Abstract
Synthetic biology is considered as an emerging research field that will bring new opportunities to biotechnology. There is an expectation that synthetic biology will not only enhance knowledge in basic science, but will also have great potential for practical applications. Synthetic biology is still in an early developmental stage in China. We provide here a review of current Chinese research activities in synthetic biology and its different subfields, such as research on genetic circuits, minimal genomes, chemical synthetic biology, protocells and DNA synthesis, using literature reviews and personal communications with Chinese researchers. To meet the increasing demand for a sustainable development, research on genetic circuits to harness biomass is the most pursed research within Chinese researchers. The environmental concerns are driven force of research on the genetic circuits for bioremediation. The research on minimal genomes is carried on identifying the smallest number of genomes needed for engineering minimal cell factories and research on chemical synthetic biology is focused on artificial proteins and expanded genetic code. The research on protocells is more in combination with the research on molecular-scale motors. The research on DNA synthesis and its commercialisation are also reviewed. As for the perspective on potential future Chinese R&D activities, it will be discussed based on the research capacity and governmental policy.
Keywords: China, Synthetic biology, Genetic circuits, Minimal genomes, Chemical synthetic biology, Protocells, DNA synthesis
1. China and biotechnology
Scientific research and development are in a fast growing pace in China, particularly in the fields of applied sciences, such as sustainable energy, nanotechnology and stem cells (Editorial, 2010). From the historical point of view, the forces driving Chinese biotechnology research are the need to fulfill agricultural demands and enhance industrial production, and to provide health care to the 1.3 billion people. Early biotechnology developments in China emphasized more on research for agricultural applications but less for medical and pharmaceutical ones (Chen et al., 2007). However, since the launch of funding projects 863 and 973 Programs, the development and utilization of molecular techniques in life science have become common. The 863 Program, the State High Technology Development Plan, took much of its shape from the US-American research system used by the National Institutes of Health and the Department of Defense. A government appointed panel of experts are responsible to draw up research priorities, call for bids, and award contracts. The 973 Program is a peer-reviewed funding for basic research. In the past Five Year Programs, the Chinese government allocated more funding for research & development (R&D). Some of these funding programs are listed in Table 1, updated from Huang et al. (Huang and Wang, 2002). This increasing expenditure on R&D resulted in an increase in science and technology publications and patents. For example, in fiscal year 2008, statistic data from the Organization for Economic Co-operation and Development (OECD), Science Citation Index (SCI) and World Intellectual Property Organization (WIPO) showed that China was in 4th place in the gross expenditure in research and development (GERD), 2nd place in publications and 5th place in granted patents (Table 2).
Table 1.
Overview of the important R&D funds for biotech in China.
| Major fund | Description |
|---|---|
| 863 Program | National High-tech R&D Program (863 Program) was approved in 1986 to promote high technology R&D in China. Biotechnology is listed as one of its eight priority fields. |
| 973 Program | National Basic Research Program of China (973 program) was approved in 1997 to support basic science and technology research. It promotes research and innovation in major frontier fields of far-reaching and strategic importance, such a life science. |
| NSFC | The National Natural Science Foundation of China (NSFC) was established in 1986 to support basic science research through internationally accepted mechanism. Some biotech related research has been funded though the General Program, Key Program, or Major Program of NSFC. |
| National Key Technologies R&D Program | Initiated in 1982 and implemented through 5 Five-year Plans. It has made remarkable contributions to the technical renovation and formation of new industries such as biotech. |
| Foundation for high-tech commercialization | A special program supported by the SDPC* since 1998 to promote application and commercialization of technologies. |
| Special Foundation for Transgenic Plant Research and Commercialization | A 5-year program launched in 1999 by MOST** to promote the research and commercialization of transgenic plants in China. The total budget of this program in the first 5 years is 500 million RMB. |
| Key Science Engineering Program | Started in the late 1990s under MOST** and SDPC* to promote basic research, including biotechnology. |
| The Climbing Program | A national program for key basic research projects, including biotechnology, which was initiated in the early 1980s. |
* SDPC stands for the State Development Planning Commission. It is now part of the National Development and Reform Commission (NDRC), a macroeconomic management agency under the Chinese State Council.
**MOST stands for Ministry of Science and Technology of the People's Republic of China.
Table 2.
Gross expenditure on R&D (GERD), SCI publications in Science & Technology, and granted patents by WIPO in 2008.
| Country | GERD |
Publication |
WIPO patent |
|||
|---|---|---|---|---|---|---|
| M USD | Rank | Paper | Rank | Total | Rank | |
| China | 64,893 | 4 | 77,813 | 5 | 48,814 | 5 |
| Germany | 94,973 | 3 | 90,556 | 2 | 53,903 | 3 |
| Japan | 166,915 | 2 | 93,691 | 1 | 239,458 | 1 |
| UK | 47,621 | 6 | 78,520 | 4 | 12,232 | 9 |
| US | 397,721 | 1 | 85,049 | 3 | 147,245 | 2 |
Among research groups that are active in biotechnology, many are engaged in genetic engineering, particularly metabolic engineering with applications in the area of biofuels, bio-chemicals and bioremediation. Taking an example from the Chinese Academy of Sciences (CAS), which is a leading Chinese research institution, has prioritized biotechnology research funding recently for “Basic Research on the Key Process of Eco-Valued Products from Straw Resources” (973 Program), which focuses on research to harness biomass to increase the added valued of agricultural productions. This may be based on the fact China is a country of abundant and diverse biological resources, as well as a wide range of climates. The unique geographic and climate setting provides the possibility to produce biomass in large quantity (Chen et al., 2007). To meet the food demand of its large population, the Chinese government emphasized research in agricultural biotechnology (Huang et al., 2004). This has made China the leading country in crop breeding. Other achievements in biotechnology are in medical science and pharmaceutical industry that has been contributed to a small portion in the national economy (Chen et al., 2007). Currently, the Chinese government sees challenges ahead in the area of sustainable development and environmental remediation (Louche et al., 2007). Developing modern technologies that enable energy generated from sustainable resources, and industrial production through environmentally friendly technologies, is important for the sustainable development in China. Biotechnological approaches developed to achieve these goals on a laboratory scale are no longer difficult: the challenge is to covert these approaches into real applications in an industrial scale. With synthetic biological techniques becoming standard practice across the life sciences, one can expect that new breakthroughs will be brought to the research in biotechnology.
2. China and SB
Similar to the situation in Western countries, synthetic biology (SB) is an emerging field in China that still lacks consensus in definition but is widely considered to bring new opportunities to biotechnology. The lack of a well-accepted definition, however, does not seem to stop the scientific community from pursuing SB, thereby leading to a quite diverse area of science and engineering. In general, the following five research activities reviewed in this section (listed in Box. 1) are usually included in SB (Bedau et al., 2010; Benner and Sismour, 2005; Deplazes and Huppenbauer, 2009; Luisi, 2007; O'Malley et al., 2008; Schmidt, 2009). In China, SB is in the list of future R&D plans (CAS roadmap). There is an expectation that SB will not only enhance knowledge in basic science, but will also have great potential for practical applications. This review aims to provide an overview of current Chinese research activities in SB and its different subfields (Table 3). It also provides a perspective on potential future initiatives, using literature reviews and personal communications with Chinese researchers. Thus, it can be regarded as a review of the current development of SB in China, and an indication that SB already exists as an emerging field with future potential.
Box 1. Subfield of SB.
-
•
Genetic circuits (based on genetic engineering but using real engineering principles)
-
•
Minimal genomes (or minimal cells)
-
•
Protocells (or synthetic cells)
-
•
Chemical synthetic biology (or xenobiology)
-
•
DNA synthesis (or synthetic genomics)
Table 3.
SB research in China by subfield.
| Subfield | Active research topics | Potentional applications |
|---|---|---|
| Genetic circuit (Section 2.1) | Genetically modifying biosysthesis pathways for ethanol. Butanol and fatty acids production | Biofuel production |
| Biosysthsis pathways for PHAs and fine chemical products (riboflavin, succinate and etc.) | Chemicals from renewable resources | |
| Biosensors to detect and/or degrade insecticides, heavy metal, and etc. | Bioremedication | |
| Genetic control elements, method for unmarked genetic modification, reporter-guided mutant selection, | Basic research | |
| Minimal genomes (Section 2.2) | Building up database on microbial metabolism, genentic parts for physiological functions | Bioinformations for rational designs |
| Identifing reduced genomes of organisms of engineering importance such as E. coli, P. putida and etc. | Chassie organisms | |
| Chemical synthetic biology (Section 2.3) | Unnatural proteins of special structure | Basic research |
| Proteins containing unnatual amino acids | Molecules with designed properties | |
| Protocells (Section 2.4) | Using basic type of procells to study energy conversion | Basic research |
| DNA based nanopore on protocells | Drug delivery system | |
| DNA synthesis (Section 2.5) | Improving DNA synthesis methods | Faciliating SB research and commercialization |
| Codon optimization | Improving activities of the molecules of interest |
2.1. Genetic circuits
Design and construction of genetic circuits (the SB variant of metabolic engineering) has been applied to a wide range of cellular regulation processes. It is known that many cellular regulatory mechanisms are encoded on the DNA level as regulatory motifs, such as promoters, repressors, oscillators etc. Gene activity, including transcriptional control and also post-transcriptional mechanisms, can be closely regulated by intrinsic and external signals. The design of tailored metabolic pathways to produce compounds of industrial interest is one of the popular research topics in China. A couple of research groups have worked on the modification of existing biosynthetic pathways, while others tried to introduce biological pathways into the model microbes. In most cases, the designed or redesigned metabolic pathways were aimed to optimize the productive capacity of microbes.
