Abstract
Purpose
To examine the feasibility of flow-independent T2-prepared inversion recovery (T2IR) black blood (BB) magnetization preparation for 3D balanced steady-state free precession (SSFP) vessel wall MRI of the popliteal artery, and to evaluate its performance relative to flow-dependent double inversion recovery (DIR), spatial presaturation (SPSAT) and motion-sensitizing magnetization preparation (MSPREP) BB techniques in healthy volunteers.
Materials and Methods
Eleven subjects underwent 3D MRI at 1.5T with four techniques performed in a randomized order. Wall and lumen signal-to-noise ratio (SNR), wall-to-lumen contrast-to-noise ratio (CNR), vessel wall area and lumen area were measured at proximal, middle and distal locations of the imaged popliteal artery. Image quality scores based on wall visualization and degree of intraluminal artifacts were also obtained.
Results
In the proximal region, DIR and SPSAT had higher wall SNR and wall-to-lumen CNR than both MSPREP and T2IR. In the middle and distal regions, DIR and SPSAT failed to provide effective blood suppression, while MSPREP and T2IR provided adequate black blood contrast with comparable wall-to-lumen CNR and image quality.
Conclusion
The feasibility of 3D SSFP imaging of the popliteal vessel wall using flow-independent T2IR was demonstrated with effective blood suppression and good vessel wall visualization. Although DIR and SPSAT are effective for thin slab imaging, MSPREP and T2IR are better suited for 3D thick slab imaging.
Keywords: black blood, vessel wall imaging, flow independent, T2 prepared inversion recovery, motion-sensitizing magnetization preparation, popliteal artery
Introduction
Vessel wall imaging is essential for accurate assessment of atherosclerosis, aneurysms and vasculitis. Clinically established imaging modalities include ultrasound, computerized tomography, and magnetic resonance imaging (MRI). High-resolution MRI of the arterial vessel wall has the advantage of characterizing atherosclerotic plaque morphology and composition noninvasively without ionizing radiation. However, MRI requires effective blood suppression to improve the contrast between the artery lumen and the surrounding vessel wall. Conventional black-blood (BB) MRI techniques including double inversion recovery (DIR) (1) and spatial presaturation (SPSAT) (2) rely on the inflow of magnetization prepared upstream blood into the imaging volume and are mainly suited for 2D imaging. However, 3D imaging is often desired because it can provide thinner slices and higher signal-to-noise ratio (SNR) than 2D imaging. To address the need for effective blood suppression within a large 3D imaging volume, several BB approaches have been developed including flow-dependent motion-sensitizing magnetization preparation (MSPREP) (3–5) which dephases the flowing blood signal using velocity-encoding gradients prior to imaging, and flow-independent T2-prepared inversion recovery (T2IR) (6,7), which exploits the difference in T1 and T2 relaxation times between blood and vessel wall to provide global blood suppression regardless of blood flow velocity and direction.
While BB imaging of the aorta (3,4,6,8), coronary (9,10) and carotid arteries (6,11,12), and the heart (1,5,6) has been widely demonstrated, BB imaging of the lower extremity vasculature has received less interest (13,14) due to several unique challenges including much slower blood flow, particularly in diseased vessels (15), and the need for much larger coverage compared to other vascular territories. Recent work has demonstrated that stagnant blood flow may induce plaque mimicking intraluminal artifacts in 2D DIR fast spin echo (FSE) images of the lower extremity vessel walls (7). Flow-independent T2IR prepared 2D FSE of the femoral and popliteal artery wall has been shown to be robust against slow flow artifacts, although this technique suffers from low SNR and long scan time.
The purpose of this study was to develop an SNR efficient 3D flow-independent T2IR balanced steady-state free precession (SSFP) vessel wall imaging sequence and compare its performance against flow-dependent DIR, SPSAT and MSPREP magnetization preparations in the popliteal artery.
Methods
Eleven healthy volunteers (8 male, mean age = 28 ± 5 years, 24–39 years) were imaged on a 1.5 T scanner (HDxt, GE Healthcare, Waukesha, WI). The study protocol was approved by the local institutional review board and a written informed consent was obtained from all subjects prior to imaging. Subjects were imaged in a supine position using a product eight-channel cardiac phased array and peripheral gating for cardiac synchronization.
