Abstract
The separation and specification of mesoderm into the notochord and somites involves members of the non-clustered δ-protocadherins. Axial (AXPC) and paraxial (PAPC) protocadherins are expressed in the early dorsal mesoderm and later become refined to the developing notochordal and somitic mesoderm respectively. The role of PAPC in this process has been studied extensively, but the role of AXPC is poorly understood. Partial knockdown of AXPC causes a specific bent axis phenotype, while more severe knockdown results in the loss of notochord formation. The inability of these embryos to develop a notochord is not due to a cell-sorting event via changes in cell adhesion during gastrulation, but rather this defect is manifested through the loss of axial mesoderm specification, but not general mesoderm induction. The results presented here show that AXPC functions in notochord morphogenesis by directing cell fate decisions rather than cell-cell adhesion.
Keywords: AXPC, protocadherin, morphogenesis, notochord, Xenopus
INTRODUCTION
The ability of the developing embryo to properly execute its morphogenetic programs is dependent, in part, on regulated changes in cell-cell adhesion (Steinberg, 2007) (Hammerschmidt and Wedlich, 2008). The cadherin super-family of adhesion molecules is integral to this process in all vertebrate and many non-vertebrate organisms (Gumbiner, 2005) (Abedin and King, 2008). Generally, cadherins constitute a family of Ca++-dependant, transmembrane cell adhesion molecules that contribute to the ability of cells to sort, and they play critical roles in the early development of embryos. Protocadherins represent the largest group of cadherin-like molecules and are widely expressed throughout development and perform numerous functions (Morishita and Yagi, 2007). Recently, the role of the non-clustered δ–protocadherins in embryonic development has begun to be explored. This subgroup contains a diverse group of molecules that are expressed in all vertebrate organisms, and recently even shown to be present in the chordates ciona (Yagi, 2008) (Noda and Satoh, 2008). There are currently 9 known members of this subgroup, which can be further divided into the δ1 (PCHD -1,-7,-9,-11) and the δ2 (PCDH -8,10,-17,-18,-19) protocadherins. Members of both subgroups have been shown to play critical roles in early embryonic development. PAPC (paraxial protocadherin, PCDH-8) is perhaps the best understood protocadherin of this group. It is expressed early in the paraxial mesoderm and its function is required for the morphogenesis of paraxial mesoderm during gastrulation including convergent-extension movements, tissue separation, and somitogenesis (Kim et al., 1998; Rhee et al., 2003; Medina et al., 2004; Unterseher et al., 2004; Chen and Gumbiner, 2006). PAPC mediates these processes through its ability to specifically down-regulate C-cadherin adhesive activity (Chen and Gumbiner, 2006; Chen et al., 2009) and its ability to interact with the Wnt receptor, Frizzled-7 (Medina et al., 2004) in order to activate downstream signaling through JNK and RhoA (Unterseher et al., 2004).
Other δ–protocadherins have also recently been identified as critical regulators of morphogenesis. For example, neural fold protocadherin (NFPC, PCDH-7) and PCDH-19 are involved specifically in the morphogenesis of neural ectoderm, however they do so through distinct mechanisms. NFPC interacts with TAF1 at its cytoplasmic tail in the neural-fold in Xenopus, an interaction that is required to direct cytoskeletal changes (Heggem and Bradley, 2003; Rashid et al., 2006). In Zebrafish, PCDH-19 interacts directly with N-cadherin to mediate changes in cell adhesion in zebrafish to drive neurulation in the anterior neural plate (Biswas et al., 2010). PCDH-18a is also involved in neural development in zebrafish, where it influences cell adhesion and migration, but not cell identity (Aamar and Dawid, 2008). In fact, none of the protocadherins examined so far have been shown to facilitate changes in cell fate.
Axial protocadherin (AXPC, PDCH-1) was shown to be involved in notochordal morphogenesis in Xenopus laevis and was suggested to do so through its adhesive function and ability to cause cell sorting (Kim et al., 1998; Kuroda et al., 2002). Its expression coincides with the onset of gastrulation, in a reciprocal pattern relative to PAPC, with AXPC in the axial notochord region and PAPC in the paraxial somitic region. This provided an interesting possibility of how protocadherins could work together to facilitate the formation of distinct tissue types. A previous report on AXPC predominately examined how over-expression of a truncated form of AXPC affected morphogenesis, with minimal investigation of loss of function effects (Kuroda et al., 2002). Therefore we investigated in more depth the AXPC loss of function phenotypes and the mechanisms by which AXPC promotes notochord morphogenesis.
RESULTS
A second allele of AXPC is predominately expressed in early Xenopus embryos
Due to the genome duplication in Xenopus laevis, many genes have two functional copies that necessitate careful analysis in morpholino (MO) design. We have found an alternate allele of AXPC in X. laevis (accession: JN029748) that has >94% identity (98% amino acid identity) with the allele previously identified (Kuroda et al., 2002). Since the sequences between the two alleles are slightly different in the region corresponding to the morpholino binding site (1A, asterisks), we compared a previously published morpholino for the first allele (Figure 1A, AXPC MO1, orange) and designed a new morpholino to the second allele of AXPC (Figure 1A, AXPC MO2, green). In order to best understand the nature of the AXPC morpholino induced phenotype, we generated an anti-AXPC antibody in order to verify the efficiency of knockdown (Figure 1C). Exogenous expression of the newly discovered allele of AXPC in Xenopus embryos yields a band of the expected size (Figure 1B, AXPC). Endogenous AXPC protein can be detected at low levels in the pre-blastula embryo (not shown) with expression reaching a maximum around stage 12.5 (Figure 1B). These results are consistent with the observations of Kuroda, et al., who show by RT-PCR that mRNA is present at low levels in the early embryo and is induced at the onset of gastrulation, also reaching its highest levels at stage 12.5 (Kuroda et al., 2002). Since endogenous AXPC levels peak at stage 12.5, we collected lysates from morpholino injected embryos at this stage for western blotting. We found that the morpholino to the newly identified allele more effectively reduces protein levels when compared to the morpholino to the previous allele (Figure 1C). Additionally, there is no additive effect when the two morpholinos are co-injected, as 20ng of each morpholino combined does not more significantly reduce AXPC protein levels than MO2 alone. Because of its better efficiency, the AXPC MO2 morpholino was used for all subsequent experiments. These results also suggest that the newly identified allele of AXPC represents the major allele expressed in the early Xenopus embryo.
