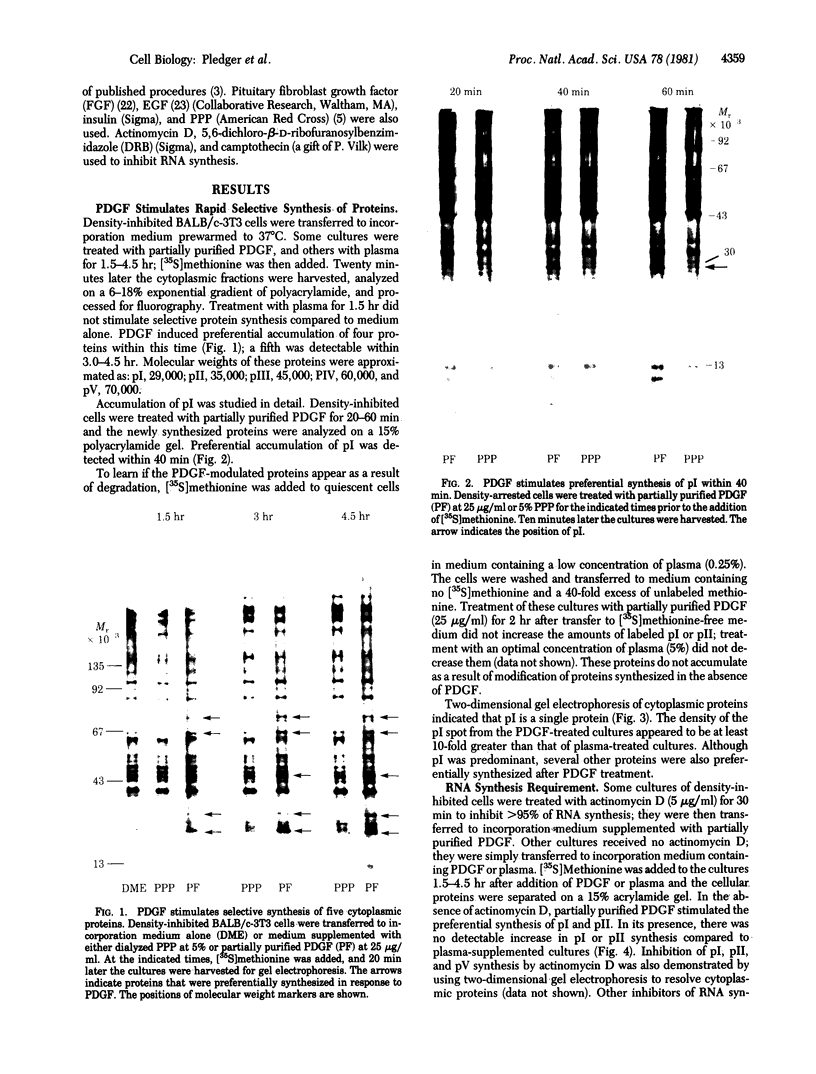
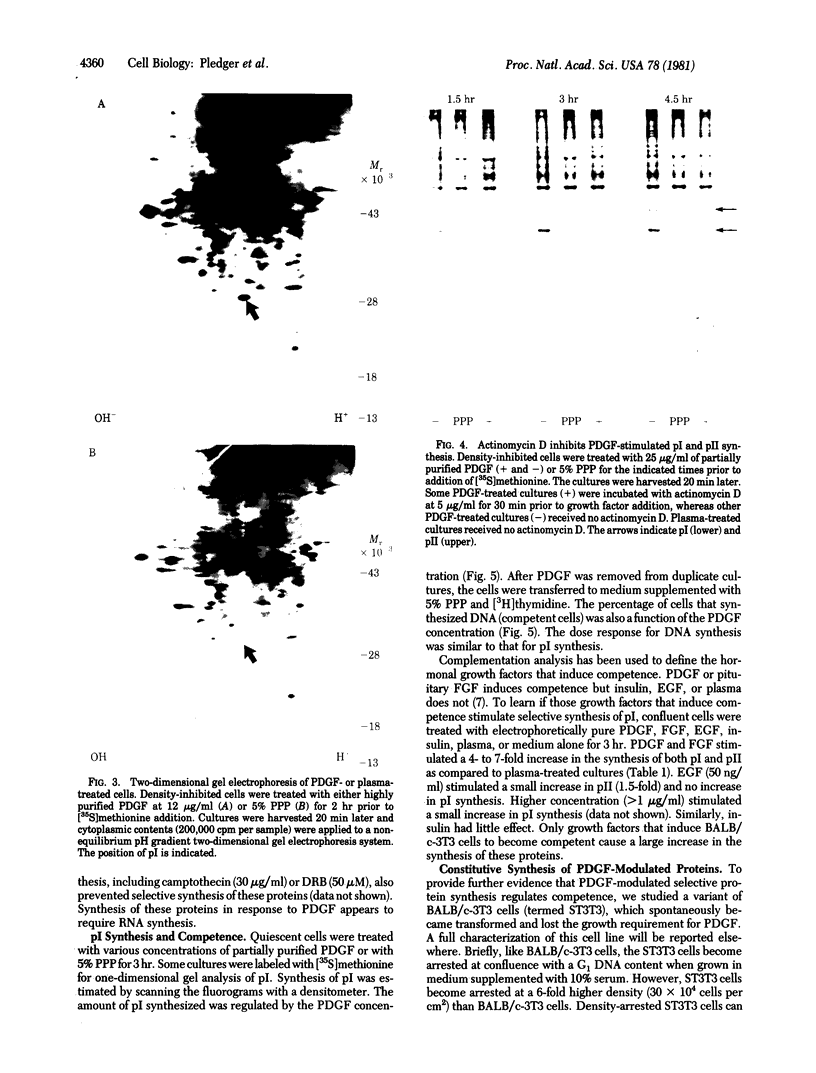
Abstract
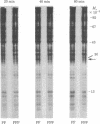
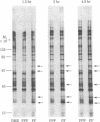
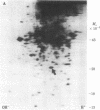
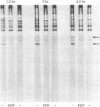
Platelet-derived growth factor (PDGF) initiates replication of density-arrested BALB/c-3T3 mouse cells by rendering them "competent" to respond to factors contained in plasma. Treatment of quiescent cells with PDGF rapidly stimulates the preferential synthesis of several cytoplasmic proteins (molecular weights 29,000 to 70,000). Four of these proteins were noted within 1.5 hr of PDGF addition and one (pI) within 40 min. Inhibitors of RNA synthesis prevented the synthesis of these proteins. Both the synthesis of pI and the stimulation of DNA synthesis displayed a similar dose response to PDGF concentration. Pituitary fibroblast growth factor, which also induces competence, stimulated pI and pII synthesis. Plasma, epidermal growth factor, or insulin, which do not induce competence, did not stimulate selective synthesis of these proteins. A transformed variant of BALB/c-3T3 cells, which has retained the growth requirement for plasma factors but lost the requirement for PDGF, synthesizes these PDGF-modulated proteins constitutively.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Scher C. D., Stiles C. D. Purification of human platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1809–1813. doi: 10.1073/pnas.76.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Benecke B. J., Ben-Ze'ev A., Penman S. The control of mRNA production, translation and turnover in suspended and reattached anchorage-dependent fibroblasts. Cell. 1978 Aug;14(4):931–939. doi: 10.1016/0092-8674(78)90347-1. [DOI] [PubMed] [Google Scholar]
- COHEN S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem. 1962 May;237:1555–1562. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons D. R., Van Wyk J. J., Pledger W. J. Sequential addition of platelet factor and plasma to BALB/c 3T3 fibroblast cultures stimulates somatomedin-C binding early in cell cycle. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6644–6648. doi: 10.1073/pnas.77.11.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates B. J., Friedkin M. Mid-G1 marker protein(s) in 3T3 mouse fibroblast cells. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4959–4961. doi: 10.1073/pnas.75.10.4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D. Localisation of a fibroblast growth factor and its effect alone and with hydrocortisone on 3T3 cell growth. Nature. 1974 May 10;249(453):123–127. doi: 10.1038/249123a0. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Wasteson A., Westermark B. Growth of normal human glial cells in a defined medium containing platelet-derived growth factor. