Abstract
Depression, a common neurological condition, is one of the leading causes of disability and suicide worldwide. Standard treatment targeting monoamine transporters selective for the neurotransmitters serotonin and noradrenalin are not able to help many patients that are poor responders. This study advances the development of sazetidine-A analogs that interact with α4β2-nAChR as partial agonists and that possess favorable antidepressant profiles. The resulting compounds that are highly selective for the α4β2 subtype of nAChR over α3β4-nAChRs are partial agonists at the α4β2 subtype and have excellent antidepressant behavioral profiles as measured by the mouse forced swim test. Preliminary ADMET studies for one promising ligand revealed an excellent plasma protein binding (PPB) profile, low CYP450 related metabolism, and low cardiovascular toxicity, suggesting it is a promising lead as well as a drug candidate to be advanced through the drug discovery pipeline.
Introduction
Depression, a common neurological condition, affects approximately 151 million people globally.1 Depressed patients suffer from various symptoms daily, such as difficulty in concentrating, insomnia, and anhedonia.2 Although there is still a dispute as to whether depression represents a syndrome associated with other illnesses or a disease of its own, this question is immaterial to the fact that depression is one of the leading causes of disability and suicide worldwide.3, 4 Today, most medications used to treat depression target serotonergic and/or noradrenergic transmitter systems, or inhibit monoamine oxidase to reduce the degradation of serotonin and noradrenaline.5 Some patients who take anti-depressant drugs experience serious side effects, with only fewer than half of patients responding well to currently available treatments.5 Clearly, there is still an urgent need to find safer and more effective drugs for treating depression.
Along with the discovery of other neurotransmitters and enzymes that relate to depression, various endeavors to find antidepressant medications based on different strategies have been pursued.5, 6 Histone deacetylases (HDAC) inhibitors,7, 8 κ opioid receptor antagonists,5and N-methyl-d-aspartic acid (NMDA) receptor antagonists9 all show an antidepressant profile in preclinical rodent models. Although there is no consensus yet, K+ channel modulators are also believed to have potential in the treatment of depression.10 Furthermore, it is likely that nAChRs provide promising targets for the treatment of depression based on the following findings. First, some classic antidepressants, including tricyclics and selective serotonin reuptake inhibitors (SSRIs), inhibit nAChRs. Second, nicotine exhibits an antidepressant behavioral profile in animals. Third, and most interestingly, presynaptic nAChRs may modulate the release of monoamines.10
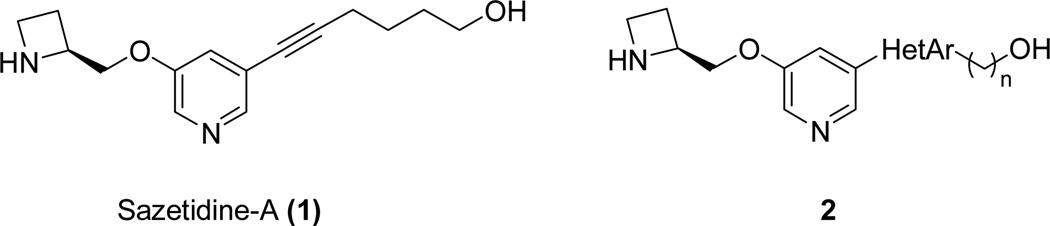
The α4β2-nAChRs are the most prevalent high affinity nicotine-binding nAChR subtypes found in the CNS. Some of the known α4β2-nAChR-selective ligands exhibit antidepressant activities in depression models.11, 12 The antidepressant effects of mecamylamine,13 and even those of the tricyclic antidepressant amitriptyline,14 are abolished in the nAChR β2 subunit knockout mice that lack α4β2-nAChR.15 Moreover, the α3β4*-nAChR-mediated autonomic nicotinic signaling could contribute to the unwanted, adverse side effects observed in vivo.16, 17 Therefore, highly selective α4β2-nAChR ligands over the α3β4 are regarded as promising antidepressant medications. Sazetidine-A (AMOP-H-OH, 1) is a potent and selective agonist at the high-sensitivity (HS) isoform of α4β2 nAChRs18 and exhibits antidepressant-like effects in rodents (Figure 1).19 Although its structural simplicity, efficacy in vivo, and high potency make compound 1 a promising lead, the presence of a metabolically unstable acetylene group diminishes the attractiveness of this compound for further development. However, compound 1 is still useful as a starting point for the design of new ligands.20, 21
Figure 1.
Structure of sazetidine-A and proposed structure with a heteroaromatic ring replacing the acetylene group
Many structural modifications of compound 1 are conceivable to create more attractive compounds. However, replacement of the acetylene with other functional groups to increase metabolic stability is a logical first choice.21Aromatic heterocycles (as in 2), for example an isoxazole ring that is present in many bioactive compounds, are potential candidates for this purpose because of their planarity, which adds a minimum of bulk compared to the acetylene group. Several bioactive compounds bearing isoxazole rings have been reported by our group, such as HDAC inhibitors22, anti-TB agents23, 24, and peroxisome proliferator-activated receptor agonists25.
In this study, several analogs of compound 1 containing an isoxazole ring replacing the acetylene are reported. These compounds are all α4β2 nAChR-selective partial agonists. Some of them exhibit promising behavioral profiles in the mouse forced swim test and appear to be leads for the development of novel antidepressants. In preliminary absorption, distribution, metabolism, excretion and toxicity (ADMET) studies, one of the active compounds was found to display a drug-like profile, thereby commending it for further development. Additionally, all compounds showed very weak binding to α3β4-nAChRs, which mediate autonomic nicotinic signaling, suggesting that few if any peripheral side-effects should be observed.26
Results and Discussion
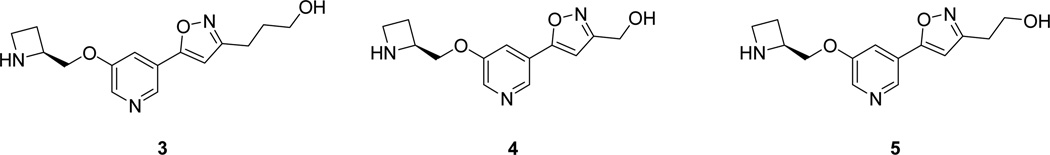
Compound 3, in which the number of carbon atoms between the pyridine ring and the terminal hydroxyl group matches that in compound 1, was synthesized first as the most likely candidate to maintain the biological activity of compound 1 (Figure 2).
Figure 2.
Analogs of sazetidine-A with an isoxazole ring in place of the acetylene group
Compound 3 was first assayed for [3H]epibatidine binding competition. Its Ki values at seven different rat nAChRs subtypes are listed in Table 1. The LogBB value was calculated and is shown in Table 1 to estimate the capability of compound 3 to cross the blood brain barrier (BBB)27, 28. With the exception of α2β2-nAChRs, the compound proved selective for α4β2-nAChRs over other subtypes, with a Ki value of 0.67 nM for α4β2-nAChRs. Moreover, this compound also exhibited high binding affinity at native α4β2*-nAChRs with a Ki value of 1.9 nM. The selectivity for α4β2- over α3β4-nAChR was nearly 15,000-fold, suggesting that ganglionic nAChR-mediated side effects26, 29 would be highly unlikely. Because nicotine has a similar affinity for α4β2*- as it does for α2β2-nAChRs, and since the expression of α2β2-nAChRs in the central nervous system (CNS) is limited, high binding affinity to that subtype is unlikely to be problematic. Having passed the nAChR subtype selectivity screen, compound 3 was subjected to a broad radioligand binding screen to evaluate off-target activity at a variety of CNS neurotransmitter receptors. As shown in Table 2, compound 3 showed no significant binding (> 50%) to other neurotransmitter receptors, including serotonergic, dopaminergic, and adrenergic receptors, with the exception of the κ opioid receptor in the initial screening. However, a secondary screen revealed that its Ki value for the κ opioid receptor was in fact greater than 10,000 nM. Taken together, the data presented in Table 2 predict that there would be few side-effects of compound 3 caused by its interaction with other neurotransmitter receptors.
Table 1.