2.1.1. Genetic circuits to harness biomass
China is a country with a population of 1.3 billion, and it also is a developing country with rapid economic growth. The increasing pace of industrialization of a country with such a large population will result in an increased demand for energy. Electrical energy in China is derived from fossil resources (mainly coal, but also partially from petroleum and natural gas), and a relatively small portion comes from sustainable resources (hydrogen, wind, solar and biomass). The dominant transport fuel is obtained mostly by refining petroleum, with only a very small part coming from biofuels. China produced about 340 million gallons of ethanol in 2005, most of it derived from corn. Several provinces have developed infrastructure for blending and refuelling E10 (10% anhydrous ethanol and 90% gasoline) (Wang, 2005). The Chinese government is now encouraging ethanol production from non-grain sources such as cassava, sweet sorghum, and sweet potato, and it is supporting R&D on lignocellulosic ethanol. Based on the roadmap of scientific development strategies from the Chinese Academy of Sciences (CAS) “Innovation 2050: Technology Renovation and the Future of China”, it is projected that 30% of the fuel used should be biofuel derived from biomass in year 2050 (CAS roadmap).
The Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT), located Qingdao, Shangdong, was co-founded by CAS, the provincial government of Shandong and the municipal government of Qingdao in 2006. This institute is intended to develop cost effective and environmentally attractive products, processes and technologies to generate biofuels from sustainable resources. It is one of the primary national research institutes and focuses on the research and development of biofuels and associated biological processes, with startup funding of US$ 46 million (RMB 315 million) . It focuses on research in catalysis and conversion for biofuel production, using SB technology that includes metabolic engineering, enzyme engineering and biological reaction engineering. One of their research interests is designing genetic circuits in algae, by metabolic engineering, to produce advanced biofuels and green chemicals. It is developing “Green Synthesis Technology” to resolve aspects of energy consumption and the pollution generated during the process of conventional chemical synthesis. One of their approaches is to design new synthetic pathways to generate bio-based chemicals, such as aromatic monomers and free fatty acids. They also try to enhance the efficiency of converting renewable carbon resources with microbial biocatalysts. In one of their recent publications, a pretreatment of cellulose was developed that used ionic liquids for enzymatic hydrolysis of wheat straw. The pretreated wheat straw can then be easily fermented by Saccharomyces cerevisiae to produce ethanol. A recent review paper from this institute (Lu, 2010) stated that one of the research interests of QIBEBT was to engineer cyanobacteria as a chassis organism for the production of high energy density fatty acid-based biofuels by use of SB based approaches, such as metabolic engineering of genetic circuits of the fatty acid pathway.
Another research institute, the Key Laboratory of Synthetic Biology (KLSB) established by CAS in 2008, aims to design functional biological parts to produce biomaterials and bioenergy through the modification and synthesis of biological systems. One of the biofuels they have been working on is butanol. (Gu et al., 2009). Butanol is produced along with acetone and ethanol in the ‘ABE’ fermentation by Clostridium acetobutylicum. But this process has the drawback of low yields due to the toxicity of butanol to the fermentative strains (Lee et al., 2008). The researchers from KLSB redesigned the butanol production pathway in C. acetobutylicum EA 2018, aiming to increase the butanol ratio by eliminating the production of other by-products, such as acetone. The acetoacetate decarboxylase gene (adc) was inactivated by TargeTron technology, and an adc-disrupted mutant (EA 2018adc) was generated. In this mutant strain, the butanol ratio increased from 70% to 80% while acetone formation was dramatically reduced. In addition to biofuel production, antibiotic biosyntheyic pathways for rifamycin and vancomycin were also investigated in some common industrial strains, such as Amycolatopsis mediterranei. They proposed that GlnR of A. mediterranei might be a global regulator with a dual functional impact upon nitrogen metabolism and related antibiotics production (Yu et al., 2007). Heterogeneous biosynthetic pathways were also investigated in other industrial strains, such as Streptomyces spp., in order to produce recombinant biomolecules. Among them, recombinant microorganisms were constructed that contained larger heterogenous genomes and that were capable of secreting correctly-folded proteins into the growth medium (Zhang et al., 2008a,b). Besides being a better host organism than Escherichia coli to produce proteins of eukaryotic origin, Streptomyces spp. are well known to produce fine chemicals, such as antibiotics, antifungals, and other bioactive compounds. A five-gene cluster cvhABCDE from Streptomyces hygroscopicus 10–22 was identified by scientists from KLSB. Within this cluster, CvhA was further characterized as a sensor histidine kinase which negatively regulated morphological differentiation in a sugar-dependent manner in S. hygroscopicus (Wang et al., 2006a).
In addition to biofuels and important secondary metabolites, chemicals derived from biomass are also among the main research topics in China. The family of polyhydroxyalkanoate (PHA) is one of the most promising biodegradable polymers. To date, there are 100 different monomer types of PHA. They can be produced in almost all bacteria in the form of intracellular inclusions and make up to 90% of the dry cell mass (Garcia et al., 2004; Haywood et al., 1990; Lee et al., 1999; Madison and Huisman, 1999; Yim et al., 1996). Unlike other ‘degradable’ polymers such as those based on petrochemicals, poly-lactic acid (PLA) and starch polymers, PHAs have useful natural properties and, therefore, it is not necessary to sacrifice their biodegradability to improve their properties further. PHAs already have properties similar to synthetic polymers, such as polyethylene and polypropylene. PHAs can be blended into a large number of copolymers that allows further engineering of the polymers to yield desired properties for a wide range of applications. A research group of Tsinghua University has worked on genetic circuits to enhance the production of PHAs in engineered microorganisms such as E. coli, Pseudomonas putida and Aeromonas hydrophila (Jian et al., 2010; Li et al., 2010; Wei et al., 2009). In order to convert laboratory scale fermentation to produce PHAs on an industrial scale, cells must be engineered to make them capable of growing in high density. It has been proposed that a limited oxygen supply is a hurdle that cells face while growing to high density. One possible solution was brought up by Jian et al. by constructing synthetic pathways that could be turned on in response to micro- or anaerobic condition (Jian et al., 2010). This approach was already applied to produce poly-3-hydroxybutyrate (PHB) (Li et al., 2009). PHB is a type of polyester used to make heat tolerant and clear packaging film, and it is produced by starch or glucose processing bacteria. The synthetic pathways were constructed to enhance PHB production from 29% to 48% of the cell dry weight under anaerobic conditions.
The reconstruction and analysis of genome-scale metabolic networks have been set as one of the main topics of the research groups in Tianjin University. A database of metabolic networks was built and named, the GSMNDB (Genome-Scale Metabolic Network DataBase) (GSMNDB). The genome-scale metabolic models of more than 50 microorganisms have been recorded. For certain model organisms such as E. coli and S. cerevisiae, two or more metabolic models have been included. The GSMNDB is to provide a central gateway to most of the published metabolic network models. A tool termed DoriC has been developed which can provide literature information and graphical views of the replication origins (oriCs) regions of bacterial genomes (Gao and Zhang, 2007). In addition, metabolic engineering approaches have been applied in the designation of genetic circuits to improve the production of chemicals from biomass, particularly the production of riboflavin (Shi et al., 2009; Zhu et al., 2006, 2007) and succinate (Wang et al., 2006b,c). DNA shuffling techniques have also been applied to improve strains for industrial production (Gong et al., 2009; Zheng et al., 2010).
2.1.2. Genetic circuits for bioremediations
Environmental pollution is an undesirable consequence of the economic growth in China. It posts a serious threat to health and sustainable development for the future. The development of cost-effective, on-site methods for environmental monitoring and remediation are critical to cope with increasing environmental problems. Among the ongoing approaches developed for bioremediation, biosensors based on synthetic biology technologies are quite attractive because they can complement both laboratory-based and field analytical methods for environmental monitoring. Future developments on more advanced engineered biosenors may enable the monitor sensors to act as bioreactors to break down the target molecules. There have been a wide range of biosensors that have been reported for potential environmental applications (Harms et al., 2006; Keenan et al., 2007; Rodriguez-Mozaz et al., 2004; Wei and Ho, 2009; Zuo et al., 2009).
China has been, and is, facing a tremendous challenge to manage environmental pollution. For example, it is estimated that by 2030 the annual amount of solid waste will increase from about 190 million tons in 2004 to over 480 million tons (Minghua et al., 2009). The Chinese environmental office admitted that there is increasing pollution of waterways in spite of continuous pollution control by standard treatments (Ansfield and Bradsher; Xinhua). The social, economical, and environmental impacts of pollution are significant. It is hoped that the advent of synthetic biology will result in useful applications to reduce environmental pollution.
Using synthetic biological approaches, genetic circuits could be designed to enable the host organisms to have the ability to act as biosensors and bioreactors to sense and break down environmental pollutants. Several unique genetic circuits for environmental applications have been developed by Chinese research groups to degrade environmental hazards, such as heavy metals, insecticides, phenols, and arsenic.
Among these hazardous compounds, pesticides represent the greatest target for bioremediation, because pesticides have been used extensively in China. They function by means of interacting with a specific biochemical target, either as a substrate (e.g., organophosphorus insecticides or organophosphate hydrolase) or as inhibitors (e.g., dithiocarbamate fungicides or aldehyde dehydrogenase; organophosphorus insecticides or acetylcholinesterase). Several enzyme systems such as organophosphorus hydrolase and acetylcholinesterase have been investigated. The State Key Laboratory of Integrated Management of Pest Insects and Rodents, Institute of Zoology, CAS is the leading institute in China for bioremediation on environmental pesticides. One of the approaches they applied is surface display of heterogeneous molecules on host microbes to enhance their ability to process pollutants thoroughly (Jiang et al., 2001). In addition, genetic circuits of the twin-arginine translocation (Tat) pathway and the ice nucleation protein (INP) display system were applied, resulting in surface display of the enzymes, organophosphorus hydrolase (OPH) and methyl parathion hydrolase, used for the detoxification of pesticides. They also integrated markers, such as fluorescent proteins, into their engineered strains to enable them to be tailored for future field applications with easy detection (Yang et al., 2010).
The engineering of Rhodococcus erythropolis for bioremediation has been investigated by a team from State Key Laboratory of Microbial Technology, Shandong University. The microbes were engineered to degrade dibenzothiophene, carbazole, and dibenzofuran to nontoxic metabolites (Yu et al., 2006). The genetic circuit encoding enzymes responsible for degrading carbazole to anthranilic acid was introduced into the targeted microbes. It is believed that such engineered stains have the potential for other applications in bioremediation.
