Abstract
Th17 cells are highly pathogenic in a variety of immune-mediated diseases, and a thorough understanding of the mechanisms of cytokine-mediated suppression of Th17 cells has great therapeutic potential. In this report, we characterize the regulation of both in vitro- and in vivo-derived Th17 cells by IL-4. We demonstrate that IL-4 suppresses re-activation of committed Th17 cells, even in the presence of TGFβ, IL-6 and IL-23. Down-regulation of IL-17 by IL-4 is dependent on STAT6 and mediated by inhibition of STAT3 binding at the Il17a promoter. Although Th1 cytokines have been shown to induce IFNγ expression by Th17 cells, IL-4 does not induce a Th2 phenotype in Th17 cells. Suppression by IL-4 is stable and long-lived when applied to immature Th17 cells, but cells that have undergone multiple rounds of stimulation, either in vivo during a Th17-mediated inflammatory disease, or in vitro, become resistant to suppression by IL-4 and lose the ability to signal through the IL-4 receptor. Thus, while IL-4 is a potent suppressor of the Th17 genetic program at early stages after differentiation, prolonged stimulation renders Th17 cells impervious to regulatory cytokines.
Introduction
Th17 cells, which express the pro-inflammatory cytokine IL-17, play an important role in the pathogenesis of a variety of autoimmune and inflammatory diseases. Naïve CD4+ T cells differentiate into Th17 cells in response to TGFβ, IL-6 and IL-21, and become more pathogenic in the presence of IL-23[1–7]. In addition, Th17 cell development depends on the transcription factors STAT3, IRF-4, RORγt and RORα [8–11]. In vitro, induction of Th17 differentiation is inhibited by the Th1 cytokines IFNγ and IL-12, as well as the Th2 cytokines IL-4 and IL-13 [12–14].
Most of what is known about the cross-regulation of Th17 cells is limited to the earliest stages of differentiation, which occur within the first few hours to days following the initial T cell activation. However, it is also important to address the role of cytokine-mediated regulation of committed Th17 cells. Little is known about the regulation of secondary stimulation, which may be more representative of a Th17 cell that has become activated and differentiated in a lymph node in the presence of TGFβ and IL-6, and then exited the lymph node and traveled to a site of inflammation with a distinct cytokine milieu, such as an inflamed joint. It has also been shown that cytokine regulatory networks can change as T cells mature. For example, IL-27 suppresses Th17 development from naïve CD4+ T cells, but fails to suppress re-activation of committed Th17 cells [15]. Understanding the regulation of pre-existing activated T cells may also have therapeutic applications in the control chronic T cell-mediated diseases such as rheumatoid arthritis (RA).
Several groups have recently demonstrated a high degree of plasticity in Th17 cells, such that stimulation with IL-12 up-regulates T-bet and IFNγ and induces a Th1-like phenotype, while stimulation with TGFβ up-regulates FoxP3 and induces a Treg-like phenotype [16, 17]. One report suggested that the Th17 phenotype is unstable, and Th17 cells will spontaneously convert to Th1 cells in lymphopenic hosts [18]. However, another group demonstrated that in vitro-generated Th17 cells quickly lose IL-17 expression unless IL-23 is added and opposing cytokines are blocked, while in vivo-generated Th17 cells continue to express IL-17 regardless of which cytokines are added [19]. Similarly, in vitro-generated Th17 cells could convert to a Th1 or Th2 phenotype when stimulated under Th1- or Th2-skewing conditions, while in vivo-generated Th17 cells were refractory to Th1- and Th2-polarizing signals.
Some Th17-to-Th1 conversion may not be surprising, considering the evidence for the close relationship between these two lineages. Th17 cells and Th1 cells were thought to share a common lineage precursor [20], and IL-17/IFNγ double-positive cells are quite common in humans[21, 22]. Similarly, the shared dependence on TGFβ implies some Treg-Th17 commonality. However, there is much less evidence of any Th2-Th17 commonality. Unlike the frequent appearance of IL-17/IFNγ double positive cells, IL-17/IL-4 double-positive cells have only rarely been encountered [23], suggesting that the relationship between Th1 and Th17 may be very different from the relationship between Th2 and Th17.
Although IL-4 has been shown to inhibit Th17 differentiation and IL-17 expression, nothing is known about the molecular mechanisms of this suppression or about the effects of IL-4 on other Th17-family genes, such as RORγt and IL-22. Similarly, the effects of IL-4 on developing versus committed Th17 cells have not been explored. In this study we demonstrate that IL-4 suppresses a subset of Th17 genes distinct from IFNγ. Suppression of IL-17 depends on STAT6, but not GATA-3, and correlates with a loss of STAT3 binding at the Il17a promoter. IL-4 induces a stable, irreversible loss of IL-17 expression without inducing conversion to a Th2 phenotype. However, repeated stimulation renders both in vivo- and in vitro-derived Th17 cells refractory to suppression by IL-4.
Materials and Methods
Mice
For in vitro Th17 differentiation and in vivo KLH-immunization, male 6- to 8-week old BALB/c mice were obtained from Jackson Laboratories. For cII/CFA immunization, male 8- to 10-week-old DBA1 mice were obtained from Jackson Laboratories. STAT6-deficient and IL-4R mutant mice on a BALB/c background were obtained from Jackson Laboratories. All animals were housed in specific pathogen free conditions and all procedures were approved by the University Committee for the Use and Care of Animals of the University of Michigan. Single-cell suspensions from spleens and thymi of CD4-Cre/STAT5a/bflox mice on a C57BL/6 background were a kind gift from the laboratory of Dr. John O’Shea at the NIH and were shipped overnight on ice. Freshly isolated spleens from GATA3 conditional knockout mice were a kind gift from the laboratory of Dr. James Engel at the University of Michigan.
Generation of BM-DC
Bone marrow was isolated from femurs and tibias, treated with hypotonic ammonium chloride buffer to lyse erythrocytes, and cultured for 6 days at 1×106 cells/mL with 10ng/mL recombinant mouse IL-4 and GM-CSF (Peprotech) in basic RPMI (10% FCS, 2% L-glutamine, 1% penicillin/streptomycin, 55µM β-mercaptoethanol). The cells were then collected using a cell scraper and CD11c+ cells were positively selected by two rounds of MACS (Miltenyi Biotech).
Purification of naïve T cells
Spleens were collected and CD4+ T cells were magnetically isolated by negative selection using the EasySep kit from Stem Cell Technologies. The purified CD4+ T cells were then labeled with CD4 FITC, CD25 PE, CD44 PE-Cy7 and CD62L APC (Biolegend). The CD4+CD25-CD44loCD62L+ cells were sorted on a FACS Vantage, Aria or Diva.
Th17 differentiation
BM-DCs and naïve T cells were plated in 6 well plates in basic RPMI at 0.125×106 BM-DCs and 0.25×106 naïve T cells per mL with 4 µg/mL anti-CD3 (145-2C11), 10 µg/mL anti-IL-4 (11B11), 10 µg/mL anti-IFNγ (R4-6A2), 1 ng/mL recombinant human TGF-β1 (Peprotech), 20ng/mL recombinant mouse IL-6 (Peprotech) and 10 ng/mL recombinant mouse IL-23 (eBioscience). For inhibition of Th17 differentiation anti-IL-4 was omitted from the culture and recombinant mouse IL-4 (Peprotech) was added at 10 ng/mL, unless stated otherwise. Alternatively, anti-IFNγ was omitted from the culture and recombinant mouse IFNγ (Peprotech) was added at 10 ng/mL, unless stated otherwise. Cells were stimulated for six days and then collected, washed twice with cold 2% NCS/PBS and put back into culture in the same volume without stimulation for two days. For inhibition of Th17 re-stimulation, IL-4 was added to the culture during the two-day rest period or during a two-day re-stimulation with anti-CD3 following the rest period.
Th17 maturation
Naïve T cells underwent six days of Th17 differentiation followed by two days of rest, according to the protocol described above. To induce maturation the cells were then expanded in a two fold culture volume with the addition of fresh BM-DCs, re-stimulated with the same cytokine and neutralizing antibody cocktail for five days, and then washed and rested for two days. This cycle of five days of stimulation and two days of rest was repeated, for a total of three weeks of culture. At the end of the three weeks the Th17 cells were re-stimulated for two days with anti-CD3 and recombinant IL-4.
ELISA
IL-17A was measured by ELISA. Plates were coated with purified anti-IL-17A (clone TC11-18H10.1, Biolegend), blocked, and then loaded with tissue culture supernatants or serum. The plates were washed and treated with biotin-conjugated anti-IL-17A (clone TC11-8H4, Biolegend) mixed with streptavidin horseradish peroxidase (Biolegend). The plates were developed with OptEIA TMB substrate (BD) and absorbance at 450 nm was quantitated with a Biorad (Hercules, CA, USA) plate reader using KC4 software (Biotek, Winooski, VT, USA).
