Abstract
Objective
Roux-en-Y gastric bypass (RYGB) surgery is the most common surgical intervention for long-term weight loss in morbidly obese patients. By reducing obesity-associated hyperfiltration, diabetes, and hypertension, RYGB is touted to stabilize if not prevent progression of chronic renal disease. To test this, we compare renal histology of diet-induced obese rats that have undergone RYGB surgery to pair-fed and sham obese controls.
Research Methods and Procedures
Sprague-Dawley rats, fed a high fat, low-oxalate diet to induce gross obesity, were randomized to RYGB (n=6), GI-intact sham-operated obese controls (Sham, n=4), or GI-intact sham-operated obese pair-fed rats (Fed, n=8). Daily body weight and food intake were recorded. On post-operative day 42, renal histology and immunohistochemistry was examined. Renal pathology was assessed by a categorical glomerular lesion score and a quantitative glomerular/tubular scoring system by experienced veterinary pathologists. Osteopontin (OPN) and ED-1 (monocyte/macrophage cell) staining were estimated on percentage of stained area and number of counted cells/high power field respectively.
Results
Compared to sham and fed controls, RYGB rats had significant reductions in body weight (p<0.001), more glomerular lesions (p=0.02), and received higher glomerular and tubular lesion scores (p<0.01). RYGB rodents had significantly stronger staining for OPN within the inner medullary region (p<0.005) and ED-1 within the outer medullary region (P<0.02) compared to sham and fed controls.
Conclusions
In this diet-induced obese rat model, RYGB is associated with chronic glomerulosclerosis and tubulointerstitial nephritis, confirmed by histology and immunohistochemistry. Prospective studies to better define the injurious mechanisms in this model are planned.
Keywords: morbid obesity, interstitial nephritis, gastric bypass, oxalate
INTRODUCTION
Roux-en-Y gastric bypass (RYGB) surgery is the most effective weight loss therapy for morbidly obese (BMI > 40 kg/m2) and severely obese patients (BMI > 35 kg/m2) with medical complications, reversing obesity-related co-morbidities such as cardiovascular failure, diabetes, hypertension, and sleep apnea [1–3]. Because of its successes, RYGB surgical cases have increased an astonishing 14-fold over the last 10 years, from 14,000 in 1998 to over 200,000 in 2007 [4]. From a renal standpoint, RYGB has been demonstrated in matched controls to decrease obesity-related hyperfiltration and proteinuria [5]. When taken with improvements in glycemic and hypertensive control, RYGB is believed by many to slow progression of chronic kidney disease (CKD) in this population [5,6]. However, its overall effect on renal structure and function is still debatable, as RYGB surgery is associated with a number of renal complications including calcium oxalate kidney stone formation due to hyperoxaluria [7–10], acute on chronic kidney failure [7,8,11], and even oxalate nephropathy leading to end stage renal disease [7,11].
To better understand the mechanisms involved in surgical weight loss, members of our group have developed and characterized a diet-induced obese (DIO) rat model of RYGB surgery similar to that performed in obese humans. Because 14 days in this model is equal to one human year [12], post-RYGB physiological changes can be studied over a prolonged period of time with minimal loss of follow-up. Weight loss patterns, metabolic alterations, and hormonal changes achieved in this model have previously been validated and are similar to those in humans, making this an extremely reliable model to investigate the effect of RYGB on a variety of organ systems [13,14]. To our knowledge, no previous study of renal glomerular or tubular histology has been undertaken in obese rodents following gastric bypass.
Through weight loss, we hypothesized that obese rodents undergoing RYGB surgery would have improved renal histology patterns compared to rats that remain obese. We tested this hypothesis in a pilot study by comparing renal glomerular and tubular histology of RYGB rodents to sham and pair-fed controls. To evaluate for associated renal inflammation or injury, renal immunohistochemical stains were performed across all groups.