Figure 1 shows the schematics of the implemented cardiac-triggered segmented-k-space 3D SSFP vessel wall MRI sequence. SSFP provides bright blood contrast and thus permits objective comparison of the four BB techniques without interference from inherent BB image contrast such as in FSE. Each BB magnetization preparation was performed every heartbeat and immediately after the peripheral trigger—typically corresponding to the period of maximum arterial blood flow in the popliteal artery—to enhance blood suppression of the flow-based DIR, SPSAT, and MSPREP techniques. The experimental MRI protocol consisted of a 3-plane 2D gradient echo localizer scan and 2D peripheral-triggered BB SSFP scout scans to determine the optimal imaging parameter (see below) for each BB preparation, followed by high-resolution 3D BB SSFP vessel wall imaging sequences performed in a randomized order using the optimized BB parameters, and concluded with a 2D phase contrast (PC) scan to measure blood flow. In each 2D BB scout scan, several images were acquired sequentially while the pertinent BB imaging parameter was automatically varied over a range of values. The BB imaging parameters were inversion time (TI) for DIR (range 275–450 ms) and T2IR (175–350 ms), field of speed (FOS) (5) in the through-plane direction for MSPREP (1–5 cm/s), and saturation flip angle for SPSAT (90°–120°.) The optimal parameter was identified by visually selecting the 2D BB scout image with the best black blood contrast.
Figure 1.
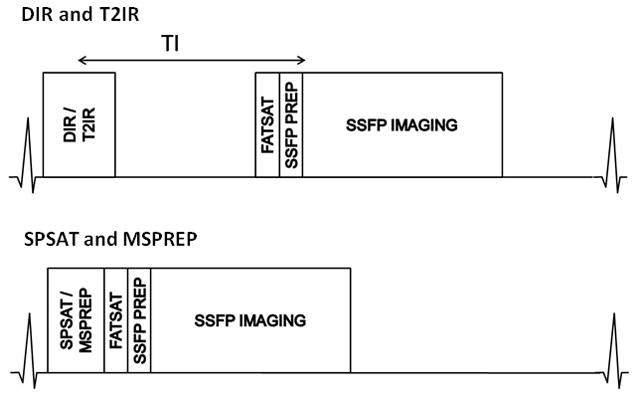
Schematic of the peripheral triggered 3D BB SSFP sequences. The four BB preparations were played out either immediately (in SPSAT and MSPREP) or after a time delay (DIR and T2IR). A spectrally selective fat saturation pulse (FATSAT) suppressed the lipid signal and 6 Kaiser-Bessel ramped “dummy” RF pulses (SSFP PREP) prepared the magnetization prior to segmented k-space SSFP data acquisition. The sequence is repeated every heartbeat until data was fully collected.
The imaging parameters were as follows: 2D BB SSFP scout scan: TR = 4.2 ms, TE = 1.7 ms, flip angle = 60°, receiver bandwidth = ±83.3 kHz, axial field of view (FOV) = 18 cm, matrix = 256×256 interpolated to 512×512, slice thickness = 12 mm, number of excitations (NEX) = 1 (DIR, SPSAT) and 2 (MSPREP, T2IR), views per segment (VPS) = 64, centric view order, scan time ~1 min (DIR, SPSAT, MSPREP) and 1.5 min (T2IR) at a nominal heart rate of 60 beats per minute (bpm). 3D BB SSFP imaging: TR = 3.8 ms, TE = 1.5 ms, flip angle = 60°, receiver bandwidth = ±83.3 kHz, axial FOV = 18 cm, matrix = 256×256 interpolated to 512×512, slice thickness = 3 mm interpolated to 1.5 mm, number of slices = 28 interpolated to 56, NEX = 2, VPS = 64, centric view order, scan time ~4 min at a nominal heart rate of 60 bpm, spectrally selective fat saturation pulse to suppress the lipid signal. 2D PC scan: TR = 8.6 ms, TE = 3.7 ms, flip angle = 25°, receiver bandwidth = ±31.25 kHz, axial FOV = 18 cm, matrix = 256×256, slice thickness = 8 mm, velocity encoding (VENC) = 50–75 cm/sec, VPS = 4, 28 reconstructed cardiac phases. The axial 3D imaging volume was prescribed to cover the distal femoral artery and the proximal popliteal artery of the right leg. The 2D BB scout scans were prescribed at the middle of the 3D imaging volume.