Flow cytometry
For intracellular cytokine staining (ICS), cells were stimulated for 6 h with 5 ng/mL PMA and 500 ng/mL ionomycin, with 10 µg/mL Brefeldin A added for the last 5 h (all chemicals from Sigma). Cells were then treated with mouse FcBlock anti-CD16/32, stained with FITC- or PE-conjugated anti-CD4 (clone GK1.5) and fixed overnight. The next day cells were permeabilized with saponin and stained with fluorescently-labeled anti-IL-17 (clone TC11-18H10.1), anti-IFNγ (clone XMG 1.2), and anti-IL-4 (clone 11B11) or the appropriate isotype control (all antibodies from Biolegend). Staining was measured with a FACS Calibur and data was analyzed using Cell Quest software (BD).
Real-time PCR
Gene expression at the mRNA level was analyzed by Taqman-based real time PCR with specific primers and probes. First, RNA was collected from frozen cell pellets with the RNEasy Mini kit and treated with DNase (Qiagen). cDNA was generated using the High Capacity cDNA archive kit (Applied Biosystems). Relative quantification using the comparative CT method was carried out using TaqMan Universal PCR Master Mix or Gene Expression Master Mix (Applied Biosystems) and run on an AB7500 machine. The following primer and probe sets were obtained from Applied Biosystems: IL-17A, IL-17F, IL-22, RORγ, IL-23R, IL-4, IL-4R, STAT6, GATA3, β-Actin and GAPDH.
Chromatin immunoprecipitation
Chromatin immunoprecipitation was carried out according to the EZ ChIP protocol (Upstate). Briefly, Th17 cells were fixed with formaldehyde and lysed with SDS. Lysates were sonicated to shear DNA and immunoprecipitated with protein G and antibodies to STAT3. Eluted DNA was quantitated by real-time PCR with SYBR green master mix (Applied Biosystems) and the following primers:
Il17a promoter forward: AGGGAGAGCTTCATCTGTGG
Il17a promoter reverse: AGATTCATGGACCCCAACAG
Il17a/f intergenic region forward: CAGACTCCAAGCACATCATG
Il17a/f intergenic region reverse: GACTGACCTACATTGTGGGC
Rorc promoter forward: AGGCTCCTGACCTTTGATTG
Rorc promoter reverse: AGGGGGTGCTGAGTAATCAC
Western blot
Th17 cells were washed and rested overnight in RPMI with 2% FCS plus cytokine-neutralizing antibodies to minimize background levels of STAT activation. The cells were then incubated with 50 ng/mL IL-4. The reaction was stopped with cold PBS plus 1 mM Na3VO4, and the cells were lysed with Phosphosafe extraction reagent (Novagen) supplemented with protease inhibitor cocktail (Calbiochem). Lysates were reduced and denatured by boiling with SDS loading buffer with 100 mM DTT. Samples were run on a 10% Precise Tris-Hepes-SDS gel (Pierce) and transferred to PVDF membrane (Millipore). Membranes were stained with the following primary antibodies at 1:1000 unless noted otherwise: anti-STAT6 (Cell Signaling), anti-phospho-Tyr641 STAT6 (Calbiochem), and anti-GAPDH (1:400, Biolegend). Secondary antibodies were goat anti-rabbit IgG HRP (1:1000, Cell Signaling) or rabbit anti-goat IgG HRP (1:10,000, Abcam). Chemiluminescence was developed with Pierce ECL Western blotting substrate and detected on blue autoradiography film (MidSci). Band intensities were quantified using Kodak 1d 3.6 software.
Immunizations
Complete Freund’s adjuvant (CFA) was prepared by mixing heat inactivated mycobacterial strain H37Ra in incomplete Freund’s adjuvant at 4 mg/ml. For KLH immunization, Imject® mcKLH subunits (Pierce, Rockford, IL) were diluted in PBS to 2mg/mL and mixed at a 1:1 ratio with CFA. Mice were immunized intraperitoneally with 100µg KLH. For collagen immunization, lyophilized chicken collagen (Chondrex, Redmond, WA, USA) was dissolved overnight in acetic acid at 4 mg/ml. CFA and collagen were mixed at a 1:1 ratio to form an emulsion and 100 µg of collagen was injected intradermally at the base of the tail.
Statistical analysis
P values were calculated by unpaired, two-way Student’s t test, one sample t test, one-way ANOVA, or two-way ANOVA with Dunnett’s post-test using Prism, as described in the figure legends.
Results
Th1 and Th2 cytokines suppress re-activation of in vivo-derived Th17 cells
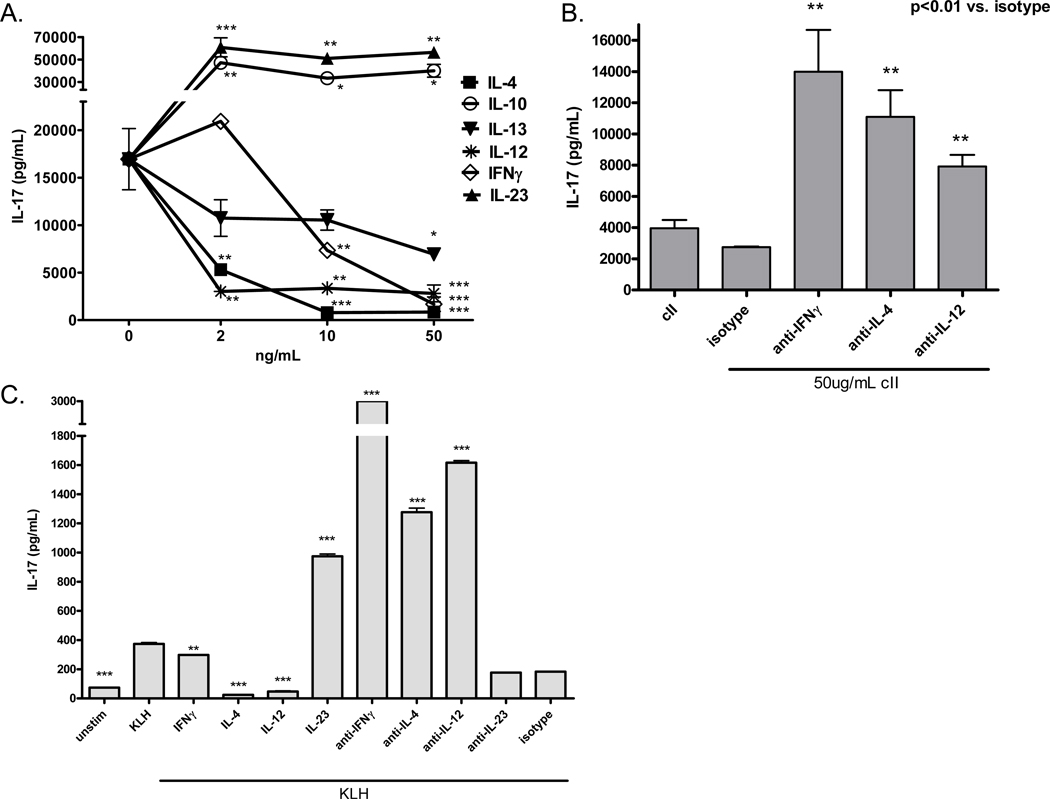
Previous work has shown that IL-4 can inhibit in vitro Th17 differentiation from naïve CD4+ T cells[12], but much less is known about the effects of IL-4 on pre-existing or memory Th17 cells, such as those that are likely to persist during many inflammatory diseases. To examine the cytokine-mediated regulation of Th17 cells generated in vivo in the context of an IL-17-dependent autoimmune disease, we immunized DBA1/LacJ mice i.d. with chick type II collagen emulsified in CFA, following the standard protocol for induction of collagen-induced arthritis, and after two weeks, spleen cells were re-stimulated in vitro with collagen, in the presence or absence of recombinant Th1 and Th2 cytokines or neutralizing antibodies. After five days of culture, IL-17 production was measured by ELISA. The results in Figure 1A demonstrate that in vivo-derived, committed Th17 cells are susceptible to counter-regulation by a number of other Th1 and Th2 cytokines. In particular, IL-4 and IL-12 were potent suppressors and IFNγ was a weak suppressor of collagen-specific IL-17 production. IL-13, another important Th2 cytokine, also inhibited IL-17 production, confirming recent data from Newcomb et al. and furthering the idea that Th2 cells can suppress Th17 activity through multiple mechanisms [14]. In addition, neutralizing antibodies to IL-4, IFNγ or IL-12 up-regulated IL-17 production, implying that the collagen-specific Th17 response is constrained by endogenous cytokines, possibly from collagen-specific T cells of opposing lineages (Figure 1B).To confirm that these observations are not restricted to DBA mice, the collagen-specific response or an autoimmune disease, we measured Th17 responses in BALB/c mice two weeks after i.p. immunization with keyhole limpet hemocyanin (KLH). Despite the well-known Th2 bias in BALB/c mice, immunization induced significant KLH-specific IL-17 production in the spleen, which was negatively regulated by both endogenous and exogenous Th1 and Th2 cytokines during antigen-specific re-challenge, recapitulating our previous findings in the collagen system (Figure 1C). These data imply that even after differentiation, Th17 cells are still susceptible to cytokine-mediated counter-regulation, which raises many interesting questions about the stability of lineage commitment, the mechanisms of suppression and the role of these regulatory pathways in chronic inflammation.