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6611–6615. doi: 10.1073/pnas.77.11.6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Platelet-derived growth factor: purification and partial characterization. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3722–3726. doi: 10.1073/pnas.76.8.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. K., Baxter J. D., Vlodavsky I., Gospodarowicz D. Epidermal growth factor and expression of specific genes: effects on cultured rat pituitary cells are dissociable from the mitogenic response. Proc Natl Acad Sci U S A. 1980 Jan;77(1):394–398. doi: 10.1073/pnas.77.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Protein synthesis: its control in erythropoiesis. Science. 1972 Mar 3;175(4025):955–961. doi: 10.1126/science.175.4025.955. [DOI] [PubMed] [Google Scholar]
- Melero J. A., Fincham V. Enhancement of the synthesis of specific cellular polypeptides in a temperature-sensitive Chinese hamster cell line (K12) defective for entry into S phase. J Cell Physiol. 1978 Jun;95(3):295–306. doi: 10.1002/jcp.1040950307. [DOI] [PubMed] [Google Scholar]
- Mendoza S. A., Wigglesworth N. M., Pohjanpelto P., Rozengurt E. Na entry and Na-K pump activity in murine, hamster, and human cells--effect of monensin, serum, platelet extract, and viral transformation. J Cell Physiol. 1980 Apr;103(1):17–27. doi: 10.1002/jcp.1041030104. [DOI] [PubMed] [Google Scholar]
- Nilsen-Hamilton M., Shapiro J. M., Massoglia S. L., Hamilton R. T. Selective stimulation by mitogens of incorporation of 35S-methionine into a family of proteins released into the medium by 3T3 cells. Cell. 1980 May;20(1):19–28. doi: 10.1016/0092-8674(80)90230-5. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E., LaTorre J. Synthesis and turnover of nuclear and cytoplasmic polyadenylic acid in mouse L cells. J Mol Biol. 1974 Jan 25;82(3):315–331. doi: 10.1016/0022-2836(74)90593-2. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. An ordered sequence of events is required before BALB/c-3T3 cells become committed to DNA synthesis. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2839–2843. doi: 10.1073/pnas.75.6.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4481–4485. doi: 10.1073/pnas.74.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle V. G., Dubrow R., Pardee A. B. Changes in the synthesis of actin and other cell proteins after stimulation of serum-arrested cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1298–1302. doi: 10.1073/pnas.76.3.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle V. G., Pardee A. B. Quiescent cells but not cycling cells exhibit enhanced actin synthesis before they synthesize DNA. J Cell Physiol. 1980 Apr;103(1):11–15. doi: 10.1002/jcp.1041030103. [DOI] [PubMed] [Google Scholar]
- Ross R., Vogel A. The platelet-derived growth factor. Cell. 1978 Jun;14(2):203–210. doi: 10.1016/0092-8674(78)90107-1. [DOI] [PubMed] [Google Scholar]
