in organ transplantation, the identification of beneficial effects of Delta1 blockade might help in the development of potential novel targets for tolerogenic strategies in alloimmunity.
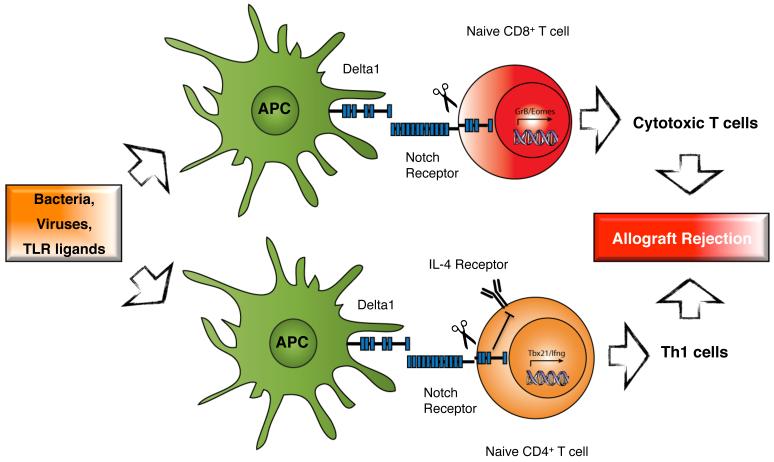
Figure 10. The role of Delta1 in T cell differentiation in alloimmunity.
Bacteria, virus and TLR ligands are some of the known stimuli that can induce the expression of Delta1 by antigen-presenting cells (APCs), which in turn promote the capacity of APCs to induce cytotoxic CD8+ T (CTL) cells and Th1-cell differentiation. When Delta1 interacts with the Notch receptor on T cells, it leads to sequential cleavage of the transmembrane region of Notch, resulting in the release of the Notch intracellular domain that translocates to the nucleus. In combination with co-factors (not shown), it activates the transcription of Notch target genes, including GrB and Eomes on CD8+ T cells; and Tbx21 and Ifng on CD4+ T cells. Delta1-Notch activation also leads to inhibition of IL-4-signaling on naïve CD4+ T cells, suppressing Th2-cell differentiation. As a result, the induction of Th1 cells and CTLs promotes rejection in the transplant setting.
Supplementary Material
Acknowledgments
We thank Mollie Jurewicz and Olaf Boenisch for invaluable technical assistance and Mohamed H. Sayegh for thoughtful discussions.
Footnotes
This work was supported by a research grant from the American Society of Transplantation to LVR, National Institute of Health (NIH) Grant RO1 AI51559, 2PO1056229 and AI70820.
Abbreviations used in this paper: DC, dendritic cell; KO, knockout; Treg, T regulatory cell; RBP, recombination signal sequence-binding protein; TLR, toll-like receptors; CREB-1, CAMP responsive element binding protein 1; MAML1, mastermind-like protein 1; CHO, Chinese hamster ovarian cells.