Figure 1.
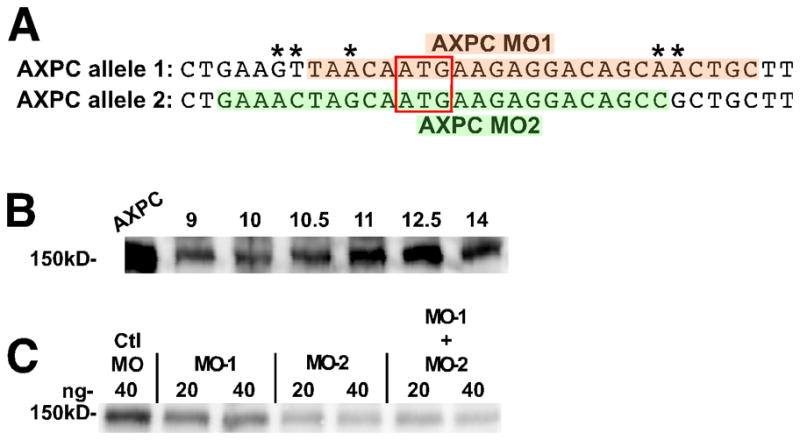
A newly identified allele of AXPC is predominantly expressed during early development.
A. Comparison of the sequence of the two alleles of AXPC surrounding the ATG start codon. MO-1 refers to a previously published construct (orange). MO-2 is a newly designed morpholino (green). Red box indicates the ATG start codon. Differences between the sequences denoted by an *. B. Western blot detection of both exogenous and endogenous expression of AXPC in lysates from embryos collected at the indicated stage. Embryos over-expressing AXPC (lane: AXPC) were collected at stage 12.5. C. Western blot of endogenous AXPC in embryo lysates at stage 12.5 injected with the indicated amount of control morpholino or AXPC morpholino. For co-injections, morpholino-1 and morpholino-2 were mixed in equal parts to give the indicated final amount. In B and C, AXPC was detected by western blotting with anti-AXPC antibody. Each lane represents the equivalent of 1 embryo.
AXPC knockdown inhibits the morphogenesis of axial mesoderm
Using the newly designed morpholino, we investigated the role of AXPC during early embryonic development. Single-sided injection of AXPC MO2 into a two-cell stage embryo results in a severely bent embryo at stage 32, whereas control embryos develop with a straight, normal A-P axis (compare Figures 2A and 2B). Mutant embryos display a range of phenotypes and therefore the bent phenotype was categorized as: strong (>45°), weak (0°< x >45°), and none (0°) (Figure 2E). Interestingly, the bend always occurred against the A-P axis (towards the right or left) and never in the dorsal-ventral plane. The directionality of the bend can be traced to the side of morpholino injection (using fluorescent dextran) in 100% of the injected embryos and is manifested during early embryogenesis, as a more subtle bend can be seen in neurula stage embryos as well (Figure 2C,D). Immunofluorescence staining on embryos bisected along the A-P axis reveals that the bend corresponds to a reduced level of AXPC in notochord cells. Control embryos show robust AXPC expression throughout the notochord (Figure 2F) whereas AXPC MO2 injected embryos exhibit normal expression on the uninjected side and greatly reduced staining on the MO injected side (Figure 2I, denoted by *). Additionally, the extracellular matrix surrounding the notochord is lost in the perturbed tissue as both fibronectin (Figures 2F and 2G, red) and laminin (not shown) are also reduced. These data indicate that AXPC is important for the normal morphogenesis of the notochord and ultimately its structural integrity.
Figure 2.
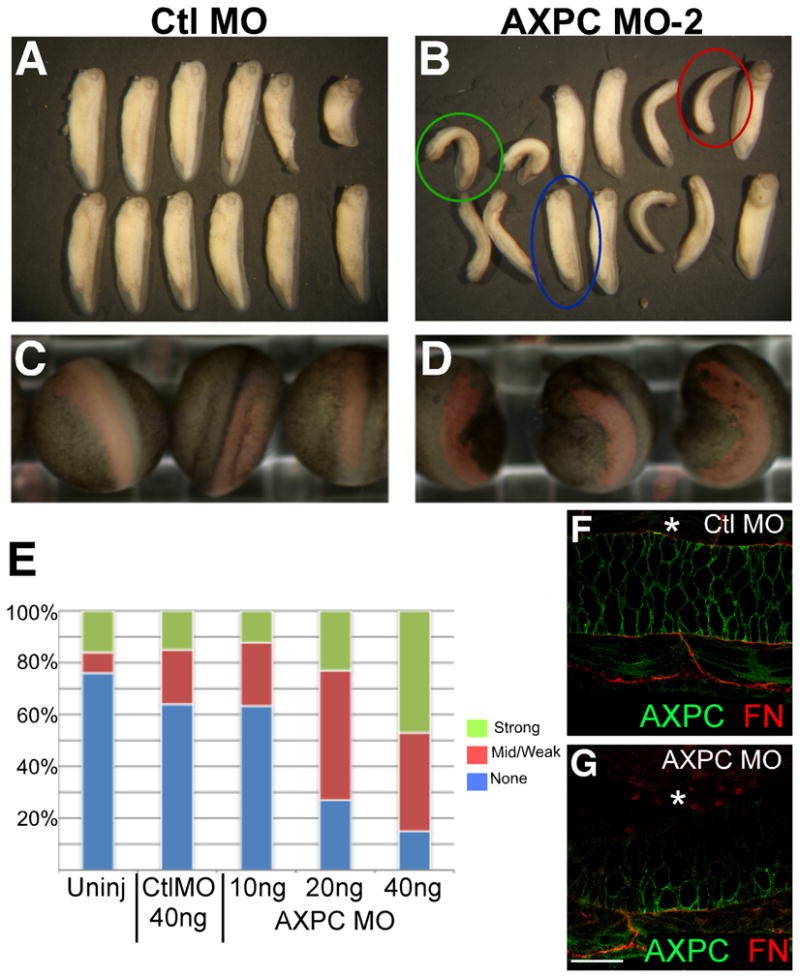
Morpholino to the second AXPC allele causes developmental axial defects.