Figure 1. Antigen-specific IL-17 production is regulated by endogenous and exogenous Th1 and Th2 cytokines.
DBA mice were immunized i.d. with cII/CFA and spleens were collected two weeks later. Single cell suspensions were re-stimulated in vitro with 50µg/mL heat-denatured collagen in the presence of recombinant cytokines (A) or 10µg/mL purified cytokine-neutralizing antibodies (B) for five days. Supernatants were collected and IL-17 was measured by ELISA. Error bars represent SEM of triplicate culture samples. *p<0.05, **p<0.01, ***p<0.001 versus 0ng/mL (A) or versus isotype control (B) by one-way ANOVA. (C) BALB/c mice were immunized i.p. with KLH in CFA and spleens were collected two weeks later. Single cell suspensions were re-stimulated in vitro with 50µg/mL heat-denatured KLH in the presence of 10ng/mL recombinant cytokines or 10µg/mL purified cytokine-neutralizing antibodies for five days. Supernatants were collected and IL-17 was measured by ELISA. Error bars represent SEM of triplicate culture samples. **p<0.01, ***p<0.001 versus KLH or isotype by one-way ANOVA.
Suppression by IL-4 and IFNγ dominates over strong stimulation of Th17 cells
Although several groups have shown that the combination of TGFβ and IL-6 acts on naïve T cells to induce Th17 differentiation [2, 3, 24], it is not clear what effect TGFβ and IL-6 have on memory Th17 cells. Therefore, we decided to re-stimulate collagen-immunized spleen cells in the presence of TGFβ and/or IL-6, with the assumption that two weeks after immunization most collagen-specific T cells have already differentiated, thus the TGFβ and IL-6 are more likely to be acting on activated or memory Th17 cells rather than inducing new Th17 differentiation in naïve T cells. The results showed that IL-6 alone, and to a much greater extent TGFβ alone, was able to up-regulate IL-17, a result consistent with the presence of some endogenous IL-6. However, the combination of TGFβ and IL-6 was significantly better at inducing IL-17 than either cytokine alone, and addition of IL-23 to the cocktail enhanced IL-17 production even further (Figure 2A). Moreover, TGFβ, IL-6 and IL-23, either alone or in combination, induced considerable IL-17 production even in the absence of exogenous collagen, suggesting that the right cytokine milieu can stimulate pre-existing Th17 cells without the need for concomitant TCR stimulation. (This inference assumes that antigen from the in vivo immunization does not persist in these cultures.) The potential for Th17 cells to become antigenin-dependent could have important implications for the development of chronic inflammation.
Figure 2. TGFβ, IL-6 and IL-23 synergistically up-regulate IL-17 production during collagen re-stimulation, but IL-4 and IFNγ continue to suppress.
Splenocytes from immunized mice were re-stimulated with or without collagen, IL-23, TGFβ, IL-6 or 10µg/mL neutralizing antibodies to IL-4 and IFNγ (A), or with collagen, TGFβ, IL-6, IL-23 and increasing concentrations of IL-4, IFNγ or IL-12 (B and C). Supernatants were collected after five days and IL-17 was measured by ELISA. Error bars represent SEM of triplicate culture samples. In (A) **p<0.01, ***p<0.001 vs. collagen alone. ##p<0.01, ###p<0.001 vs. unstim by one-way ANOVA. In (B) all points are p<0.01 vs. 0ng/mL IL-4 by two-way ANOVA. In (C) ***p<0.001 vs. 0ng/mL cytokine by two-way ANOVA.
Given that TGFβ, IL-6 and IL-23 can greatly enhance the activation of pre-existing Th17 cells, we wondered how these positive signals might interact with negative signals coming from Th1 and Th2 cytokines. Thus we asked whether IL-4, IFNγ, and IL-12 would continue to suppress IL-17 production in the presence of TGFβ, IL-6 and IL-23. ELISA assays of cytokines produced in spleen cell cultures showed that the suppressive signals from IL-4 overpowered any combination of activating signals, and even very elevated production of IL-17 was still potently down-regulated (Figure 2B). Interestingly, IFNγ continued to suppress IL-17 in the presence of TGFβ, IL-6 and IL-23, while IL-12 did not (Figure 2C). These results point to different mechanisms of suppression downstream of the IFNγ and IL-12 receptors, and may reflect competition for shared signaling pathways by IL-12 and IL-23.
IL-4 and IFNγ selectively inhibit unique subsets of Th17 genes
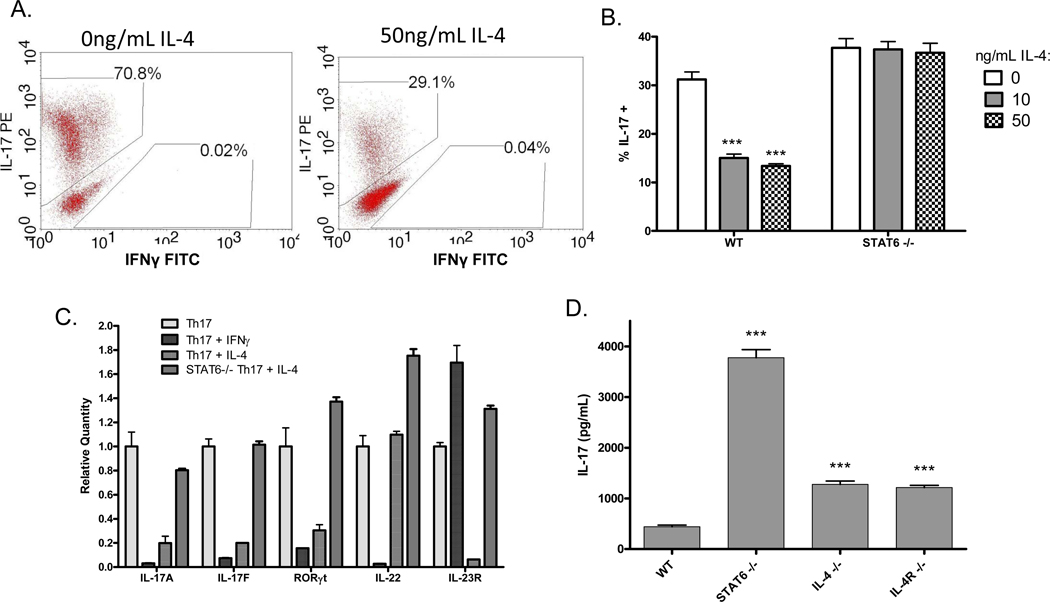
To more carefully interrogate the regulatory mechanisms at work during different stages of Th17 development, we next analyzed in vitro-derived Th17 cells. Purified naive CD4+ T cells were differentiated into Th17 cells for five or six days as described in materials and methods. The cells were then rested for two days, followed by challenge with recombinant Th1 and Th2 cytokines in the presence or absence of anti-CD3 for two days. This protocol of five day priming – 2 day rest – 2 day re-stimulation allows us to specifically focus on the effects of opposing cytokines on activation of pre-existing Th17 cells, rather than the effects on differentiation of naïve T cells into Th17. Th17 activity was measured by intracellular flow cytometry (ICS), ELISA and real-time PCR. As predicted, the results clearly demonstrate that adding IL-4 or IFNγ during re-stimulation inhibits the expression of IL-17 protein, sometimes by as much as 95%, and with a more potent inhibition by IL-4 (Figure 3A). We also found that IL-4 and IFNγ inhibited expression of IL-17A, IL-17F and RORγt mRNA (Figure 3C). Interestingly, however, these cytokines selectively inhibited the expression of a unique subset of Th17 genes: IL-4 suppressed IL-23R but not IL-22, while IFNγ suppressed IL-22 but not IL-23R. The differential regulation of IL-22 and IL-23R implies that the Th17 gene expression program is not completely reversed in the presence of IFNγ and IL-4, leaving open the possibility that these conflicting cytokine milieus may yield either a mixed population of cells or cells of a mixed phenotype.
Figure 3. IL-4 suppresses a different subset of Th17 genes than IFNγ, and the effect of IL-4 is mediated by STAT6.
Naïve CD4+ T cells from wildtype or STAT6-deficient mice were cultured under Th17-skewing conditions for five days, followed by two days rest and two days re-stimulation in the presence or absence of 50ng/mL IL-4 or IFNγ. (A) Cells were treated for six hours with PMA, ionomycin and brefeldin A, and IL-17 expression was measured by ICS. (B) Cells were treated for six hours with PMA, ionomycin and brefeldin A, and IL-17 expression was measured by ICS. Error bars represent SEM of triplicate cultures. *** p<0.001 vs. 0ng/mL IL-4 by one-way ANOVA. (C) RNA was collected and IL-17A, IL-17F, RORγt, IL-22 and IL-23R expression was measured by real-time PCR with primers and probes from Applied Biosystems. Data is normalized first to â-actin, the internal control, and then to the matched sample without IL-4 or IFNγ. Error bars represent SEM of triplicate PCRs. (D) Augmented IL-17 expression in STAT6-deficient mice. Spleen cells from wildtype BALB/c, STAT6 knockout, IL-4 knockout, or IL-4R knockout mice were stimulated in vitro with anti-CD3. Five days later supernatant was collected and IL-17 expression was measured by ELISA. Bars represent mean SEM of triplicate samples. ***p<0.001 vs. wildtype by one-way ANOVA.