References
- 1.Trambley J, Bingaman AW, Lin A, Elwood ET, Waitze SY, Ha J, Durham MM, Corbascio M, Cowan SR, Pearson TC, Larsen CP. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. 1999;104:1715–1722. doi: 10.1172/JCI8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vu MD, Amanullah F, Li Y, Demirci G, Sayegh MH, Li XC. Different costimulatory and growth factor requirements for CD4+ and CD8+ T cell-mediated rejection. J Immunol. 2004;173:214–221. doi: 10.4049/jimmunol.173.1.214. [DOI] [PubMed] [Google Scholar]
- 3.Halamay KE, Kirkman RL, Sun L, Yamada A, Fragoso RC, Shimizu K, Mitchell RN, McKay DB. CD8 T cells are sufficient to mediate allorecognition and allograft rejection. Cell Immunol. 2002;216:6–14. doi: 10.1016/s0008-8749(02)00530-0. [DOI] [PubMed] [Google Scholar]
- 4.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 5.Osborne BA, Minter LM. Notch signalling during peripheral T-cell activation and differentiation. Nat Rev Immunol. 2007;7:64–75. doi: 10.1038/nri1998. [DOI] [PubMed] [Google Scholar]
- 6.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 7.Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annu Rev Immunol. 2005;23:945–974. doi: 10.1146/annurev.immunol.23.021704.115747. [DOI] [PubMed] [Google Scholar]
- 8.Yuan JS, Kousis PC, Suliman S, Visan I, Guidos CJ. Functions of notch signaling in the immune system: consensus and controversies. Annu Rev Immunol. 2010;28:343–365. doi: 10.1146/annurev.immunol.021908.132719. [DOI] [PubMed] [Google Scholar]
- 9.Radtke F, Fasnacht N, Macdonald HR. Notch signaling in the immune system. Immunity. 2010;32:14–27. doi: 10.1016/j.immuni.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Maekawa Y, Minato Y, Ishifune C, Kurihara T, Kitamura A, Kojima H, Yagita H, Sakata-Yanagimoto M, Saito T, Taniuchi I, Chiba S, Sone S, Yasutomo K. Notch2 integrates signaling by the transcription factors RBP-J and CREB1 to promote T cell cytotoxicity. Nat Immunol. 2008;9:1140–1147. doi: 10.1038/ni.1649. [DOI] [PubMed] [Google Scholar]
- 11.Sun J, Krawczyk CJ, Pearce EJ. Suppression of Th2 cell development by Notch ligands Delta1 and Delta4. J Immunol. 2008;180:1655–1661. doi: 10.4049/jimmunol.180.3.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamaguchi E, Chiba S, Kumano K, Kunisato A, Takahashi T, Hirai H. Expression of Notch ligands, Jagged1, 2 and Delta1 in antigen presenting cells in mice. Immunol Lett. 2002;81:59–64. doi: 10.1016/s0165-2478(01)00326-1. [DOI] [PubMed] [Google Scholar]
- 13.Kubo M. Notch: filling a hole in T helper 2 cell differentiation. Immunity. 2007;27:3–5. doi: 10.1016/j.immuni.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Cho OH, Shin HM, Miele L, Golde TE, Fauq A, Minter LM, Osborne BA. Notch regulates cytolytic effector function in CD8+ T cells. J Immunol. 2009;182:3380–3389. doi: 10.4049/jimmunol.0802598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sho M, Yamada A, Najafian N, Salama AD, Harada H, Sandner SE, Sanchez-Fueyo A, Zheng XX, Strom TB, Sayegh MH. Physiological mechanisms of regulating alloimmunity: cytokines, CTLA-4, CD25+ cells, and the alloreactive T cell clone size. J Immunol. 2002;169:3744–3751. doi: 10.4049/jimmunol.169.7.3744. [DOI] [PubMed] [Google Scholar]
- 16.Waaga AM, Gasser M, Kist-van Holthe JE, Najafian N, Muller A, Vella JP, Womer KL, Chandraker A, Khoury SJ, Sayegh MH. Regulatory functions of self-restricted MHC class II allopeptide-specific Th2 clones in vivo. J Clin Invest. 2001;107:909–916. doi: 10.1172/JCI11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waaga-Gasser AM, Grimm MR, Lutz J, Lange V, Lenhard SM, Aviles B, Kist-van Holthe JE, Lebedeva T, Samsonov D, Meyer D, Hancock WW, Heemann U, Gasser M, Chandraker A. Regulatory allospecific T cell clones abrogate chronic allograft rejection. J Am Soc Nephrol. 2009;20:820–830. doi: 10.1681/ASN.2008020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16:343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Moriyama Y, Sekine C, Koyanagi A, Koyama N, Ogata H, Chiba S, Hirose S, Okumura K, Yagita H. Delta-like 1 is essential for the maintenance of marginal zone B cells in normal mice but not in autoimmune mice. Int Immunol. 2008;20:763–773. doi: 10.1093/intimm/dxn034. [DOI] [PubMed] [Google Scholar]