Binding Affinities of Compounds 3–5 at Seven Rat nAChR Subtypes
| Compd. | Ki (nM)a | LogBBd | ||||||
|---|---|---|---|---|---|---|---|---|
| α2β2 | α2β4 | α3β2 | α3β4 | α4β2 | α4β4 | α4β2*b | ||
| 3 | 0.90±0.05 | 1410e | 16±4 | >10,000 | 0.67±0.20 | 182 | 1.9±0.3 | −0.93 |
| 4 | 0.32±0.04 | 310 | 2.9±0.5 | 6660 | 0.31±0.10 | 81±7 | 0.61±0.10 | −0.99 |
| 5 | 0.76±0.20 | 518 | 8.4±1.5 | >10,000 | 0.37±0.10 | 219 | 1.8±0.2 | −0.99 |
| Nicotinec | 5.5 | 70 | 29 | 260 | 4.9 | 23 | 9.8 | 0.03 |
See Experimental Section.
The asterisk means that other unidentified subunits also may be present, because membrane fractions prepared from rat forebrain contain nAChR subtypes whose subunit composition has not been precisely determined, although they have features of nAChRs containing α4 and β2 subunits.
The binding data for nicotine are from the PDSP Assay Protocol Book (http://pdsp.med.unc.edu/).
LogBB was calculated using the following equation: LogBB = −0.0148PSA + 0.152CLogP + 0.139.
SEM values are not provided for Ki values >100 nM.
Table 2.
Binding Competition Efficacies of Compound 3 at Various Neurotransmitter Receptors at 10 µMa
| Receptors | Serotonergic Receptors | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 5-HT1A | 5-HT1B | 5-HT1D | 5-HT1E | 5-HT2A | 5-HT2B | 5-HT2C | 5-HT3 | 5-HT5A | |
| %Inhibition | 0.3 | −9.9b | 7.1 | 4.1 | 22.2 | 17.7 | 14.6 | 18.8 | −17.8 |
| Receptors | Serotonergic Receptors | GABA | Dopaminergic Receptors | BZPRc | |||||
| 5-HT6 | 5-HT7 | GABAA | D1 | D2 | D3 | D4 | D5 | ||
| %Inhibition | 22.3 | 16.2 | 26.9 | −3.3 | 19.8 | 15.3 | 19.8 | −1.8 | 21.7 |
| Receptors | Adrenergic Receptors | ||||||||
| α1A | α1B | α1D | α2A | α2B | α2C | β1 | β2 | β3 | |
| %Inhibition | −9.5 | −0.4 | 2.9 | −0.5 | 10.8 | 22.0 | −3.2 | −1.0 | −17.7 |
| Receptors | Histaminergic Receptors | Muscarinic Receptors | |||||||
| H1 | H2 | H3 | H4 | M1 | M2 | M3 | M4 | M5 | |
| %Inhibition | −19.9 | 42.6 | 6.2 | −4.4 | 2.0 | 11.4 | −6.8 | 2.0 | −18.9 |
| Receptors | Opioid Receptorsd | Transporterse | Sigma Receptors | ||||||
| DOR | KOR | MOR | DAT | NET | SERT | σ1 | σ2 | ||
| %Inhibition | 6.4 | 20f | 14.2 | −10.1 | 14.2 | −0.6 | 25.9 | −4.3 | |
The default concentration for primary binding experiments is 10 µM (n=4). The inhibition data were generously provided by the National Institute of Mental Health's Psychoactive Drug Screening Program, Contract # HHSN-271-2008-00025-C (NIMH PDSP).
Negative inhibition represents a stimulation of binding.
BZPR: Benzodiazepine Receptor (rat brain site).
DOR: Delta Opioid Receptor; KOR: Kappa Opioid Receptor; MOR: Mu Opioid Receptor.
DAT: Dopamine Transporter; NET: Norepinephrine Transporter; SERT: Serotonin Transporter.
Replication of the binding assay for the this receptor did not confirm the initial result, and the more accurate figure from the secondary assay run is provided instead.
The functional activity of compound 3 was determined using the 86Rb+ ion flux assay30 in SH-EP1-hα4β2 cells (Table 3).31–33 Its nanomolar EC50 and low efficacy characterized compound 3 as a partial agonist of α4β2-nAChRs.
Table 3.
Sensitivities and Efficacies of Ligand Agonism and Inactivation of Human α4β2-nAChRa
| Compd. | Agonism | Inactivation | Ki (nM) | ||
|---|---|---|---|---|---|
| EC50 (nM) | Efficacy (%) | IC50 (nM) | Efficacy (%) | α4β2 | |
| 3 | 36 | 13 | 61 | 68 | 0.67±0.20 |
| 4 | 25 | 13 | 16 | 66 | 0.31±0.10 |
| 5 | 110 | 20 | 44 | 85 | 0.37±0.10 |
| (−)-Nicotine | 290 | 88 | 430 | 93 | 4.9 |
The indicated compounds were used in 86Rb+ efflux assays to define their intrinsic activities as agonists defined by their EC50 values (nM) and efficacies (normalized to that of a full agonist at a maximally efficacious concentration; see Experimental Section, “Agonism”) when acting at human α4β2-nAChRs heterologously and stably expressed in transfected SH-EP1 human epithelial cells. The compounds also were used in efflux assays to define their abilities to inactivate responses to a full agonist at its EC90 concentration as defined by inactivation IC50 values (nM) and inhibitory efficacy (normalized to complete functional inhibition; see Experimental Section, “Inactivation”) when acting at human α4β2-nAChRs. The term “inactivation” is used because compounds may be acting to desensitize receptors and/or as competitive or non-competitive antagonists, and further work is needed to make such a distinction. Also shown for reference are Ki values (nM) for blockade of specific binding of [3H]epibatidine to membrane fractions prepared from (α4β2) HEK cells transfected to express rat α4 and β2 subunits. SEM values were determined for each parameter and although not presented here typically are less than 15% for efficacy measures and no more than a factor of 2 for molar EC50 or IC50 values. Results for compounds 3, 4, and 5 are from 4 independent determinations. Results for nicotine are from Reference 13.
Encouraged by these findings, we pursued additional modifications of compound 3. In the case of analogs of compound 1, it is known that shortening of the oligomethylene chain generally results in retention of affinity and agonist efficacy, while extending the chain reduces these characteristics. Therefore, compounds 4 and 5 featuring shorter chains were synthesized. As is evident from Table 1, both of these compounds exhibit high binding affinity at α4β2-nAChRs and native α4β2*-nAChRs with Ki values in the nanomolar range, and possess high selectivity for α4β2-nAChRs over other nAChRs subtypes, with the non-problematic exception of α2β2-nAChRs. The selectivity for α4β2-over α3β4-nAChRs of compound 4 is an impressive 20,000-fold. The functional characterization reported in Table 3 shows that both compounds 4 and 5 are partial agonists at α4β2-nAChRs.