A–D. Embryos were injected on one side of 2-cell embryo with 10, 20, or 40ng (shown) of morpholino along with a Dextran-Ruby tracer. Embryos were cultured and observed at stages 22 (C,D) and 30 (A,B). Fluorescent Dextran-Ruby (red) images were superimposed onto brightfield images in C and D. E. Embryos were scored at stage 30 as a strong (>45°), mid/weak (0°<X<45°), or no (0°) phenotype. Anterior is up. F,G. Whole mount immunofluorescence on embryos bisected sagitally at stage 30, stained with anti-AXPC (green) and anti-fibronectin (red). Asterisks denote side of morpholino injection. Confocal images obtained from ventral aspect at 600X magnification. Scale bar = 50μm.
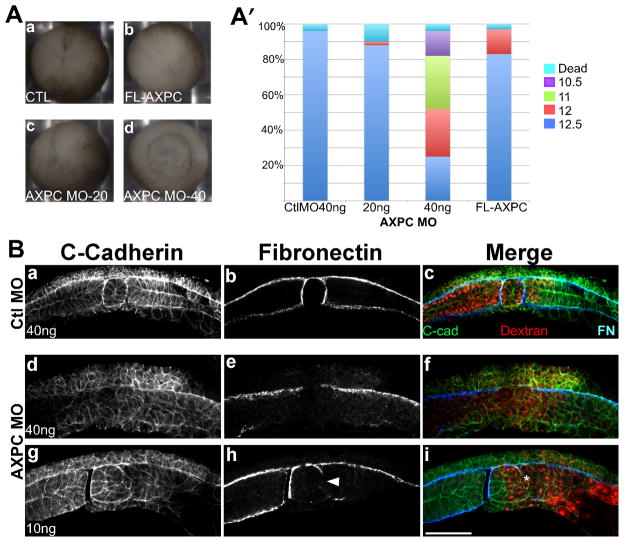
In order to address where AXPC is functioning in the embryo, we examined earlier gastrula staged embryos with more complete knockdown of AXPC for morphogenetic defects. For these experiments, AXPC MO2 was injected into both blastomeres at the two-cell stage into the dorsal marginal zone (DMZ). Embryos were first observed for their ability to begin gastrulation and close their blastopores. Control embryos are able to sufficiently execute blastopore closure and gastrulate (Figure 3Aa, n=53). 40ng of AXPC MO2 caused a severe delay in the ability of embryos to close their blastopores (n=56). At the time point where control embryos reached stage 12.5, the AXPC MO2 treated embryos exhibited delayed blastopore closure that resemble embryos in stages ranging from 10.5 to 12.5 (Figure 3A′). In many of these embryos the blastoporal ring eventually closed and covered the yolk plug. The more severely affected embryos ceased to develop shortly after gastrulation.
Figure 3.
Loss of AXPC expression perturbs notochordal morphogenesis
A. 2-cell stage embryos were injected bilaterally in the dorsal marginal zone with 40ng control morpholino (a), 20 or 40ng AXPC morpholino (c,d) or 1ng AXPC mRNA (d) and cultured until uninjected controls reached stage 12.5. Stage was determined by blastopore size and percentage was calculated by: # in given stage/total # embryos (A′, n>50). B. Whole mount immunofluorescence of bisected embryos injected with either control morpholino (CtlMO, a–c) or AXPC morpholino-2 (AXPC MO2, d–i). Sections were stained to detect C-cadherin to outline cell borders (a,d,g) and fibronectin to delineate tissue boundaries (b,e,h). Dextran tracer can be observed as red in c,f, and i. Arrowhead in h marks a localized loss of FN staining. Confocal images acquired at 200X magnification. Scale bar = 150μm.
One of the hallmarks of gastrulation is the separation of axial mesoderm from paraxial mesoderm, in order to form the notochord and somites respectively (Wilson et al., 1989; Wilson and Keller, 1991). We examined notochord-somite separation in embryos that were ultimately able to reach mid-gastrula after a significant delay compared to control embryos. Control embryos exhibited the expected morphology in both notochordal and somitic tissues, as observed through C-cadherin immunostaining, which outlines the membranes of all cells (Figure 3B). Both tissues are completely separated from one another and each is ensheathed by a dense extracellular matrix, as indicated by fibronectin immunostaining (Figure 3Bb). Conversely, in embryos lacking AXPC, the mesoderm fails to separate into axial and paraxial tissues (Figure 3Bd–f). Cadherin immunostaining shows that the cells are poorly organized within the mesoderm and the embryo lacks a discernable notochord or somitic tissue. An ECM rich boundary surrounding the area where the notochord should be is also lacking in these embryos (Figure 3Be). Embryos injected laterally with 10ng of MO show a localized, sided disruption in the boundary between axial and paraxial mesoderm (Figure 3Bi, *) and an associated loss of the fibronectin-rich notochord-somite boundary (Figure 3Bj, arrowhead). However, in both cases the boundary between the ectoderm and mesoderm remains intact and there appears to be no mixing of these cell populations. Together these data indicate that AXPC is required for the dorsal mesoderm to develop into axial and paraxial tissues.
AXPC does not modulate cell sorting in vivo
Because some of the δ-protocadherins can influence changes in cell-cell adhesion in a variety of ways, we reasoned that AXPC could affect notochordal morphogenesis through changes in cell adhesion to drive the separation of axial from paraxial mesoderm. A previous report claimed that a truncated, tail-less form of AXPC (TL-AXPC, lacking the entire cytoplasmic tail) could mediate changes in cell adhesion (Kuroda et al., 2002). However, the cytoplasmic tail has been shown to be functionally important for both classical cadherins and protocadherins, so we tested our hypothesis using the wild-type form of AXPC.