- Rudert W. A., Graves M. W., Chadwick D. E., Lieberman I. Two candidate G1 polypeptides in chick embryo fibroblasts. J Cell Physiol. 1980 Jul;104(1):73–81. doi: 10.1002/jcp.1041040111. [DOI] [PubMed] [Google Scholar]
- Rudland P. S., Seifert W., Gospodarowicz D. Growth control in cultured mouse fibroblasts: induction of the pleiotypic and mitogenic responses by a purified growth factor. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2600–2604. doi: 10.1073/pnas.71.7.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford R. B., Ross R. Platelet factors stimulate fibroblasts and smooth muscle cells quiescent in plasma serum to proliferate. J Cell Biol. 1976 Apr;69(1):196–203. doi: 10.1083/jcb.69.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs L. Constitutive uncoupling of pathways of gene expression that control growth and differentiation in myeloid leukemia: a model for the origin and progression of malignancy. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6152–6156. doi: 10.1073/pnas.77.10.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D., Shepard R. C., Antoniades H. N., Stiles C. D. Platelet-derived growth factor and the regulation of the mammalian fibroblast cell cycle. Biochim Biophys Acta. 1979 Aug 10;560(2):217–241. doi: 10.1016/0304-419x(79)90020-9. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Willingham M. C., Pastan I. Direct visualization of binding, aggregation, and internalization of insulin and epidermal growth factor on living fibroblastic cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2659–2663. doi: 10.1073/pnas.75.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter Y., Hernaez L., Cuatrecasas P. Epidermal growth factor: biological activity requires persistent occupation of high-affinity cell surface receptors. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5788–5791. doi: 10.1073/pnas.75.12.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. C., Stiles C. D. Cytoplasmic transfer of the mitogenic response to platelet-derived growth factor. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4363–4367. doi: 10.1073/pnas.78.7.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles C. D., Isberg R. R., Pledger W. J., Antoniades H. N., Scher C. D. Control of the Balb/c-3T3 cell cycle by nutrients and serum factors: analysis using platelet-derived growth factor and platelet-poor plasma. J Cell Physiol. 1979 Jun;99(3):395–405. doi: 10.1002/jcp.1040990314. [DOI] [PubMed] [Google Scholar]
- Tucker R. W., Scher C. D., Stiles C. D. Centriole deciliation associated with the early response of 3T3 cells to growth factors but not to SV40. Cell. 1979 Dec;18(4):1065–1072. doi: 10.1016/0092-8674(79)90219-8. [DOI] [PubMed] [Google Scholar]
- Wrann M., Fox C. F., Ross R. Modulation of epidermal growth factor receptors on 3T3 cells by platelet-derived growth factor. Science. 1980 Dec 19;210(4476):1363–1365. doi: 10.1126/science.6254158. [DOI] [PubMed] [Google Scholar]