In vivo Behavioral Pharmacology
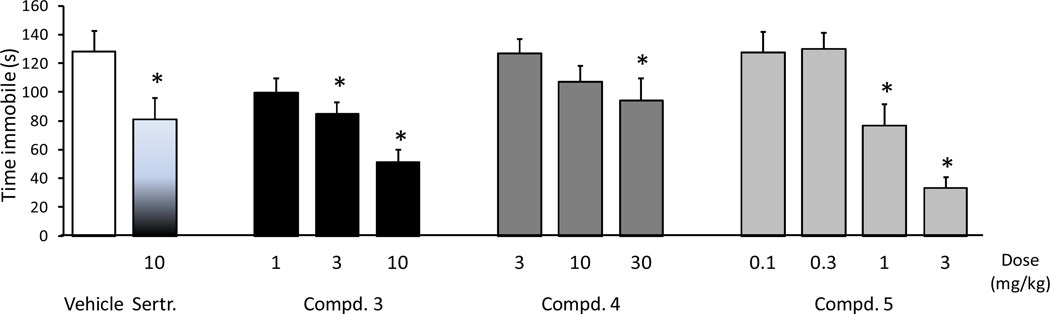
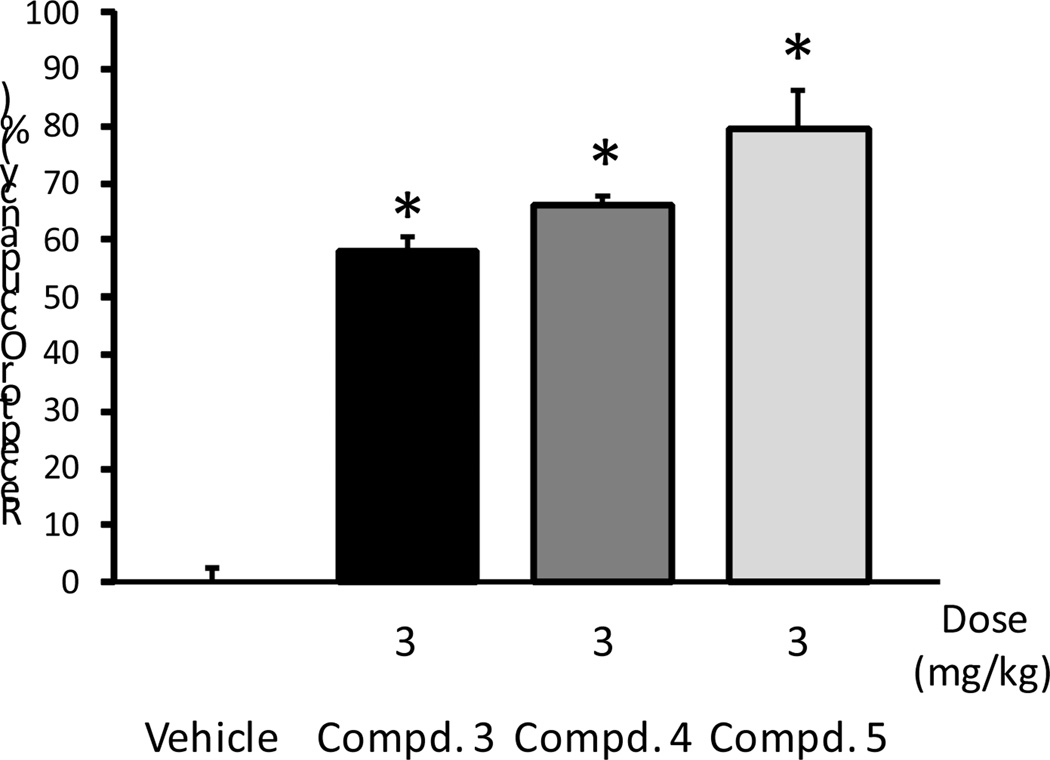
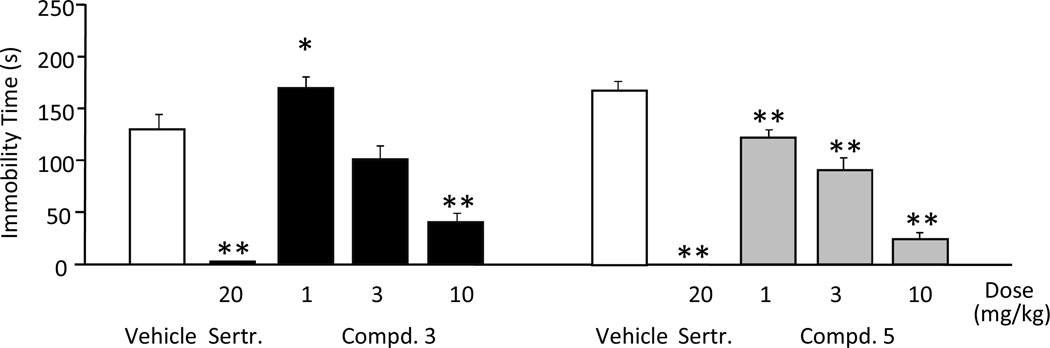
To determine whether the high activity of our compounds at α4β2-nAChR would translate into antidepressant-like efficacy in a behavioral model, compounds 3–5 were investigated in the mouse forced swim test. In this assay antidepressants decrease the amount of time mice spent immobile when forced to swim in a confined space.34 When our compounds were injected intraperitoneally (Figure 3), compound 5 showed the best antidepressant-like response, with a significant reduction in immobility seen at 1 and 3 mg/kg, while compound 3 produced a significant reduction in immobility at 3 mg/kg but only an insignificant trend at 1 mg/kg. Compound 4 was surprisingly weakly active only at 30 mg/kg. Compound 5 showed the highest level of ex vivo receptor occupancy at β2* receptors at 3 mg/kg (around 80%) compared to compounds 3 and 4, which showed approximately 60–70% occupancy at that dosage (Figure 4). The greater efficacy of compound 5 as compared to the other two compounds in the forced swim test may be related to the higher level of receptor occupancy of β2* receptors. Despite the high level of receptor occupancy observed, the poor antidepressant behavior of compound 4 in the forced swim test may indicate subtle differences between ligands that could be missed in a 86Rb+ efflux assay, such as the in vivo elevation of cholinergic tone. As compounds 3 and 5 were both active at 3 mg/kg when administered intraperitoneally in the forced swim test, they were next tested for their antidepressant activity following oral administration. As shown in Figure 5 both compounds 3 and 5 decreased the time immobile at 10 mg/kg in the forced swim test, but 3 failed to show significant activity when tested at the 1 and 3 mg/kg level.
Figure 3.
Compounds 3 and 5 reduced immobility in the forced swim test in mice at the medium and highest dose tested. Compound 4 showed an insignificant trend at the highest dose only. The SSRI sertraline, produced the expected decrease in immobility. (ANOVAs: F (11,108) = 6.9, p < 0.001. *Fisher’s PLSD post-hoc test: ps < 0.05 vs vehicle). All drugs were injected intraperitoneally; n = 10/group).
Figure 4.
Receptor occupancy studies of compounds 3–5 in mice showed a significant occupancy level. Compound 5 showed a higher receptor occupancy than compounds 3 and 4. (*Mann-Whitney U: p < 0.05). All drugs were injected intraperitoneally; n = 3/group.
Figure 5.
Compounds 3 reduced immobility in the forced swim test in mice when given orally at 10 mg/kg. Immobility was slightly higher at the lowest dose. Compound 5 was efficacious at all doses tested. The SSRI sertraline, produced the expected decrease in immobility. (ANOVAs: Fs > 34.5; ps < 0.001. *Fisher’s PLSD post-hoc tests: ps < 0.05 vs vehicle). N = 9−10/group.
Preliminary ADMET Study35
Encouraged by these favorable biological data, we submitted compound 3 for a preliminary ADMET test. In vitro metabolism studies with human liver microsomes and mouse liver microsomes at a concentration of 1 µM showed no detectable metabolism. Assays of the compound’s inhibitory potential towards nine different CYP450 enzymes also revealed no significant inhibition. Plasma protein binding (PPB) assays were conducted with both human plasma and mouse plasma (CD-1) at 10 µM. In human plasma, only 0.4% binding was observed, while in mouse plasma the bound fraction was as high as 26%. The weak PPB translates to a high concentration of free drug in the blood, suggesting that compound 3 should easily diffuse through cell membranes and cross the BBB to reach its biological target. Cardiotoxicity associated with the inhibition of human ether-a-go-go-related gene (hERG)36 was also evaluated, and no obvious inhibition of hERG-related tail current was seen even at the high concentration of 10 µM (8.4% inhibition). These results suggest that compound 3 and its side-chain homologs represent promising drug candidates for further preclinical study.