MATERIALS AND METHODS
Diet, surgery, and weight loss
Animal protocols were approved by institutional review board animal ethics committee. Starting at 4 weeks of age, 18 Sprague Dawley pups were given a low-oxalate, high-fat and carbohydrate diet (Levin diet [15]) that provided 4.41 kcal/g (17% protein, 32% fat, 51% carbohydrate). All diet-induced obese (DIO) rats were then randomized to RYGB (n=6), sham-operated obese controls (Sham, n=4), or sham-operated obese pair-fed rats (Fed, n=8). Gastric bypass, described in detail elsewhere [13,16], involved creation of a small gastric pouch with a proximal gastrojejunostomy. A 30 cm biliary-pancreatic limb was then anastomosed to the distal jejunum (jejuno-jejunostomy), creating a 10 cm Roux limb and 36 cm common channel for digestion and absorption. Sham and pair-fed (Fed) rat controls underwent gastric mobilization without transection. Post-operatively, sham and RYGB continued on high fat and low oxalate diet ad libitum while pair feds ingested a similar amount of food as a paired-RYGB rat from the previous day, modeling a restrictive-only procedure. Daily weights were averaged from each group with standard deviation. Total weight loss was estimated by dividing total weight lost by pre-surgical weight. On post-operative day 42, rodents were sacrificed. Kidneys were harvested, weighed, and immediately submerged in liquid nitrogen for cryopreservation purposes.
Renal histochemistry
To evaluate histology, kidneys were fixed in 10% buffered formalin and paraffin-embedded. Three-micrometer thick sagittal sections were cut, deparaffinized, stained with Periodic acid-Schiff (PAS), and viewed by light microscopy. Two separate veterinary pathologists, blinded to each others’ results, examined glomeruli and tubules across all three rat groups and scored lesions by two different quantitative methods. First, based on a human jejunoileal bypass (JIB) study from the 1970’s [17], 300 randomly selected glomeruli were evaluated for presence or absence of tuft atrophy, Bowman’s capsule changes, peri-glomerular fibrosis, or membranoproliferative changes. Total results (number positive out of 300) from both pathologists were averaged and analyzed by Fisher’s exact test. Second, histologic alterations were evaluated by a lesion scoring system developed by Reinhard et al [18]. Glomerular lesions were again evaluated for the presence or absence of tuft atrophy, Bowman’s capsule changes, peri-glomerular fibrosis, membranoproliferative changes, tuft adhesions, sclerosis and/or atrophy to include all the glomeruli on the slide. Lesion scores were assigned as follows: 0 = no lesions, 1 = less than 10%, 2 = 10 to 25%, 3 = 25 to 50%, and 4 = greater than 50% of total slide area affected. Similarly, tubular lesions were scored from across the entire section, including interstitial fibrosis, cellular infiltrates, and tubular atrophy with thickening of the basement membrane. Lesion scores were assigned as follows: 0 = no lesions, 1 = <25%, 2 = 25 to 50%, and 3 = >50% of total slide area affected. All lesion scores were analyzed with Kruskall-Wallis test for statistical significance.
Renal immunohistochemistry
Immunohistochemical stains for osteopontin (OPN), a known secretory glycoprotein upregulated during tubular injury and experimental hyperoxaluria, and ED-1 (macrophage-derived mononuclear cell stain) were performed on all samples. To better estimate location of staining, kidneys were divided into renal cortex, outer medulla, and inner medulla. OPN stain was estimated based on percentage of stained area compared to the kidney section size on 30 separate high power fields (HPF) from each section of one kidney. ED-1 stain was estimated on the number of positive-stained cells counted in a 10x field (using high power as necessary) divided by the number of fields counted (~30/each section of kidney). Counts were then scored as follows: 0–10 cells = 1; 11–20 cells = 2; 21–30 cells = 3; >30 cells = 4. Data were compared using ANOVA and t-test. For all results, a p-value of ≤0.05 was considered significant.