Heavy metals can be highly toxic as environmental contaminants. Heavy metals, particularly lead (Pb) and mercury (Hg), can cause a number of adverse effects on health, and at certain concentrations can lead to diseases or even death. A DNAzyme-based microarray method was developed by Zuo et al. to detect multiple metal ions (Zuo et al., 2009). The DNA substrates (Cu-Sub and Pb-Sub) of metal dependent DNAzymes (Cu-Enz and Pb-Enz) were first immobilized on the surface of aldehyde-coated slides. In the presence of Cu2+ and Pb2+ metal ions, the substrate was irreversibly cleaved into two fragments triggering the releasing of a fluorescent-labelled probe (Cy3-probe). The cleavage event was measured by the intensity of fluorescent signal. When no metal ions were present, the Cy3-probe would remain hybridized with the substrate resulting in a strong fluorescence signal, while in presence of metal ions, cleavage resulted in a weakening of the fluorescent signal. A control probe of Cy5 labelled was used as a reference for data normalization. This method can detect heavy metal ions in aqueous solution at parts-per-billion concentration, and be extended to carry out multi-component detection that will provide further applications of environmental monitoring. The host institute of this research group is the State Key Laboratory of Bioreactor Engineering (SKLBE) of East China University of Science and Engineering. It is a specified key laboratory where research is focused on bioreactor engineering-related disciplines, including bioprocess engineering (industrial scale fermentation), metabolic engineering, biological catalysis and enzyme engineering, and biomass energy and bio-based chemicals (SKLBE).
Synthetic microbial ecosystems will have wide applications in bioremediation. Yet the interactions of organisms within these systems need to be clarified due to their complex compositions and structures. A recent study carried by researchers from Tianjin University may provide a new approach to illustrate the complex interactions within a system by construction of an artificial ecosystem via SB (Hu et al., 2010). The interactions of the species within this designed system are well defined. Two quorum-sensing signal transduction circuits have been designed that simulate a synthetic ecosystem and mimic the plausible response that a natural system would have in the dynamics formed by changing environmental factors. The synthetic ecosystem constructed through this study has provided valuable insights in ecology, and may act as a chassis for construction of more complex microbial ecosystems.
2.1.3. Genetic circuits for basic research
Positive and negative feedback loops are important genetic elements in gene regulatory networks, acting as switches, oscillators, and excitable devices. Genetic circuits that are a combination of different feedback loops can act as more precise regulation modulators. A minimal model system was developed in Nanjing University, and its dynamics and performance advantage in response to stimuli were explored in a unifying framework (Tian et al., 2009). This system was tunable from mono-stability to bi-stability by increasing the strength of positive feedback, and the bi-stability regime was modulated by the strength of negative feedback. The transitions from bi-stability to excitability and oscillation were achieved by increasing the strength of negative feedback, while the reverse conversion occurred by enhancing the strength of positive feedback. In addition, this system was more flexible than a single feedback loop that could produce robust oscillations over a wider stimulus regime compared with a single time-delayed negative feedback loop. It has been suggested that the coupled feedback loops can act as toolboxes for engineering diverse functional circuits in SB.
A method for unmarked genetic modification was developed in the methylotrophic yeast Pichia pastoris through a knock-out of the endogenous genes (such as arg1 and met2) and a knock-in of the targeted genes (a green fluorescent protein expression cassette) (Yang et al., 2009). Site-directed mutagenesis on the ARG1 gene was also performed in this study. Remarkably, all these genetic modifications have been achieved without introducing unwanted selection markers. In this approach, the E. coli toxin gene mazF was used as a counter-selectable marker that was controlled by AOX1 promoter, and the induced expression of MazF in P. pastoris halted cell growth. A genetic module was constructed in which mazF and a zeocin resistance gene acted as counter-selectable and active-selectable markers. When the AOX1 promoter was activated, the markers were recycled efficiently via homologous recombination between the direct repeats. This method allows the selectable marker gene to be recycled for multiple modifications, a useful approach for other genetic manipulations in the yeasts.
A reporter-guided mutant selection (RGMS) method was developed recently by a research team from Institute of Microbiology CAS to facilitate the selection of mutants for over-expressing targets of interest (Xiang et al., 2009). This protocol was applied to improve clavulanic acid (CA) production in Streptomyces clavuligerus. In a single-reporter design, the transcriptional activator ccaR for biosynthesis of CA was chosen as the target of interest, and neo (resistance to kanamycin) as the reporter. Subsequently, a double-reporter circuit was configured, in which a xylE-neo double-reporter cassette was used to monitor ccaR expression in order to reduce the high false positive rate of the single-reporter method. Up to 90% of mutants selected by the modified method showed improvement in CA titer. The RGMS approach can generate a pool of mutants with genetic diversity and provide a library for further screening by inverse metabolic engineering. The same approach can be applied to engineer strains that produce biofuels and other molecules of interest.
After exposure to an antibiotic, it is common to find surviving microbes, and this phenomenon may be due to phenotypic heterogeneity. A toxin–antitoxin (TA) module composed of two gene pairs, hipA and hipB, was identified by researchers from Peking University, and it was suggested that they are responsible for generating the antibiotic resistant subpopulation (Lou et al., 2008). A simple genetic regulation model was built to study the phenotypic heterogeneity. This molecular model illustrated the emergence of a resistant strain, suggesting that genetic circuits might be built to investigate the molecular mechanisms of the phenotypes of microbes.
Synthetic genetic circuits for programming cell populations and coordinating behaviour across a population have been studied by a research group of the Third Military Medical University (Wang et al., 2008). An artificial cell-to-cell communication system was studied in mammalian cells, using nitric oxide signalling elements, by integrating nitric oxide synthesis with the c-fos promoter. The intercellular messenger, nitric oxide, was synthesized by the engineered ‘sender’ cells, and it diffused into the environment and activated the c-fos promoter of the receiver cells. Once the nitric oxide signal was received, expression of a green fluorescence protein (GFP) reporter was activated in the engineered 'receiver' cells. This sender–receiver system was under positive-feedback regulation, which resulted in population density-dependent GFP expression in a quorum-sensing pattern. It was proposed that such artificial cell-to-cell communication in mammalian cells could serve as a versatile tool for regulated gene expression, and as a building block for complex artificial gene regulatory networks for use in gene therapy, tissue engineering, and other applications.
Microbial chemotaxis is the ability of motile microorganisms to direct their movement in response to chemical gradients in the environment. Flagellum rotation of E. coli is driven by a molecular motor switch between two operational modes. Specific receptors, which are located in the very beginning of the chemotaxis pathway, are involved in the sensing of attractants and transducing the signal to the motor to control its rotation mode. Essential features of the chemotaxis pathway are the amplification rate, adaptation robustness, relaxation time and feedback loop. The research team led by ZR Sun has proposed a model of the chemotaxis dynamic pathway, in which these essential features are assembled together by adapting reverse-engineering methodology (Luo et al., 2010). Reverse engineering is the process of discovering the technological principles of a device, object or system through analysis of its structure, function and operation. It involves disassembly of the object, detailed analysis of its workings, and the manufacture of a new object with similar function to the original. The kinetic relationship between the input and output in the wild type chemotaxis pathway was set as the transfer function of a biological controller. The optimized transfer function was applied to a designed pathway that was evaluated by computational simulation. Their work provides an example that reverse engineering is a powerful approach to abstract the design principles from native pathways, and for designing new pathways without knowledge of detailed pathway mechanisms.
2.2. Minimal genomes
One of the subfields of SB is research on minimal genomes that contain only a minimal DNA sequence essential for life. The ideal minimal genome will be composed only of genes that are essential for the survival of the respective organism under defined conditions. The non-essential genes and non-encoding regions are usually removed, for example, genetic elements of alternative metabolic pathways or those encoding responses to stress situations. It is believed that minimal cells – built on minimal genomes – can serve as the efficient platforms to develop microbes with new functions.
A minimal genome can be achieved via top-down approach by trimming the existing genome. It is believed that cells of minimal genomes can provide better chasses to host genetic components for desired metabolic functions and efficient production of targets of interest. Currently, several research groups in China are working on minimal genomes, and most of them utilize top-down approaches. Among them, a top–down approach was applied to obtain a reduced Pseudomonas putida genome on strain K224. The engineered P putida with reduced oxidation activities on fatty acids by deleting β-oxidation-related genes has then been used as a chassis for better production of PHAs (Li et al., 2011; Liu et al., 2011; Wang et al., 2011). The genome of E. coli, one of the common engineered microbes for industrial application, has been studied in order to map essential and non-essential genes for the induced expression of an exogenous endonuclease gene. A research group from Institute of Psychology, CAS has established a genetic database MyBASE, for genome polymorphism and gene function studies of Mycobacterium (Zhu et al., 2009), and MethyCancer, a database of human DNA methylation and cancer (He et al., 2008). This research group also worked on modeling the transcriptome, based on transcript-sampling data (Zhu et al., 2008). In addition, they are one of the very few recipients currently funded by the Natural Science Foundation of China (NSFC) to work on SB-related projects, including a project on minimal genome research that is based on comparative genomics and large-scale deletion of genome fragments. One of the international cooperation in which the group participated was the 6th Framework Program of the European Commission on PROgrammable BActeria CaTalYzing reSearch (PROBACTYS). Another group from Tianjin University has built a database of essential genes (DEG), to record the currently available genes that are indispensable for the survival of an organism. The DEG contains essential genes of a wide range of bacteria, such as E. coli, B. subtilis, H. pylori, S. pneumoniae, M. genitalium and H. influenzae (Zhang and Lin, 2009; Zhang and Zhang, 2008; Zhang et al., 2004). These microbial genes can be used to construct a minimal genome as a functional module, which would serve as a chassis for other research in SB. Besides essential genes for prokaryotes, DEG contains genes of eukaryotes as well, such as yeast, human, mouse, worms, fruit flies, zebra fish and Arabidopsis thaliana. Furthermore, this database can provide information for essential genes that can act as targets for drug development.