Suppression by IL-4 depends on STAT6 but not GATA3
Because IL-4R signaling is mediated primarily by STAT6, we hypothesized that STAT6 was required for suppression of IL-17 by IL-4, and to address this question we repeated our previous in vitro experiments using STAT6-deficient Th17 cells. In the re-stimulation of in vitro-derived Th17 cells, IL-4 had no effect on IL-17 expression in the absence of STAT6 (Figure 3B). Similarly, by real-time RT-PCR, IL-4 failed to robustly suppress IL-17A, IL-17F, RORγt and IL-23R expression in STAT6-deficient Th17 cells (Figure 3C). Spleen cells from STAT6-deficient mice stimulated with anti-CD3 in the absence of exogenous IL-4 also had increased IL-17 expression by ELISA as compared to wildtype T cells, indicating that endogenous cytokines also inhibit IL-17 expression via STAT6 (Figure 3D). STAT5 can also be activated by IL-4 and has been reported to mediate suppression of IL-17 downstream of IL-2 [25, 26]. Using STAT5-deficient spleen cells stimulated with anti-CD3, however, we found that STAT5 was dispensable for suppression of IL-17 by IL-4 (Supplementary Figure S3). Similarly, IL-4R signaling has been shown to activate IRS-2, but when we used mice bearing a point mutation in the IL-4R at the IRS-2 binding site, thus preventing recruitment and activation of IRS-2 by IL-4 without impacting STAT6 activation, recombinant IL-4 continued to inhibit IL-17 expression, demonstrating that IRS-2 activation is not required for suppression of IL-17(Supplementary Figure S1).
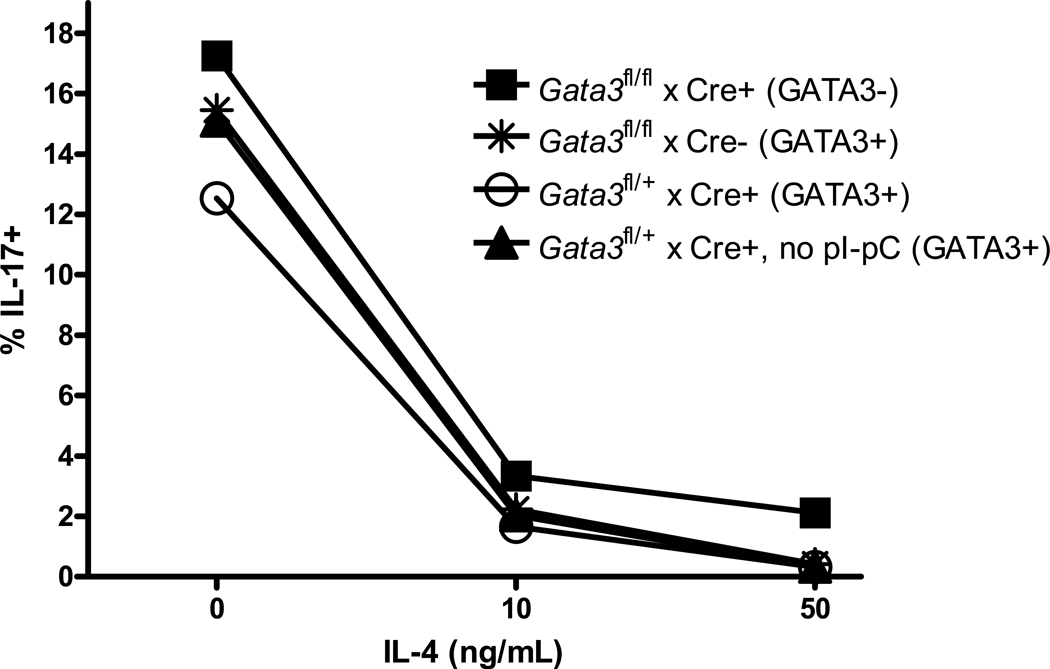
Previous data from other groups suggests that suppression of Th1 development by IL-4 is mediated by GATA-3, the transcription factor induced by IL-4 that acts as the master regulator of Th2 differentiation [27–30]. In addition, we found that GATA-3 mRNA is up-regulated in Th17 cells as early as two hours after the addition of IL-4, prior to down-regulation of IL-17 mRNA (data not shown). Thus we hypothesized that GATA-3 may play a role in suppression of IL-17 by IL-4. Mice bearing a floxed Gata3 gene with inducible Crerecombinase under control of the IFN-inducible Mx1 promoter (Gata3fl/fl:TgMx1cre mice) were treated with pI-pC to induce excision of the Gata3 gene, which resulted in a 70% decrease in total splenic levels of GATA3 mRNA (Supplementary Figure S2). While the in vivo conditional deletion was less than ideal, published reports using these mice demonstrate a near complete suppression of early T lineage progenitor development, suggesting that the incomplete deletion is sufficient to significantly alter T cell function[31]. Spleen cells from these mice and control mice that are unable to delete GATA3 were cultured with anti-CD3 in the presence or absence of Th17-skewing cytokines, and the effect of IL-4 on IL-17 expression was measured by ICS and real-time PCR. Contrary to our prediction, however, the results in Figure 4 show that deletion of Gata3 had no effect on suppression of IL-17 by IL-4.
Figure 4. GATA3 is not required for suppression of IL-17 by IL-4.
Spleen cells from mice with inducible Gata3 deletion or controls without deletion were stimulated with anti-CD3 and increasing concentrations of IL-4. After five days cells were treated with PMA, ionomycin and brefeldin A for six hours and IL-17 expression was measured by ICS.
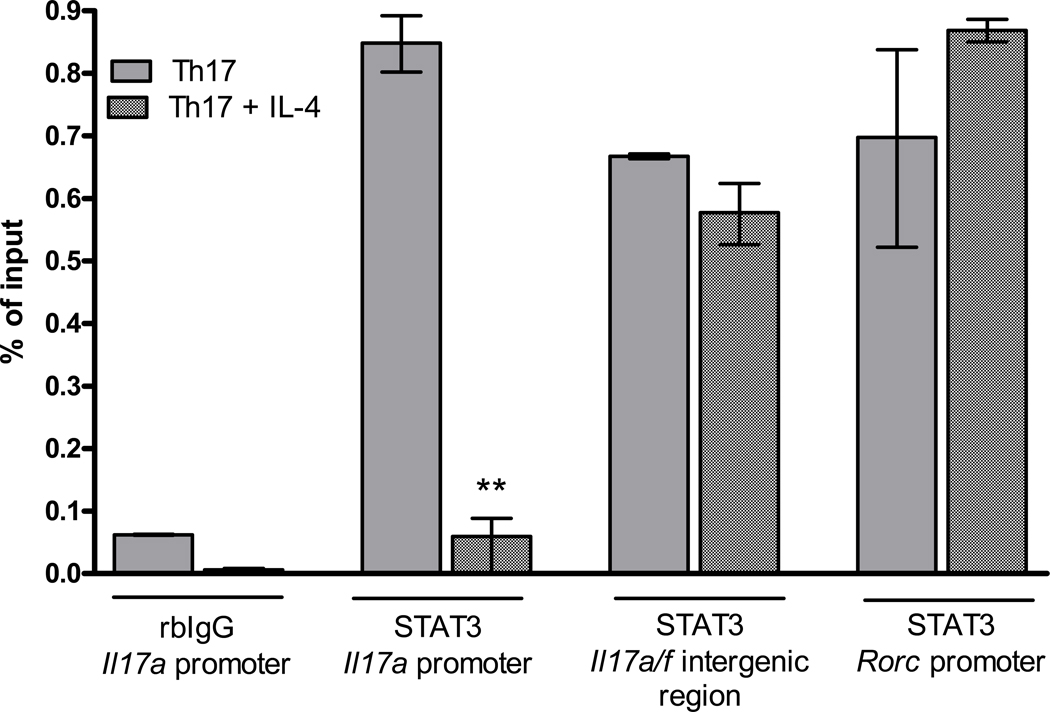
Suppression by IL-4 results in loss of STAT3 binding at the Il17a promoter
Th17 development in mice depends on the transcription factor signal transducer and activator of transcription 3 (STAT3), which is activated by IL-6 and IL-23. STAT3 has multiple roles in Th17 development: in activated Th17 cells stimulated with IL-23, it binds directly to the Il17a promoter and induces IL-17 expression, and in naïve T cells stimulated with TGFβ and IL-6, it is required for induction of RORγt expression [10, 32–34]. Thus we hypothesized that IL-4 may mediate suppression of Th17 activity by inhibiting STAT3 binding at Th17 gene loci. To test this hypothesis we re-stimulated Th17 cells with anti-CD3 and Th17-skewing cytokines in the presence or absence of IL-4 for 6 or 24 hours and measured STAT3 binding at the Il17a promoter, Il17a/f intergenic region and Rorcpromoter by chromatin immunoprecipitation (ChIP). The results in Figure 5 demonstrate very strong STAT3 binding at all three regions.