RESULTS
Caloric intake, weight loss, and kidney weight
After 16 weeks on high fat, low-oxalate diet [13,16], DIO rodents weighed 613 g (STD 31 g), roughly twice the weight of standard non-obese rodents. At 21 days post-operative, average estimate caloric intake was: Sham = 63.6±4.4 kcal/day; RYGB = 18.9±4.5 kcal/day; and Fed = 23.2±3.9 kcal/day. At 42 days post-operative, average estimated caloric intake was: Sham = 68.3±4.4 kcal/day; RYGB = 49.3±5.4 kcal/day; and Fed = 42.4±4.9 kcal/day. By postoperative day 42, Sham controls weighed on average 620 g (±12 g) compared to Fed (587±25 g; p<0.1) and RYGB (479±18 g; p<0.001). Pair fed rodents lost ~5% of total body weight while RYGB rodents lost ~23% of total body weight. The average kidney weight was 3.5 gm with no significant differences seen among groups (p=0.496).
Glomerular and tubular lesion scoring
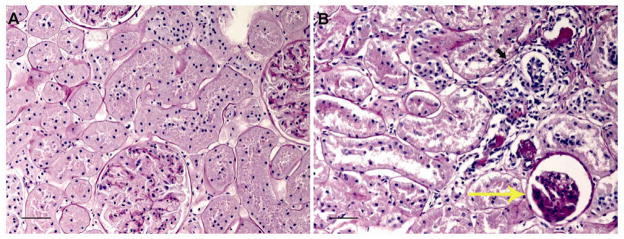
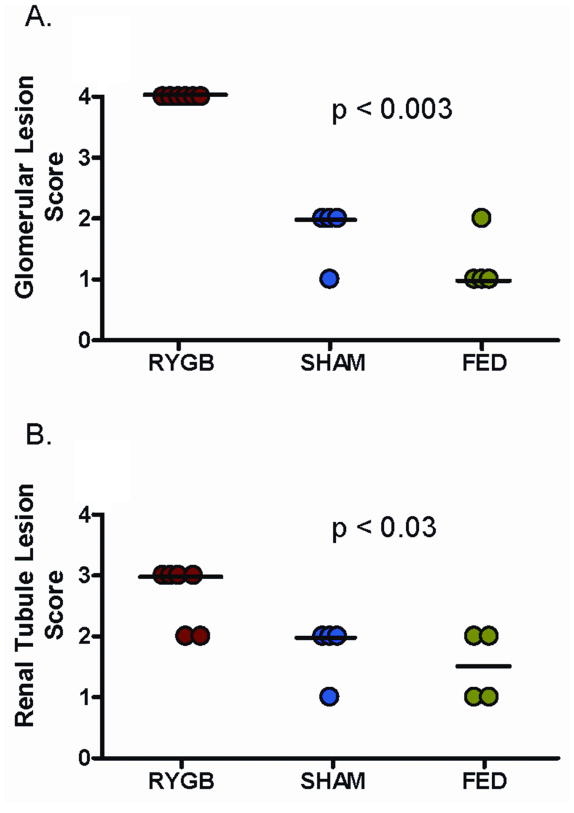
Quantitatively, more glomerular lesions (Table 1) were identified in RYGB rodents than in either Sham or Fed controls (p<0.02). These lesions included tuft atrophy (21%; p<0.0001), Bowman’s capsule thickening (20%; p<0.001), and membranoproliferative changes (32.7%; p<0.001). No differences were noted in number of glomeruli with periglomerular fibrosis across all three groups. Fed group was found to have more membranoproliferative changes (4.7% vs 12.3%, p=0.0011) when compared to Sham (Table 1). For percentage area scoring, RYGB rodents demonstrated higher glomerular (p=0.0033) and tubular (p=0.0257) scores compared to Sham and Fed controls (Figure 1). Representative light-microscopy sections are demonstrated in Figure 2. This particular Fed rodent (2A) received a total percentage area glomerular and tubular score of 1. In comparison, RYGB rodent (2B) received a total percentage area glomerular score of 4 and tubular score of 3. Renal cortical and medullary calcifications were present in low numbers across all groups with no statistically significant difference notable.
