Abstract
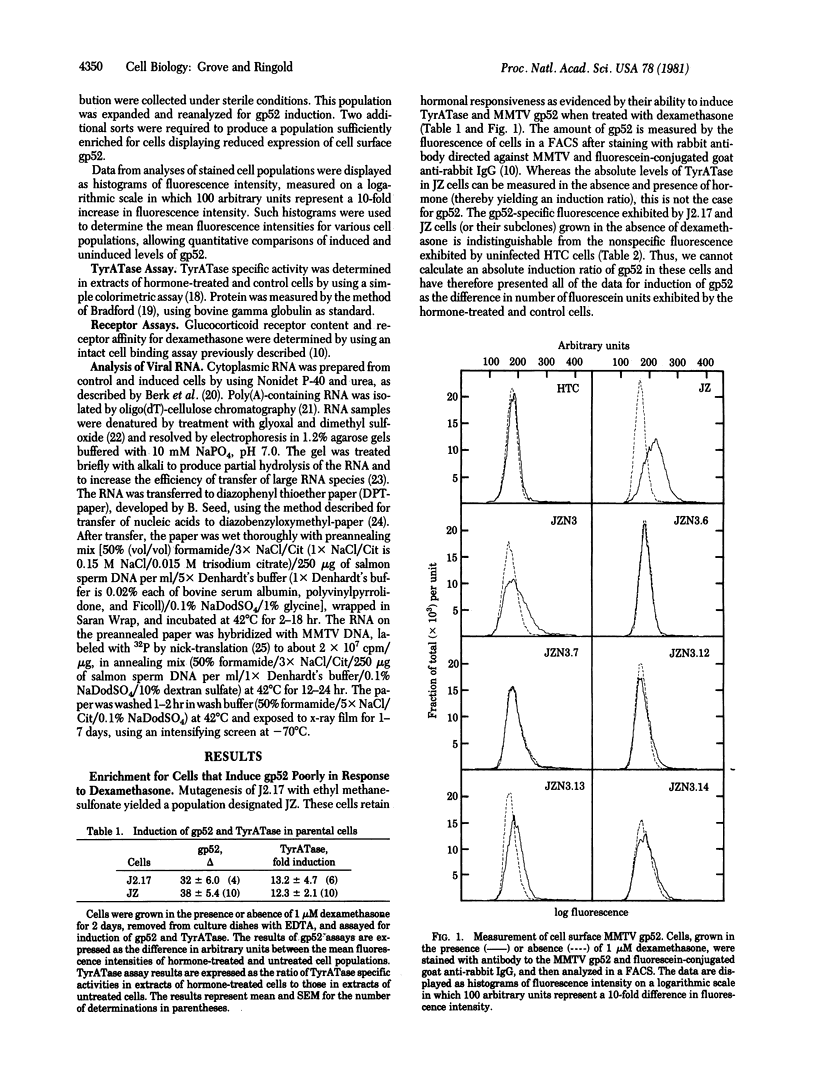
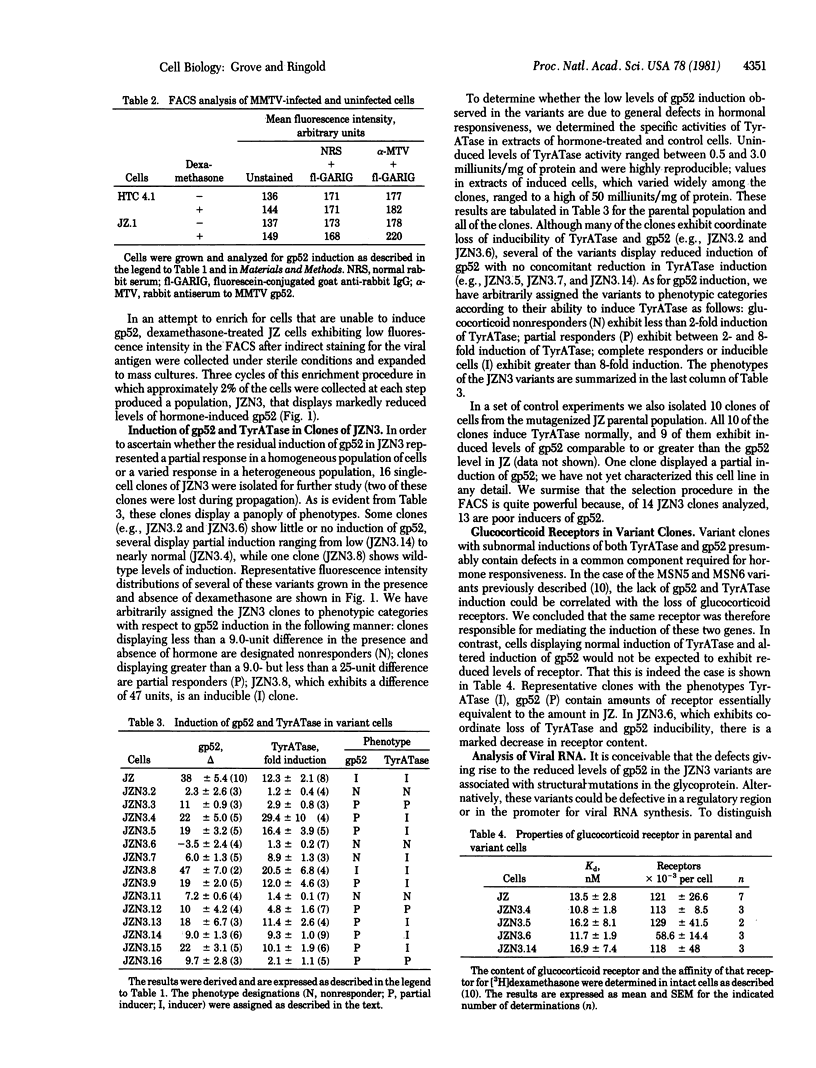
We have been studying the mechanism of glucocorticoid hormone action by using mouse mammary tumor virus (MMTV)-infected rat hepatoma cells as a model system. J2.17, a clonal cell line that contains one MMTV provirus, induces tyrosine aminotransferase (TyrATase; L-tyrosine:2-oxoglutarate aminotransferase, EC 2.6.1.5), viral RNA, and the cell surface viral glycoprotein gp52 in response to dexamethasone. Using a fluorescence-activated cell sorter and a rabbit antiserum directed against gp52, we selected a cell population that displays a reduced hormone-mediated increase in cell surface gp52. Fourteen clones of this population were assayed for induction of viral gp52 and RNA and of cellular TyrATase. The results of these assays revealed that the clones display a variety of responses to hormone. One clone has retained wild-type responses of both TyrATase and gp52. Six clones exhibit coordinately reduced or abolished responses of both markers. Seven clones show normal induction of TyrATase but reduced or undetectable induction of gp52. These latter clones exhibit reduced production of MMTV RNA and thus may represent a unique class of variants defective in the regulation of MMTV gene expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Lee F., Harrison T., Williams J., Sharp P. A. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell. 1979 Aug;17(4):935–944. doi: 10.1016/0092-8674(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chan L., Means A. R., O'Malley B. W. Rates of induction of specific translatable messenger RNAs for ovalbumin and avidin by steroid hormones. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1870–1874. doi: 10.1073/pnas.70.6.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J., Gannon F. Current models of steroid hormone action: a critique. Annu Rev Physiol. 1976;38:425–450. doi: 10.1146/annurev.ph.38.030176.002233. [DOI] [PubMed] [Google Scholar]
- Gronemeyer H., Pongs O. Localization of ecdysterone on polytene chromosomes of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2108–2112. doi: 10.1073/pnas.77.4.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner B., Hynes N. E., Diggelmann H. Identification of mouse mammary tumor virus-specific mRNA. J Virol. 1979 Apr;30(1):417–420. doi: 10.1128/jvi.30.1.417-420.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S. J., Gehring U. Molecular mechanisms of steroid hormone action. Adv Cancer Res. 1978;28:313–397. doi: 10.1016/s0065-230x(08)60650-8. [DOI] [PubMed] [Google Scholar]