For each 3D data set, a region of interest (ROI) analysis was performed at proximal (2.4 cm from the superior edge of the imaging volume), middle (4.8 cm), and distal (7.2 cm) locations using ImageJ (NIH, Bethesda, MD) to measure vessel wall and lumen SNR, and wall-to-lumen contrast-to-noise ratio (CNR). The inner and outer boundaries of the vessel wall were manually traced from which lumen area, total vessel area, and vessel wall area were obtained. Additionally, the image quality was scored based on the depiction of the vessel wall and the degree of intraluminal artifacts for the proximal, middle, and distal regions. Specifically, two subscores (0=very poor, 1=poor, 2=fair, 3=good, 4=excellent) were assigned to two images 1.2cm apart in each region and were then averaged to obtain the final score. The reader was blinded to the BB techniques. Blood flow measurements were carried out using Flow 3.2 (Medis Medical Imaging Systems, Leiden, The Netherlands). Statistical analysis was performed with a two-tailed paired-sample t-test to assess differences in SNR, CNR, and area measurements, and a two-tailed Wilcoxon paired-sample signed rank test to compare image quality scores. Values are shown as mean ± standard deviation. P values of less than 0.05 were considered to indicate statistical significance.
Results
Images were acquired successfully in all eleven subjects. The optimal BB imaging parameters determined by 2D BB scout scans were as follows: TI = 366 ± 49 ms for DIR, saturation flip angle = 97° ± 5° for SPSAT, FOS = 2.0 ± 1.3 cm/s for MSPREP, and TI = 298 ± 47 ms for T2IR. The average heart rate was 55 ± 6 bpm, average blood flow rate was 90 ± 31 mL/min, and average peak blood velocity was 35 ± 6 cm/s.
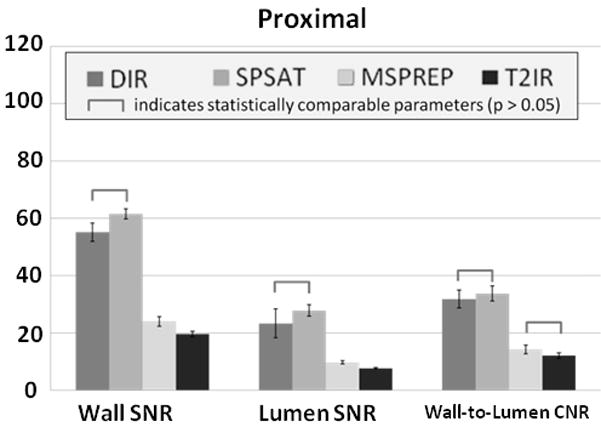
Figure 2 demonstrates typical source images obtained with the four BB techniques at proximal, middle and distal locations and a reformatted coronal view of the popliteal artery in one subject. Note that DIR and SPSAT yielded poor blood suppression in the distal half of the 9 cm axial imaging volume, while MSPREP and T2IR provided effective blood suppression and good vessel wall depiction throughout the volume. Compared to both MSPREP and T2IR, DIR and SPSAT preparations provided significantly higher wall SNR (P<0.0001) at the cost of significantly higher lumen SNR (P<0.05 at proximal, P<0.005 at middle and distal locations) (Fig.3). As a result, for DIR and SPSAT, wall-to-lumen CNR from proximal to middle locations decreased by 7% (P=0.42) and 49% (P=0.0001), respectively, and further decreased by 57% (P=0.001) and 44% (P=0.04), respectively, from middle to distal locations. While DIR yielded lower wall SNR than SPSAT, it suppressed the blood signal more effectively, leading to significantly higher wall-to-lumen CNR in the middle and distal locations. Compared to T2IR, MSPREP had higher wall SNR, although statistically significant difference was found only in the proximal location (P=0.047). T2IR provided approximately a 22% lower lumen SNR compared to MSPREP at all three locations (P<0.005). The wall-to-lumen CNR difference between T2IR and MSPREP was not found to be statistically significant (P>0.2).