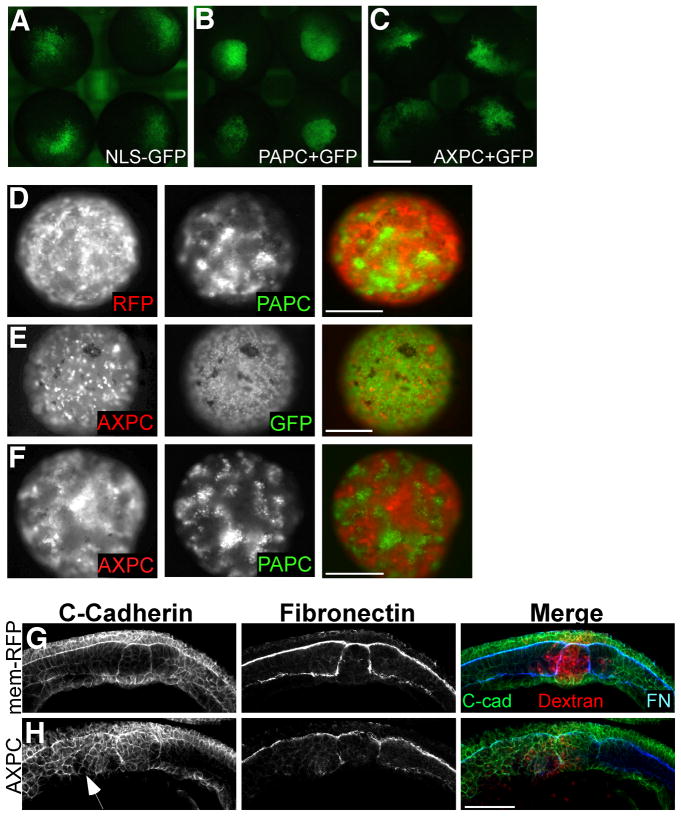
Cell dispersal and blastomere reaggregation assays were used to examine the effect of full-length AXPC on cell adhesion. In cell dispersal assays, embryos are injected in one cell with fluorescent tracer either with or without a test mRNA at the 32-cell stage and cultured until the late blastula. The population of cells derived from the original injected cell tends to stay in close proximity to one another, making them easy to track. Differences in adhesion between the injected and uninjected cells can easily be observed, as they will form smooth boundaries between one another. As previously shown, this can be seen by carefully comparing the cohesiveness of the patch of labeled cells expressing PAPC (Figure 4B) to the more dispersed patch containing GFP-only labeled cells (Figure 4A) (Chen and Gumbiner, 2006). Embryos that express full-length AXPC exhibit no sorting activity when examined in cell dispersal assays. In contrast to PAPC, AXPC expressing cells lack any cohesion and freely mix with the uninjected cells, similar to GFP control cells (compare Figures 4C and 4A, and Table 1).
Figure 4.
AXPC does not mediate cell sorting.
A–C. In cell dispersal assays, embryos were injected into one cell at 32-cell stage with nuclear-GFP alone (A), GFP+PAPC (B) or GFP+AXPC (C) and cultured to stage 9. Scale bar = 0.5mm. D–F. Reaggregation assay performed using blastomeres isolated from excised animal caps expressing RFP, GFP, AXPC-mCherry, and PAPC+GFP. Cells were mixed as indicated and images were acquired once aggregates formed (5–6 hours). Scale Bar = 1mm. G,H. AXPC constructs have functional activity in perturbing notochordal morphogenesis. Sagittal cryosections of stage 12.5 embryos from injected dorsally at 2-cell stage, with 1ng RFP (I) or 1ng AXPC (J). C-cadherin, fibronectin (FN), dextran-TRITC (red). Confocal images taken at 200X magnification. Scale bar = 150μm.
Table 1.
Percentage of embryos displaying a cohesive population of cells in dispersal assays
| Injected mRNA | Total n | % of embryos with a cohesive patch |
|---|---|---|
| GFP only | 53 | 2 |
| PAPC+GFP | 104 | 96 |
| AXPC+GFP | 190 | 0.5 |
Similar results are obtained when blastomere reaggregation experiments are performed from mixed populations of blastomeres, either expressing or not expressing AXPC. Cells expressing both AXPC and RFP (as red tracer) are evenly distributed throughout the aggregate when mixed with cells expressing GFP alone and fail to exhibit sorting (defined as a clustered population of labeled cells) (Figure 4E, Table 2). In contrast, as shown previously, blastomeres expressing both PAPC and GFP form cohesive populations that sort away from the control (RFP) cells (Figure 4D) (Chen and Gumbiner, 2006). Since AXPC and PAPC are expressed in juxtaposed tissues, we tested the ability of AXPC to abrogate PAPC function in trans. In this assay, AXPC fails to alter the ability of PAPC to mediate sorting when blastomeres are mixed together (4F). The AXPC-PAPC aggregates resemble the RFP-PAPC aggregates, where the PAPC cells form isolated populations. The appearance of AXPC populations is likely due to their exclusion from the PAPC islands rather than a separate sorting event.
Table 2.
Percentage of aggregates exhibiting sorting in blastomere reaggregation assays
| Mixed Blastomeres | Total number of aggregates | % aggregates exhibiting sorting |
|---|---|---|
| PAPC+RFP | 15 | 100 |
| AXPC+GFP | 13 | 8 |
| AXPC-RFP + PAPC-GFP | 11 | 82 |
Whereas AXPC fails to mediate changes in cell sorting, its over-expression shows that it has a biological activity, because embryos injected with AXPC mRNA (targeted to DMZ), exhibit a perturbation in notochord-somite structure (Figure 4H). In control embryos, the axial and paraxial tissues have a well-defined epithelial-like architecture, as exhibited by C-cadherin immunostaining (Figure 4G). However, the defined architecture of the somite and notochord that is over-expressing AXPC (Figure 4H, red cells) is lost as cells appear to be disorganized throughout the tissues (Figure 4H, arrow). It is interesting to note that despite these observed defects, embryos over-expressing AXPC do not have problems with blastopore closure (Figure 3Bb), in contrast to AXPC knockdown (Figure 3Bd). While we find that AXPC does not mediate changes in cell adhesion, our data is similar to the results from Kuroda, et al (Kuroda et al., 2002), in that over-expression of AXPC can affect some aspect of tissue morphogenesis.