Table 1.
Frequency of glomerular lesions from obese rats. Scores represent percentage positive for each specific lesion out of 300 randomly chosen glomeruli across all groups.
| Lesion | Treatment Group Scores | ||
|---|---|---|---|
| RYGB | Sham | Fed | |
| Tuft atrophy | 21% † | 8.3% | 7.7% |
|
| |||
| Bowman’s capsule thickening | 20% † | 5.3% | 6.3% |
|
| |||
| Periglomerular fibrosis | 8% | 5% | 4.7% |
|
| |||
| Membranoproliferative changes | 32.7% † | 4.7% | 12.3% ‡ |
p<0.0001 compared to both Sham and Fed by Fisher’s exact
p=0.0011 compared to Sham by Fisher’s exact
Figure 1.
Glomerular and renal tubule lesion scoring system. RYGB rodents had significantly higher glomerular (p=0.0033) and tubular (p=0.0257) scores than Sham and Fed controls. Dark line represents median value.
Figure 2.
Representative PAS stained rodent kidney sections. Pair-fed control (A) received a glomerular score of 1 and a tubular score of 1. RYGB (B) received a glomerular score 4 (large yellow arrow) and tubular score 3 (small dark arrow). 600x magnification with scale bar = 10 μm.
Immunohistochemistry
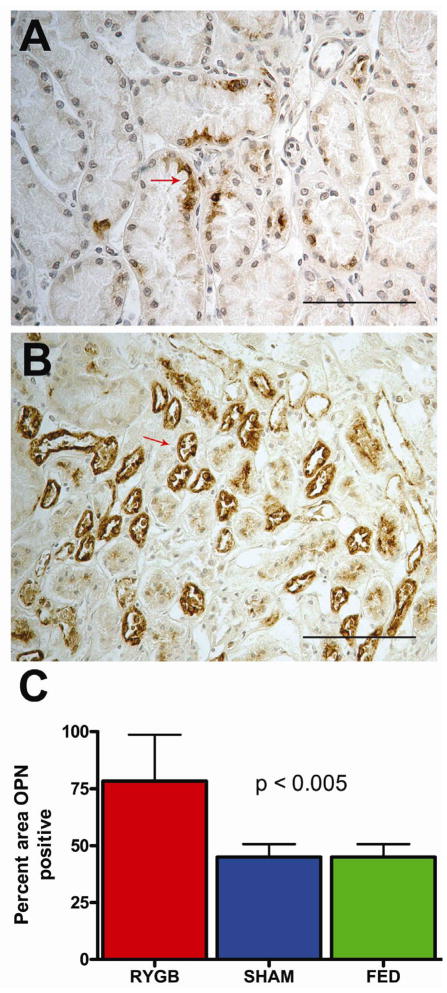
Representative osteopontin staining from Sham controls (A) compared to RYGB (B) is demonstrated in Figure 3. More prominent OPN staining was generally seen throughout all regions of RYGB kidneys, but statistically significant staining differences were only identified within the renal tubular epithelium of the inner medullary region of RYGB rodents compared to Sham and Fed controls (Figure 3C, p<0.005).
Figure 3.
Immunohistochemical detection of osteopontin (OPN) from obese rodent kidney sections. Representative OPN staining from Sham control (A) compared to RYGB surgery (B). 400x magnification with scale bar = 100 μm. RYGB group had more % area positive OPN staining (C) from renal inner medulla than Sham or Fed controls (P<0.005). ). Values represent mean ± SD of the total OPN stained renal tissue area analyzed by ANOVA (p<0.01) and Fisher’s (p<0.01).