Chemistry
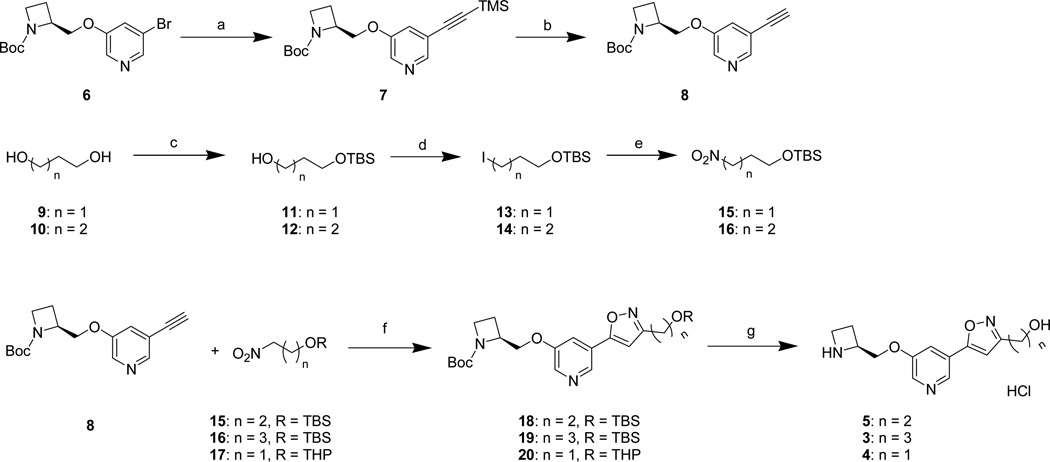
The known starting material 637 was first transformed to alkyne 8 through a Sonogashira coupling reaction with ethynyltrimethylsilane, followed by deprotection with TBAF to remove the trimethylsilyl (TMS) group (Scheme 1). Nitro compounds 15 and 16 were synthesized by monoprotection of diols 9 and 10, followed by conversion of the remaining free hydroxyl groups to nitro groups through iodides 13 and 14 as intermediates, respectively. Protected isoxazoles 18–20 were synthesized through [3+2]-cycloaddition reactions of alkyne 8 with nitrile oxides derived from the above nitro compounds and from their commercially available, tetrahydropyranyl-protected lower homolog 17. Deprotection with acid of the penultimate precursors 18–20 yielded compounds 3–5 as their HCl salts.
Scheme 1.
a Reagents and conditions: (a) ethynyltrimethylsilane, CuI, PPh3, PdCl2 (PPh3)2, Et3N; (b) (n-Bu)4NF, THF; (c) NaH, TBSCl, THF; (d) PPh3, imidazole, I2, Et2O/MeCN; (e) AgNO2, Et2O; (f) PhNCO, Et3N, toluene; (g) HCl, ether/MeOH.
Conclusion
In spite of the plethora of antidepressant drugs on the market, there is still the need to identify improved drug therapies that have a faster onset of action and provide effective treatment of refractory depression. As is now well understood, nAChRs represent promising targets in the search for new antidepressants. Based on rational drug design principles, analogs of compound 1 bearing an isoxazole ring in place of its metabolically unstable acetylene group were designed and tested. The compounds in this series were found to be highly selective α4β2-nAChRs partial agonists. Compounds 3 and 5 exhibit excellent behavioral profiles in the mouse forced swim test, whether administered intraperitoneally or orally, consistent with the antidepressant-like efficacy of these analogs. Moreover, compound 3 exhibited no significant binding affinity for other neurotransmitter receptors or transporters, suggesting that is may be free of undesirable side effects associated with off-target activity. A preliminary ADMET study for the isoxazole 3 revealed that this compound causes no pronounced CYP450 inhibition, possesses low plasma protein binding, and has low hERG inhibitory activity. The compelling activity profile shown by the α4β2-nAChR partial agonist, isoxazole 3, both at the pharmacological and in vivo levels recommend this compound and its analogs for further study in the quest for new antidepressant drug candidates.
Experimental Section
General
Proton and carbon NMR spectra were recorded on a 300 MHz or a 400 MHz spectrometer (1H frequency). NMR chemical shifts are reported in δ (ppm) using the δ 7.26 signal of CDCl3 (1H NMR), δ 4.80 signal of D2O (1H NMR) and the δ 77.2 signal of CDCl3 (13C NMR) as internal standards. 13C NMR spectra in D2O were not adjusted. Optical rotation was detected on an Autopol IV automatic polarimeter. Mass spectra were acquired in the ESI mode at an ionization potential of 70 eV with a LC-MS MSD (Hewlett Packard). Column chromatography was performed using Merck silica gel (40–60 mesh). Purity of the final compounds (>98%) was established using an Agilent 1100 HPLC system equipped with a Synergi 4 μ Hydro-RP 80A column, with detection at 254 (and 280) nm, using a variable wavelength detector G1314A; flow rate = 1.4 mL/min; gradient elution over a time span of 20–29 min, from 30% MeOH–H2O to 100% MeOH (both containing 0.05 vol% of CF3COOH). PL-HCO3 MP-Resin, StratoSphere SPE purchased from Agilent technologies, was used to remove the extra CF3COOH and to transform the compound to its free amine form after HPLC purification.
General Procedure for the [3+2] Cycloaddition to Form Isoxazoles (Method A)
To a solution of nitro compound (2.0–3.0 equiv) and alkyne 8 (1.0 equiv.) in dry toluene (0.2 M in alkyne) were added phenyl isocyanate (1.0 equiv.) and triethylamine (1.0 equiv.). The reaction mixture was stirred at 60 °C for 24–48 h. After the reaction mixture was cooled to room temperature, it was diluted with water and stirred vigorously for 2 h. The mixture was extracted with CH2Cl2, dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The residue was purified by column chromatography with hexane/EtOAc (1:1) to give the isoxazoles in yields of 50–80%.
General Procedure for the Deprotection of N-Boc Precursors to Afford Amines as Hydrochloride Salts (Method B)
To a solution of N-Boc protected precursor (1.0 equiv.) in MeOH was added 2N anhydrous HCl/ether (1 mL) under argon protection at room temperature. The mixture was stirred overnight. After the solvent was evaporated, the residue was dissolved in distilled water (about 20–30 mL), the solution was filtered over a cotton plug, and the water was removed under reduced pressure at 35 °C. The crude product was purified by HPLC (see HPLC conditions below), and the resulting TFA salt was treated with AAA resin to afford the free amine. This material was dissolved in MeOH and treated again with 2 N anhydrous HCl/ether (1 mL) under argon protection at room temperature. The mixture was stirred overnight. After the solvent was evaporated, the residue was dissolved in distilled water (about 20– 30 mL), the solution was filtered over a cotton plug, and water was removed under reduced pressure at 35 °C. Pure HCl salt was obtained after lyophilization.
Preparative HPLC conditions (H2O/MeCN system–gradient A)
ACE AQ 150×21.2 mm column; UV detection at both 254 nm and 280 nm; flow 17.0 mL/min; gradient of 8 to 100% acetonitrile in water [both containing 0.05 vol% of CF3COOH] in 25 min, isocratic 100% for another 5 min, return to 8% in the next 5 min, and final equilibration at 8% for the final 5 min.
Preparative HPLC conditions (H2O/MeOH system–gradient B)
ACE AQ 150×21.2 mm column; UV detection at both 254 nm and 280 nm; flow 17.0 mL/min; gradient of 8 to 100% MeOH in water [both containing 0.05 vol% of CF3COOH] in 25 min, isocratic 100% for another 5 min, return to 8% in the next 5 min, and final equilibration at 8% for the final 5 min.
3-[5-[5-(2(S)-Azetidinylmethoxy)-3-pyridyl]-3-isoxazolyl]-1-propanol Hydrochloride (3)
Method B was used. Yield: 63% (white solid); purity: 98.8%; 1H NMR (400 MHz, D2O) δ 8.93 (s, 1H), 8.68 (s, 1H), 8.59 (m, 1H), 7.11 (s, 1H), 5.00 (m, 1H), 4.66 (m, 2H), 4.13 (m, 2H), 3.64 (t, J = 6.4 Hz, 2H), 2.83 (t, J = 7.6 Hz, 2H), 2.72 (m, 2H), 1.95 (m, 2H); 13C NMR (100 MHz, D2O) δ 165.5, 162.4, 156.3, 131.6, 130.0, 127.6, 127.5, 104.2, 67.7, 60.2, 58.2, 43.4, 29.2, 21.5, 19.9; LC-MS m/z 290.2 (M+H)+; (c = 0.37, MeOH).
5-[5-(2(S)-Azetidinylmethoxy)-3-pyridyl]-3-isoxazolylmethanol Hydrochloride (4)
Method B was used. Yield: 46% (white solid); purity: 99.0%; 1H NMR (400 MHz, D2O) δ 8.93 (s, 1H), 8.66 (d, J = 2.4 Hz, 1H), 8.50 (s, 1H), 7.16 (s, 1H), 5.04 (m, 1H), 4.82 (m, 2H), 4.65 (d, J = 4.0 Hz, 2H), 4.16 (m, 2H), 2.76 (m, 2H); 13C NMR (100 MHz, D2O) δ 165.0, 163.5, 156.6, 132.3, 130.8, 127.8, 127.6, 103.4, 68.1, 58.6, 55.2, 43.8, 20.3; LC-MS m/z 262.1 (M+H)+; (c = 0.64, MeOH). Anal. Calcd. for C13H15N3O3•2.05HCl: C, 46.47; H, 5.11; N, 12.51; Cl, 21.63. Found: C, 46.41; H, 4.95; N, 12.36; Cl, 21.83.