Essential genes encode biological functions critical for cell survival. Consequently, their null mutants are often difficult to obtain, thereby impeding subsequent genetic and functional analysis. A theophylline-responsive riboswitch that enables target gene expression to be specifically “tuned” from low to high levels has been developed, and it can be used to generate conditional hypomorphic mutants (Jin et al., 2009). In this ligand-responsive riboswitch system, low levels of gene activity in the absence of the ligand (theophylline) permit cell survival, thus the gene activities can be investigated. While supplementing the ligand, the normal gene expression levels and wild-type phenotypes will be restored. The team demonstrated that the gene encoding an RNA binding protein (CsrA) for carbon storage regulation, csrA, is the essential gene in E. coli that encodes a global regulatory protein CsrA. A mutant, named switch-csrA, was constructed by placing the theophylline-responsive riboswitch immediately upstream of the csrA ribosome binding site. In the absence of ligand, the mutant switch-csrA produced low levels of CsrA, resulting in the aggregation of cells. Theophylline binding induced conformational changes in the riboswitch, thereby activating it and resulting in an efficient csrA translation (i.e., autoaggregation did not occur). It revealed that autoaggregation was regulated by the CsrA module via the polysaccharide adhesin, poly-beta-1,6-N-acetyl-D-glucosamine. The ligand-responsive riboswitches approach that they developed represents a new means to construct conditional hypomorphic mutants to investigate the activities of essential genes, which effectively complements traditional genetic approaches.
2.3. Chemical synthetic biology (Xenobiology)
The SB subfields described above involve biochemical similarities to natural life forms. However, the biochemistry of synthetic life could be entirely different. Such systems arise from chemical SB, where the very basics of life biochemistry are changed in order to create biological systems that are truly different, both in metabolism and on the genetic information level. Examples include altered or non-naturally occurring bases within the DNA, a concept that involves entirely different genetic information storage molecules, so-called xeno-nucleic acids (XNA) that cannot interact with naturally occurring DNA (Benner and Sismour, 2005; Herdewijn and Marliere, 2009; Marliere, 2009; Yang et al., 2006, 2007). Another example is the use of non-natural building blocks such as non-canonical amino acids. More visionary ideas involve replacing carbon with silicon in essential biomolecules. Other visions encompass possible life forms that use not only non-natural elements but also architectures entirely different from the genome–ribosome–protein architecture of “life as we know it” (Schmidt, 2010).
Researchers from Peking University have synthesized several unnatural proteins. A monomer protein has been synthesized composed of beta-alpha-beta folds (two parallel beta strands connected by an alpha helix). Beta-alpha-beta structural motifs are commonly used building blocks in protein structures containing parallel beta-sheets (Liang et al., 2009). The beta-alpha-beta fold structure showed high thermal stability. Prior to constructing the monomer protein, another small protein, with an independent beta-alpha-beta structure (DS119), was synthesized and its folding mechanism was studied by all-atom molecular dynamics and coarse-grained simulations in order to investigate its folding pathways and energy landscape. Results from the structural studies revealed that DS119 was a parallel beta-sheet. This group also works on designing unnatural protein-protein interaction pairs (Liu et al., 2007). They have designed an algorithm for de novo design of an unnatural protein–protein interaction pair by scanning the Protein Data Bank for suitable scaffold proteins that can be used for grafting key interaction residues and that can form stable complexes with the target protein after additional mutations (Qi et al., 2010).
Proteins containing unnatural amino acids usually have new chemical, physical, and biological properties. It has been shown that proteins containing unnatural amino acids can have improved stability, specificity, and catalytic properties. Tremendous efforts have been made to incorporate unnatural amino acids into proteins, and to introduce new functional groups through chemical or biosynthetic means (Wang and Schultz, 2004). A research group from China Pharmaceutical University (Nanjing, Jiangsu) has provided an example how this can be done with biosynthetic pathways; involving the combination of homology modelling and molecular docking for rational mutant design (Chen et al., 2008). The Methanococcus jannaschii tRNA(Tyr)/tyrosyl-tRNA synthetase pair has been engineered to incorporate unnatural amino acids into proteins in E. coli. The amino acid binding site of M. jannaschii tyrosyl-tRNA synthetase has been mutated to design novel synthetases specific for unnatural amino acids, in this case, to corporate p-acetyl-L-phenylalanine into proteins. Among 60 mutated aminoacyl-tRNA synthetases, 15 of them showed binding ability to p-acetyl-L-phenylalanine, of which two had considerable binding affinities. An orthogonal tyrosyl suppressor tRNA/aminoacyl-tRNA synthetase system was established to selectively incorporate p-azido-L-phenylalanine into the amber nonsense codon TAG of uricase in E. coli (Sun et al., 2010). It has been found that optimal suppressor tRNAs can optimize site-specific modification of unnatural amino acids in target proteins by substituting p-azido-L-phenylalanine for two Phe of uricase at position 170 and 281. These proteins were further modified as polyethylene glycol (PEG) derivates for improving their pharmacological properties, by reducing their antigenicity and immunogenicity. This approach was based on a method developed by Schultz et al. for site-specific incorporation of unnatural amino acids into proteins in vivo to obtain mutant uricase carrying unnatural amino acids (Wang and Schultz, 2004; Xie et al., 2004).
2.4. Protocells
The research on protocells deals with the bottom–up approach to construct synthetic cell-like vesicles assembled from non-living chemical components (Hanczyc and Szostak, 2004; Mansy and Szostak, 2008; Mansy et al., 2008). Protocells can be designed to perform desired cell functions within a stabilized internal cytoplasm-like environment (Murtas, 2009; Rasmussen et al., 2004; Szostak et al., 2001). It has been shown that protocells can be used to study energy conversion in cells (Xu et al., 2010). The basic type of protocells is composed of a lipid bilayer membrane and membrane proteins that can serve as a unique platform to study the interaction of membrane receptors with the molecules of interests (Zepik and Walde, 2008; Zepik et al., 2008). Among all these membrane proteins, the ion channels play different roles in cellular circuits. They form nano sized pores, which can only work in the environment of a lipid membrane. Researchers from Peking University and Institute of Chemistry CAS have reported a synthetic DNA based nanopore system where the gates of single solid-state conical ion channel-like nanopores can be controlled by DNA switches immobilized inside the nanopores (Xia et al., 2008). The researchers found that the high- (on-state) and low- (off-state) conductance states of a nanopore-DNA system corresponded to the single-stranded and i-motif structures of the attached DNA motors. This novel nanopore–DNA system, which was gated by collective folding of structured DNA molecules in response to an external stimulus, provided an artificial oscillatory counterpart of protein nanopore channels. This DNA motor-driven nanopore switch can be used to construct a protocell with more precisely controlled functions. In the near future, the DNA molecules can be replaced by other functional biomolecules, such as polypeptides or protein enzymes.
2.5. DNA synthesis
In contrast to traditional recombinant DNA technologies, such as plasmid based gene cloning, chemical synthesis of DNA is essential in synthetic genomes and biosynthetic pathways of rational design, and to create any new DNA sequence. DNA synthesis has been used extensively in life science, for example, to study gene functions of template DNAs that are difficult to obtain, or to optimize the codons of genes to be expressed in heterogeneous systems. For example, the reconstruction of the 1918 Spanish Influenza Pandemic Virus was achieved by chemically synthesizing the genome without a template (Tumpey et al., 2005). Recent innovations in chemical synthesis technology have enabled the assembly of a whole genome, and the creation of a bacterial cell controlled by a chemically synthesized genome containing 1,077,947 base pairs of Mycoplasma mycoides JCVI-syn1.0 (Gibson et al., 2010).
The prevailing synthesis methods are PCR-based and ligase-based DNA synthesis (Liang et al., 2011). To enhance the power of these methods, new synthesis and assembly techniques are needed to meet the increasing demands of SB. A couple of research groups in China are currently working on this topic. A PCR-based, two-step DNA synthesis (PTDS) method for synthesis of long segments of DNA was modified by Xiong et al. This involved synthesis of individual fragments of the DNA of interest, 60mer oligonucleotides with 20 bp overlap to produce DNA fragment of 500 bp in length, and PCR amplification to assemble the entire sequence of the DNA of interest with the two outermost primers. This modified method can produce DNA fragments of 5–6 kb with high G + C contents within 5–7 days (Xiong et al., 2004). The same research group also developed a method for assembly and PCR-based accurate synthesis (PAS) of long DNA sequences. The PAS protocol was developed based on the PTDS method with additional steps, such as the purification on the synthetic oligonucleotides by PAGE prior to the first PCR and error correction using an overlap-extension PCR, if needed. The process took approximately 7 days to synthesize DNA fragments up to 12 kb (Xiong et al., 2006). An isothermal DNA synthesis method-isothermal unidirectional elongation method (IUEM) has been developed by Lin et al., which can synthesize DNA fragments up to 300 bp for use as building blocks for the synthesis of longer DNA sequences, which is an isothermal synthesis process and involves the cooperation of three enzymes (Lin et al., 2007).
Besides research on DNA synthesis, codon optimization is another topic of interest among Chinese researchers. Xylanase is an enzyme that can degrade the linear polysaccharide beta-1, 4-xylan into xylose. The hydrolysed product has broad applications in food processing. A codon-optimized recombinant xylanase gene from Streptomyces sp. S38 was synthesized and extracellularly expressed in P. pastoris (Fu et al., 2010). Phytases catalyze the release of phosphate from phytic acid. These enzymes are important to the farming industry. The synthetic gene with codon optimization on phyCs (the gene encoding neutral phytase) has yielded a 90-fold increase when expressed in P. pastoris (Zou et al., 2006).