Figure 5. IL-4 inhibits STAT3 binding at the Il17a promoter but not at other sites.
Th17 cells were generated in vitro as described. After one week the cells were re-stimulated for 6 hours with anti-CD3 and Th17-polarizing cytokines in the presence or absence of 50ng/mL IL-4. ChIPs were carried out using the EZ-ChIP kit according to manufacturer’s instructions (Millipore), with antibody specific for STAT3 or isotype control. Eluted DNA was quantitated by SYBR green real time PCR with primers specific for the Il17a promoter, Il17a/f intergenic region or Rorc promoter. Data is normalized to the corrected Ct values of the 1% input sample. Error bars represent SEM of triplicate PCR reactions. *** p<0.01 versus Th17 conditions by Student’s t test.
Interestingly, IL-4 inhibited STAT3 binding at the Il17a promoter but had no effect at the Il17a/f intergenic region or the Rorc promoter.
Suppression by IL-4 is stable but does not induce Th2 conversion
Several groups have recently demonstrated a high degree of plasticity in Th17 cells[17, 35], thus we decided to ask whether prolonged culture with IL-4, the chief Th2-skewing cytokine, would induce Th17-to-Th2 conversion. Th17 cells were generated in vitro, with five days of differentiation followed by two days of rest. Following the rest period, the Th17 cells were re-stimulated in Th0, Th2 or Th17 conditions for two, four or six days. Th2 conversion was assessed by real-time PCR for IL-4 and GATA-3, as well as IL-17 and RORγt. The results in Figure 6A show that low levels of mRNA for IL-4 and GATA-3 were expressed in Th2-stimulated Th17 cultures, but similar levels of IL-4 and GATA-3 message were also expressed in Th17 cells stimulated with anti-CD3 alone. While IL-4 mRNA expression persisted longer in cultures re-stimulated with Th2 conditions versus anti-CD3, these results may indicate a small number of contaminating Th0 or Th2 cells in the culture rather than induction of Th17-Th2 conversion. In addition, the levels of IL-4 mRNA expressed by Th17 cells re-stimulated in Th2 conditions were significantly lower than the levels of IL-4 mRNA expressed by naïve T cells differentiated under Th2 conditions in vitro(Supplementary Figure S3). We also examined IL-4 expression in Th17 cells by ICS and found that less than two percent of the cells expressed IL-4 after two days re-stimulation in Th0 or Th2 conditions. Furthermore, the IL-4 expression on day two was extinguished by day four or six of Th2 culture, and no cells co-expressed IL-4 and IL-17, implying that there was no Th17-Th2 conversion (Figure 6B and data not shown). Thus even though low levels of IL-4 and GATA-3 mRNA persist, IL-4 protein expression is quickly extinguished, suggesting a role for post-transcriptional regulation.
Figure 6. Th17 cells re-stimulated in Th2 conditions do not convert to Th2 cells.
Th17 cells were re-stimulated for two, four or six days in Th0, Th2 or Th17 conditions and expression of IL-4 and GATA-3 were measured by real time PCR (A). Results were normalized to â-actin expression. Error bars represent the SEM of triplicate PCRs. Alternatively, in (B) IL-17A and IL-4 expression were measured by ICS.
Although we demonstrated that Th17 cells stimulated in the presence of IL-4 do not convert to Th2 cells, it was not clear whether IL-17 expression was only temporarily down-regulated, requiring constant IL-4R signaling to maintain suppression, or persistently extinguished even after removal of IL-4. Thus we again generated Th17 cells in vitro(primary culture), which were re-stimulated in secondary culture, with Th2 conditions (anti-CD3, 10ng/mL IL-4, anti-IFNγ), Th0 conditions (anti-CD3, anti-IL-4, anti-IFNγ), or “Th23 conditions” (anti-CD3, 20ng/mL IL-23, anti-IL-4, anti-IFNγ) to maintain the existing Th17 cell population without inducing new differentiation. After two days of secondary culture the cells were washed and rested in tertiary culture for one, two or three days to allow the Th17 cells to regain IL-17 expression. After each phase of culture cells were re-stimulated with PMA, ionomycin and brefeldin A for ICS. The results in Figure 7 show that secondary stimulation in Th0 or Th23 conditions imprinted Th17 cells for increasing levels of IL-17 expression after stimulation was removed, while secondary stimulation in Th2 conditions resulted in acontinual decline in IL-17 expression even after IL-4 was removed. These results demonstrate that IL-4-mediated suppression of IL-17 is stable and does not require continual exposure to IL-4, suggesting that IL-4 may induce heritable changes in chromatin structure at the Il17a locus. Cell numbers and viability were consistent within groups and over time during tertiary culture, implying that there was no selective proliferation or cell death. Thus, not only is IL-17 expression easily suppressed by low doses of IL-4 despite the presence of activating cytokines, but the suppression by IL-4 is also persistent and not passively reversed.
Figure 7. Suppression of IL-17 expression persists after removal of IL-4.
Th17 cells were generated in vitro and treated with IL-4 to suppress IL-17 expression as described, then washed and put back into culture for one, two or three days to allow cells to regain IL-17 expression. Primary culture = five days Th17 differentiation and two days rest, as described. Secondary culture = two days re-stimulation in Th0 conditions (anti-CD3, anti-IL-4, anti-IFNγ), Th23 conditions (anti-CD3, anti-IL-4, anti-IFNγ, 20ng/mL IL-23) or Th2 conditions (anti-CD3, 10ng/mL IL-4, anti-IFNγ). Tertiary culture = one, two or three days resting culture. After each phase of culture cells were re-stimulated with PMA, ionomycin and brefeldin A and IL-17 expression was assessed by ICS.
Repeated stimulation renders Th17 cells resistant to suppression by IL-4
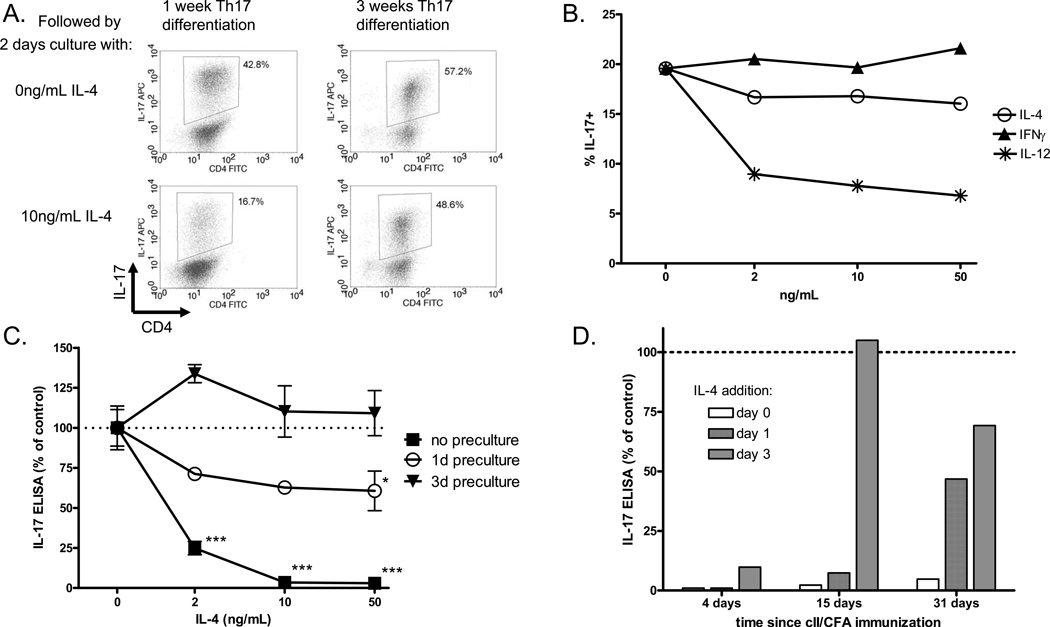
The results shown thus far suggest that Th17 cells are very sensitive to suppression by IL-4, which raises the question of how Th17 cells manage to persist in vivo during an inflammatory disease where they may encounter many suppressive cytokines. One explanation may be the process of maturation or stabilization. Previous reports from other groups have demonstrated that developing Th1 cells progress through several stages of maturation, gradually stabilizing their phenotype in response to cytokine stimulation. Early in the culture, IL-4 suppresses Th1 differentiation and IFNγ expression, but after prolonged stimulation, Th1 cells lose the ability to respond to IL-4 and become resistant to suppression [29, 36, 37]. Previous data from our lab on the effect of IL-4-transduced dendritic cells on IL-17 production during CIA suggested that Th17 cells are less susceptible to regulation by IL-4 after the onset of arthritis [38]. Thus we decided to address the maturation of Th17 cells in vitro and in vivo, as measured by insensitivity to suppression by IL-4. Th17 cells were generated in vitro with five days of differentiation and two days of rest, as in previous experiments. To induce maturation we repeated this process of five-day stimulation and two-day rest two additional times, for a total of three weeks of culture, and then assayed the effect of IL-4 on IL-17 expression during a two-day re-stimulation with anti-CD3 followed by ICS. Figure 8A shows representative dot plots for IL-17 expression in Th17 cells treated with IL-4 after one week or three weeks of culture. The results show that after three rounds of stimulation, Th17 cells are largely resistant to suppression by IL-4. Interestingly, the results in Figure 8B demonstrate that Th17 cells cultured for three weeks become resistant to suppression by IL-4 and IFNγ but remain sensitive to suppression by IL-12.