2-[5-[5-(2(S)-Azetidinylmethoxyl)-3-pyridyl]-3-isoxazolyl]ethanol Hydrochloride (5)
Method B was used. Yield: 68% (white solid); purity: 98.9%; 1H NMR (400 MHz, D2O) δ 8.91 (s, 1H), 8.67 (s, 1H), 8.58 (s, 1H), 7.12 (s, 1H), 4.98 (m, 1H), 4.64 (m, 2H), 4.11 (m, 2H), 3.91 (t, J = 6.0 Hz, 2H), 2.97 (t, J = 6.0 Hz, 2H), 2.71 (m, 2H); 13C NMR (100 MHz, D2O) δ 164.1, 163.3, 157.0, 132.4, 130.7, 128.3, 128.2, 105.0, 68.4, 59.7, 58.9, 44.1, 28.7, 20.6; LC-MS m/z 276.1 (M+H)+; (c = 0.65, MeOH).
3-[[1-(tert-Butoxycarbonyl)-2(S)-azetidinyl]methoxy]-5-[(trimethylsilyl)ethynyl]pyridine (7)
To a stirred solution of starting material 6 (550 mg, 1.62 mmol), PPh3 (139 mg, 0.53 mmol), and CuI (137 mg, 0.72 mmol) in triethylamine (7.0 mL) was added PdCl2 (PPh3)2 (112 mg, 0.16 mmol). The mixture was stirred at room temperature for 20 min under argon, and then ethynyltrimethylsilane (0.68 mL, 4.8 mmol) was added. The reaction mixture was stirred at 60 °C for 24 h, quenched with saturated NH4Cl aq., and extracted with CH2Cl2. The organic phase was dried over anhydrous MgSO4, filtered, and concentrated in vacuo. The residue was purified by column chromatography with hexane/EtOAc (10:1–2:1) to give the trimethylsilylalkyne 7 (560 mg, 97%). 1H NMR (400 MHz, CDCl3) δ 8.13 (m, 2H), 7.17 (s, 1H), 4.36 (m, 1H), 4.19 (m, 1H), 3.97 (m, 1H), 3.73 (t, J = 7.6 Hz, 2H), 2.15 (m, 2H), 1.26 (s, 9H), 0.09 (s, 9H). 13C NMR (100 MHz, CDCl3) δ 156.0, 154.3, 144.9, 138.1, 123.1, 120.3, 101.2, 97.9, 79.6, 65.6, 59.9, 46.8, 28.3, 18.9, −0.3.
3-[[1-(tert-Butoxycarbonyl)-2(S)-azetidinyl]methoxy]-5-ethynylpyridine (8)
To a solution of trimethylsilylalkyne 7 (160 mg, 0.444 mmol) in 14 mL of anhydrous THF at 0 °C was added 1.0M tetrabutylammonium fluoride in THF (1.3 mL, 1.3 mmol). The mixture was stirred at 0 °C for 1 h. quenched with saturated NH4Cl aqueous solution, and extracted with CH2Cl2. The organic phase was dried over anhydrous Na2SO4, filtered, and concentrated in vacuo. The residue was purified by chromatography with hexane/EtOAc (1:1) to give alkyne 8 as an oil (125 mg, 98%). 1H NMR (300 MHz, CDCl3) δ 8.24 (m, 2H), 7.27 (s, 1H), 4.46 (m, 1H), 4.30 (m, 1H), 4.06 (m, 1H), 3.82 (t, J = 7.5 Hz, 2H), 3.18 (s, 1H), 2.24 (m, 2H), 1.35 (s, 9H).
3-(tert-Butyldimethylsilyloxy)-1-propanol (11)
To a solution of 1,3-propanediol (1.0 mL, 14 mmol) in anhydrous DME (30 mL) was added NaH (60% dispersion in mineral oil, 560 mg, 14 mmol) in one portion at 0 °C under Ar. The reaction mixture was allowed to warm to room temperature, and after stirring at room temperature for 30 min, tert-butyldimethylsilyl chloride (2.1 g, 14 mmol) was added. The mixture was set aside for 2 days before it was quenched by adding saturated aqueous NH4Cl solution. The mixture was extracted with EtOAc (3 times), and the combined organic layers were washed with brine. After drying over anhydrous Na2SO4, the solvent was removed. The residue was purified by column chromatography with hexane/EtOAc (4:1) to afford compound 11 (1.28 g, 48%) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 3.80 (m, 4H), 2.66 (br, 1H), 1.76 (quint, J = 5.6 Hz, 2H), 0.89 (s, 9H), 0.06 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 63.0, 62.5, 34.4, 26.1, 18.4, 5.3.
4-(tert-Butyldimethylsilyloxy)-1-butanol (12)
Following the same procedure as for the preparation of compound 11, compound 12 was obtained in a yield of 71% as a pale-yellow oil. 1H NMR (400 MHz, CDCl3) δ 3.66 (m, 4H), 2.47 (br, 1H), 1.63 (m, 4H), 0.90 (s, 9H), 0.07 (s, 6H).
1-(tert-Butyldimethylsilyloxy)-3-iodopropane (13)
To a solution of compound 11 (2.80 g, 14.7 mmol), Ph3P (4.63 g, 17.6 mmol) and imidazole (2.00 g, 29.4 mmol) in ether/MeCN (1:1, 80 mL) was added I2 (4.67 g, 18.4 mmol) at 0 °C. The mixture was allowed to stand at room temperature overnight, before it was quenched with sat. NaHSO3 solution. The product was extracted with hexane/EtOAc (4:1), and the organic layer was washed with brine and dried over Na2SO4. After concentration, the residue was purified by column chromatography with hexane/EtOAc (10:1) to afford compound 13 (1.83 g, 41%) as a pale-yellow oil. 1H NMR (400 MHz, CDCl3) δ 3.67 (t, J = 6.0 Hz, 2H), 3.28 (t, J = 6.8 Hz, 2H), 1.99 (m, 2H), 0.90 (s, 9H), 0.07 (s, 6H).
1-(tert-Butyldimethylsilyloxy)-4-iodobutane (14)
Followed the same procedure as for the preparation of compound 13, crude compound 14 was obtained as pale-yellow oil and used directly in the next step without further purification.
1-(tert-Butyldimethylsilyloxy)-3-nitropropane (15)
To a solution of compound 13 (300 mg, 1.0 mmol) in ether (7 mL) in a bottle wrapped with aluminum foil was added AgNO2 (purchased from Aldrich, 170 mg, 1.1 mmol) in one portion. The mixture was stirred at room temperature for 2 days. After filtration followed by solvent removal, the crude compound 15 (174 mg, 79%) was obtained as a colorless oil and used directly in the next step without further purification. 1H NMR (400 MHz, CDCl3) δ 4.50 (t, J = 6.8 Hz, 2H), 3.71 (t, J = 6.0 Hz, 2H), 2.18 (m, 2H), 0.88 (s, 9H), 0.05 (s, 6H); 13C NMR (100 MHz, CDCl3) δ 72.8, 59.4, 30.4, 26.0, 18.4, 5.4.
1-(tert-Butyldimethylsilyloxy)-4-nitrobutane (16)
Following the same procedure as for the preparation of compound 15, compound 16 was obtained (yield: 34% over two steps) as a pale-yellow oil.
3-[[1-(tert-Butoxycarbonyl)-2(S)-azetidinyl]methoxy]-5-[3-[(2-tert-butyldimethylsilyloxy)ethyl]-5-isoxazolyl]pyridine (18)
Method A was followed. Yield: 54% (pale-yellow oil); 1H NMR (400 MHz, CDCl3) δ 8.47 (m, 2H), 7.59 (s, 1H), 6.56 (s, 1H), 4.53 (m, 1H), 4.39 (m, 1H), 4.16 (m, 1H), 3.89 (m, 4H), 2.92 (t, J = 6.0 Hz, 2H), 2.30 (m, 2H), 1.39 (s, 9H), 0.84 (s, 9H), 0.03 (s, 6H).