Directed evolution in vitro is a powerful molecular tool for designing new biological parts. A few Chinese research groups are using DNA synthesis methods to investigate the function of some enzymes of interest. A strategy for directed in vitro evolution of reporter genes, based on semi-rational design and high-throughput screening, was developed by Xiong et al., and it involves DNA shuffling and screening (Xiong et al., 2010). Such an approach was applied in an in vitro directed evolution strategy through DNA shuffling to investigate beta-galactosidase of E. coli. Five mutants were obtained resulting from two rounds of DNA shuffling and screening with higher beta-glucuronidase activity than wild-type beta-galactosidase. The sequencing on these mutants reveals variants at fourteen nucleic acid sites, resulting in changes in ten amino acids (Xiong et al., 2007b). A strategy of a semi-rational design of directed evolution was developed of which a gene encoding the thermostable beta-galactosidase of Pyrococcus woesei was chemically synthesized with optimized G + C content and mRNA secondary structures. The synthetic sequence was subjected to DNA shuffling, library construction and screening. One mutant with higher activity was identified, and sequencing revealed eight sites deduced from the original sequence. Subsequently, 16 degenerate oligonucleotides were designed corresponding to the eight amino acids, and used to screen in the second round of DNA shuffling. Another mutated sequence was identified exhibiting a lower specific activity (Xiong et al., 2007a).
To date, commercial DNA synthesis services are capable of synthesizing DNA in lengths up to tens of kilobase. There are a few Chinese companies, such as Generay Biotechology (http://www.generay.com.cn), Sangon Biotech (http://www.sangon.com) and ShineGene (http://www.shinegene.org.cn), which provide synthetic genes using PCR-based technologies. Given the rapid development in synthesis techniques and a reduction in cost, more Chinese research groups will utilize synthetic genes and genomes obtained from Chinese companies.
3. Perspectives
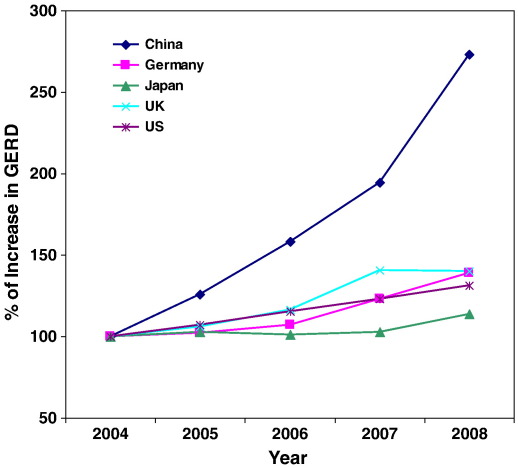
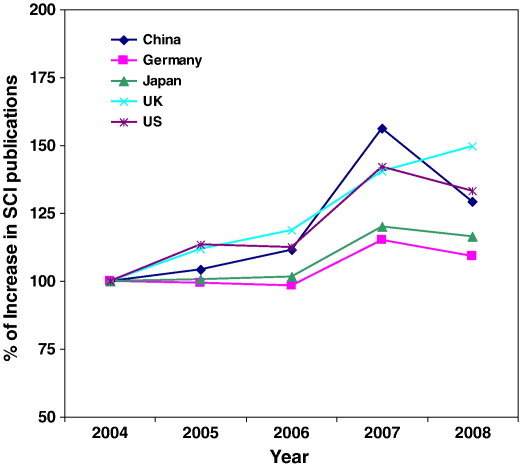
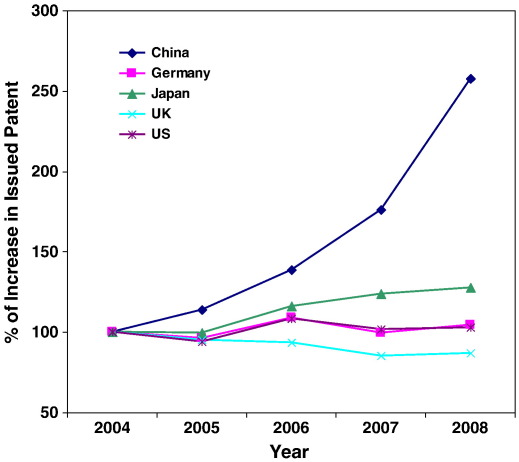
The United States is in the lead position in SB research: they host most of the research entities (institutes or universities), and conduct most of the R&D activities (Synthetic Biology Project). Other global players are Germany, Switzerland, France and UK (Pei et al., 2011). China, however, has now a couple of groups active in research on SB. Although SB has already been established in China, more funding is needed to make China an internationally acknowledged player in the field. In China, SB-related research started as early as the 1960s when Chinese scientists made the first protein by chemical synthesis – synthetic insulin (Kung et al., 1965). China has been a key participant in the “Human Genome Project” and remains as one of the major contributors to genome databases. Furthermore, China has been active in the most of the emerging research fields such as genomics, bioinformatics, nanotechnology, and lately, in the stem cell research. China is one of the leading countries in the gross expenditure on R&D, issued patents and scientific publications (shown in Figs. 1, 2 and 3). All these have laid the foundation for the development of SB.
Fig. 1.
Increase in R&D expenditures (GERD) compared with those in year 2004 (Source: OECD). The GERD of the five selected countries were obtained from the statistics database of OECD. The annual increase of the GERD was calculated by its ratio to those in year 2004.
Fig. 2.
Increase in SCI publications in S&T compared with those of year 2004 (Source: ISI). The SCI publications of Science and Technology (S&T) of the five selected countries were obtained from the ISI Web of Knowledge. The annual increase of the publications was calculated by its ratio to those in year 2004.
Fig. 3.
Increase in granted patents compared with those of year 2004 (Source: WIPO). The issued patents of the five selected countries were obtained from the statistics database of WIPO. The annual increase of the patents was calculated by its ratio to those in year 2004.
SB is still in an early developmental stage, both internationally and in China, and no consensus has been achieved regarding its exact definition, as shown by our recent survey among Chinese researchers. The driving force behind SB in China was primarily scientific curiosity to learn more about biological systems, and how they might be manipulated and controlled through an engineering approach. One of the goals of future SB research is to develop engineering principles for biological systems. As knowledge of natural systems accumulates, SB research will move from genetic circuits to improved regulatory systems for synthetic devices, and further to synthetic cells, eventually leading to multiple cell systems. In natural systems, there are regulated interactions between DNA, RNA, proteins and metabolites. Identifying modular regulatory elements such as promoters and other regulators has been essential to the progress of SB. There is considerable evidence that genomic rearrangements and horizontal gene transfer have driven the evolution of new biological capabilities. Similarly, the identification of biological modules that confer new functionalities, when assembled in different contexts, will drive the progress of SB (Schmidt and Pei, 2011).
In consideration of increasing environmental concerns and the depletion of fossil fuel reserves, chemicals derived from biomass are considered as the promising environmental and economic alternatives. Many scientists believe that SB will contribute to the conversion of biomass to useful biochemicals. There are high expectations of SB to produce biofuels and biochemicals, and to develop environmental and medical applications. In the 10th five-year plan of China's National Key Technologies R&D Program, research on the mechanism of microbial degradation of lignocellulose was proposed to be further strengthened (SKLMT). Design and reconstruction of lignocellulose-degrading microbes has been one of the research priorities, including using the direct evolution technique to improve the activity of cellulase and its enzymatic characteristics (e.g., alkali-resistance and surfactant-resistance). It indicates that more research in China will be focused on applied science or industrial biotechnology such as biocatalysts to reduce the cost of processing cellulose biomass. Innovations in bioremediations from SB are also widely expected. Within a couple of years, it is believed that applications will be achieved in the medical sciences, such as reprogramming mammalian cells for treatment purposes (i.e., tissue engineering, improved cell cultures for medicine production, etc.). The latest release from 973 Program confirmed that a multi-million dollar project on an artificial synthetic cell factory, which would be funded with an estimated yearly budget of 40 million CNY or 4.3 Mio Euros for two years with a possibility for constitutive funding if evaluated positively by 2013 (Ministry of Science and Technology). The research on the minimal genome of E coli and its synthesis has also gained support from 973 Program recently (Shanghai Institute of Life Science). A couple of other proposals applying SB approaches, for the development of a microbial chassie for antibiotics production and other biopolymers, had also been proposed. The current GERD of China in year 2010 is 1.5% which is projected to 2.5% in year 2020 (Editorial, 2011). With such an increase of R&D expenditure, there is no doubt that more research will be funded not only in the traditional disciplines but also in emerging ones like SB.
Technological innovations in the synthesis of nucleic acids and DNA sequencing have accelerated the development of SB. The synthesis of DNA, with any sequence and without a template, makes possible the de novo synthesis of genes and even whole genomes. This means that new biological functions can be designed and used for research and application purposes. Technical advances of DNA synthesis have increased the productivity and quality of the processes with a continuous decrease in costs. A couple of companies in China currently offer commercial DNA synthesis, which in the long term will reduce labor intense DNA synthesis in research laboratories. For example, in the field of commercialized DNA sequencing, the Beijing Genomics Institute (BGI) hired > 1000 bioinformatics specialists and purchased 128 HiSeq 2000 Illumina genome sequencers, thereby doubling the world's sequencing capacity and turning China into a major service provider in this sector (Editorial, 2011). One of technology and platforms in BGI is oligonucleotide synthesis (BGI). It can be expected that BGI and other Chinese biotech companies have the potential to be one of the major providers of DNA synthesis in the future.
With few private investors, the Chinese government plays an important role in fostering innovations. The current funding for R& D of SB in China is mainly through the government's Project 873, Project 973, or NSFC. In 2008, a dedicated research funding scheme for SB was proposed to foster new research into the development of new biofuels and biomaterials, as well as novel approaches to bioremediation and medical applications. However, it was delayed due to the lack of consensus definition of SB (personal communication). In addition to funding from the state, provincial and municipal governments are the other constant sources that contribute to the budget for research (Editorial, 2011). Industry or private funding is more relevant in the US, while there are only a few private sources in China. These include, for example, the cooperation between Royal Dutch Shell Group and QIBEBT (QIBEBT Project) and Boeing Inc. and QIBEBT for biofuel (Pangea), and the China Petroleum & Chemical Corporation (Sinopec) and Novozymes for cellulosic biofuel (WSJ). It has been reported that the first industrial production of the 2nd generation of biofuel will be commercialized in 2011 (Novozymes). In the roadmap of scientific development developed by 300 scientists of CAS, 22 key scientific issues have been brought up. Among them, synthetic life and SB, as well as 20 other scientific issues, have been considered to be important for energy resources, the environment, public health, and traditional or non-traditional security (CAS roadmap).