Figure 2.
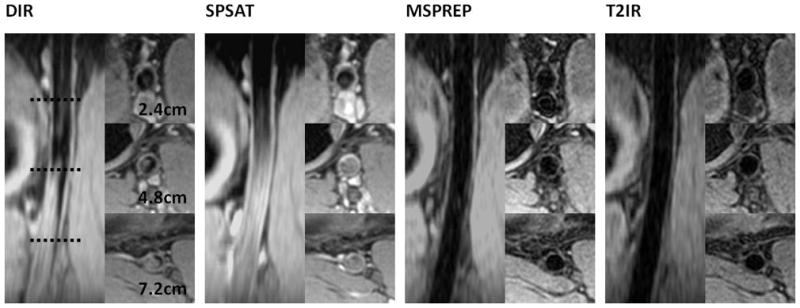
Axial source and coronal reformatted vessel wall images of the popliteal artery obtained with the four BB techniques in the same subject. This case followed the general trends observed in this study.
Figure 3.

Comparison of four BB techniques with respect to wall SNR, lumen SNR, and wall-to-lumen CNR measured at the proximal (2.4 cm from the superior end), middle (4.8 cm), and distal (7.2 cm) regions of the imaging volume (n = 11). The error bars demonstrate the standard error. MSPREP and T2IR provided significantly better blood suppression than both DIR and SPSAT.
Area measurements are summarized in Table 1. T2IR and MSPREP provided statistically comparable lumen area, total vessel area, and vessel wall area at all three locations. While all techniques yielded statistically comparable vessel wall area at all locations, lumen area and total vessel wall area from DIR and SPSAT were generally significantly smaller than those of MSPREP and T2IR, except in the proximal region.
Table 1.
Lumen area, total vessel area, and vessel wall area measurements obtained with BB 3D SSFP in the popliteal artery (n = 11).
| DIR | SPSAT | MSPREP | T2IR | |
|---|---|---|---|---|
| Lumen area (mm2) | ||||
| Proximal | 29 ± 8†* | 27 ± 10†* | 33 ± 10 | 33 ± 10 |
| Middle | 23 ± 7†* | 20 ± 7†* | 29 ± 8 | 29 ± 8 |
| Distal | 16 ± 5†* | 17 ± 5†* | 24 ± 8 | 23 ± 6 |
|
| ||||
| Total vessel area (mm2) | ||||
| Proximal | 52 ± 11* | 51 ± 12†* | 55 ± 15 | 55 ± 14 |
| Middle | 42 ± 11†* | 39 ± 10†* | 47 ± 11 | 48 ± 11 |
| Distal | 31 ± 8†* | 32 ± 8 †* | 39 ± 10 | 39 ± 9 |
|
| ||||
| Vessel wall area (mm2) | ||||
| Proximal | 23 ± 4 | 24 ± 6 | 22 ± 6 | 21 ± 5 |
| Middle | 19 ± 5 | 20 ± 3 | 19 ± 3 | 19 ± 3 |
| Distal | 15 ± 3 | 15 ± 3 | 15 ± 3 | 16 ± 3 |
indicates significance compared to T2IR (P < 0.05).
indicates significance compared to MSPREP (P < 0.05).
T2IR and MSPREP received higher image quality scores than either SPSAT or DIR in middle and distal regions, while the differences among all four techniques were not significant or only marginally significant in the proximal region (Fig.4). Image quality difference between MSPREP and T2IR was not statistically significant except in the middle region, where T2IR had higher average image quality (P=0.02). DIR and SPSAT received an average score of approximately 2 (“fair”) for all regions. The middle and distal regions receive a better image quality score (2.5–3 or “good”) than the proximal region (2 or “fair”) for both T2IR and MSPREP (P<0.01).
Figure 4.
Comparison of four BB techniques with respect to image quality scores obtained for the proximal, mid and distal regions of the 9 cm axial imaging volume. T2IR and MSPREP provided the best overall image quality.