AXPC is required for normal expression of chordin during gastrulation
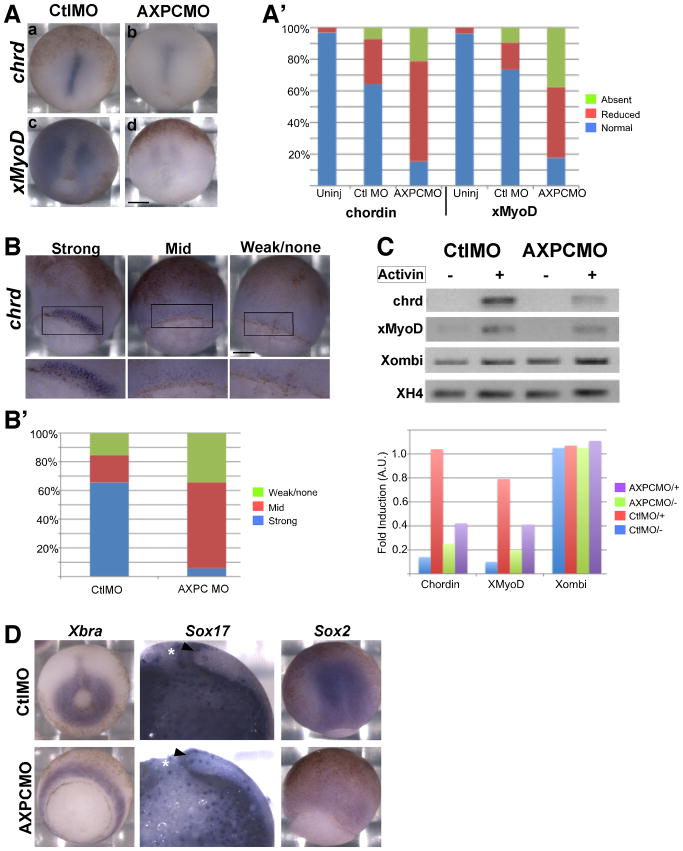
Since AXPC does not seem to mediate cell sorting through changes in cell adhesion, yet it is required for mesodermal morphogenesis, we examined the possibility that AXPC influences cell fate decisions. Chordin is a well-established marker of axial mesoderm and is initially expressed generally throughout the presumptive dorsal mesoderm in the early gastrula and is later refined to the axial mesoderm and notochord (Sasai et al., 1994; Wessely et al., 2001). Chordin staining in control embryos shows the refined notochordal expression pattern (Figure 5A). The AXPC MO2 injected embryos that are able to close their blastopore exhibit a large reduction in, or loss of, chordin expression in the notochord (~85%) (Figure 5A,A′). The reduction of chordin expression is accompanied by a reduced expression of the paraxial mesodermal marker, MyoD (85%) (Figure 5A,A′).
Figure 5.
AXPC is specifically required for specification of axial mesoderm but not general mesoderm induction.
A. Embryos were injected bilaterally in the dorsal marginal zone at the 2-cell stage with 40ng of either control (CtlMO) or AXPC morpholino (AXPCMO), then cultured until stage 12.5 and prepared for in situ hybridization with either chordin or MyoD. A′. Embryos were scored by using the categories of ‘normal’, ‘reduced’, and ‘absent’ to describe the staining intensity. A. Examples of ‘normal’ and ‘reduced’ are depicted (a,b or c,d respectively) (n > 30). Anterior is up. B. Embryos were prepared as described above for the detection of chordin at stage 10.5. B′. The categories ‘strong’, ‘mid’, and ‘weak’ represent the degree of chordin staining and used to score the control and AXPC MO injected embryos. Lower image is a higher magnification of the dorsal lip (boxed region) (n>30). C. RT-PCR analysis of animal caps from embryos injected with either control or AXPC MO and treated with or without 100ng activin to induce mesodermal gene expression. Data was normalized to histone 4 (XH4). D. Mesoderm is not respecified to nueroectoderm or endoderm due to loss of AXPC. Embryos were injected as described in A and probed for Xbra, Sox17 (endoderm), or Sox2 (neuroectoderm). Asterisks denote the blastopore. Arrowhead indicates dorsal lip. Scale Bar in A and B = 250μm.
Since AXPC, like chordin, is induced by nodal signaling at the onset of gastrulation (Kim et al., 1998; Kuroda et al., 2002), its initial expression coincides with the onset of chordin expression. At stage 10.5, chordin is normally expressed throughout the dorsal mesoderm (Figure 5B, strong). In AXPC MO2 treated embryos, expression of chordin is severely reduced at the onset of gastrulation (Figure 5B, mid and weak/none). Over 95% of the AXPC MO2 treated embryos have a much a lower intensity of expression when compared to control embryos (Figure 5B′, mid and weak). These results indicate that perhaps AXPC is necessary for mesodermal cells to properly initiate chordin expression as a result of events arising from nodal induction. To test this hypothesis, we examined the ability of animal cap tissue from AXPC MO2 treated embryos to induce gene expression in response to activin treatment. As expected, animal caps from embryos injected with control morpholino show a substantial increase in both chordin (10-fold) and MyoD (8-fold) induction when treated with activin (Figure 5C,C′). Conversely, animal caps from embryos treated with AXPC MO2 and induced with activin exhibit a diminished ability to induce chordin and MyoD, roughly 2-fold induction compared to the 10- and 8-fold induction in control embryos, respectively (Figure 5C′). Xombi (VegT) expression remains unchanged in both control and AXPC morpholino animal caps (Figure 5C,C′) indicating that the reduced gene expression is not simply a product of a gross defect in gene expression (Lustig et al., 1996). In addition, the mesodermal marker Xbra, a known target of Xombi (Kavka and Green, 2000), is robustly expressed in embryos injected with AXPC MO2 indicating that early mesoderm induction still occurs (Figure 5D). Although the axial and paraxial mesodermal markers are considerably reduced, the tissue does not appear to adopt the fate of other tissue types. Neither Sox17 (endodermal) nor Sox2 (neural) are expressed in the prospective mesoderm of AXPC MO2 injected embryos (Figure 5D). In fact, overall Sox2 levels are reduced compared to the control MO, probably secondary to reduced chordin expression.
DISCUSSION
Protocadherin involvement in the morphogenesis of the early embryo is a topic of great interest. Here we show that AXPC, a member of the subgroup of δ-protocadherin molecules, plays an important role in notochordal morphogenesis, through its ability to regulate gene expression in the dorsal mesoderm. We identified a second allele of AXPC and established that this allele is the predominantly expressed allele during early embryogenesis. Using a newly designed morpholino to this allele, we show that AXPC expression is required for the morphogenesis of the embryo and in particular the axial mesoderm. Owing to the differences in allelic expression, it is likely that the previously published data showing a milder phenotype with MO-1 (Kuroda et al., 2002) is due to an insufficient reduction of AXPC protein levels.
The bent axis phenotype in the sided loss of AXPC is reflective of a structural defect in the notochord. At the tailbud stage notochordal cells become highly vacuolated and by the tadpole stage increase the osmotic pressure of the notochord, thereby generating a very stiff rod-like structure running along the A-P axis (Adams et al., 1990). If there are localized defects in the notochord itself, such as the loss of ECM components, then the notochord would ‘buckle’ to towards the weakened side as the pressure built up (Skoglund and Keller, 2007). The fact that AXPC MO2 treated embryos always bend towards the side of AXPC loss (and corresponding reduction in ECM components) supports the idea that AXPC ultimately contributes to the structural integrity of the notochord. Since notochordal cells are not initially specified and are unable to establish a boundary with paraxial tissue, then the loss of matrix proteins probably reflects a secondary effect of AXPC loss, leading to the observed structural defects.