As an emerging scientific field, SB is largely known only within academia in China. Due to the media coverage of the so-called “synthetic bacteria” in 2010, the term, synthetic biology, has received public exposure. The Chinese people tend to embrace new innovations easily and may show great enthusiasm for SB, viewing it as a science with tremendous potential. However, they are not aware of the associated concerns of biosafety, biosecurity, and ethics. Debates on the societal impact of emerging science in China are always delayed until the maturation of the technology. For example, the now soaring public concerns on genetically modified crops came after the official approval of its commercialization. To avoid the same scenario for future applications of SB, it would be better to conduct SB research in collaboration with social scientists, and in a manner of ongoing public engagement in order to raise awareness on the societal issues. Our continuing project, which is funded by FWF and NSFC, is aimed to provide better scientific insight into the challenges of SB to biosafety and risk assessment, and will address some of the concerns on ethical, legal and social issues (ELSI). We will look into the Chinese regulations for SB, and keep up to date with the SB related activities in China.
Acknowledgements
The authors would like to thank Dr. David Wade for English proof reading. This project is supported by the Austrian Science Fund (FWF, Fonds zur Förderung der Wissenschaftlichen Forschung) project “Investigating the biosafety and risk assessment needs of SB in Austria (Europe) and China” Project No. I215-B17; and the National Natural Science Foundation of China (NSFC) Grant No. 30811130544.
References
- 973 Program Multidisciplinary and interdisciplinary science. http://www.973.gov.cn/English/AreaItem.aspx?catid=07
- Ansfield J., Bradsher K. China Report shows more pollution in waterways. http://www.nytimes.com/2010/02/10/world/asia/10pollute.html?_r=2
- Bedau M.A., McCaskill J.S., Packard N.H., Rasmussen S. Living technology: exploiting life's principles in technology. Artif Life. 2010;16(1):89–97. doi: 10.1162/artl.2009.16.1.16103. [DOI] [PubMed] [Google Scholar]
- Benner S.A., Sismour A.M. Synthetic biology. Nat Rev Genet. 2005;6:533–543. doi: 10.1038/nrg1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BGI Technology&Platforms: oligonucleotide synthesis. http://www.genomics.cn/en/platform.php?id=195
- CAS roadmap Innovation 2050: Technology Revolution and the Future of China. http://www.edu.cn/html/rd/z/cxlx.shtml
- Chen M., Cai L., Fang Z., Tian H., Gao X., Yao W. Site-specific incorporation of unnatural amino acids into urate oxidase in Escherichia coli. Protein Sci. 2008;17:1827–1833. doi: 10.1110/ps.034587.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Wang H.G., Wen Z.J., Wang Y. Life sciences and biotechnology in China. Philos Trans R Soc Lond B Biol Sci. 2007;362:947–957. doi: 10.1098/rstb.2007.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deplazes A., Huppenbauer M. Synthetic organisms and living machines: positioning the products of synthetic biology at the borderline between living and non-living matter. Syst Synth Biol. 2009;3:55–63. doi: 10.1007/s11693-009-9029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Editorial Domestic science. Nature. 2010;466:667. doi: 10.1038/466667a. [DOI] [PubMed] [Google Scholar]
- Editorial China calling. Nat Biotechnol. 2011;29:89. doi: 10.1038/nbt.1784. [DOI] [PubMed] [Google Scholar]
- Fu X.Y., Zhao W., Xiong A.S., Tian Y.S., Peng R.H. High expression of recombinant Streptomyces sp. S38 xylanase in Pichia pastoris by codon optimization and analysis of its biochemical properties. Mol Biol Rep. 2010 doi: 10.1007/s11033-010-0644-7. (Electronic publication ahead of print) [DOI] [PubMed] [Google Scholar]
- Gao F., Zhang C.T. DoriC: a database of oriC regions in bacterial genomes. Bioinformatics. 2007;23:1866–1867. doi: 10.1093/bioinformatics/btm255. [DOI] [PubMed] [Google Scholar]
- Garcia B., Olivera E.R., Sandoval A., Arias-Barrau E., Arias S., Naharro G. Strategy for cloning large gene assemblages as illustrated using the phenylacetate and polyhydroxyalkanoate gene clusters. Appl Environ Microbiol. 2004;70:5019–5025. doi: 10.1128/AEM.70.8.5019-5025.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson D.G., Glass J.I., Lartigue C., Noskov V.N., Chuang R.Y., Algire M.A. Creation of a bacterial cell controlled by a chemically synthesized genome. Science. 2010;329:52–56. doi: 10.1126/science.1190719. [DOI] [PubMed] [Google Scholar]
- Gong J., Zheng H., Wu Z., Chen T., Zhao X. Genome shuffling: progress and applications for phenotype improvement. Biotechnol Adv. 2009;27:996–1005. doi: 10.1016/j.biotechadv.2009.05.016. [DOI] [PubMed] [Google Scholar]
- GSMNDB http://202.113.12.55/GSMNDB/gsmndb.htm
- Gu Y., Hu S., Chen J., Shao L., He H., Yang Y. Ammonium acetate enhances solvent production by Clostridium acetobutylicum EA 2018 using cassava as a fermentation medium. J Ind Microbiol Biotechnol. 2009;36:1225–1232. doi: 10.1007/s10295-009-0604-1. [DOI] [PubMed] [Google Scholar]
- Hanczyc M.M., Szostak J.W. Replicating vesicles as models of primitive cell growth and division. Curr Opin Chem Biol. 2004;8:660–664. doi: 10.1016/j.cbpa.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Harms H., Wells M.C., van der Meer J.R. Whole-cell living biosensors–are they ready for environmental application? Appl Microbiol Biotechnol. 2006;70:273–280. doi: 10.1007/s00253-006-0319-4. [DOI] [PubMed] [Google Scholar]
- Haywood G.W., Anderson A.J., Ewing D.F., Dawes E.A. Accumulation of a polyhydroxyalkanoate containing primarily 3-hydroxydecanoate from simple carbohydrate substrates by Pseudomonas sp. strain NCIMB 40135. Appl Environ Microbiol. 1990;56:3354–3359. doi: 10.1128/aem.56.11.3354-3359.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Chang S., Zhang J., Zhao Q., Xiang H., Kusonmano K. MethyCancer: the database of human DNA methylation and cancer. Nucleic Acids Res. 2008;36:D836–D841. doi: 10.1093/nar/gkm730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdewijn P., Marliere P. Toward safe genetically modified organisms through the chemical diversification of nucleic acids. Chem Biodivers. 2009;6:791–808. doi: 10.1002/cbdv.200900083. [DOI] [PubMed] [Google Scholar]
- Hu B., Du J., Zou R.Y., Yuan Y.J. An environment-sensitive synthetic microbial ecosystem. PLoS One. 2010;5:e10619. doi: 10.1371/journal.pone.0010619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Hu R., Rozelle S. China's agricultural research system and reforms: challenges and implications for developing countries Asian. J Agric Dev. 2004;1:98–112. [Google Scholar]
- Huang J., Wang Q. Agricultural biotechnology development and policy in China. AgBioForum. 2002;5:122–135. [Google Scholar]
- Jian J., Li Z.J., Ye H.M., Yuan M.Q., Chen G.Q. Metabolic engineering for microbial production of polyhydroxyalkanoates consisting of high 3-hydroxyhexanoate content by recombinant Aeromonas hydrophila. Bioresour Technol. 2010;101:6096–6102. doi: 10.1016/j.biortech.2010.02.089. [DOI] [PubMed] [Google Scholar]
- Jiang G., Chen R., Yan H., Ouyang Q. A new method of preparing fiber-optic DNA biosensor and its array for gene detection. Sci China C Life Sci. 2001;44:33–39. doi: 10.1007/BF02882070. [DOI] [PubMed] [Google Scholar]
- Jin Y., Watt R.M., Danchin A., Huang J.D. Use of a riboswitch-controlled conditional hypomorphic mutation to uncover a role for the essential csrA gene in bacterial autoaggregation. J Biol Chem. 2009;284:28738–28745. doi: 10.1074/jbc.M109.028076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan P.O., Knight A.W., Billinton N., Cahill P.A., Dalrymple I.M., Hawkyard C.J. Clear and present danger? The use of a yeast biosensor to monitor changes in the toxicity of industrial effluents subjected to oxidative colour removal treatments. J Environ Monit. 2007;9:1394–1401. doi: 10.1039/b710406e. [DOI] [PubMed] [Google Scholar]
- Kung Y.T., Du Y.C., Huang W.T., Chen C.C., Ke L.T. Total synthesis of crystalline bovine insulin. Sci Sin. 1965;14:1710–1716. [PubMed] [Google Scholar]
- Lee S.Y., Choi J., Wong H.H. Recent advances in polyhydroxyalkanoate production by bacterial fermentation: mini-review. Int J Biol Macromol. 1999;25:31–36. doi: 10.1016/s0141-8130(99)00012-4. [DOI] [PubMed] [Google Scholar]
- Lee S.Y., Park J.H., Jang S.H., Nielsen L.K., Kim J., Jung K.S. Fermentative butanol production by Clostridia. Biotechnol Bioeng. 2008;101:209–228. doi: 10.1002/bit.22003. [DOI] [PubMed] [Google Scholar]
- Li S.Y., Dong C.L., Wang S.Y., Ye H.M., Chen G.Q. Microbial production of polyhydroxyalkanoate block copolymer by recombinant Pseudomonas putida. Appl Microbiol Biotechnol. 2011;90:659–669. doi: 10.1007/s00253-010-3069-2. [DOI] [PubMed] [Google Scholar]