Discussion
Our preliminary results in healthy volunteers demonstrated the feasibility of 3D T2IR SSFP vessel wall imaging of the popliteal artery. The technique provided good quality BB images within a clinically reasonable scan time. Compared to conventional inflow-based DIR and SPSAT techniques, flow-independent T2IR and velocity-based MSPREP were found to provide superior blood suppression over a 9 cm axial segment of the popliteal artery, particularly in the distal region where the inflow effect diminishes substantially. Unlike DIR and SPSAT, T2IR and MSPREP do not rely on the inflow of blood with nulled signal into the imaging volume and therefore are also suited for coronal and sagittal acquisitions which can cover the lower extremity vasculature more efficiently than the axial acquisition used in this study (14,16). Our experience indicates that T2IR can provide reliable blood suppression in other image plane orientations such as in the coronal (16) or oblique planes (17). While the weakness of DIR and SPSAT for thick slab BB MRI has been well recognized (18,19) and reported in previous comparison studies (7,19), this work directly compares these techniques against the more recently developed MSPREP and T2IR techniques using the same imaging pulse sequence, scanner setup and subjects. As the effectiveness of blood suppression in DIR and SPSAT degrades with increasing slab thickness, the results presented here may provide practical guidance for thin slab targeted vessel wall imaging of the lower extremities.
Compared to DIR and SPSAT, MSPREP and T2IR provided lower wall SNR, which may be overcome by imaging at higher field strengths such as 3T (16), especially when high spatial resolution is desired. Compared to MSPREP, T2IR provided similar wall-to-lumen CNR, and received comparable or higher image quality scores. While T2IR can provide global blood suppression regardless of blood flow velocity, direction, and spatial resolution, MSPREP relies on velocity-induced signal dephasing within the imaging voxel. It therefore depends on voxel size and may be suboptimal in regions with slow blood flow such as along the wall-lumen boundary (7). This difference may be important in diseased vessels with slower blood flow, although this remains to be investigated.
In a previous study, a single image of the popliteal vessel wall was acquired in 6.5 min using a 2D T2IR FSE sequence. Here, 56 high resolution popliteal vessel wall images were acquired in about 4 minutes, representing almost two orders of magnitude scan time reduction on a per slice basis. This substantial time saving can be attributed to the higher SNR efficiency of 3D imaging and the SSFP acquisition (20). This SNR advantage also permits acquisitions with higher resolution, potentially leading to the improved visualization of small plaque components (16,19).
The optimal FOS value of 2.0 cm/s for MSPREP in the popliteal artery was comparable with that reported by Koktzoglou et al (3.3 cm/s calculated from reported b-value = 1.1 s/mm2 and gradient area = 231 ms mT/m) (3) and Wang et al (3.0 cm/s calculated from reported first-order gradient moment m1 = 784 mT ms2/m) (4) in the carotid artery. However, it was substantially smaller than that reported by Fan et al (135 cm/s calculated from m1 = 17.4 mT ms2/m) for non-contrast MRA of the lower extremity (21). This large discrepancy may be explained by the different purposes of MSPREP: whereas small velocity-encoding gradients may be sufficient to generate good quality angiograms, larger gradients are needed to suppress intraluminal signal.
In this study, we noted a significant decrease in image quality score in the proximal region compared to the middle and distal region scores for both T2IR and MSPREP. This may be due to the increased curvature of the popliteal artery with respect to the axial imaging volume in the proximal region, leading to lower wall SNR (Fig.3) and increased wall blurring when compared to the middle and distal regions where the artery is fairly straight. The effects of vessel orientation on vessel wall depiction and SNR have been demonstrated in the carotid bifurcation (22). Potential remedies include using higher field strengths to improve SNR and higher resolution to reduce partial volume effect.