- 20.Yang J, Riella LV, Boenisch O, Popoola J, Robles S, Watanabe T, Vanguri V, Yuan X, Guleria I, Turka LA, Sayegh MH, Chandraker A. Paradoxical Functions of B7: CD28 Costimulation in a MHC Class II-Mismatched Cardiac Transplant Model. Am J Transplant. 2009 doi: 10.1111/j.1600-6143.2009.02839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan X, Paez-Cortez J, Schmitt-Knosalla I, D’Addio F, Mfarrej B, Donnarumma M, Habicht A, Clarkson MR, Iacomini J, Glimcher LH, Sayegh MH, Ansari MJ. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J Exp Med. 2008;205:3133–3144. doi: 10.1084/jem.20081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Gong N, Huang X, Reynolds AD, Mosley RL, Gendelman HE. Neuromodulatory activities of CD4+CD25+ regulatory T cells in a murine model of HIV-1-associated neurodegeneration. J Immunol. 2009;182:3855–3865. doi: 10.4049/jimmunol.0803330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Correia MP, Costa AV, Uhrberg M, Cardoso EM, Arosa FA. IL-15 induces CD8+ T cells to acquire functional NK receptors capable of modulating cytotoxicity and cytokine secretion. Immunobiology. 2011;216:604–612. doi: 10.1016/j.imbio.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, Andersen CB, Angelini A, Berry GJ, Burke MM, Demetris AJ, Hammond E, Itescu S, Marboe CC, McManus B, Reed EF, Reinsmoen NL, Rodriguez ER, Rose AG, Rose M, Suciu-Focia N, Zeevi A, Billingham ME. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–1720. doi: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Harada H, Salama AD, Sho M, Izawa A, Sandner SE, Ito T, Akiba H, Yagita H, Sharpe AH, Freeman GJ, Sayegh MH. The role of the ICOS-B7h T cell costimulatory pathway in transplantation immunity. J Clin Invest. 2003;112:234–243. doi: 10.1172/JCI17008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimizu K, Schonbeck U, Mach F, Libby P, Mitchell RN. Host CD40 ligand deficiency induces long-term allograft survival and donor-specific tolerance in mouse cardiac transplantation but does not prevent graft arteriosclerosis. J Immunol. 2000;165:3506–3518. doi: 10.4049/jimmunol.165.6.3506. [DOI] [PubMed] [Google Scholar]
- 27.Shuai K, Liu B. Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol. 2003;3:900–911. doi: 10.1038/nri1226. [DOI] [PubMed] [Google Scholar]
- 28.Mukherjee S, Schaller MA, Neupane R, Kunkel SL, Lukacs NW. Regulation of T cell activation by Notch ligand, DLL4, promotes IL-17 production and Rorc activation. J Immunol. 2009;182:7381–7388. doi: 10.4049/jimmunol.0804322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischbein MP, Yun J, Laks H, Irie Y, Fishbein MC, Bonavida B, Ardehali A. Role of CD8+ lymphocytes in chronic rejection of transplanted hearts. J Thorac Cardiovasc Surg. 2002;123:803–809. doi: 10.1067/mtc.2002.120008. [DOI] [PubMed] [Google Scholar]
- 30.Maekawa Y, Tsukumo S, Chiba S, Hirai H, Hayashi Y, Okada H, Kishihara K, Yasutomo K. Delta1-Notch3 interactions bias the functional differentiation of activated CD4+ T cells. Immunity. 2003;19:549–559. doi: 10.1016/s1074-7613(03)00270-x. [DOI] [PubMed] [Google Scholar]