- Li Z.J., Cai L., Wu Q., Chen G.Q. Overexpression of NAD kinase in recombinant Escherichia coli harboring the phbCAB operon improves poly(3-hydroxybutyrate) production. Appl Microbiol Biotechnol. 2009;83:939–947. doi: 10.1007/s00253-009-1943-6. [DOI] [PubMed] [Google Scholar]
- Li Z.J., Shi Z.Y., Jian J., Guo Y.Y., Wu Q., Chen G.Q. Production of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) from unrelated carbon sources by metabolically engineered Escherichia coli. Metab Eng. 2010;12:352–359. doi: 10.1016/j.ymben.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Liang H., Chen H., Fan K., Wei P., Guo X., Jin C. De novo design of a beta alpha beta motif. Angew Chem Int Ed Engl. 2009;48:3301–3303. doi: 10.1002/anie.200805476. [DOI] [PubMed] [Google Scholar]
- Liang J., Luo Y., Zhao H. Synthetic biology: putting synthesis into biology. Wiley Interdiscip Rev Syst Biol Med. 2011;3:7–20. doi: 10.1002/wsbm.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J.W., Zhang X.D., Cao X.Y., Hu J., Ji C.L. Isothermal unidirectional elongation method of gene synthesis. Yi Chuan. 2007;29:765–770. doi: 10.1360/yc-007-0765. [DOI] [PubMed] [Google Scholar]
- Liu Q., Luo G., Zhou X.R., Chen G.Q. Biosynthesis of poly(3-hydroxydecanoate) and 3-hydroxydodecanoate dominating polyhydroxyalkanoates by beta-oxidation pathway inhibited Pseudomonas putida. Metab Eng. 2011;13:11–17. doi: 10.1016/j.ymben.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Liu S., Zhu X., Liang H., Cao A., Chang Z., Lai L. Nonnatural protein–protein interaction-pair design by key residues grafting. Proc Natl Acad Sci U S A. 2007;104:5330–5335. doi: 10.1073/pnas.0606198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou C., Li Z., Ouyang Q. A molecular model for persister in E. coli. J Theor Biol. 2008;255:205–209. doi: 10.1016/j.jtbi.2008.07.035. [DOI] [PubMed] [Google Scholar]
- Louche C., Lambkin A., Oliver P. EU-China Trade and Investment Relations – Study 11 of 12: Sustainable Technologies and Services. European Commission Trade. 2007 [Google Scholar]
- Lu X. A perspective: photosynthetic production of fatty acid-based biofuels in genetically engineered cyanobacteria. Biotechnol Adv. 2010;28(6):742–746. doi: 10.1016/j.biotechadv.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Luisi P.L. Chemical aspects of synthetic biology. Chem Biodivers. 2007;4:603–621. doi: 10.1002/cbdv.200790053. [DOI] [PubMed] [Google Scholar]
- Luo J., Wang J., Ma T.M., Sun Z. Reverse engineering of bacterial chemotaxis pathway via frequency domain analysis. PLoS One. 2010;5:e9182. doi: 10.1371/journal.pone.0009182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison L.L., Huisman G.W. Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic. Microbiol Mol Biol Rev. 1999;63:21–53. doi: 10.1128/mmbr.63.1.21-53.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansy S.S., Schrum J.P., Krishnamurthy M., Tobe S., Treco D.A., Szostak J.W. Template-directed synthesis of a genetic polymer in a model protocell. Nature. 2008;454:122–125. doi: 10.1038/nature07018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansy S.S., Szostak J.W. Thermostability of model protocell membranes. Proc Natl Acad Sci U S A. 2008;105:13351–13355. doi: 10.1073/pnas.0805086105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marliere P. The farther, the safer: a manifesto for securely navigating synthetic species away from the old living world. Syst Synth Biol. 2009;3:77–84. doi: 10.1007/s11693-009-9040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minghua Z., Xiumin F., Rovetta A., Qichang H., Vicentini F., Bingkai L. Municipal solid waste management in Pudong New Area, China. Waste Manag. 2009;29:1227–1233. doi: 10.1016/j.wasman.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Ministry of Science and Technology National Key Basic Research Program (973 Program) budget proposed (translated) http://www.most.gov.cn/tztg/201012/W020101209353614372751.pdf
- Murtas G. Artificial assembly of a minimal cell. Mol Biosyst. 2009;5:1292–1297. doi: 10.1039/b906541e. [DOI] [PubMed] [Google Scholar]
- Novozymes Novozymes adds granulation capacity in Tianjin, China. http://www.novozymes.com/en/news/news-archive/Pages/Novozymes-adds-granulation-capacity-in-Tianjin.aspx
- O'Malley M.A., Powell A., Davies J.F., Calvert J. Knowledge-making distinctions in synthetic biology. Bioessays. 2008;30:57–65. doi: 10.1002/bies.20664. [DOI] [PubMed] [Google Scholar]
- Pangea Boeing and Chinese energy officials announce sustainable biofuel initiatives. http://www.worldofrenewables.com/renewables_news/bioenergy/biofuels_ethanol/boeing_and_chinese_energy_officials_announce_sustainable_biofuel.html
- Pei, L., Gaisser, S., Schmidt, M. Synthetic biology in the view of European public funding organisations Public Understanding of Science; 2011. (Electronic publication ahead of print). [DOI] [PMC free article] [PubMed]
- Qi Y., Huang Y., Liang H., Liu Z., Lai L. Folding simulations of a de novo designed protein with a betaalphabeta fold. Biophys J. 2010;98:321–329. doi: 10.1016/j.bpj.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QIBEBT http://english.qibebt.cas.cn/au/bi/
- QIBEBT Project Project List of Chinese Academy of Sciences-Qingdao Institute of Bioenergy and Bioprocess Technology. http://english.qibebt.cas.cn/rh/rps/
- Rasmussen S., Chen L., Deamer D., Krakauer D.C., Packard N.H., Stadler P.F. Evolution. Transitions from nonliving to living matter. Science. 2004;303:963–965. doi: 10.1126/science.1093669. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Mozaz S., Marco M.P., Lopez de Alda M.J., Barcelo D. Biosensors for environmental monitoring of endocrine disruptors: a review article. Anal Bioanal Chem. 2004;378:588–598. doi: 10.1007/s00216-003-2385-0. [DOI] [PubMed] [Google Scholar]
- Schmidt M. Special issue: societal aspects of synthetic biology. Syst Synth Biol. 2009;3:1–2. doi: 10.1007/s11693-009-9043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. Xenobiology: a new form of life as the ultimate biosafety tool. Bioessays. 2010;32:322–331. doi: 10.1002/bies.200900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M., Pei L. Synthetic toxicology: where engineering meets biology and toxicology. Toxicol Sci. 2011;120(Suppl. 1):S204–S224. doi: 10.1093/toxsci/kfq339. [DOI] [PubMed] [Google Scholar]
- Shanghai Institute of Life Science Annual meeting (2010) of the key laboratory of Synthetic biology. http://www.bio.cas.cn/yjjz/sjdt/201101/t20110119_3064813.html
- Shi S., Chen T., Zhang Z., Chen X., Zhao X. Transcriptome analysis guided metabolic engineering of Bacillus subtilis for riboflavin production. Metab Eng. 2009;11:243–252. doi: 10.1016/j.ymben.2009.05.002. [DOI] [PubMed] [Google Scholar]
- SKLBE about SKLBE. http://sklbe.ecust.edu.cn/English/jianjie.php
- SKLMT State Key Laboratory of Microbial Technology Developing Programming. http://www.mbtech.sdu.edu.cn/eng/plan.htm
- Sun R., Zheng H., Fang Z., Yao W. Rational design of aminoacyl-tRNA synthetase specific for p-acetyl-L-phenylalanine. Biochem Biophys Res Commun. 2010;391:709–715. doi: 10.1016/j.bbrc.2009.11.125. [DOI] [PubMed] [Google Scholar]
- Synthetic biology Project. Mapping the Emerging Synthetic Biology Landscape. http://www.synbioproject.org/library/inventories/map/
- Szostak J.W., Bartel D.P., Luisi P.L. Synthesizing life. Nature. 2001;409:387–390. doi: 10.1038/35053176. [DOI] [PubMed] [Google Scholar]
- Tian X.J., Zhang X.P., Liu F., Wang W. Interlinking positive and negative feedback loops creates a tunable motif in gene regulatory networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2009;80:011926. doi: 10.1103/PhysRevE.80.011926. [DOI] [PubMed] [Google Scholar]
- Tumpey T.M., Basler C.F., Aguilar P.V., Zeng H., Solorzano A., Swayne D.E. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science. 2005;310:77–80. doi: 10.1126/science.1119392. [DOI] [PubMed] [Google Scholar]
- Wang H.A., Qin L., Lu P., Pang Z.X., Deng Z.X., Zhao G.P. cvhA gene of Streptomyces hygroscopicus 10–22 encodes a negative regulator for mycelia development. Acta Biochim Biophys Sin (Shanghai) 2006;38:271–280. doi: 10.1111/j.1745-7270.2006.00160.x. [DOI] [PubMed] [Google Scholar]
- Wang H.H., Zhou X.R., Liu Q., Chen G.Q. Biosynthesis of polyhydroxyalkanoate homopolymers by Pseudomonas putida. Appl Microbiol Biotechnol. 2011;89:1497–1507. doi: 10.1007/s00253-010-2964-x. [DOI] [PubMed] [Google Scholar]
- Wang L., Schultz P.G. Expanding the genetic code. Angew Chem Int Ed Engl. 2004;44:34–66. doi: 10.1002/anie.200460627. [DOI] [PubMed] [Google Scholar]
- Wang M. 2005. Energy and greenhouse gas emissions impacts of fuel ethanol. NGCA Renewable Fuels Forum. [Google Scholar]