This study has several limitations. First, BB preparations were synchronized with the peripheral trigger to maximize the blood suppression of the flow-based techniques. As a consequence, each technique imaged the vessel wall at a different phase in the cardiac cycle, which may have affected the vessel wall area measurements. Second, while electrocardiographic gating may have provided improved flow timing for flow-sensitive BB techniques, peripheral gating was used to simplify the experimental setup and was found to adequately capture the period of systolic peak flow in all subjects as evidenced by the phase contrast flow measurements. Third, BB parameter optimization was performed on a single slice at the middle of the imaging volume to simplify the scout scan procedures, which may lead to suboptimal blood suppression elsewhere within the volume. For flow-based techniques in particular, blood suppression depends on blood velocity and it is generally difficult to determine a single optimal value for the BB parameter that would provide uniform blood suppression. Fourth, patients with peripheral arterial occlusive disease were not enrolled in this preliminary study. The performance of T2IR and MSPREP remains to be evaluated in the presence of slower blood flow in diseased arteries. Fifth, other recently developed BB techniques such as FSE with variable refocusing flip angles (14) were not included in this comparison study but are the subject of ongoing research. Sixth, plaque characterization generally requires a multi-contrast imaging protocol. While SPSAT does not alter the image contrast of stationary tissues, DIR, MSPREP and T2IR magnetization preparations induce T1, T2, and mixed T1 and T2 weighting, respectively. The utility of T2IR for plaque characterization remains therefore to be evaluated.
In conclusion, flow-independent 3D T2IR SSFP imaging of the popliteal vessel wall is feasible with effective blood suppression across a thick imaging volume. MSPREP and T2IR provide similar image quality and are better suited for thick slab 3D BB vessel wall imaging than conventional DIR and SPSAT techniques.
Acknowledgments
Grant Support: This work was partially funded by NIH grant R01 HL-060879.
References
- 1.Edelman RR, Chien D, Kim D. Fast selective black blood MR imaging. Radiology. 1991;181(3):655–660. doi: 10.1148/radiology.181.3.1947077. [DOI] [PubMed] [Google Scholar]
- 2.Felmlee JP, Ehman RL. Spatial presaturation: a method for suppressing flow artifacts and improving depiction of vascular anatomy in MR imaging. Radiology. 1987;164(2):559–564. doi: 10.1148/radiology.164.2.3602402. [DOI] [PubMed] [Google Scholar]
- 3.Koktzoglou I, Li D. Diffusion-prepared segmented steady-state free precession: Application to 3D black-blood cardiovascular magnetic resonance of the thoracic aorta and carotid artery walls. J Cardiovasc Magn Reson. 2007;9(1):33–42. doi: 10.1080/10976640600843413. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Yarnykh VL, Hatsukami T, Chu B, Balu N, Yuan C. Improved suppression of plaque-mimicking artifacts in black-blood carotid atherosclerosis imaging using a multislice motion-sensitized driven-equilibrium (MSDE) turbo spin-echo (TSE) sequence. Magn Reson Med. 2007;58(5):973–981. doi: 10.1002/mrm.21385. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen TD, de Rochefort L, Spincemaille P, et al. Effective motion-sensitizing magnetization preparation for black blood magnetic resonance imaging of the heart. J Magn Reson Imaging. 2008;28(5):1092–1100. doi: 10.1002/jmri.21568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu C, Bley T, Wieben O, Brittain J, Reeder S. Flow-independent T2-prepared inversion recovery black-blood MR imaging. Journal of Magnetic Resonance Imaging. 2010;31(1):248–254. doi: 10.1002/jmri.21986. [DOI] [PubMed] [Google Scholar]
- 7.Brown R, Nguyen T, Spincemaille P, et al. Effect of Blood Flow on Double Inversion Recovery Vessel Wall MRI of the Peripheral Arteries: Quantitation with T2 Mapping and Comparison with Flow-Insensitive T2-Prepared Inversion Recovery Imaging. MRM. 2010;63(3):736–744. doi: 10.1002/mrm.22227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mani V, Itskovich VV, Szimtenings M, et al. Rapid extended coverage simultaneous multisection black-blood vessel wall MR imaging. Radiology. 2004;232(1):281–288. doi: 10.1148/radiol.2321031022. [DOI] [PubMed] [Google Scholar]