Figure 8. Th17 cells become resistant to suppression by IL-4 both in vitro and ex vivo.
(A.) Th17 cells were generated by five days stimulation and two days rest as described, followed by two days re-stimulation in the presence or absence of IL-4 and ICS for IL-17. Alternatively, cells were cultured for two more rounds of five days Th17 stimulation and two days rest, for a total of three weeks of culture. After three weeks, the cells were re-stimulated for two days in the presence or absence of IL-4, followed ICS for IL-17. (B) Th17 cells cultured for three weeks to induce maturation were re-stimulated for two days with increasing concentrations of IL-4, IFNã or IL-12, followed by ICS for IL-17. (C) DBA mice were immunized i.d. with cII/CFA and spleens were collected two weeks later. Total spleen cells were re-stimulated with heat-denatured collagen for zero, one or three days, and then collected, washed and re-plated with collagen and increasing concentrations of IL-4 for two days. Supernatants were collected and IL-17 was measured by ELISA. Bars represent mean +/− SEM of triplicate culture samples, normalized to 0ng/mL IL-4. *p<0.05, *** p<0.001 vs. 0ng/mL IL-4 by one-way ANOVA. (D) DBA mice were immunized with cII/CFA and spleens were collected 4, 15 or 31 days laters. Spleen cells were re-stimulated with collagen and 50ng/mL IL-4 was added on day 0, 1 or 3. Supernatants were collect on day five and IL-17 was measured by ELISA. IL-17 expression is normalized to the sample with no IL-4.
We have already shown that IL-4 suppresses IL-17 expression by collagen-immunized splenocytes, thus demonstrating that antigen-specific Th17 cells in the spleen two weeks after immunization have not fully matured. However, we decided to look for ex vivo maturation of splenocytes from immunized mice by stimulating with antigen for one or three days to induce maturation, and then washing and challenging with antigen in the presence or absence of IL-4 for two days, followed by IL-17 ELISA of the supernatants. As the results in Figure 8C show, we found that three days of ex vivo re-stimulation with antigen was sufficient to induce Th17 maturation and resistance to suppression by IL-4 in whole spleen cultures from two-week immunized mice. Similar results were observed for KLH-immunized BALB/c (data not shown).
The ability of Th17 cells to become resistant to suppression could have important implications for the development of autoimmune disease, and IL-4-transduced dendritic cells have been shown to suppress collagen-specific IL-17 production from mice before the onset of arthritis but not after [38]. Thus we asked whether Th17 maturation correlated with disease progression or severity in CIA. To address this question we collected spleens from mice at different time points after immunization with cII/CFA and assessed the Th17 sensitivity to suppression by IL-4 on day zero, one or three of culture. We found that six weeks after immunization, splenic Th17 cells were moderately sensitive to suppression by IL-4 directly ex vivo. However, Th17 responses of cells from mice that had undergone prolonged immunizations matured more quickly ex vivo than Th17 responses of cells from very short immunizations (Figure 8D and Table 1).
Table 1. In vivo experience correlates with ex vivo development of resistance to IL-4.
DBA mice were immunized with cII/CFA and spleens were collected after one, two, four or six weeks. Spleen cells were re-stimulated with collagen and IL-4 was added on day zero, one or three of culture. IL-17 was measured by ELISA on day five. A designation of resistance required that IL-17 production in the presence of IL-4 was at least 70% of IL-17 production in the absence of IL-4. Spleens from one week immunized mice continued to respond to IL-4 when it was added after three days of culture, while spleens from six week immunized mice failed to respond when IL-4 was added on day zero or one.
| Weeks since immunization |
Days culture until IL-4 resistance |
|---|---|
| 1 | >3 |
| 2 | 2 |
| 4 | 1 |
| 6 | 0–1 |
Mature Th17 cells express IL-4R but lose the ability to phosphorylate STAT6
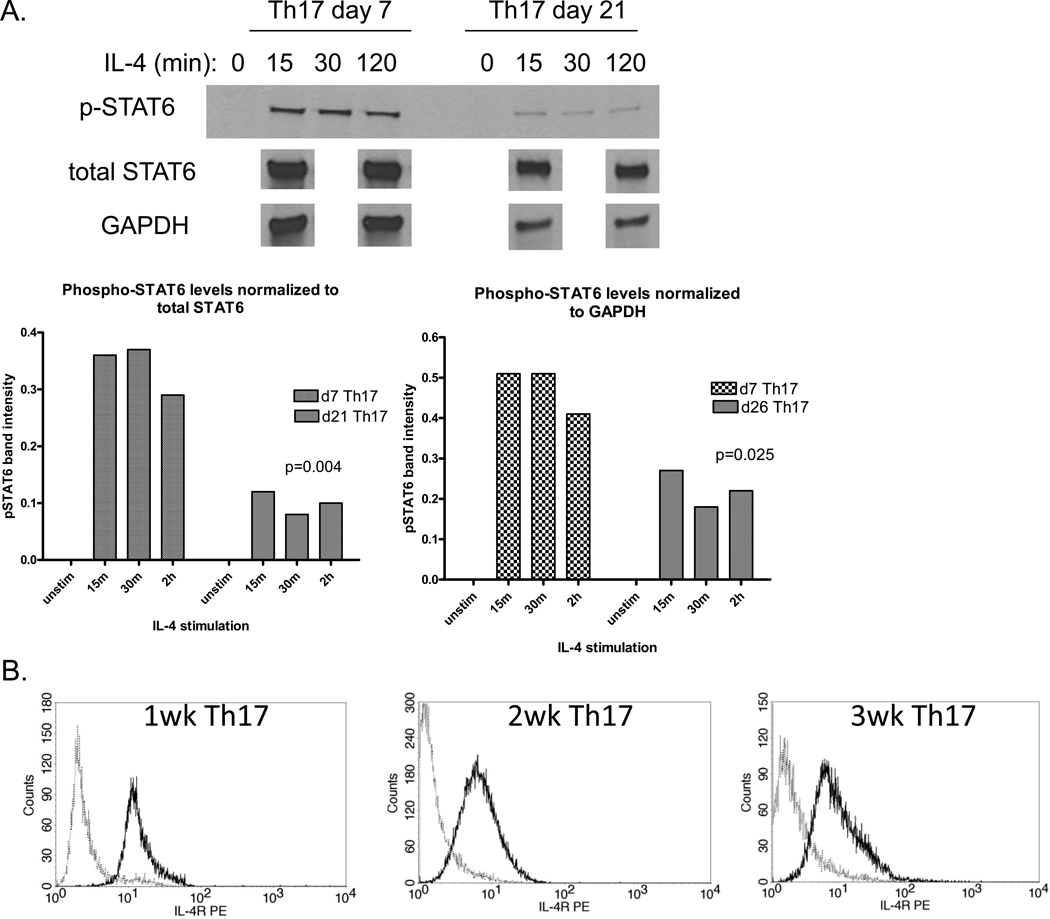
Our observation of desensitization of Th17 cells to challenge with IL-4 begged the question of whether signaling through the IL-4R remained intact following maturation. To measure IL-4R signaling, Th17 cells were rested in low serum media with cytokine-neutralizing antibodies overnight to minimize the background level of activation, stimulated with IL-4 for 15, 30 or 120 min, and lysed in the presence of protein phosphatase inhibitors. STAT6 activation was measured by Western blot with phospho-STAT6 specific antibodies. The results in Figure 9A show that IL-4 induced significantly less STAT6 activation in mature Th17 cells cultured for three weeks versus immature Th17 cells cultured for one week, regardless of whether phospho-STAT6 levels are normalized to total STAT6 or to the loading control GAPDH. In addition, when total STAT6 expression was normalized to GAPDH, we found that STAT6 was actually up-regulated in mature Th17 cells (data not shown). Thus the loss of STAT6 activation was not simply due to down-regulation of STAT6 expression.
Figure 9. Impaired IL-4R signaling in mature Th17 cells.