Representative ED-1 staining from Fed controls (A) compared to RYGB (B) is demonstrated in Figure 4. Increased numbers of ED-1 stained cells were seen throughout RYGB kidneys. Statistically significant differences in ED-1 staining cells were only identified within the outer medullary region of RYGB rodents compared to controls (Figure 4C, p<0.02).
Figure 4.
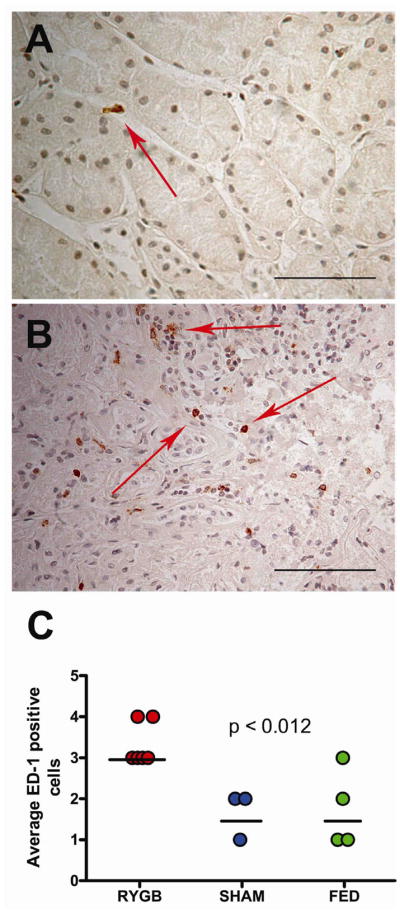
Immunohistochemical detection of ED-1 from obese rodent kidney sections. Representative ED-1 staining from Fed control (A) compared to RYGB surgery (B). 400x magnification with scale bar = 100 μm. RYGB group had higher ED-1 scoring (C) from renal outer medulla than Sham or Fed controls (p<0.02). Dark line represents median value.
DISCUSSION
Obesity has been implicated as a cause of chronic renal disease primarily through hyperfiltration [19] as well as through its co-morbid conditions diabetes and hypertension. Logically, RYGB-associated weight loss should reverse these conditions and stabilize if not improve renal histology. The 26% total body weight loss for RYGB, in our study, is comparable to weight loss noted in long term human studies [2]. A 5% weight loss for pair feds, while slightly less than expected, is well within reported success rates for long-term gastric restrictive procedures [2]. The most remarkable finding of this study was the amount and degree of diverse microscopic alterations within renal glomeruli, tubules, and interstitium in our RYGB rats 42 days following surgery (~3½ human years) compared to controls. Because control groups included both obese sham and obese pair-feds, the degenerative and inflammatory lesions noted in the RYGB group cannot solely be explained by the presence of obesity nor of weight loss/caloric restriction.
The considerable uniformity of abnormal features within RYGB rodent kidneys suggests a common pathogenesis related to some bypass-induced alteration. Glomerular and membranoproliferative changes could be explained by the presence of hypertension or diabetes, but one would expect the changes to be reversed by a weight loss procedure - not caused by it. Moreover, this DIO model is not hypertensive, and the mild insulin resistance associated with dietary obesity in these rodents has previously been shown to reverse as soon as two weeks following RYGB [13,14,20]. The infiltration of the renal interstitium with lymphocytes and monocytes was highest in the outer medulla and well characterized by ED-1 staining. This is most consistent with interstitial nephritis. Increased tubular expression of osteopontin, a highly potent chemokine, was noted within the inner medulla, a major site of OPN-production within the renal tubules. Additionally, the RYGB kidneys were characterized by increased amounts of tubular atrophy, tubular basement membrane thickening, and glomerulosclerosis compared to controls – all of which describe chronic tubulointerstitial nephritis (TIN). Although speculative, the pathogenesis of this TIN is most likely to be explained by RYGB-associated hyperoxaluria.