Examination of embryos with a more severe phenotype indicates that AXPC is actually involved in the early stages of notochordal morphogenesis, where it likely functions in the dorsal mesoderm to help initiate the morphogenesis of axial and paraxial mesodermal tissues. The observed delays in blastopore closure can easily be explained by the fact that defects in mesodermal patterning have been shown to cause delays in gastrulation, including blastopore closure (Kofron et al., 1999; Yasuo and Lemaire, 2001). Since the dorsal mesoderm in AXPC knockdown embryos lacks any discernable axial or paraxial structure and, in fact, is completely disorganized, it is not surprising that there is a severe delay in this process.
We found no evidence that AXPC functions as a cell adhesion molecule, because of its inability to mediate cell sorting events. Unlike PAPC, which down-regulates the adhesive activity of C-cadherin and facilitates cell sorting (Chen and Gumbiner, 2006; Chen et al., 2009), AXPC shows little ability to cause sorting events in similar naïve blastomere cells. Since AXPC and PAPC are expressed in neighboring tissues, and presumably together in an initial mesodermal population in the early gastrula, it was possible that perhaps they function together to mediate this separation. However, when expressed in opposing cells, there appears to be no compounded effect, beyond simple PAPC mediated sorting, indicating that AXPC does not affect the cell sorting function of PAPC in trans. While the ‘non-sorting’ result differs with a previously published observation using a truncated AXPC (a deletion of the entire cytoplasmic tail), we believe that the use of full-length AXPC provides the most accurate representation of the biological function of AXPC.
Despite this lack of sorting activity, targeted AXPC over-expression does result in defective mesodermal development, as seen in the disorganization of the somitic mesoderm exogenously expressing AXPC, showing that AXPC does have biological activity. Similar disruptions in embryos expressing the truncated AXPC protein were observed by Kuroda, et al (Kuroda et al., 2002), who attributed this perturbation to changes in cell sorting, without a corresponding loss of marker gene expression. In both instances, the exogenous expression of AXPC may allow target gene expression, while disregulating the spatial and/or temporal patterning and therefore disturb notochord and somite development.
Our results support the hypothesis that AXPC directs notochordal morphogenesis through the regulation of mesodermal cell identity. The reduced amounts of chordin and MyoD are sufficient to explain the loss mesodermal derivatives, since both are necessary in the specification of axial and paraxial mesoderm, respectively (Harvey, 1992; Oelgeschlager et al., 2003). The effect of AXPC knockdown is specific to axial and paraxial mesoderm specification, since general early mesodermal markers (Xbra and Xombi) are robustly expressed. In support of the specificity, endodermal gene expression appears normal in AXPC MO injected embryos. However, the expression of Sox2, a neuroectoderm specific gene, is significantly reduced. This reduction of Sox2 can be explained by the fact that chordin is required to induce neural tissue (Schulte-Merker et al., 1997; Oelgeschlager et al., 2003) and a loss of chordin will necessarily result in a loss of neural gene expression. Since the ‘unspecified’ axial mesoderm does not adopt a new fate as either endoderm or ectoderm, it is likely that the developmental program is inhibited at the conversion of general to axial mesoderm.
Robust AXPC expression begins at the onset of gastrulation, during the time of general mesoderm induction (Kuroda et al., 2002). Since the loss of AXPC results in the inability of mesodermal cells to express either chordin or MyoD, we propose that AXPC functions upstream of both genes. The fact that chordin and MyoD are markers of axial and paraxial mesoderm, respectively, leads to the question of how AXPC can be involved with the specification of both axial and paraxial cell identity at the same time? It is possible that AXPC is required early for a transition from a general to a specific mesodermal identity as well as a later role in maintaining notochord fate. Alternatively, AXPC could be specifically required for promoting notochord cell identity, which then generates signals required for paraxial mesoderm development. In either case, if AXPC were lost, then the entire mesodermal population would be unable to be properly specified and the morphogenetic plan would fail.
It has recently been shown that protocadherins play an important role in directing different aspects embryo morphogenesis, through a variety of biological functions. Clustered protocadherins may or may not regulate cell adhesion during neuronal development (Yagi, 2008), but few, if any, of the non-clustered protocadherins have been shown to directly regulate cell-cell adhesion. However, some can indirectly regulate cell-cell adhesion and cell sorting (Chen and Gumbiner, 2006; Rashid et al., 2006; Aamar and Dawid, 2008; Biswas et al., 2010). Here we demonstrate that AXPC functions in morphogenesis by modulating gene expression, rather than a cell-sorting event. The ability of AXPC to regulate gene expression is a unique function among the δ-protocadherins. No other member has been shown to directly affect gene expression. For example, PAPC is also expressed in the early gastrula, but unlike AXPC, the loss of PAPC only perturbs the morphogenesis of the mesoderm, it does not lead to defects in gene expression (Unterseher et al., 2004). PAPC has been shown to possess signaling abilities, as it functions to direct tissue separation through XFz7 and the JNK pathway via its cytoplasmic tail (Medina et al., 2004), but resulting changes in gene expression have not been established. The results here add to the growing body of protocadherin function and highlight the importance of these molecules in critical embryological processes.
EXPERIMENTAL PROCEDURES
X. laevis embryo care and microinjection
All experimental protocols that involved the use of X. laevis were approved by the Animal Care and Use Committee at the University of Virginia. X. laevis eggs and embryos were obtained and handled by standard techniques. Standard Nieuwkoop staging of embryos was used (Nieuwkoop and Faber, 1994). Microinjection of mRNAs or morpholinos was performed at the 2– or 4–cell stage. Typically 10nL were used to inject either 1–2 ng mRNA or 20–40 ng of morpholinos.
Cell dispersal and reaggregation assays
Both assays were performed as described previously (Chen et al., 2009). Either NLS-GFP or mem-RFP mRNA (0.3ng) was co-injected with AXPC or PAPC as a lineage tracer. For the cell dispersal assay, 0.5ng mRNA was injected into one cell at the 32-cell stage and cultured to stage 9 in 0.1X MBS. Embryos were then examined by epi-fluorescence for the distribution of fluorescently labeled cells.