(A) Th17 cells were generated in vitro with one or three weeks of stimulation and rested overnight in low-serum media with cytokine-neutralizing antibodies to reduce the background level of STAT activation. Cells were then washed and stimulated with 50ng/mL IL-4 for 15, 30 or 120 min and lysed with PhosphoSafeLysis Buffer (Novagen). Lysates were reduced and run on 10% SDS-PAGE gels and stained with antibodies for phospho-STAT6, total STAT6 or GAPDH. Band intensities in part (A) were quantitated with Kodak software and phospho-STAT6 intensity was normalized either to total STAT6 intensity or GAPDH intensity; p<0.05 for the average band intensity between mature and immature Th17 cells via two-way t test. In (B), IL-4R expression in immature and mature Th17 cells was measured by flow cytometry. Bold lines represent IL-4R, dotted lines represent isotype control.
One plausible explanation for the loss of STAT6 activation could be down-regulation of the IL-4R. We therefore decided to measure IL-4Rα expression at different stages of Th17 maturation by flow cytometry. The results in Figure 9B show that IL-4R expression was not significantly down-regulated in three-week Th17 cultures versus one- or two-week cultures.
Discussion
The data presented herein demonstrate a remarkable degree of complexity in the cytokine-mediated regulation of Th17 cells. Initial reports suggested that T helper cell cross-regulation is rather black and white: TGFβ, IL-6 and IL-23 promote Th17 cells while IL-4, IFNγ and IL-12 inhibit Th17 cells. Upon closer inspection, however, we see many shades of grey. For instance, Th17 cells developing in the presence of IL-4 may continue to express IL-22, while Th17 cells developing in the presence of IFNγ may continue to express IL-23R, suggesting that there may be an array of T helper cell subset phenotypes that include intermediate states that are not fully polarized to one of the well-defined Th subsets. It is also particularly interesting that IL-4 and IFNγ continue to suppress IL-17 production in the presence of TGFβ, IL-6 and IL-23 while IL-12 does not. Another important difference appears in the process of Th17 maturation, which results in resistance to suppression by IL-4 and IFNγ but not to IL-12.
Th1 cytokines can suppress IL-17 expression but also induce a Th1-like phenotype in Th17 cells. IL-4, on the other hand, induces potent and stable suppression without inducing any Th2 conversion, even in the face of strong Th17 stimuli. This is consistent with previous work from our lab demonstrating that a single injection of IL-4-transduced dendritic cells induces long-lasting protection from CIA, which is likely mediated by suppression of collagen-specific Th17 responses [38, 39]. These results suggest a fundamental difference in the mechanisms underlying regulation of Th17 cells by Th1 and Th2 cytokines. How Th17 cells integrate a complex array of positive and negative signals is an interesting area for future research and may depend on the ability of key transcription factors such as T-bet and GATA-3 to bind to Th17 gene loci and interact with RORγt.
The molecular mechanisms mediating Th17 suppression and/or lineage conversion downstream of Th1 and Th2 cytokines remains largely unknown. We have shown that IL-4-mediated suppression is dependent on STAT6 and independent of STAT5, IRS-2 and GATA-3. One potential mechanism of suppression downstream of the IL-4R could be direct binding of STAT6 to Th17 gene loci and inhibition of transcription. However, we performed multiple STAT6 ChIPs, scanning wide regions of the Il17a and Rorc promoters and found no evidence for direct binding (Supplementary Figure S4). Given that IL-17 expression is dependent on STAT3, we hypothesized that IL-4R signaling may suppress IL-17 expression by inhibiting STAT3 DNA-binding activity, which we confirmed by ChIP for STAT3 at the Il17a promoter. Interestingly, STAT3 binding was specifically down-regulated at the Il17a promoter, while there was no loss of STAT3 binding at the Rorc promoter or Il17a/f intergenic region, suggesting that STAT3 may be displaced from the Il17a promoter by a STAT6-induced transcriptional repressor that specifically binds this region. We have screened several candidates and as yet have been unable to identify the direct target of STAT6 that ultimately mediates IL-17 gene silencing.
Although much attention has been given to the cytokines that regulate T helper differentiation from naïve T cells, there is a paucity of data on how cells are regulated beyond this early window. For instance, current dogma states that TGFβ and IL-6 act on naïve T cells to induce Th17 differentiation, while IL-23 acts on existing Th17 cells, but our observations suggest that TGBβ and IL-6 may be equally as important as IL-23 for augmenting cytokine production by effector or memory Th17 cells. In addition, recent observations demonstrate a remarkable degree of fluidity within T helper cell lineages in vivo, with cells converting from one lineage to another or stably expressing multiple cytokines characteristic of different lineages. Thus, much more work is needed to address the role of cytokine-mediated regulation throughout the full lifespan of each of the T helper cell subsets, as well as the ways in which our notions of divergent T helper lineages break down and the lines between distinct subsets become blurred.
In our experiments, Th17 cells cultured under Th2 conditions did not take on a Th2 phenotype and were unable to re-express IL-17 after the suppressive signals were removed. However, we also found that three rounds of in vitro stimulation rendered Th17 cells resistant to suppression by IL-4 as a result of desensitization of the IL-4R, suggesting that there may be a specific window of opportunity for Th17 suppression. Importantly, we observed a similar maturation process in Th17 cells generated in vivo, and maturation state correlated with disease progression. The in vitro maturation kinetics closely mirrored what was demonstrated for Th1 cells, suggesting that this process is not Th17-specific, but rather may be a universal property of chronically activated T helper cells (to our knowledge, there is no evidence to date regarding Th2 maturation). Although we found that Th17 cells generated both in vitro and in vivo gradually become more stable and resistant to suppression, data from other groups suggest that in vitro-derived Th17 cells quickly lose their IL-17 expression and can be converted to other lineages, even after three rounds of stimulation. However, in vivo-derived Th17 cells maintained their phenotype and were resistant to suppression [17–19]. The conflicting observations of lineage stability following in vitro differentiation may be due to differences in culture conditions or APC populations. For example, others have used peptide plus irradiated spleens cells while we used anti-CD3 and BM-DCs. Several groups have already shown that in vitro-derived Th17 cells differ greatly from in vivo-derived Th17 cells, and one of these reports suggests that in vivo-derived memory Th17 cells are more resistant to suppression and lineage conversion than in vitro-derived Th17 cells [19, 40, 41]. In this regard, our in vitro culture conditions may more closely approximate the natural setting with respect to Th17 cell maturation, compared to more short term Th17 cultures.
Similar to published data on mature Th1 cells [36, 37, 42], we were able to demonstrate a loss of STAT6 activation in response to IL-4 in mature Th17 cells, despite normal levels of all of the IL-4R signaling components. One potential mechanism for loss of STAT6 activation in mature Th17 cells is up-regulation of a member of the suppressor of cytokine signaling (SOCS) family of proteins. Most SOCS family proteins preferentially interact with one or more specific cytokine receptors, thereby inhibiting activation of unique STAT molecules. SOCS3, for example, has been shown to suppress Th17 differentiation by binding to the IL-6R and inhibiting STAT3 activation, while SOCS5 has been shown to bind to the IL-4R and inhibit STAT6 activation in Th1 cells [42]. There can be some redundancy, however, because previous work has also demonstrated that inhibition of IL-4R signaling in Th1 cells may be mediated by SOCS1 [43, 44]. In fact, there is disagreement on the underlying mechanisms of IL-4R desensitization in mature Th1 cells: Seki et al. demonstrated selective up-regulation of SOCS5 in and SOCS5-dependent inhibition of IL-4R signaling [42], while Huang et al. found no increase in expression of SOCS1, SOCS3 or SOCS5 and suggested that recruitment of STAT6 to the IL-4R was impaired through an unknown mechanism [37]. In our in vitro-matured Th17 cells there was up-regulation of both SOCS1 and SOCS5; however, Th17 cells from SOCS5-deficient mice (a kind gift from Dr. Sandra Nicholson at the Walter and Eliza Hall Institute) showed no loss of IL-4R desensitization upon maturation (data not shown). Future experiments are needed to address the role of SOCS1 in Th17 maturation. This line of inquiry is made difficult by the lethal phenotype of SOCS1−/− mice, which has been attributed to over-expression of IFNγ[45].
The simple observation that inflamed joints from arthritic mice co-express large quantities of IL-17, IFNγ and IL-4 [46] suggests that Th17 cells at the site of inflammation are resistant to suppression, and that more work is needed to determine the role of Th17 maturation in disease. Similarly, much work is needed to assess the sensitivity and resistance to suppression by IL-4 in human Th17 cells from patients with various immune-mediated diseases. The concept of distinct stages of human Th17 maturation raises many exciting new questions and ideas about the etiology of Th17-mediated disease. Better understanding of the molecular mechanisms mediating stabilization of committed cytokine production may lead to new approaches for targeted therapies.
Supplementary Material
Acknowledgements
We would like to thank the following people for kind donation of mutant mouse tissues and reagents: Arian Laurence, John O’Shea, Chia-Jui Ku, Tomonori Hosoya, Douglas Engel, and Sandra Nicholson. We would also like to thank the staff at the University of Michigan hybridoma core facility and the BRCF flow cytometry core facility for technical assistance. We are grateful to Donna Cash for assistance with preparation of the manuscript.
Grant support: Supported by a grant from the Arthritis Foundation and by NIH grants AR38477 and P30 AR048310. LAC was supported by a NIH immunopathology training grant, a NIH regenerative sciences training grant, and a University of Michigan Rackham Postdoctoral Fellowship.