- 31.Kaczorowski DJ, Nakao A, Mollen KP, Vallabhaneni R, Sugimoto R, Kohmoto J, Tobita K, Zuckerbraun BS, McCurry KR, Murase N, Billiar TR. Toll-like receptor 4 mediates the early inflammatory response after cold ischemia/reperfusion. Transplantation. 2007;84:1279–1287. doi: 10.1097/01.tp.0000287597.87571.17. [DOI] [PubMed] [Google Scholar]
- 32.Tesar BM, Jiang D, Liang J, Palmer SM, Noble PW, Goldstein DR. The role of hyaluronan degradation products as innate alloimmune agonists. Am J Transplant. 2006;6:2622–2635. doi: 10.1111/j.1600-6143.2006.01537.x. [DOI] [PubMed] [Google Scholar]
- 33.Hrabe de Angelis M, McIntyre J, 2nd, Gossler A. Maintenance of somite borders in mice requires the Delta homologue DII1. Nature. 1997;386:717–721. doi: 10.1038/386717a0. [DOI] [PubMed] [Google Scholar]
- 34.Hozumi K, Negishi N, Suzuki D, Abe N, Sotomaru Y, Tamaoki N, Mailhos C, Ish-Horowicz D, Habu S, Owen MJ. Delta-like 1 is necessary for the generation of marginal zone B cells but not T cells in vivo. Nat Immunol. 2004;5:638–644. doi: 10.1038/ni1075. [DOI] [PubMed] [Google Scholar]
- 35.Shin HM, Minter LM, Cho OH, Gottipati S, Fauq AH, Golde TE, Sonenshein GE, Osborne BA. Notch1 augments NF-kappaB activity by facilitating its nuclear retention. EMBO J. 2006;25:129–138. doi: 10.1038/sj.emboj.7600902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palaga T, Miele L, Golde TE, Osborne BA. TCR-mediated Notch signaling regulates proliferation and IFN-gamma production in peripheral T cells. J Immunol. 2003;171:3019–3024. doi: 10.4049/jimmunol.171.6.3019. [DOI] [PubMed] [Google Scholar]
- 37.Adler SH, Chiffoleau E, Xu L, Dalton NM, Burg JM, Wells AD, Wolfe MS, Turka LA, Pear WS. Notch signaling augments T cell responsiveness by enhancing CD25 expression. J Immunol. 2003;171:2896–2903. doi: 10.4049/jimmunol.171.6.2896. [DOI] [PubMed] [Google Scholar]
- 38.Stallwood Y, Briend E, Ray KM, Ward GA, Smith BJ, Nye E, Champion BR, McKenzie GJ. Small interfering RNA-mediated knockdown of notch ligands in primary CD4+ T cells and dendritic cells enhances cytokine production. J Immunol. 2006;177:885–895. doi: 10.4049/jimmunol.177.2.885. [DOI] [PubMed] [Google Scholar]
- 39.Lunsford KE, Horne PH, Koester MA, Eiring AM, Walker JP, Dziema HL, Bumgardner GL. Activation and maturation of alloreactive CD4-independent, CD8 cytolytic T cells. Am J Transplant. 2006;6:2268–2281. doi: 10.1111/j.1600-6143.2006.01479.x. [DOI] [PubMed] [Google Scholar]
- 40.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 41.Wong KK, Carpenter MJ, Young LL, Walker SJ, McKenzie G, Rust AJ, Ward G, Packwood L, Wahl K, Delriviere L, Hoyne G, Gibbs P, Champion BR, Lamb JR, Dallman MJ. Notch ligation by Delta1 inhibits peripheral immune responses to transplantation antigens by a CD8+ cell-dependent mechanism. J Clin Invest. 2003;112:1741–1750. doi: 10.1172/JCI18020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elyaman W, Bradshaw EM, Wang Y, Oukka M, Kivisakk P, Chiba S, Yagita H, Khoury SJ. JAGGED1 and delta1 differentially regulate the outcome of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:5990–5998. doi: 10.4049/jimmunol.179.9.5990. [DOI] [PubMed] [Google Scholar]
- 43.Minter LM, Turley DM, Das P, Shin HM, Joshi I, Lawlor RG, Cho OH, Palaga T, Gottipati S, Telfer JC, Kostura L, Fauq AH, Simpson K, Such KA, Miele L, Golde TE, Miller SD, Osborne BA. Inhibitors of gamma-secretase block in vivo and in vitro T helper type 1 polarization by preventing Notch upregulation of Tbx21. Nat Immunol. 2005;6:680–688. [PubMed] [Google Scholar]
- 44.Zhu J, Min B, Hu-Li J, Watson CJ, Grinberg A, Wang Q, Killeen N, Urban JF, Jr., Guo L, Paul WE. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol. 2004;5:1157–1165. doi: 10.1038/ni1128. [DOI] [PubMed] [Google Scholar]
- 45.Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989;170:2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.