For reaggregation assays, all four cells at the 4-cell stage were injected with 0.5ng mRNA. Animal caps were excised at stage 9 and blastomeres were dissociated by incubating in Ca++/Mg++ free 1X MBS. Blastomeres from differentially injected embryos were mixed 3:3 in the presence of Ca++/Mg++ and allowed to reaggregate for at least 5 hours on a rotating orbital platform.
DNA constructs/cloning
The plasmids pCS2+/AXPC and pCS2+/nls-GFP were kindly provided by E.M. DeRobertis (University of California, Los Angeles, Los Angeles, CA) and L. Davidson (University of Pittsburgh, Pittsburgh PA) respectively. pCS2+/PAPC was described previously. In order to clone the second allele of AXPC, two partial, overlapping partial clones were identified (OpenBiosystems, Accession no: CA791551.1 and BC079761) and cloned together into pCS2+. The following primers were used (restriction sites in bold): For CA791551.1: forward 5′CGGGA-GAGTGGATCCATGCAGAGC-3′, reverse 5′-CCGCTGTTTAGATCTCTCATACTCTGGG-3′; and for BC079761: forward 5′-CCCAGAGTATGAGAGATCTAAACAGCGG-3′, reverse 5′-CCCACATCACTGGCTCGAGGTACC-3′.
mRNA and MO
Capped mRNA for AXPC, PAPC, and GFP were in vitro transcribed with SP6 using the Riboprobe in vitro Transcription System (Promega) and purified using the RNeasy Mini kit (Qiagen). All morpholinos were designed by Genetools, LLC Co. AXPC MO-1: 5′-GCAGTTGCTGTCCTCTT-CATTGTTA-3′ (previously described), AXPC MO-2: 5′-GGCTGTCCTCTTCATTGCTAGTTTC-3′, Control MO 5- CCTCTTACCTCAGTTACAATTTATA-3′. Morpholinos were diluted in water and mixed with Dextran fluoro-ruby (Invitrogen, 1mg/mL) immediately before injection.
Antibodies and immunofluorescence
The rabbit polyclonal anti-AXPC antibody was raised against three co-injected peptides that span the extra-cellular domain of AXPC: CTLKFSVIAKDKGANPKIA, CDSKHLYKLELGHPYLRVDGK, and CLDREQREHYDFHVVAVDKG (Covance). An N-terminal cysteine residue was added to aid in purification. Affinity purification was performed with the SulphoLink kit (Pierce) using the LDRE- peptide. Anti-AXPC was used at 1:100 for immunofluorescence and 1:500 for western blotting. Other antibodies used for IF were: rabbit anti-XC-cadherin (XCad) 1:1000 and mouse anti-fibronectin (4H2) 1:2000.
For whole mount immunofluorescence embryos were fixed in 3.6% paraformaldehyde for 2 hours at room temperature. Post-fix, the vitelline membrane was removed and the embryos were bisected along the transverse axis with a razor. Embryos were blocked overnight at 4°C in Blocking solution (1XPBS, 0.1%Tween-20, 0.5%BSA, 0.5% goat serum, 1%DMSO). After antibody staining, embryos were dehydrated in methanol and cleared in BB:BA (benzyl benzoate:benzyl alcohol) for imaging. Cryosections were performed as described previously (Fagotto and Brown, 2008), with the exception of using 12μm sections. Confocal images were acquired using a Nikon C1 confocal microscope.
in situ hybridization
In situ hybridization was performed as described in (Sive, 2000), with the exception that embryos were prepared by fixation in 3.6% paraformaldehyde. cDNA templates for chordin, MyoD, Xbra, Sox2, and Sox17 were generously provided by R. Granger. Images were obtained with a Leica MZ16F stereo-microscope.
Animal cap induction and RT-PCR
Animal caps were prepared as described previously (Chen and Gumbiner, 2006) and treated with 100ng/mL activin for 75 minutes at RT in 1X MBS, then further incubated for 5 hours in 1X MBS (when sister embryos reached stage 10.5). RNA was extracted using the Trizol Reagent (Invitrogen) and cDNA was synthesized using the Retroscript Kit (Ambion).
RT-PCR was performed at with the following primers and cycling conditions: Chrd (58°C, 22 cycles, forward, 5′-CCTCCATCCAAGACTCCAGCAG-3′; reverse 5′-GGAGGAGGAGGAGCTTTGGGACAAG-3′ ), MyoD (58°C, 22 cycles, forward 5′-CCTCAACTAACCCCAACCAA-3′; reverse 5′-TATTGCTGGGAGAAGGGATG-3′), Xombi (55°C, 25 cycles, forward 5′-CTAAAGGATTCCGGGAGC-AG-3′; reverse 5′-CTCCTTCGATAATGGCTCTTG-3′), and XH4 (55°C, 20 cycles, 5′-GCGGGATAA-CATTCAGGGTA-3′; reverse 5′-CCATGCGGTAACTGTCTT-3′).
Acknowledgments
We would like to thank Ray Keller for technical support and meaningful discussions, and Eddy De Robertis and Doug DeSimone for reagents.
Grant Support: T32-NIH-NIAMS AR050960-05 to M.D.Y. and NIH-NIGMS R01 GM52717 to B.M.G.