3-[[1-(tert-Butoxycarbonyl)-2(S)-azetidinyl]methoxy]-5-[3-[(3-tert-butyldimethylsilyloxy)propyl]-5-isoxazolyl]pyridine (19)
Method A was followed. Yield: 59% (pale yellow oil); 1H NMR (400 MHz, CDCl3) δ 8.54 (s, 1H), 8.32 (s, 1H), 7.55 (s, 1H), 6.45 (s, 1H), 4.48 (m, 1H), 4.34 (m, 1H), 4.13 (dd, J = 4.0, 12.0, 1H), 3.83 (t, J = 6.0 Hz, 2H), 3.64 (t, J = 6.0 Hz, 2H), 2.74 (t, J = 6.0 Hz, 2H), 2.27 (m, 2H), 1.86 (m, 2H), 1.34 (s, 9H), 0.83 (s, 9H), −0.01 (s, 6H); 13C NMR (75 MHz, CDCl3) δ 166.5, 164.6, 156.2, 155.3, 139.6, 139.4, 124.3, 117.5, 100.8, 79.8, 68.9, 62.1, 60.1, 47.2, 31.2, 28.3, 26.0, 22.7, 19.1, 18.3.
3-[[1-(tert-Butoxycarbonyl)-2(S)-azetidinyl]methoxy]-5-[3-[(tetrahydro-2H-pyran-2-yloxy)methyl]-5-isoxazolyl]pyridine (20)
Method A was followed. Yield: 89% (pale-yellow oil); 1H NMR (300 MHz, CDCl3) δ 8.42 (s, 1H), 8.18 (s, 1H), 7.43 (s, 1H), 6.56 (s, 1H), 4.60 (m, 1H), 4.47 (m, 1H), 4.35 (m, 1H), 4.22 (m, 1H), 4.02 (m, 1H), 3.99 (m, 1H), 3.69 (m, 3H), 3.36 (m, 1H), 2.14 (m, 2H), 1.66-1.41 (m, 6H), 1.20 (s, 9H); 13C NMR (75 MHz, CDCl3) δ 166.7, 162.1, 155.8, 154.9, 139.5, 139.0, 123.7, 117.1, 100.3, 98.0, 79.3, 68.6, 61.8, 59.9, 59.8, 30.0, 28.6, 25.0, 18.9, 18.8.
In vitro studies
[3H]Epibatidine competition study: For experimental details please refer to the PDSP web site http://pdsp.med.unc.edu/
Cell lines and culture
Cell lines naturally or heterologously expressing specific, functional, human nAChR subtypes were used. The human clonal cell line TE671/RD naturally expresses human muscle-type a1*-nAChRs, containing α1, β1, γ, and δ subunits, with function detectable using 86Rb+ efflux assays.38 The human neuroblastoma cell line SH-SY5Y naturally expresses autonomic α3β4*-nAChRs, containing α3, β4, probably α5, and sometimes β2 subunits, and also displays function detectable using 86Rb+ efflux assays.39 SH-SY5Y cells also express homopentameric α7-nAChR, however, their function is not detected in the 86Rb+ efflux assay under the conditions used. SH-EP1 human epithelial cells stably transfected with human α4 and β2 subunits (SH-EP1-hα4β2 cells) have been established and characterized with both ion flux and radioligand binding assays.40 SH-EP1-hα4β2 cells have further been shown to express a mixture of high sensitivity and low sensitivity α4β2 nAChRs, for which the ratio of functional expression on the cell surface can be approximated by the response to a fully efficacious dose of compound 1, which is fully efficacious at high sensitivity α4β2 nAChR and negligibly efficacious at low sensitivity α4β2.
TE671/RD, SH-SY5Y, and transfected SH-EP1 cell lines were maintained as low passage number (1–26 from our frozen stocks) cultures to ensure stable expression of native or heterologously expressed nAChRs as previously described.38 Cells were passaged once a week by splitting just-confluent cultures 1/300 (TE671/RD), 1/10 (SH-SY5Y) or 1/40 (transfected SH-EP1) in serum-supplemented medium to maintain log-phase growth.
86Rb+ efflux assays
Function of nAChR subtypes was investigated using an established 86Rb+ efflux assay protocol.38 The assay is specific for nAChR function under the conditions used, for example, giving identical results in the presence of 100 nM atropine to exclude possible contributions of muscarinic acetylcholine receptors. Cells harvested at confluence from 100 mm plates under a stream of fresh medium only (SH-SY5Y cells) or after mild trypsinization (Irvine Scientific, USA; for TE671/RD or transfected SH-EP1 cells) were then suspended in complete medium and evenly seeded at a density of 1.25–2 confluent 100 mm plates per 24-well plate (Falcon; ~100–125 mg of total cell protein per well in a 500 µL volume; poly-l-lysine-coated for SH-SY5Y cells). After cells had adhered generally overnight, but no sooner than 4 h later, the medium was removed and replaced with 250 µL per well of complete medium supplemented with ~350000 cpm of 86Rb+ (NEN; counted at 40% efficiency using Cerenkov counting and the Packard TriCarb 1900 Liquid Scintillation Analyzer). After at least 4 h and typically overnight, 86Rb+ efflux was measured using the “flip-plate” technique.40 Briefly, after aspiration of the bulk of 86Rb+ loading medium from each well of the “cell plate,” each well containing cells was rinsed with 2 mL of fresh 86Rb+ efflux buffer (130 mM NaCl, 5.4 mM KCl, 2 mM CaCl2, 5 mM glucose, 50 mM HEPES, pH 7.4) to remove extracellular 86Rb+. Following removal of residual rinse buffer by aspiration, the flip-plate technique was used again to simultaneously introduce 1.5 mL of fresh efflux buffer containing drugs of choice at indicated final concentrations from a 24-well “efflux/drug plate” into the wells of the cell plate. After a 9.5 min incubation, the solution was “flipped” back into the efflux/drug plate, and any remaining buffer in the cell plate was removed by aspiration. 10 min after the initiation of the first drug treatment, a second efflux/drug plate was used to reintroduce the same concentrations of drugs of choice with the addition of an ~EC90 concentration of the full agonist carbamylcholine for 5 min (~EC90 concentrations were 200 µM for SH-EP1-hα4β2 cells, 2 mM for SHSY5Y cells, and 464 mM for TE671/RD cells). The second drug treatment was then flipped back into its drug plate, and the remaining cells in the cell plate were lysed and suspended by addition of 1.5 mL of 0.1 M NaOH, 0.1% sodium dodecyl sulfate to each well. Suspensions in each well were then subjected to Cerenkov counting (Wallac Micobeta Trilux 1450; 25% efficiency) after placement of inserts (Wallac 1450–109) into each well to minimize cross-talk between wells.
For quality control and normalization purposes, the sum of 86Rb+ in cell plates and efflux/drug plates was defined to confirm material balance (i.e., that the sum of 86Rb+ released into the efflux/drug plates and 86Rb+ remaining in the cell plate were the same for each well). Similarly, the sum of 86Rb+ in cell plates and efflux/drug plates also determined the efficiency of 86Rb+ loading (the percentage of applied 86Rb+ actually loaded into cells). Furthermore, the sum of 86Rb+ in cell plates and the second efflux/drug plates defined the amount of intracellular 86Rb+ available at the start of the second, 5 min assay and were used to normalize nAChR function assessed.
For each experiment, in one set of control samples, total 86Rb+ efflux was assessed in the presence of a fully efficacious concentration of carbamylcholine alone (1 mM for SH-EP1-hα4β2 and TE671/RD cells, or 3 mM for SH-SY5Y cells). Nonspecific 86Rb+ efflux in another set of control samples was measured either in the presence of the fully efficacious concentration of carbamylcholine plus 100 µM mecamylamine, which gave full block of agonist-induced and spontaneous nAChR-mediated ion flux, or in the presence of efflux buffer alone. Both determinations of nonspecific efflux were equivalent. Specific efflux was then taken as the difference in control samples between total and nonspecific 86Rb+-efflux. The same approaches were used to define total, nonspecific, and specific ion flux responses in samples subjected to the second, 5 min, exposure to test drug with or without carbamylcholine at its ~EC90 concentration. For the purpose of determining the approximate ratio of high sensitivity and low sensitivity α4β2 nAChRs for a given experiment, a fully efficacious dose of 1 µM compound 1 was used for quality control.