Historical jejunoileal bypass (JIB) surgery achieved significant weight loss for the morbidly obese until it was banned in the late 1970’s due to undesirable side effect profile, including hyperoxaluria-related renal failure (10%), calcium oxalate nephrolithiasis (29%), and chronic diarrhea due to malabsorption (29%) [21]. The mechanism of JIB hyperoxaluria has long been presumed to be enhanced gut mucosal permeability to oxalate (due to excess colonic bile salts) combined with increased intraluminal oxalate availability, also known as “enteric hyperoxaluria.” Once absorbed, the excess oxalate is subsequently excreted by the kidneys. Several human cohort series have described persistent enteric hyperoxaluria years following RYGB surgery, averaging 80 mg/day in RYGB kidney stone formers [7,8,22,23] and 58 mg/day in RYGB non-stone formers [24–27]. This moderate hyperoxaluria pales in comparison to primary hyperoxaluria (>100 mg/day) or JIB surgery (>120 mg/day). Despite the modest increase, reports of oxalate nephropathy have also been also described for RYGB, although to a much lesser degree than JIB [7,11]. In the most thorough human case series to date, Nasr et al [11] reviewed renal histology from 11 patients who underwent RYGB and developed oxalate nephropathy. In addition to mild to moderate amounts of interstitial inflammation and tubular atrophy (TIN) seen throughout the biopsy specimens, more than 30% of all glomeruli (mean of 13.7 per biopsy) were considered “sclerotic.”[11] Oxalate deposits, predominantly intraluminal, were observed with a mean of 40 deposits per biopsy.
If the pathogenesis of TIN in this model is due to hyperoxaluria, how is it that no crystal deposits were noted in the RYGB group of rats? The rat and mouse family is notorious for their resistance to renal crystal deposition and stone formation, even in the face of severe hyperoxaluria. Our group and others have previously documented hyperoxaluria, crystalluria, and renal tubular damage without renal crystal deposition in rats using hydroxyproline [28,29]. Additionally, interstitial nephritis due to oxalosis without tissue oxalate deposition has been described in hyperoxaluric human JIB studies, both in patients who died from JIB oxalate nephropathy and in hyperoxaluric JIB patients with normal renal function [17,30]. Lastly, oxalate crystals within tissue specimens are often lost during histologic slide processing. Prospective studies in this model, in particular urine chemistries, may yield future insights into the mechanisms behind this injury pattern.
One of this study’s largest limitations is the lack of serum and urinary data. We do not know baseline or post-operative renal function or urinary oxalate levels in these animals. Therefore, any conclusion made from the renal histology must be considered speculative. Additionally, given the small number of rodents and single time point for tissue analysis, the findings are primarily hypothesis generating. Even with these limitations in mind, the deterioration of glomerular and tubular histology in RYGB rodents is significant and noteworthy. Future prospective studies in this animal model are being developed to address both renal function and oxalate concerns. Clinicians are encouraged to continue renal surveillance in this potentially high risk population.
CONCLUSIONS
Bariatric surgery and renal phenomena are irrevocably linked. Compared to controls, our model of RYGB surgery led to a significant deterioration in renal histology, including chronic tubulointerstitial nephritis and glomerulosclerosis. Based on past and present human data, we speculate that excessive urinary oxalate contributed to these injury patterns. Further studies in this model as well as in humans are necessary to determine the etiology and best means of preventing this type of renal complication.
Acknowledgments
The authors acknowledge Pat Glenton for technical laboratory assistance.
Funding for this study was obtained through NIH K08 DK089000 (BKC) and American Diabetes Association grant 1-05-RA-81 (MMM).