Bibliography
- 1.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 3.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGF[beta] in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature. 2007;448(7152):484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448(7152):480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 6.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O'Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10(3):314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs TH-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8(9):967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 8.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor ROR[gamma]t directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 9.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR[alpha] and ROR[gamma] Immunity. 2008;28(1):29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282(13):9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 11.Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. The development of inflammatory TH-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8(9):958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 12.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 13.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang Y-H, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newcomb DC, Zhou W, Moore ML, Goleniewska K, Hershey GKK, Kolls JK, Peebles RS., Jr A functional IL-13 receptor is expressed on polarized murine CD4+ Th17 cells and IL-13 signaling attenuates Th17 cytokine production. J Immunol. 2009;182(9):5317–5321. doi: 10.4049/jimmunol.0803868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-behi M, Ciric B, Yu S, Zhang G-X, Fitzgerald DC, Rostami A. Differential effect of IL-27 on developing versus committed Th17 cells. J Immunol. 2009;183(8):4957–4967. doi: 10.4049/jimmunol.0900735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Sem Immunol. 2007;19(6):409–417. doi: 10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30(1):92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nurieva R, Yang XO, Chung Y, Dong C. Cutting Edge: in vitro generated Th17 cells maintain their cytokine expression program in normal but not lymphopenic hosts. J Immunol. 2009;182(5):2565–2568. doi: 10.4049/jimmunol.0803931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lexberg M, Taubner A, Förster A, Albrecht I, Richter A, Kamradt T, Radbruch A, Chang H-D. Th memory for interleukin-17 expression is stable in vivo. Eur J Immunol. 2008;38(10):2654–2664. doi: 10.1002/eji.200838541. [DOI] [PubMed] [Google Scholar]
- 20.McKenzie BS, Kastelein RA, Cua DJ. Understanding the IL-23-IL-17 immune pathway. Trends Immunol. 2006;27(1):17–23. doi: 10.1016/j.it.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204(8):1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boniface K, Blumenschein WM, Brovont-Porth K, McGeachy MJ, Basham B, Desai B, Pierce R, McClanahan TK, Sadekova S, de Waal Malefyt R. Human Th17 cells comprise heterogeneous subsets including IFN-gamma producing cells with distinct properties from the Th1 lineage. J Immunol. 2010;185(1):679–687. doi: 10.4049/jimmunol.1000366. [DOI] [PubMed] [Google Scholar]
- 23.Raymond M, Van VQ, Wakahara K, Rubio M, Sarfati M. Lung dendritic cells induce TH17 cells that produce TH2 cytokines, express GATA-3, and promote airway inflammation. J Allergy Clin Immunol. doi: 10.1016/j.jaci.2011.04.029. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 24.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-[beta] induces development of the TH17 lineage. Nature. 2006;441(7090):231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 25.Lischke A, Moriggl R, Brändlein S, Berchtold S, Kammer W, Sebald W, Groner B, Liu X, Hennighausen L, Friedrich K. The interleukin-4 receptor activates STAT5 by a mechanism that relies upon common gamma-chain. J Biol Chem. 1998;273(47):31222–31229. doi: 10.1074/jbc.273.47.31222. [DOI] [PubMed] [Google Scholar]
- 26.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank Rebecca B, Meylan F, Siegel R, Hennighausen L, Shevach EM, O'Shea John J. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26(3):371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Fields PE, Kim ST, Flavell RA. Cutting Edge: changes in histone acetylation at the IL-4 and IFN-{gamma} coci accompany Th1/Th2 cifferentiation. J Immunol. 2002;169(2):647–650. doi: 10.4049/jimmunol.169.2.647. [DOI] [PubMed] [Google Scholar]
- 28.Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated iInteraction of T-bet with GATA-3. Science. 2005;307(5708):430–433. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- 29.Ouyang W, Ranganath SH, Weindel K, Bhattacharya D, Murphy TL, Sha WC, Murphy KM. Inhibition of Th1 development mediated by GATA-3 through an IL-4-independent mechanism. Immunity. 1998;9(5):745–755. doi: 10.1016/s1074-7613(00)80671-8. [DOI] [PubMed] [Google Scholar]
- 30.Usui T, Preiss J, Kanno Y, Yao Z, Bream J, O'Shea J, Strober W. T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. J Exp Med. 2006;203(3):755–766. doi: 10.1084/jem.20052165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosoya T, Kuroha T, Moriguchi T, Cummings D, Maillard I, Lim K, Engel J. GATA-3 is required for early T lineage progenitor development. J Exp Med. 2009;206(13):2987–3000. doi: 10.1084/jem.20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu B-M, Tato C, Yoshimura A, Hennighausen L, O'Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci. 2006;103(21):8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathur AN, Chang H-C, Zisoulis DG, Stritesky GL, Yu Q, O'Malley JT, Kapur R, Levy DE, Kansas GS, Kaplan MH. STAT3 and STAT4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178(8):4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 34.Nishihara M, Ogura H, Ueda N, Tsuruoka M, Kitabayashi C, Tsuji C, Aono H, Ishihara K, Huseby E, Betz UAK, Murakami M, Hirano T. IL-6-gp130-STAT3 in T cells directs the development of IL-17+ Th with a minimum effect on that of Treg in the steady state. Int Immunol. 2007;19(6):695–702. doi: 10.1093/intimm/dxm045. [DOI] [PubMed] [Google Scholar]
- 35.Mukasa R, Balasubramani A, Lee YK, Whitley SK, Weaver BT, Shibata Y, Crawford GE, Hatton RD, Weaver CT. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity. 2010;32(5):616–627. doi: 10.1016/j.immuni.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang H, Paul W. Impaired interleukin 4 signaling in T helper type 1 cells. J Exp Med. 1998;187(8):1305–1313. doi: 10.1084/jem.187.8.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Z, Xin J, Coleman J, Huang H. IFN-{gamma} suppresses STAT6 phosphorylation by inhibiting its recruitment to the IL-4 receptor. J Immunol. 2005;174(3):1332–1337. doi: 10.4049/jimmunol.174.3.1332. [DOI] [PubMed] [Google Scholar]
- 38.Sarkar S, Tesmer LA, Hindnavis V, Endres DA, Fox DA. Interleukin-17 as a molecular target in immune-mediated arthritis: Immunoregulatory properties of genetically modified murine dendritic cells that secrete interleukin-4. Arthritis Rheum. 2007;56(1):89–100. doi: 10.1002/art.22311. [DOI] [PubMed] [Google Scholar]
- 39.Morita Y, Yang J, Gupta R, Shimizu K, Shelden EA, Endres J, Mule JJ, McDonagh KT, Fox DA. Dendritic cells genetically engineered to express IL-4 inhibit murine collagen-induced arthritis. J Clin Invest. 2001;107(10):1275–1284. doi: 10.1172/JCI11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, Sakaguchi N, Sakaguchi S. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J Exp Med. 2007;204(12):2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou L, Chong MMW, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30(5):646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Seki Y, Hayashi K, Matsumoto A, Seki N, Tsukada J, Ransom J, Naka T, Kishimoto T, Yoshimura A, Kubo M. Expression of the suppressor of cytokine signaling-5 (SOCS5) negatively regulates IL-4-dependent STAT6 activation and Th2 differentiation. Proc Natl Acad Sci U S A. 2002;99(20):13003–13008. doi: 10.1073/pnas.202477099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu C-R, Mahdi RM, Ebong S, Vistica BP, Chen J, Guo Y, Gery I, Egwuagu CE. Cell proliferation and STAT6 pathways are negatively regulated in T cells by STAT1 and suppressors of cytokine signaling. J Immunol. 2004;173(2):737–746. doi: 10.4049/jimmunol.173.2.737. [DOI] [PubMed] [Google Scholar]
- 44.Cornish A, Chong M, Davey G, Darwiche R, Nicola N, Hilton D, Kay T, Starr T, Alexander W. Suppressor of cytokine signaling-1 regulates signaling in response to interleukin-2 and other gamma c-dependent cytokines in peripheral T cells. J Biol Chem. 2003;278(25):22755–22761. doi: 10.1074/jbc.M303021200. [DOI] [PubMed] [Google Scholar]
- 45.Alexander WS, Starr R, Fenner JE, Scott CL, Handman E, Sprigg NS, Corbin JE, Cornish AL, Darwiche R, Owczarek CM, Kay TWH, Nicola NA, Hertzog PJ, Metcalf D, Hilton DJ. SOCS1 Is a critical inhibitor of interferon [gamma] signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell. 1999;98(5):597–608. doi: 10.1016/s0092-8674(00)80047-1. [DOI] [PubMed] [Google Scholar]
- 46.Sarkar S, Cooney L, White P, Dunlop D, Endres J, Jorns J, Wasco M, Fox D. Regulation of pathogenic IL-17 responses in collagen-induced arthritis: roles of endogenous interferon-gamma and IL-4. Arthritis Res Ther. 2009;11(5):R158. doi: 10.1186/ar2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.