Intrinsic agonist activity of test drugs was ascertained during the first 9.5 min of the initial 10 min exposure period using samples containing test drug only at different concentrations and was normalized, after subtraction of nonspecific efflux, to specific efflux in carbamylcholine control samples. Specific 86Rb+ efflux elicited by test drug as a percentage of specific efflux in carbamylcholine controls was the same in these samples whether measured in absolute terms or as a percentage of loaded 86Rb+. Even in samples previously giving an efflux response during the initial 10 min exposure to a partial or full agonist, residual intracellular 86Rb+ was adequate to allow assessment of nAChR function in the secondary, 5 min assay. However, care was needed to ensure that data were normalized to the amount of intracellular 86Rb+ available at the time of the assay, as absolute levels of total, nonspecific, or specific efflux varied in cells partially depleted of intracellular 86Rb+ due to action of any agonist present during the 10 min drug exposure period. That is, calculations of specific efflux as a percentage of loaded 86Rb+ were typically corrected for any variation in the electrochemical gradient of 86Rb+ created by intracellular ion depletion after the first (agonism/pretreatment) drug treatment.
Ion flux assays (n ≥ 3 separate studies for each drug and cell line combination) were fit to the Hill equation, F=Fmax/(1+(X/EC50)n), where F is the percentage of control, Fmax, for EC50 (n > 0 for agonists) or IC50 (n < 0 for antagonists) values using Prism 4 (GraphPad, San Diego, USA). Most ion flux data were fit allowing maximum and minimum ion flux values to be determined by curve fitting but in some cases, where antagonists or agonists had weak functional potency, minimum ion flux was set at 0% of control or maximum ion flux was set at 100% of control, respectively.
General Procedures for Behavioral Studies
Animals
BALB/cJ male mice (8−10 weeks old at testing) were obtained from Jackson Laboratory (Bar Harbor, ME USA). Mice were housed four to a cage in a colony room maintained at 22±2 °C on a 12 h light–dark cycle. All animal experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the PsychoGenics Animal Care and Use Committee.
Drugs
Compounds 3–5 were synthesized according to procedures described in the text, and sertraline was purchased from Toronto Research Chemicals (Ontario, Canada). All compounds were dissolved in injectable water and administered by intraperitoneal (IP) injection or oral gavage (PO) in a volume of 10 mL/kg.
Mouse forced swim test
Procedures were based on those previously described34. Mice were individually placed into clear glass cylinders (15 cm tall × 10 cm wide, 1 L beakers) containing 23±1 °C water 12 cm deep (approximately 800 mL). Mice were administered vehicle, the SSRI sertraline (10 or 20 mg/kg) as a positive control, or compounds 3–5. Thirty minutes following IP or PO administration, mice were placed in the water, and the time the animal spent immobile was recorded over a 6 min trial. Immobility was defined as the postural position of floating in the water.
Statistical Analysis
Data were analyzed with Analysis of Variance (ANOVA) with treatment group (vehicle, sertraline, compounds 3–5) as the between group variable and total time immobile in sec (over the 6 min trial) as the dependent variable. Significant main effects were followed up with the post hoc Newman-Keuls test.
β2* nAchR ex vivo receptor occupancy
Compound 3, 4 and 5 (3 mg/kg) or water was administered 30 min before brain collection (the same time point as in forced swim testing) for analysis of β2 nAChR occupancy in the thalamus (for compound 3 and 5, n=3; for compound 4, n=4) as described before.41
Acknowledgments
This research was supported by Award Number U19MH085193 from the National Institute of Mental Health. The Phoenix research component was also supported in part by the Barrow Neurological Foundation and was conducted in part in the Charlotte and Harold Simensky Neurochemistry of Alzheimer’s Disease Laboratory. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. We thank the PDSP program for performing binding affinity assays. We thank Dr. Werner Tueckmantel of PsychoGenics, Inc. for his assistance in proofreading the manuscript.
Abbreviations
- CNS
central nervous system
- BBB
blood-brain barrier
- HS
high-sensitivity
- nAChR(s)
nicotinic acetylcholine receptor(s)
- PPB
plasma protein binding
- ADMET
absorption, distribution, metabolism, excretion (and toxicity)
- HDAC
histone deacetylases
- NMDA
N-methyl-d-aspartic acid
- SSRI
selective serotonin reuptake inhibitor
- hERG
human ether-a-go-go-related gene
References
- 1.WHO. Mental health and development: targeting people with mental health conditions as a vulnerable group. WHO Press; 2010. [Google Scholar]
- 2.Henn F, Vollmayr B, Sartorius A. Mechanisms of depression: the role of neurogenesis. Drug Discovery Today: Disease Mechanisms. 2004;1:407–411. [Google Scholar]
- 3.WHO. Global health risks: mortality and burden of disease attributable to selected major risks. WHO Press; 2009. [Google Scholar]
- 4.Reeves RR, Ladner ME. Antidepressant-induced suicidality: an update. CNS Neurosci Ther. 2010;16:227–234. doi: 10.1111/j.1755-5949.2010.00160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 6.Zheng CJ, Han LY, Yap CW, Ji ZL, Cao ZW, Chen YZ. Therapeutic targets: progress of their exploration and investigation of their characteristics. Pharmacol Rev. 2006;58:259–279. doi: 10.1124/pr.58.2.4. [DOI] [PubMed] [Google Scholar]
- 7.Abel T, Zukin RS. Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr Opin Pharmacol. 2008;8:57–64. doi: 10.1016/j.coph.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schroeder FA, Lin CL, Crusio WE, Akbarian S. Antidepressant-like effects of the histone deacetylase inhibitor, sodium butyrate, in the mouse. Biol Psychiatry. 2007;62:55–64. doi: 10.1016/j.biopsych.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 9.Miyamoto Y, Yamada K, Noda Y, Mori H, Mishina M, Nabeshima T. Lower sensitivity to stress and altered monoaminergic neuronal function in mice lacking the NMDA receptor epsilon 4 subunit. J Neurosci. 2002;22:2335–2342. doi: 10.1523/JNEUROSCI.22-06-02335.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shytle RD, Silver AA, Lukas RJ, Newman MB, Sheehan DV, Sanberg PR. Nicotinic acetylcholine receptors as targets for antidepressants. Mol Psychiatry. 2002;7:525–535. doi: 10.1038/sj.mp.4001035. [DOI] [PubMed] [Google Scholar]
- 11.Buckley MJ, Surowy C, Meyer M, Curzon P. Mechanism of action of A-85380 in an animal model of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:723–730. doi: 10.1016/j.pnpbp.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson SM, Brodkin JD, Lloyd GK, Menzaghi F. Antidepressant-like effects of the subtype-selective nicotinic acetylcholine receptor agonist, SIB-1508Y, in the learned helplessness rat model of depression. Psychopharmacology (Berl) 2000;152:295–303. doi: 10.1007/s002130000531. [DOI] [PubMed] [Google Scholar]
- 13.Rabenstein RL, Caldarone BJ, Picciotto MR. The nicotinic antagonist mecamylamine has antidepressant-like effects in wild-type but not beta2- or alpha7-nicotinic acetylcholine receptor subunit knockout mice. Psychopharmacology (Berl) 2006;189:395–401. doi: 10.1007/s00213-006-0568-z. [DOI] [PubMed] [Google Scholar]