Footnotes
Conception and design of the study: MMM, CGG, BKC. Generation/Collection/Assembly: MMM, CGG, BKC, LR, MKR. Analysis: BKC, LR, MKR, SRK. Interpretation of data: BKC, LR, MKR, SRK, MMM. Drafting/Revision/Manuscript Approval: BKC, LR, MKR, SRK, CGG, MMM.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 2.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 3.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005;294:1909–1917. doi: 10.1001/jama.294.15.1909. [DOI] [PubMed] [Google Scholar]
- 4.Blackburn GL. The 2008 Edward E. Mason Founders lecture: interdisciplinary teams in the development of “best practice” obesity surgery. Surg Obes Relat Dis. 2008;4:679–684. doi: 10.1016/j.soard.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Navarro-Diaz M, Serra A, Romero R, Bonet J, Bayes B, Homs M, et al. Effect of drastic weight loss after bariatric surgery on renal parameters in extremely obese patients: long-term follow-up. J Am Soc Nephrol. 2006;17:S213–217. doi: 10.1681/ASN.2006080917. [DOI] [PubMed] [Google Scholar]
- 6.Saliba J, Kasim NR, Tamboli RA, Isbell JM, Marks P, Feurer ID, et al. Roux-en-Y gastric bypass reverses renal glomerular but not tubular abnormalities in excessively obese diabetics. Surgery. 2010;147:282–287. doi: 10.1016/j.surg.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson WK, Houghton SG, Milliner DS, Lieske JC, Sarr MG. Enteric hyperoxaluria, nephrolithiasis, and oxalate nephropathy: potentially serious and unappreciated complications of Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2005;1:481–485. doi: 10.1016/j.soard.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Sinha MK, Collazo-Clavell ML, Rule A, Milliner DS, Nelson W, Sarr MG, et al. Hyperoxaluric nephrolithiasis is a complication of Roux-en-Y gastric bypass surgery. Kidney Int. 2007;72:100–107. doi: 10.1038/sj.ki.5002194. [DOI] [PubMed] [Google Scholar]
- 9.Durrani O, Morrisroe S, Jackman S, Averch T. Analysis of stone disease in morbidly obese patients undergoing gastric bypass surgery. J Endourol. 2006;20:749–752. doi: 10.1089/end.2006.20.749. [DOI] [PubMed] [Google Scholar]
- 10.Matlaga BR, Shore AD, Magnuson T, Clark JM, Johns R, Makary MA. Effect of gastric bypass surgery on kidney stone disease. J Urol. 2009;181:2573–2577. doi: 10.1016/j.juro.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 11.Nasr SH, D’Agati VD, Said SM, Stokes MB, Largoza MV, Radhakrishnan J, et al. Oxalate nephropathy complicating Roux-en-Y Gastric Bypass: an underrecognized cause of irreversible renal failure. Clin J Am Soc Nephrol. 2008;3:1676–1683. doi: 10.2215/CJN.02940608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quinn R. Comparing rat’s to human’s age: how old is my rat in people years? Nutrition. 2005;21:775–777. doi: 10.1016/j.nut.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Meguid MM, Ramos EJ, Suzuki S, Xu Y, George ZM, Das UN, et al. A surgical rat model of human Roux-en-Y gastric bypass. J Gastrointest Surg. 2004;8:621–630. doi: 10.1016/j.gassur.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Guijarro A, Suzuki S, Chen C, Kirchner H, Middleton FA, Nadtochiy S, et al. Characterization of weight loss and weight regain mechanisms after Roux-en-Y gastric bypass in rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1474–1489. doi: 10.1152/ajpregu.00171.2007. [DOI] [PubMed] [Google Scholar]