References
- Aamar E, Dawid IB. Protocadherin-18a has a role in cell adhesion, behavior and migration in zebrafish development. Dev Biol. 2008;318:335–346. doi: 10.1016/j.ydbio.2008.03.040. [DOI] [PubMed] [Google Scholar]
- Abedin M, King N. The premetazoan ancestry of cadherins. Science. 2008;319:946–948. doi: 10.1126/science.1151084. [DOI] [PubMed] [Google Scholar]
- Adams DS, Keller R, Koehl MA. The mechanics of notochord elongation, straightening and stiffening in the embryo of Xenopus laevis. Development. 1990;110:115–130. doi: 10.1242/dev.110.1.115. [DOI] [PubMed] [Google Scholar]
- Biswas S, Emond MR, Jontes JD. Protocadherin-19 and N-cadherin interact to control cell movements during anterior neurulation. J Cell Biol. 2010;191:1029–1041. doi: 10.1083/jcb.201007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Gumbiner BM. Paraxial protocadherin mediates cell sorting and tissue morphogenesis by regulating C-cadherin adhesion activity. J Cell Biol. 2006;174:301–313. doi: 10.1083/jcb.200602062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Koh E, Yoder M, Gumbiner BM. A protocadherin-cadherin-FLRT3 complex controls cell adhesion and morphogenesis. PLoS One. 2009;4:e8411. doi: 10.1371/journal.pone.0008411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F, Brown CM. Detection of nuclear beta-catenin in Xenopus embryos. Methods Mol Biol. 2008;469:363–380. doi: 10.1007/978-1-60327-469-2_23. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Wedlich D. Regulated adhesion as a driving force of gastrulation movements. Development. 2008;135:3625–3641. doi: 10.1242/dev.015701. [DOI] [PubMed] [Google Scholar]
- Harvey RP. MyoD protein expression in Xenopus embryos closely follows a mesoderm induction-dependent amplification of MyoD transcription and is synchronous across the future somite axis. Mech Dev. 1992;37:141–149. doi: 10.1016/0925-4773(92)90076-v. [DOI] [PubMed] [Google Scholar]
- Heggem MA, Bradley RS. The cytoplasmic domain of Xenopus NF-protocadherin interacts with TAF1/set. Dev Cell. 2003;4:419–429. doi: 10.1016/s1534-5807(03)00036-4. [DOI] [PubMed] [Google Scholar]
- Kavka AI, Green JB. Evidence for dual mechanisms of mesoderm establishment in Xenopus embryos. Dev Dyn. 2000;219:77–83. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1025>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Kim SH, Yamamoto A, Bouwmeester T, Agius E, Robertis EM. The role of paraxial protocadherin in selective adhesion and cell movements of the mesoderm during Xenopus gastrulation. Development. 1998;125:4681–4690. doi: 10.1242/dev.125.23.4681. [DOI] [PubMed] [Google Scholar]
- Kofron M, Demel T, Xanthos J, Lohr J, Sun B, Sive H, Osada S, Wright C, Wylie C, Heasman J. Mesoderm induction in Xenopus is a zygotic event regulated by maternal VegT via TGFbeta growth factors. Development. 1999;126:5759–5770. doi: 10.1242/dev.126.24.5759. [DOI] [PubMed] [Google Scholar]
- Kuroda H, Inui M, Sugimoto K, Hayata T, Asashima M. Axial protocadherin is a mediator of prenotochord cell sorting in Xenopus. Dev Biol. 2002;244:267–277. doi: 10.1006/dbio.2002.0589. [DOI] [PubMed] [Google Scholar]
- Lustig KD, Kroll KL, Sun EE, Kirschner MW. Expression cloning of a Xenopus T-related gene (Xombi) involved in mesodermal patterning and blastopore lip formation. Development. 1996;122:4001–4012. doi: 10.1242/dev.122.12.4001. [DOI] [PubMed] [Google Scholar]
- Medina A, Swain RK, Kuerner KM, Steinbeisser H. Xenopus paraxial protocadherin has signaling functions and is involved in tissue separation. Embo J. 2004;23:3249–3258. doi: 10.1038/sj.emboj.7600329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H, Yagi T. Protocadherin family: diversity, structure, and function. Curr Opin Cell Biol. 2007;19:584–592. doi: 10.1016/j.ceb.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal table of Xenopus laevis (Daudin) : a systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. New York: Garland Pub; 1994. p. 252. 210 leaves of plates pp. [Google Scholar]
- Noda T, Satoh N. A comprehensive survey of cadherin superfamily gene expression patterns in Ciona intestinalis. Gene Expr Patterns. 2008;8:349–356. doi: 10.1016/j.gep.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Oelgeschlager M, Kuroda H, Reversade B, De Robertis EM. Chordin is required for the Spemann organizer transplantation phenomenon in Xenopus embryos. Dev Cell. 2003;4:219–230. doi: 10.1016/s1534-5807(02)00404-5. [DOI] [PubMed] [Google Scholar]
- Rashid D, Newell K, Shama L, Bradley R. A requirement for NF-protocadherin and TAF1/Set in cell adhesion and neural tube formation. Dev Biol. 2006;291:170–181. doi: 10.1016/j.ydbio.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Rhee J, Takahashi Y, Saga Y, Wilson-Rawls J, Rawls A. The protocadherin papc is involved in the organization of the epithelium along the segmental border during mouse somitogenesis. Dev Biol. 2003;254:248–261. doi: 10.1016/s0012-1606(02)00085-4. [DOI] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Merker S, Lee KJ, McMahon AP, Hammerschmidt M. The zebrafish organizer requires chordino. Nature. 1997;387:862–863. doi: 10.1038/43092. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early Development of Xenopus laevis. Cold Spring Harbor; 2000. [DOI] [PubMed] [Google Scholar]
- Skoglund P, Keller R. Xenopus fibrillin regulates directed convergence and extension. Dev Biol. 2007;301:404–416. doi: 10.1016/j.ydbio.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MS. Differential adhesion in morphogenesis: a modern view. Curr Opin Genet Dev. 2007;17:281–286. doi: 10.1016/j.gde.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Unterseher F, Hefele JA, Giehl K, De Robertis EM, Wedlich D, Schambony A. Paraxial protocadherin coordinates cell polarity during convergent extension via Rho A and JNK. Embo J. 2004;23:3259–3269. doi: 10.1038/sj.emboj.7600332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessely O, Agius E, Oelgeschlager M, Pera EM, De Robertis EM. Neural induction in the absence of mesoderm: beta-catenin-dependent expression of secreted BMP antagonists at the blastula stage in Xenopus. Dev Biol. 2001;234:161–173. doi: 10.1006/dbio.2001.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson P, Keller R. Cell rearrangement during gastrulation of Xenopus: direct observation of cultured explants. Development. 1991;112:289–300. doi: 10.1242/dev.112.1.289. [DOI] [PubMed] [Google Scholar]
- Wilson PA, Oster G, Keller R. Cell rearrangement and segmentation in Xenopus: direct observation of cultured explants. Development. 1989;105:155–166. doi: 10.1242/dev.105.1.155. [DOI] [PubMed] [Google Scholar]
- Yagi T. Clustered protocadherin family. Dev Growth Differ. 2008;50(Suppl 1):S131–140. doi: 10.1111/j.1440-169X.2008.00991.x. [DOI] [PubMed] [Google Scholar]
- Yasuo H, Lemaire P. Role of Goosecoid, Xnot and Wnt antagonists in the maintenance of the notochord genetic programme in Xenopus gastrulae. Development. 2001;128:3783–3793. doi: 10.1242/dev.128.19.3783. [DOI] [PubMed] [Google Scholar]