- Wang Q., Chen X., Yang Y., Zhao X. Genome-scale in silico aided metabolic analysis and flux comparisons of Escherichia coli to improve succinate production. Appl Microbiol Biotechnol. 2006;73:887–894. doi: 10.1007/s00253-006-0535-y. [DOI] [PubMed] [Google Scholar]
- Wang Q., Wu C., Chen T., Chen X., Zhao X. Expression of galactose permease and pyruvate carboxylase in Escherichia coli ptsG mutant increases the growth rate and succinate yield under anaerobic conditions. Biotechnol Lett. 2006;28:89–93. doi: 10.1007/s10529-005-4952-2. [DOI] [PubMed] [Google Scholar]
- Wang W.D., Chen Z.T., Kang B.G., Li R. Construction of an artificial intercellular communication network using the nitric oxide signaling elements in mammalian cells. Exp Cell Res. 2008;314:699–706. doi: 10.1016/j.yexcr.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Wei F., Ho C.M. Aptamer-based electrochemical biosensor for Botulinum neurotoxin. Anal Bioanal Chem. 2009;393:1943–1948. doi: 10.1007/s00216-009-2687-y. [DOI] [PubMed] [Google Scholar]
- Wei X.X., Shi Z.Y., Yuan M.Q., Chen G.Q. Effect of anaerobic promoters on the microaerobic production of polyhydroxybutyrate (PHB) in recombinant Escherichia coli. Appl Microbiol Biotechnol. 2009;82:703–712. doi: 10.1007/s00253-008-1816-4. [DOI] [PubMed] [Google Scholar]
- WSJ Novozymes teams up with Sinopec and Cofco in China demo plant. http://online.wsj.com/article/BT-CO-20100527-702156.html
- Xia F., Guo W., Mao Y., Hou X., Xue J., Xia H. Gating of single synthetic nanopores by proton-driven DNA molecular motors. J Am Chem Soc. 2008;130:8345–8350. doi: 10.1021/ja800266p. [DOI] [PubMed] [Google Scholar]
- Xiang S.H., Li J., Yin H., Zheng J.T., Yang X., Wang H.B. Application of a double-reporter-guided mutant selection method to improve clavulanic acid production in Streptomyces clavuligerus. Metab Eng. 2009;11:310–318. doi: 10.1016/j.ymben.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Xie J., Wang L., Wu N., Brock A., Spraggon G., Schultz P.G. The site-specific incorporation of p-iodo-L-phenylalanine into proteins for structure determination. Nat Biotechnol. 2004;22:1297–1301. doi: 10.1038/nbt1013. [DOI] [PubMed] [Google Scholar]
- Xinhua Water, air pollution in China still serious. http://www.chinadaily.com.cn/china/2009-02/24/content_7508856.htm
- Xiong A.S., Peng R.H., Zhuang J., Liu J.G., Gao F., Xu F. A semi-rational design strategy of directed evolution combined with chemical synthesis of DNA sequences. Biol Chem. 2007;388:1291–1300. doi: 10.1515/BC.2007.153. [DOI] [PubMed] [Google Scholar]
- Xiong A.S., Peng R.H., Zhuang J., Liu J.G., Xu F., Cai B. Directed evolution of beta-galactosidase from Escherichia coli into beta-glucuronidase. J Biochem Mol Biol. 2007;40:419–425. doi: 10.5483/bmbrep.2007.40.3.419. [DOI] [PubMed] [Google Scholar]
- Xiong A.S., Yao Q.H., Peng R.H., Cheng Z.M. Directed in vitro evolution of reporter genes based on semi-rational design and high-throughput screening. Methods Mol Biol. 2010;634:239–256. doi: 10.1007/978-1-60761-652-8_18. [DOI] [PubMed] [Google Scholar]
- Xiong A.S., Yao Q.H., Peng R.H., Duan H., Li X., Fan H.Q. PCR-based accurate synthesis of long DNA sequences. Nat Protoc. 2006;1:791–797. doi: 10.1038/nprot.2006.103. [DOI] [PubMed] [Google Scholar]
- Xiong A.S., Yao Q.H., Peng R.H., Li X., Fan H.Q., Cheng Z.M. A simple, rapid, high-fidelity and cost-effective PCR-based two-step DNA synthesis method for long gene sequences. Nucleic Acids Res. 2004;32:e98. doi: 10.1093/nar/gnh094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Sigworth F.J., LaVan D.A. Synthetic protocells to mimic and test cell function. Adv Mater. 2010;22:120–127. doi: 10.1002/adma.200901945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Freudl R., Qiao C., Mulchandani A. Cotranslocation of methyl parathion hydrolase to the periplasm and of organophosphorus hydrolase to the cell surface of Escherichia coli by the Tat pathway and ice nucleation protein display system. Appl Environ Microbiol. 2010;76:434–440. doi: 10.1128/AEM.02162-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Jiang W., Yang S. mazF as a counter-selectable marker for unmarked genetic modification of Pichia pastoris. FEMS Yeast Res. 2009;9:600–609. doi: 10.1111/j.1567-1364.2009.00503.x. [DOI] [PubMed] [Google Scholar]
- Yang Z., Hutter D., Sheng P., Sismour A.M., Benner S.A. Artificially expanded genetic information system: a new base pair with an alternative hydrogen bonding pattern. Nucleic Acids Res. 2006;34:6095–6101. doi: 10.1093/nar/gkl633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Sismour A.M., Sheng P., Puskar N.L., Benner S.A. Enzymatic incorporation of a third nucleobase pair. Nucleic Acids Res. 2007;35:4238–4249. doi: 10.1093/nar/gkm395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim K.S., Lee S.Y., Chang H.N. Synthesis of poly-(3-hydroxybutyrate-co-3-hydroxyvalerate) by recombinant Escherichia coli. Biotechnol Bioeng. 1996;49:495–503. doi: 10.1002/(SICI)1097-0290(19960305)49:5<495::AID-BIT2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Yu B., Ma C., Zhou W., Zhu S., Wang Y., Qu J. Simultaneous biodetoxification of S, N, and O pollutants by engineering of a carbazole-degrading gene cassette in a recombinant biocatalyst. Appl Environ Microbiol. 2006;72:7373–7376. doi: 10.1128/AEM.01374-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Yao Y., Liu Y., Jiao R., Jiang W., Zhao G.P. A complex role of Amycolatopsis mediterranei GlnR in nitrogen metabolism and related antibiotics production. Arch Microbiol. 2007;188:89–96. doi: 10.1007/s00203-007-0228-7. [DOI] [PubMed] [Google Scholar]
- Zepik H.H., Walde P. Achievements and challenges in generating protocell models. Chembiochem. 2008;9:2771–2772. doi: 10.1002/cbic.200800557. [DOI] [PubMed] [Google Scholar]
- Zepik H.H., Walde P., Kostoryz E.L., Code J., Yourtee D.M. Lipid vesicles as membrane models for toxicological assessment of xenobiotics. Crit Rev Toxicol. 2008;38:1–11. doi: 10.1080/10408440701524519. [DOI] [PubMed] [Google Scholar]
- Zhang C.T., Zhang R. Gene essentiality analysis based on DEG, a database of essential genes. Methods Mol Biol. 2008;416:391–400. doi: 10.1007/978-1-59745-321-9_27. [DOI] [PubMed] [Google Scholar]
- Zhang L., Ou X., Zhao G., Ding X. Highly efficient in vitro site-specific recombination system based on streptomyces phage phiBT1 integrase. J Bacteriol. 2008;190:6392–6397. doi: 10.1128/JB.00777-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Lin Y. DEG 5.0, a database of essential genes in both prokaryotes and eukaryotes. Nucleic Acids Res. 2009;37:D455–D458. doi: 10.1093/nar/gkn858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Ou H.Y., Zhang C.T. DEG: a database of essential genes. Nucleic Acids Res. 2004;32:D271–D272. doi: 10.1093/nar/gkh024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Zeng A., Fang P., Qin Z. Characterization of replication and conjugation of Streptomyces circular plasmids pFP1 and pFP11 and their ability to propagate in linear mode with artificially attached telomeres. Appl Environ Microbiol. 2008;74:3368–3376. doi: 10.1128/AEM.00402-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H., Gong J., Chen T., Chen X., Zhao X. Strain improvement of Sporolactobacillus inulinus ATCC 15538 for acid tolerance and production of D-lactic acid by genome shuffling. Appl Microbiol Biotechnol. 2010;85:1541–1549. doi: 10.1007/s00253-009-2243-x. [DOI] [PubMed] [Google Scholar]
- Zhu J., He F., Wang J., Yu J. Modeling transcriptome based on transcript-sampling data. PLoS One. 2008;3:e1659. doi: 10.1371/journal.pone.0001659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Chang S., Fang K., Cui S., Liu J., Wu Z. MyBASE: a database for genome polymorphism and gene function studies of Mycobacterium. BMC Microbiol. 2009;9:40. doi: 10.1186/1471-2180-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Chen X., Chen T., Shi S., Zhao X. Over-expression of glucose dehydrogenase improves cell growth and riboflavin production in Bacillus subtilis. Biotechnol Lett. 2006;28:1667–1672. doi: 10.1007/s10529-006-9143-2. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Chen X., Chen T., Zhao X. Enhancement of riboflavin production by overexpression of acetolactate synthase in a pta mutant of Bacillus subtilis. FEMS Microbiol Lett. 2007;266:224–230. doi: 10.1111/j.1574-6968.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- Zou L.K., Wang H.N., Pan X., Xie T., Wu Q., Xie Z.W. Design and expression of a synthetic phyC gene encoding the neutral phytase in Pichia pastoris. Acta Biochim Biophys Sin (Shanghai) 2006;38:803–811. doi: 10.1111/j.1745-7270.2006.00231.x. [DOI] [PubMed] [Google Scholar]
- Zuo P., Yin B.C., Ye B.C. DNAzyme-based microarray for highly sensitive determination of metal ions. Biosens Bioelectron. 2009;25:935–939. doi: 10.1016/j.bios.2009.08.024. [DOI] [PubMed] [Google Scholar]