- 15.Levin BE, Dunn-Meynell AA, Routh VH. Brain glucose sensing and body energy homeostasis: role in obesity and diabetes. Am J Physiol. 1999;276:R1223–1231. doi: 10.1152/ajpregu.1999.276.5.R1223. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y, Ohinata K, Meguid MM, Marx W, Tada T, Chen C, et al. Gastric bypass model in the obese rat to study metabolic mechanisms of weight loss. J Surg Res. 2002;107:56–63. doi: 10.1006/jsre.2002.6508. [DOI] [PubMed] [Google Scholar]
- 17.Drenick EJ, Stanley TM, Border WA, Zawada ET, Dornfeld LP, Upham T, et al. Renal damage with intestinal bypass. Ann Intern Med. 1978;89:594–599. doi: 10.7326/0003-4819-89-5-594. [DOI] [PubMed] [Google Scholar]
- 18.Reinhard MK, Hottendorf GH, Powell ED. Differences in the sensitivity of Fischer and Sprague-Dawley rats to aminoglycoside nephrotoxicity. Toxicol Pathol. 1991;19:66–71. doi: 10.1177/019262339101900108. [DOI] [PubMed] [Google Scholar]
- 19.Chagnac A, Weinstein T, Korzets A, Ramadan E, Hirsch J, Gafter U. Glomerular hemodynamics in severe obesity. Am J Physiol Renal Physiol. 2000;278:F817–822. doi: 10.1152/ajprenal.2000.278.5.F817. [DOI] [PubMed] [Google Scholar]
- 20.Guijarro A, Osei-Hyiaman D, Harvey-White J, Kunos G, Suzuki S, Nadtochiy S, et al. Sustained weight loss after Roux-en-Y gastric bypass is characterized by down regulation of endocannabinoids and mitochondrial function. Ann Surg. 2008;247:779–790. doi: 10.1097/SLA.0b013e318166fd5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Requarth JA, Burchard KW, Colacchio TA, Stukel TA, Mott LA, Greenberg ER, et al. Long-term morbidity following jejunoileal bypass. The continuing potential need for surgical reversal. Arch Surg. 1995;130:318–325. doi: 10.1001/archsurg.1995.01430030088018. [DOI] [PubMed] [Google Scholar]
- 22.Asplin JR, Coe FL. Hyperoxaluria in kidney stone formers treated with modern bariatric surgery. J Urol. 2007;177:565–569. doi: 10.1016/j.juro.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 23.Whitson JM, Stackhouse GB, Stoller ML. Hyperoxaluria after modern bariatric surgery: case series and literature review. Int Urol Nephrol. 2009;42:369–374. doi: 10.1007/s11255-009-9602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel BN, Passman CM, Fernandez A, Asplin JR, Coe FL, Kim SC, et al. Prevalence of hyperoxaluria after bariatric surgery. J Urol. 2009;181:161–166. doi: 10.1016/j.juro.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park AM, Storm DW, Fulmer BR, Still CD, Wood GC, Hartle JE., 2nd A prospective study of risk factors for nephrolithiasis after Roux-en-Y gastric bypass surgery. J Urol. 2009;182:2334–2339. doi: 10.1016/j.juro.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 26.Maalouf NM, Tondapu P, Guth ES, Livingston EH, Sakhaee K. Hypocitraturia and hyperoxaluria after Roux-en-Y gastric bypass surgery. J Urol. 2010;183:1026–1030. doi: 10.1016/j.juro.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duffey BG, Alanee S, Pedro RN, Hinck B, Kriedberg C, Ikramuddin S, et al. Hyperoxaluria is a long-term consequence of Roux-en-Y Gastric bypass: a 2-year prospective longitudinal study. J Am Coll Surg. 2010;211:8–15. doi: 10.1016/j.jamcollsurg.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 28.Khan SR, Shevock PN, Hackett RL. Urinary enzymes and calcium oxalate urolithiasis. J Urol. 1989;142:846–849. doi: 10.1016/s0022-5347(17)38928-0. [DOI] [PubMed] [Google Scholar]
- 29.Evan AP, Bledsoe SB, Smith SB, Bushinsky DA. Calcium oxalate crystal localization and osteopontin immunostaining in genetic hypercalciuric stone-forming rats. Kidney Int. 2004;65:154–161. doi: 10.1111/j.1523-1755.2004.00396.x. [DOI] [PubMed] [Google Scholar]
- 30.Cohen AH. Massive obesity and the kidney. A morphologic and statistical study. Am J Pathol. 1975;81:117–130. [PMC free article] [PubMed] [Google Scholar]