- 14.Caldarone BJ, Harrist A, Cleary MA, Beech RD, King SL, Picciotto MR. High-affinity nicotinic acetylcholine receptors are required for antidepressant effects of amitriptyline on behavior and hippocampal cell proliferation. Biol Psychiatry. 2004;56:657–664. doi: 10.1016/j.biopsych.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Picciotto MR, Zoli M, Lena C, Bessis A, Lallemand Y, Le Novere N, Vincent P, Pich EM, Brulet P, Changeux JP. Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature. 1995;374:65–67. doi: 10.1038/374065a0. [DOI] [PubMed] [Google Scholar]
- 16.Romanelli MN, Gratteri P, Guandalini L, Martini E, Bonaccini C, Gualtieri F. Central nicotinic receptors: structure, function, ligands, and therapeutic potential. ChemMedChem. 2007;2:746–767. doi: 10.1002/cmdc.200600207. [DOI] [PubMed] [Google Scholar]
- 17.Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol. 2009;78:703–711. doi: 10.1016/j.bcp.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 18.Zwart R, Carbone AL, Moroni M, Bermudez I, Mogg AJ, Folly EA, Broad LM, Williams AC, Zhang D, Ding C, Heinz BA, Sher E. Sazetidine-A is a potent and selective agonist at native and recombinant alpha 4 beta 2 nicotinic acetylcholine receptors. Mol. Pharmacol. 2008;73:1838–1843. doi: 10.1124/mol.108.045104. [DOI] [PubMed] [Google Scholar]
- 19.Xiao Y, Fan H, Musachio JL, Wei ZL, Chellappan SK, Kozikowski AP, Kellar KJ. Sazetidine-A, a novel ligand that desensitizes alpha4beta2 nicotinic acetylcholine receptors without activating them. Mol Pharmacol. 2006;70:1454–1460. doi: 10.1124/mol.106.027318. [DOI] [PubMed] [Google Scholar]
- 20.Kozikowski AP, Eaton JB, Bajjuri KM, Chellappan SK, Chen Y, Karadi S, He R, Caldarone B, Manzano M, Yuen PW, Lukas RJ. Chemistry and pharmacology of nicotinic ligands based on 6-[5-(azetidin-2-ylmethoxy)pyridin-3-yl]hex-5-yn-1-ol (AMOP-H-OH) for possible use in depression. ChemMedChem. 2009;4:1279–1291. doi: 10.1002/cmdc.200900079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Eaton JB, Caldarone B, Lukas RJ, Kozikowski AP. Chemistry and pharmacological characterization of novel nitrogen analogues of AMOP-H-OH (Sazetidine-A, 6-[5- (azetidin-2-ylmethoxy)pyridin-3-yl]hex-5-yn-1-ol) as alpha4beta2-nicotinic acetylcholine receptor-selective partial agonists. J. Med. Chem. 2010;53:6973–6985. doi: 10.1021/jm100765u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tapadar S, He R, Luchini DN, Billadeau DD, Kozikowski AP. Isoxazole moiety in the linker region of HDAC inhibitors adjacent to the Zn-chelating group: effects on HDAC biology and antiproliferative activity. Bioorg Med Chem Lett. 2009;19:3023–3026. doi: 10.1016/j.bmcl.2009.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang Q, Mao J, Wan B, Wang Y, Brun R, Franzblau SG, Kozikowski AP. Searching for new cures for tuberculosis: design, synthesis, and biological evaluation of 2-methylbenzothiazoles. J. Med. Chem. 2009;52:6757–6767. doi: 10.1021/jm901112f. [DOI] [PubMed] [Google Scholar]
- 24.Lilienkampf A, Pieroni M, Wan B, Wang Y, Franzblau SG, Kozikowski AP. Rational design of 5-phenyl-3-isoxazolecarboxylic acid ethyl esters as growth inhibitors of Mycobacterium tuberculosis. a potent and selective series for further drug development. J. Med. Chem. 2010;53:678–688. doi: 10.1021/jm901273n. [DOI] [PubMed] [Google Scholar]
- 25.Wei ZL, Petukhov PA, Bizik F, Teixeira JC, Mercola M, Volpe EA, Glazer RI, Willson TM, Kozikowski AP. Isoxazolyl-serine-based agonists of peroxisome proliferator-activated receptor: design, synthesis, and effects on cardiomyocyte differentiation. J. Am. Chem. Soc. 2004;126:16714–16715. doi: 10.1021/ja046386l. [DOI] [PubMed] [Google Scholar]
- 26.Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem. Pharmacol. 2009;78:703–711. doi: 10.1016/j.bcp.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Nicolazzo JA, Charman SA, Charman WN. Methods to assess drug permeability across the blood-brain barrier. J. Pharm. Pharmacol. 2006;58:281–293. doi: 10.1211/jpp.58.3.0001. [DOI] [PubMed] [Google Scholar]
- 28.Fan Y, Unwalla R, Denny RA, Di L, Kerns EH, Diller DJ, Humblet C. Insights for predicting blood-brain barrier penetration of CNS targeted molecules using QSPR approaches. J. Chem. Inf. Model. 2010;50:1123–1133. doi: 10.1021/ci900384c. [DOI] [PubMed] [Google Scholar]
- 29.Romanelli MN, Gratteri P, Guandalini L, Martini E, Bonaccini C, Gualtieri F. Central nicotinic receptors: structure, function, ligands, and therapeutic potential. ChemMedChem. 2007;2:746–767. doi: 10.1002/cmdc.200600207. [DOI] [PubMed] [Google Scholar]
- 30.Lukas RJFJD, Eaton JB, Gentry CL. Some methods for studies of nicotinic acetylcholine receptor pharmacology. In: Levin ED, editor. Nicotinic Receptors and the Nervous System. Boca Raton, FL: CRC Press; 2002. pp. 3–27. [Google Scholar]
- 31.Paterson NE, Min W, Hackett A, Lowe D, Hanania T, Caldarone B, Ghavami A. The high-affinity nAChR partial agonists varenicline and sazetidine-A exhibit reinforcing properties in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1455–1464. doi: 10.1016/j.pnpbp.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 32.Turner JR, Castellano LM, Blendy JA. Nicotinic partial agonists varenicline and sazetidine-A have differential effects on affective behavior. J Pharmacol Exp Ther. 2010;334:665–672. doi: 10.1124/jpet.110.166280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozikowski AP, Eaton JB, Bajjuri KM, Chellappan SK, Chen Y, Karadi S, He R, Caldarone B, Manzano M, Yuen PW, Lukas RJ. Chemistry and pharmacology of nicotinic ligands based on 6-[5-(azetidin-2-ylmethoxy)pyridin-3-yl]hex-5-yn-1-ol (AMOP-H-OH) for possible use in depression. ChemMedChem. 2009;4:1279–1291. doi: 10.1002/cmdc.200900079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- 35.The Preliminary ADMET studies were carried out by Cerep, Inc.
- 36.Jamieson C, Moir EM, Rankovic Z, Wishart G. Medicinal chemistry of hERG optimizations: Highlights and hang-ups. J. Med. Chem. 2006;49:5029–5046. doi: 10.1021/jm060379l. [DOI] [PubMed] [Google Scholar]
- 37.Lin N-HHY, Holladay MW, Ryther K, Li Y. 3-Pyridyloxymethyl heterocyclic ether compounds useful in controlling chemical synaptic transmission. 1997 May 13; [Google Scholar]
- 38.Lukas RJFJD, Eaton JB, Gentry CL. In: Nicotinic receptors and the nervous system. Edward DL, editor. 2002. pp. 3–27. [Google Scholar]
- 39.Lukas RJ, Norman SA, Lucero L. Characterization of Nicotinic Acetylcholine Receptors Expressed by Cells of the SH-SY5Y Human Neuroblastoma Clonal Line. Mol. Cell Neurosci. 1993;4:1–12. doi: 10.1006/mcne.1993.1001. [DOI] [PubMed] [Google Scholar]
- 40.Eaton JB, Peng JH, Schroeder KM, George AA, Fryer JD, Krishnan C, Buhlman L, Kuo YP, Steinlein O, Lukas RJ. Characterization of human alpha 4 beta 2-nicotinic acetylcholine receptors stably and heterologously expressed in native nicotinic receptor-null SH-EP1 human epithelial cells. Mol. Pharmacol. 2003;64:1283–1294. doi: 10.1124/mol.64.6.1283. [DOI] [PubMed] [Google Scholar]
- 41.Caldarone BJ, Wang D, Paterson NE, Manzano M, Fedolak A, Cavino K, Kwan M, Hanania T, Chellappan SK, Kozikowski AP, Olivier B, Picciotto MR, Ghavami A. Dissociation between duration of action in the forced swim test in mice and nicotinic acetylcholine receptor occupancy with sazetidine, varenicline, and 5-I-A85380. Psychopharmacology (Berl) 2011;217:199–210. doi: 10.1007/s00213-011-2271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]