- Honma Y., Kasukabe T., Okabe J., Hozumi M. Glucocorticoid binding and mechanism of resistance in some clones of mouse myeloid leukemic cells resistant to induction of differentiation by dexamethasone. J Cell Physiol. 1977 Nov;93(2):227–235. doi: 10.1002/jcp.1040930208. [DOI] [PubMed] [Google Scholar]
- McKnight G. S. The induction of ovalbumin and conalbumin mRNA by estrogen and progesterone in chick oviduct explant cultures. Cell. 1978 Jun;14(2):403–413. doi: 10.1016/0092-8674(78)90125-3. [DOI] [PubMed] [Google Scholar]
- Rhoads R. E., McKnight G. S., Schimke R. T. Quantitative measurement of ovalbumin messenger ribonucleic acid activity. Localization in polysomes, induction by estrogen, and effect of actinomycin D. J Biol Chem. 1973 Mar 25;248(6):2031–2039. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Cardiff R. D., Varmus H. E., Yamamoto K. R. Infection of cultured rat hepatoma cells by mouse mammary tumor virus. Cell. 1977 Jan;10(1):11–18. doi: 10.1016/0092-8674(77)90134-9. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Shank P. R., Varmus H. E., Ring J., Yamamoto K. R. Integration and transcription of mouse mammary tumor virus DNA in rat hepatoma cells. Proc Natl Acad Sci U S A. 1979 Feb;76(2):665–669. doi: 10.1073/pnas.76.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G., Lasfargues E. Y., Bishop J. M., Varmus H. E. Production of mouse mammary tumor virus by cultured cells in the absence and presence of hormones: assay by molecular hybridization. Virology. 1975 May;65(1):135–147. doi: 10.1016/0042-6822(75)90014-8. [DOI] [PubMed] [Google Scholar]
- Robertson D. L., Varmus H. E. Structural analysis of the intracellular RNAs of murine mammary tumor virus. J Virol. 1979 May;30(2):576–589. doi: 10.1128/jvi.30.2.576-589.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenau W., Baxter J. D., Rousseau G. G., Tomkins G. M. Mechanism of resistance to steroids: glucocorticoid receptor defect in lymphoma cells. Nat New Biol. 1972 May 3;237(70):20–24. doi: 10.1038/newbio237020a0. [DOI] [PubMed] [Google Scholar]