- 9.Kim WY, Stuber M, Bornert P, Kissinger KV, Manning WJ, Botnar RM. Three-dimensional black-blood cardiac magnetic resonance coronary vessel wall imaging detects positive arterial remodeling in patients with nonsignificant coronary artery disease. Circulation. 2002;106(3):296–299. doi: 10.1161/01.cir.0000025629.85631.1e. [DOI] [PubMed] [Google Scholar]
- 10.Fayad ZA, Fuster V, Fallon JT, et al. Noninvasive in vivo human coronary artery lumen and wall imaging using black-blood magnetic resonance imaging. Circulation. 2000;102(5):506–510. doi: 10.1161/01.cir.102.5.506. [DOI] [PubMed] [Google Scholar]
- 11.Yuan C, Beach KW, Smith LH, Jr, Hatsukami TS. Measurement of atherosclerotic carotid plaque size in vivo using high resolution magnetic resonance imaging. Circulation. 1998;98(24):2666–2671. doi: 10.1161/01.cir.98.24.2666. [DOI] [PubMed] [Google Scholar]
- 12.Makhijani M, Hu H, Pohost G, Nayak K. Improved blood suppression in three-dimensional (3D) fast spin-echo (FSE) vessel wall imaging using a combination of double inversion-recovery (DIR) and diffusion sensitizing gradient (DSG) preparations. Journal of Magnetic Resonance Imaging. 2010;31(2):398–405. doi: 10.1002/jmri.22042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isbell D, Meyer CH, Rogers WJ, et al. Reproducibility and reliability of atherosclerotic plaque volume measurements in peripheral arterial disease with cardiovasculr magnetic resonance. J Cardiovasc Magn Reson. 2007;9:71–76. doi: 10.1080/10976640600843330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Z, Fan Z, Carrol T, et al. Three-Dimensional T2-Weighted MRI of the Human Femoral Arterial Vessel Wall at 3.0 Tesla. Investigative Radiology. 2009;44(9):619–626. doi: 10.1097/RLI.0b013e3181b4c218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohajer K, Zhang H, Gurell D, et al. Superficial femoral artery occlusive disease severity correlates with MR cine phase-contrast flow measurements. J Magn Reson Imaging. 2006;23(3):355–360. doi: 10.1002/jmri.20514. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen T, Kawaji K, Spincemaille P, Prince M, Wang Y. Large field-of-view submillimeter isotropic resolution bilateral peripheral vessel wall MRI using 3D fast spin echo with flow-insensitive blood suppression at 3 Tesla. ISMRM; Stockholm, Sweden. 2010. p. 3691. [Google Scholar]
- 17.Nguyen T, Kawaji K, Spincemaille P, et al. Three dimensional black blood MRI with extensive cardiothoracic coverage: A feasibility study in healthy volunteers. ISMRM; Stockholm, Sweden. 2010. p. 3650. [Google Scholar]
- 18.Simonetti O, Finn J, White R, Laub G, Henry D. Black blood T2-weighted inversion-recovery MR imaging of the heart. Radiology. 1996;199(1):49–57. doi: 10.1148/radiology.199.1.8633172. [DOI] [PubMed] [Google Scholar]
- 19.Balu N, Chu B, Hatsukami T, Yuan C, Yarnykh V. Comparison between 2D and 3D high-resolution black-blood techniques for carotid artery wall imaging in clinically significant atherosclerosis. Journal of Magnetic Resonance Imaging. 2008;27(4):918–924. doi: 10.1002/jmri.21282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawaji K, Nguyen T, Spincemaille P, et al. High-Resolution 3D Isotropic Black-Blood Imaging with T2prep Inversion Recovery: Comparison between FSE and SSFP. ISMRM; Stockholm, Sweden. 2010. p. 3027. [Google Scholar]
- 21.Fan Z, Sheehan J, Bi X, Liu X, Carr J, Li D. 3D noncontrast MR angiography of the distal lower extremities using flow-sensitive dephasing (FSD)-prepared balanced SSFP. Magnetic Resonance in Medicine. 2009;62(6):1523–1532. doi: 10.1002/mrm.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Antiga L, Wasserman BA, Steinman DA. On the overestimation of early wall thickening at the carotid bulb by black blood MRI, with implications for coronary and vulnerable plaque imaging. Magnetic Resonance in Medicine. 2008;60(5):1020–1028. doi: 10.1002/mrm.21758. [DOI] [PubMed] [Google Scholar]