- Rousseau G. G., Baxter J. D., Higgins S. J., Tomkins G. M. Steroid-induced nuclear binding of glucocorticoid receptors in intact hepatoma cells. J Mol Biol. 1973 Sep 25;79(3):539–554. doi: 10.1016/0022-2836(73)90405-1. [DOI] [PubMed] [Google Scholar]
- Rousseau G. G., Baxter J. D., Tomkins G. M. Glucocorticoid receptors: relations between steroid binding and biological effects. J Mol Biol. 1972 Jun 14;67(1):99–115. doi: 10.1016/0022-2836(72)90389-0. [DOI] [PubMed] [Google Scholar]
- Rousseau G. G., Higgins S. J., Baxter J. D., Gelfand D., Tomkins G. M. Binding of glucocorticoid receptors to DNA. J Biol Chem. 1975 Aug 10;250(15):6015–6021. [PubMed] [Google Scholar]
- Seifert S. C., Gelehrter T. D. Isolation of rat hepatoma cell variants selectively resistant to dexamethasone inhibition of plasminogen activator. J Cell Physiol. 1979 Jun;99(3):333–341. doi: 10.1002/jcp.1040990308. [DOI] [PubMed] [Google Scholar]
- Sen G. C., Smith S. W., Marcus S. L., Sarkar N. H. Identification of the messenger RNAs coding for the gag and env gene products of the murine mammary tumor virus. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1736–1740. doi: 10.1073/pnas.76.4.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley C. H., Tomkins G. M. Isolation of lymphoma cell variants resistant to killing by glucocorticoids. Cell. 1974 Aug;2(4):213–220. doi: 10.1016/0092-8674(74)90013-0. [DOI] [PubMed] [Google Scholar]
- Sibley C. H., Tomkins G. M. Mechanisms of steroid resistance. Cell. 1974 Aug;2(4):221–227. doi: 10.1016/0092-8674(74)90014-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thompson E. B., Aviv D., Lippman M. E. Variants of HTC cells with low tyrosine aminotransferinase inducibility and apparently normal glucorticoid receptors. Endocrinology. 1977 Feb;100(2):406–419. doi: 10.1210/endo-100-2-406. [DOI] [PubMed] [Google Scholar]
- Toft D., Gorski J. A receptor molecule for estrogens: isolation from the rat uterus and preliminary characterization. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1574–1581. doi: 10.1073/pnas.55.6.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M. Steroid receptors: elements for modulation of eukaryotic transcription. Annu Rev Biochem. 1976;45:721–746. doi: 10.1146/annurev.bi.45.070176.003445. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. On the specificity of the binding of the estradiol receptor protein to deoxyribonucleic acid. J Biol Chem. 1974 Nov 25;249(22):7076–7086. [PubMed] [Google Scholar]




